Abstract
Background
Hippocampal volume (HV) decline is an important marker of psychosis and has been associated with hypothalamus–pituitary–adrenal (HPA) axis dysregulation in various disorders. Given recent findings of sex differences in HPA axis function in psychosis, the current study investigated differences in HV in male and female first episode psychosis (FEP) patients and controls and the interaction of HV with the cortisol awakening response (CAR) and symptoms.
Methods
Fifty-eight patients with a diagnosis of FEP (39 men, 19 women) and 27 healthy community controls (15 men, 12 women) underwent structural magnetic resonance imaging (MRI) on a 1.5 T scanner. Hippocampal volume was determined using previously established segmentation protocols. Saliva samples for cortisol assessment were collected at 0, 30 and 60 min after awakening. Psychotic symptoms were assessed with the Scale for Assessment of Positive Symptoms (SAPS), the Scale for Assessment of Negative Symptoms (SANS) and the Global Assessment of Functioning (GAF) scale.
Results
Male patients had significantly smaller left and right HVs compared to male controls, which appeared to be secondary to global brain volume differences. However, even when controlling for overall brain size, male patients showed smaller HV compared to female patients. The CAR was significantly lower in male patients compared to male controls and female patients. Only in male patients, smaller left HV was significantly associated with a blunted CAR, and smaller HV bilaterally was related to positive psychotic symptoms and lower levels of functioning.
Conclusions
We propose that reduced hippocampal volume and an attenuated cortisol awakening response are related markers of increased stress vulnerability in male psychosis patients and that both contribute to the unfavorable clinical picture in men.
Keywords: First episode psychosis, Hippocampus, Hypothalamus–pituitary–adrenal axis, Cortisol awakening response, Sex differences
Highlights
-
•
We examined sex differences in neurobiological markers of stress in psychosis.
-
•
Hippocampal volume and cortisol levels to awakening are reduced in male patients.
-
•
Male first episode psychosis patients show markers of high stress vulnerability.
-
•
Neurobiological deficits relate to poor outcome in male but not female patients.
-
•
The neural-diathesis stress model of schizophrenia is particularly valid for men.
1. Introduction
Reduced hippocampal volume (HV) is a common finding in psychotic disorders (Adriano et al., 2012; Nelson et al., 1998; Pantelis et al., 2003; Steen et al., 2006; Velakoulis et al., 2006). It is apparent early in the course of psychosis, and further progression of structural abnormalities is observed as the illness evolves (Pantelis et al., 2003; Steen et al., 2006). Hippocampal volume reduction has been implicated in various aspects of the pathophysiology of psychosis including symptom severity (Bodnar et al., 2010; Watson et al., 2012), cognitive function and insight (Buchy et al., 2010; Harrison, 2004).
Smaller HV has been associated with abnormal regulation of the hypothalamus–pituitary–adrenal (HPA) axis in various neuropsychiatric conditions (Sapolsky, 2000). A dysregulation of HPA axis function is increasingly observed in psychosis, characterized by diurnal hyperactivity and a blunted cortisol response to awakening and to acute stress (Borges et al., 2013; Mondelli et al., 2010b; Pruessner et al., 2008; Pruessner et al., 2013; Ritsner et al., 2007; Ryan et al., 2004; van Venrooij et al., 2012). Still, very few researchers have investigated the relationship between HPA axis function and HV in psychosis. A recent study reported an association between higher diurnal cortisol levels and smaller HV in first episode psychosis (FEP) patients (Mondelli et al., 2010b), but a previous study had not found such a relationship (Gunduz-Bruce et al., 2007).
Research in patients with hippocampal damage suggests that reduced hippocampal integrity specifically compromises the cortisol awakening response (CAR), with diurnal cortisol secretion remaining intact (Buchanan et al., 2004; Wolf et al., 2005). Our research group has recently demonstrated a blunted CAR particularly in male FEP (Pruessner et al., 2008; Pruessner et al., 2013), and we had argued that this finding might be related to hippocampal and other gray matter abnormalities. Indeed, male psychosis patients show significantly greater structural brain abnormalities compared to female patients, such as larger ventricles (Nopoulos et al., 1997), smaller frontal lobes (Bora et al., 2012), smaller medial temporal lobe volumes (Gur et al., 2000) and reduced hippocampal volume (Adriano et al., 2012; Bogerts et al., 1990a; Bora et al., 2012; Bryant et al., 1999; Exner et al., 2008; Irle et al., 2011).
The present study was designed to assess sex differences in hippocampal volume and their relationship with the cortisol awakening response and symptoms of psychosis. We hypothesized that reduced hippocampal volume will be associated with an attenuated cortisol awakening response and that both these measures will be related to symptom severity particularly in male patients.
2. Material and methods
2.1. Subjects
Fifty-eight patients with a first episode of psychosis (39 men, 19 women) were recruited from the Prevention and Early Intervention Program for Psychosis (PEPP) (Malla et al., 2003) at the Douglas Mental Health University Institute in Montreal. All patients were within the first 2 years of treatment and follow-up for a first episode of psychosis, had less than 30 days of exposure to antipsychotic medication prior to admission, and were recruited to the present study when they were deemed clinically stable to participate. Since patients usually stabilize within 3 months of admission, most were recruited within the first 6 months, assuring limited exposure to antipsychotic medications. Overlap of patients with our previous report (Pruessner et al., 2013) was 62%. Twenty-seven healthy community controls (15 men, 12 women) were recruited through advertisements in local free newspapers. Control subjects were screened with a telephone interview followed by a diagnostic interview with the Structured Clinical Interview for DSM IV, non-patient edition (SCID-NP) (First et al., 2002) to rule out a diagnosis of a mental disorder in the subjects themselves or in their first degree relatives, as well as use of psychotropic or other medication that could affect HPA axis functioning. All study procedures were approved by the McGill Institutional Review Board, and participants provided written informed consent prior to participation in the study.
2.2. Hippocampal volume assessment
All participants underwent structural high-resolution (isotropic 1 mm) MRI on a Siemens 1.5 T scanner. Hippocampal volume was determined using an appearance model-based automatic segmentation method with patch based local refinement (Hu et al., 2011) and was quality-controlled by a validated rater employing our manual segmentation protocol for this structure (Pruessner et al., 2000). As a measure of total brain volume differences, the individual scaling factor used to transform native into normalized brain volumes based on the MNI 152 template (Mazziotta et al., 1995) was employed.
2.3. The cortisol awakening response
All participants received oral and written instructions for saliva sampling with the Salivette© sampling device (Sarstedt, Quebec City, Canada) at 0, 30 and 60 min after awakening. Participants were instructed not to eat or drink before and during the sampling time and to refrain from brushing their teeth. Samples were stored in a −20°C freezer until analysis. Cortisol was analyzed using a time-resolved immunoassay with fluorescence detection (Dressendorfer et al., 1992). Intra- and inter- assay coefficients of variation were smaller than 10% and 12%, respectively.
2.4. Symptom assessment
Psychotic symptoms were assessed with the Scale for Assessment of Positive Symptoms (SAPS) (Andreasen, 1984) and the Scale for Assessment of Negative Symptoms (SANS) (Andreasen, 1983). Attention items were excluded for the SANS. Functioning was assessed with the Global Assessment of Functioning (GAF) scale (Luborsky, 1962), and depression was assessed with the Calgary Depression Scale (CDS) (Addington et al., 1990).
2.5. Statistical analyses
Differences in all demographic and biological measures were assessed first between the patient and control group as a whole, then in the male and female subgroups, and finally between male and female patients. Clinical and treatment related variables were compared in male and female patients. T-tests were employed for normally distributed data, Mann–Whitney U-tests for skewed data and Chi-Square tests for binary data. For biological variables, univariate ANOVAs were employed to assess group (patients, controls) differences stratified by sex in total brain and left and right hippocampal volume. Repeated measures (0, 30, 60 min) ANOVAs were used to determine group and sex differences in the CAR. These analyses were repeated with ANCOVAs controlling for potential confounders. Both native HV and HV adjusted for total brain size were employed as dependent variables to demonstrate actual volume differences and the impact of global brain volume. In order to assess sex differences in the patient and control groups, we conducted ANCOVAs with hippocampal volume corrected for total brain size as dependent variable, controlling for medication dose and other relevant confounders. Chlorpromazine equivalents (CPZEs) of individual medication dosages were calculated according to Bezchlibnik-Butler and Jeffries (2006). Paired t-tests were used to assess hemisphere differences in HV. In order to obtain a single value for correlational analyses including the CAR, we calculated the area under the curve with respect to ground (Pruessner et al., 2003). Spearman correlations were utilized to assess associations between HV, scaling factor, CAR, and symptoms. Demographic and treatment related variables were included as covariates in partial correlations where applicable.
3. Results
3.1. Demographic and clinical characteristics
Patients were younger than controls at trend level, reported higher rates of cannabis use and cigarette smoking and were more likely to be single. Table 1 provides details on demographic variables in male and female patients and controls. No significant differences between male and female patients were observed in the ratio of non-affective versus affective psychosis, duration of untreated psychosis, duration of untreated illness, positive and negative symptom severity, global functioning and treatment with antipsychotic medication. Table 2 provides details on these patient characteristics for men and women. Higher medication dose was significantly related to impairment in global functioning and more severe negative symptoms in male (rho = −.49; p = .006 and rho = .59; p = .005; respectively) but not female patients (rho = .49; p = .087 and p > .75; respectively). In male patients, higher medication dose was also related to smaller right hippocampal volume (rho = −.38; p = .037). No such relationship with medication dose was observed for left HV (p > .12), total brain volume (p > .66) or the CAR (p > .75). No association between biological variables and medication dose was observed in female patients (all p > .55). Age, cannabis use, cigarette smoking and relationship status were included as covariates in subsequent analyses comparing patients and controls where applicable. Medication dose was included as covariate when comparing male and female patients.
Table 1.
Group and sex differences in socio-demographic variables and smoking.
| Total group | Patients (N = 58) | Controls (N = 27) | Statistic | p-Value |
|---|---|---|---|---|
| Age, N (SD) | 23.87 (3.71) | 22.26 (3.61) | t = 1.88 | .064 |
| Sex, male, N (%) | 39 (67.2) | 15 (55.6) | χ2 = 1.09 | .297 |
| Education > high school, N (%) | 28 (48.3) | 18 (66.7) | χ2 = 2.51 | .113 |
| Ethnicity, white, N (%) | 45 (77.6) | 21 (77.8) | χ2 = 0.00 | .984 |
| Relationship status, single, N (%) | 54 (93.1) | 19 (70.4) | χ2 = 7.85 | .005 |
| Cannabis use past 3 months, N (%) | 25 (43.1) | 5 (18.5) | χ2 = 4.88 | .027 |
| Tobacco smoking, >5 cigarettes/day, N (%) | 32 (55.2) | 5 (18.5) | χ2 = 10.1 | .002 |
| Patients only | Men (N = 39) | Women (N = 19) | Statistic | p-Value |
| Age, N (SD) | 23.79 (3.69) | 24.04 (3.83) | t = –.24 | .812 |
| Education > high school, N (%) | 17 (43.6) | 11 (57.9) | χ2 = 1.05 | .306 |
| Ethnicity, white, N (%) | 30 (76.9) | 15 (78.9) | χ2 = .030 | .862 |
| Relationship status, single, N (%) | 37 (94.9) | 17 (89.5) | χ2 = .580 | .446 |
| Cannabis use past 3 months, N (%) | 18 (46.2) | 7 (36.8) | χ2 = .452 | .502 |
| Tobacco smoking, >5 cigarettes/day, N (%) | 23 (59.0) | 9 (47.4) | χ2 = .696 | .404 |
| Men only | Patients (N = 39) | Controls (N = 15) | Statistic | p-Value |
| Age, N (SD) | 23.79 (3.69) | 21.60 (3.62) | t = 1.96 | .055 |
| Education > high school, N (%) | 17 (43.6) | 10 (66.7) | χ2 = 2.31 | .129 |
| Ethnicity, white, N (%) | 30 (76.9) | 11 (73.3) | χ2 = 0.08 | .782 |
| Relationship status, single, N (%) | 37 (94.9) | 13 (86.7) | χ2 = 1.06 | .302 |
| Cannabis use past 3 months, N (%) | 18 (46.2) | 3 (20.0) | χ2 = 3.12 | .077 |
| Tobacco smoking, >5 cigarettes/day, N (%) | 23 (59.0) | 3 (20.0) | χ2 = 6.59 | .010 |
| Women only | Patients (N = 19) | Controls (N = 12) | Statistic | p-Value |
| Age, N (SD) | 24.04 (3.83) | 23.08 (3.58) | t = 0.69 | .494 |
| Education > high school, N (%) | 11 (57.9) | 8 (66.7) | χ2 = 0.24 | .625 |
| Ethnicity, white, N (%) | 15 (78.9) | 10 (83.3) | χ2 = 0.09 | .763 |
| Relationship status, single, N (%) | 17 (89.5) | 6 (50.0) | χ2 = 5.98 | .014 |
| Cannabis use past 3 months, N (%) | 7 (36.8) | 2 (16.7) | χ2 = 1.45 | .228 |
| Tobacco smoking, >5 cigarettes/day, N (%) | 9 (47.4) | 2 (16.7) | χ2 = 3.03 | .082 |
Table 2.
Patient characteristics: Diagnoses and treatment.
| Male (N = 39) | Female (N = 19) | Statistic | p-Value | |
|---|---|---|---|---|
| Non-affective psychosisa, N (%) | 29 (74.4) | 13 (68.4) | χ2 = 0.23 | .635 |
| Duration of untreated illness (DUI), weeks (median) | 211.1 | 282.0 | Z = –1.64 | .101 |
| Duration of untreated psychosis (DUP), weeks (median) | 17.14 | 15.71 | Z = –0.52 | .603 |
| Symptom ratings: | ||||
| Positive symptoms (SAPS), mean (SE) | 11.48 (2.25) | 7.94 (2.08) | T(56) = 1.00 | .322 |
| Negative symptoms (SANS), mean (SE) | 22.69 (2.09) | 18.56 (2.23) | T(56) = 1.34 | .185 |
| Global assessment of functioning (GAF), mean (SE) | 48.69 (3.11) | 56.32 (4.01) | T(56) = –1.45 | .153 |
| Depression (CDS), mean (SE) | 3.72 (0.82) | 4.94 (1.61) | T(55) = –0.75 | .455 |
| Time treated with antipsychotics, weeks (median) | 16.71 | 17.26 | Z = –0.42 | .673 |
| Dosage of antipsychotic medication (CPZE)b, mean (SD) | 180.8 (159.6) | 141.5 (112.1) | T(41) = 0.80 | .427 |
| Antipsychotic medication prescribed, N (%) | ||||
| Olanzapine | 12 (30.8) | 7 (35.9) | ||
| Risperidone (oral) | 10 (25.6) | 4 (21.1.) | ||
| Risperidone (injectable)b | 7 (18.0) | 1 (5.3) | ||
| Quetiapine | 4 (10.3) | 1 (5.3) | ||
| Aripiprazoleb | 1 (2.6) | 3 (15.8) | ||
| Paliperidoneb | 1 (2.6) | 2 (10.5) | ||
| Clozapine | 1 (2.6) | 0 (0.0) | ||
| Ziprasidone | 1 (2.6) | 0 (0.0) | ||
| No antipsychotic medication | 2 (5.1) | 1 (5.3) |
According to the SCID, as apposed to affective psychosis.
Chlorpromazine equivalent doses; could not be calculated for patients treated with paliperidone, aripiprazole and long-acting injectable risperidone.
3.2. Group and sex differences in biological measures
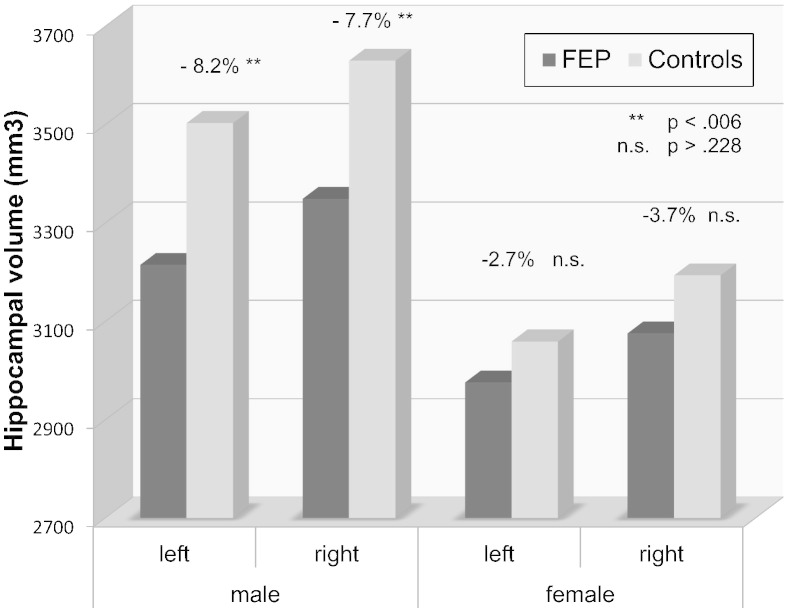
Univariate ANOVAs revealed that left and right hippocampal volume were significantly smaller in patients compared to controls. When stratifying the groups by sex, the differences between patients and controls were only significant in men (F(1) = 8.88; p = .004 and F(1) = 8.26; p = .006, respectively; Fig.1). Global brain volume was also smaller in male patients compared to male controls. Those group differences were still significant when ANCOVAs were conducted which included age, cannabis use, cigarette smoking and relationship status as covariates (see Table 3 for details). When repeating these analyses with hippocampal volumes corrected for total brain size, differences between patients and controls were not significant anymore (all p > .40), suggesting that the observed volume differences in the hippocampus were secondary to global brain volume differences.
Fig. 1.
Hippocampal volume in male and female FEP patients and controls.
Table 3.
Hippocampal volume and cortisol awakening response in male and female patients and controls.
| Total group | FEP (N = 58) | Controls (N = 27) | ANOVA (F/p-value) | ANCOVA (F/p-value)e |
|---|---|---|---|---|
| Left HV, mean (SD)a | 3136.5 (323.1) | 3305.9 (343.9) | 4.86/.030 | 3.99/.049 |
| Right HV, mean (SD)a | 3259.6 (343.9) | 3435.9 (327.8) | 4.98/.028 | 5.71/.019 |
| Scaling factor (SD)b | 1.249 (0.130) | 1.202 (0.137) | 2.30/.133 | 4.86/.030 |
| CAR AUCg, mean (SD)c | 693.0 (398.4) | 932.6 (341.4) | 8.03/.006 | 8.02/.006 |
| Time of awakening, mean (SD)d | 8:51 a.m. (1:53) | 8:23 a.m. (1:31) | 1.31/.255 | 1.26/.272 |
| Men | FEP (N = 39) | Controls (N = 15) | ANOVA (F/p-value) | ANCOVA (F/p-value)e |
| Left HV, mean (SD)a | 3214.7 (336.9) | 3503.0 (261.6) | 8.88/.004 | 4.09/.049 |
| Right HV, mean (SD)a | 3349.2 (351.5) | 3629.9 (219.3) | 8.26/.006 | 4.71/.035 |
| Scaling factorb | 1.194 (0.095) | 1.109 (0.036) | 10.23/.002 | 7.18/.010 |
| CAR AUCg, mean (SD)c | 620.1 (368.6) | 861.3 (298.1) | 5.65/.021 | 6.09/.021 |
| Time of awakening, mean (SD)d | 9:01 a.m. (2:01) | 8:13 (1:33) | 1.97/.166 | 2.31/.135 |
| Women | FEP (N = 19) | Controls (N = 12) | ANOVA (F/p-value) | ANCOVA (F/p-value)e |
| Left HV, mean (SD)a | 2975.9 (224.8) | 3059.5 (270.7) | 0.87/.359 | 1.87/.184 |
| Right HV, mean (SD)a | 3075.7 (246.6) | 3193.3 (277.6) | 1.52/.228 | 5.41/.028 |
| Scaling factorb | 1.363 (0.119) | 1.319 (0.111) | 1.03/.319 | 2.53/.124 |
| CAR AUCg, mean (SD)c | 842.7 (425.1) | 1021.7 (383.2) | 1.62/.214 | 1.67/.208 |
| Time of awakening, mean (SD)d | 8:31 a.m. (1:36) | 8:36 (1:30) | 0.02/ .898 | 2.64/.612 |
Hippocampal volume, native space.
Transformation factor from native into standard space, indicates total brain volume differences.
Cortisol awakening response (area under the curve with respect to ground).
At the time of cortisol assessment.
Controlling for age, cigarette smoking, cannabis use and relationship status.
In order to compare the difference in hippocampal volume in male and female patients, we conducted ANCOVAs within the patient group with sex (male, female) as independent variable and hippocampal volume corrected for total brain size as dependent variable while controlling for medication dose, age, cannabis use, smoking and relationship status. Male patients showed significantly smaller left and right hippocampal volumes compared to female patients (F(1) = 19.14; p < .001 and F(1) = 16.84; p < .001, respectively). In controls, relative left and right volume of the hippocampus was also smaller in men compared to women, but the difference was only significant for the right and not for the left hippocampus (F(1) = 4.41; p = .048 and p = .34, respectively). Paired sample t-tests revealed smaller left compared to right HV in both patients and controls (t(57) = –5.96; p < .001 and t(26) = –4.02; p < .001), and both men and women. The strongest difference between left and right HVs was observed in male patients (t(38) = –5.74; p < .001).
The CAR was significantly smaller in patients compared to controls (F(1) = 8.03; p = .006), but further analysis revealed that this difference was only significant in male but not female participants (F(1) = 5.65; p = .021 and F(1) = 1.62; p = .214, respectively; see Table 3 for details). The CAR was significantly smaller in men compared to women in both patients and controls (F = 4.26; p = .044 and F = 4.96; p = .037, respectively).
3.3. Associations between hippocampal volume and the CAR
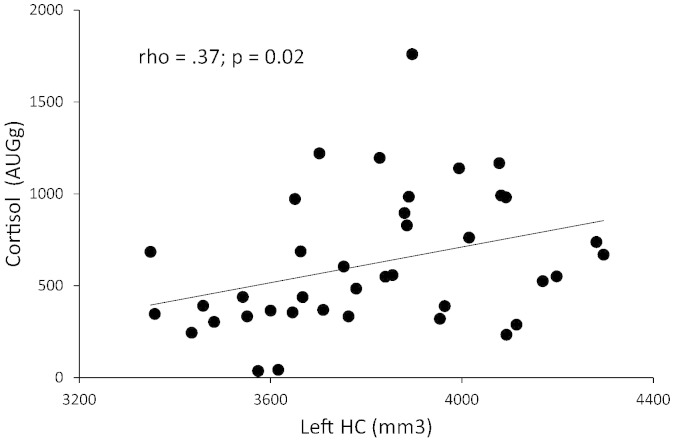
In the total patient group, both left and right HVs (relative to total brain size) were related to the CAR at trend level (rho = .24; p = .073 and rho = .25; p = .059; respectively). No such association was observed in controls (p > .222). When separating the groups by sex, we observed a significant correlation between the CAR and left hippocampal volume only in male patients (rho = .37; p = .021; see Fig. 2). This correlation was still significant when an outlier with cortisol levels more than three standard deviations above the group mean was excluded from the analysis (rho = .37; p = .024). The correlation between right HV and the CAR in male patients was significant at trend level (p = .10). No significant association between left or right HV and the CAR was observed in female patients (both p > .50) and in male or female controls (all p > .13). When controlling for medication dose, cannabis use and cigarette smoking employing partial correlations in a subgroup of patients for which CPZE medication dose could be calculated (30 men, 13 women), the association between the CAR and left and right HV in male patients was still significant (r(24) = .39; p = .011 and r(24) = .40; p = .034; respectively). Again, no association was observed in female patients (all p > .21) and in male or female controls, controlling for cannabis use and smoking (all p > .50). No association was observed between the CAR and total brain volume in either group or sex (all p > .14).
Fig. 2.
Spearman correlation between left hippocampal volume and the cortisol awakening response in male first episode psychosis patients.
3.4. Association between biological measures and symptoms
In the total patient group, smaller left and right HVs (adjusted for total brain size) were associated with lower global functioning (rho = .29; p = .026 and rho = .32; p = .016; respectively). Total brain volume differences were not related to symptoms in the total group (p > .22). When separated by sex, smaller left and right HVs in male patients were significantly associated with lower global functioning (both rho = .38; p = .017) and more positive symptoms of psychosis (rho = −.39; p = .014 and rho = −.37; p = .020; respectively). No such associations with symptoms were observed in female patients (all p > .18) and for total brain volume (p > .10). Hippocampal and total brain volumes were not associated with negative symptoms and depression in either sex (all p > .18). Hippocampal volume was also not significantly related to DUP or DUI (all p > .14). Controlling for cannabis use and cigarette smoking in subgroups of patients using partial correlations confirmed the association between left and right HVs and positive symptoms in men (r(36) = –40; p = .012 and r(36) = –36; p = .029; respectively). However, when controlling for medication dose in a subgroup of patients, the previously observed associations between hippocampal volume and symptoms were not significant anymore (all p > .11). A more blunted CAR in patients was associated with a lower level of global functioning at trend level (rho = .24; p = .069). The CAR was not related to any other symptoms in the total patient group or when separated by sex (all p > .12).
4. Discussion
The present study investigated sex differences in hippocampal volume and their association with the cortisol awakening response and symptoms in patients with a first episode of psychosis. As hypothesized, HV was significantly reduced in male patients both in comparison to male controls and to female patients. The observed HV differences between male patients and controls appeared to be secondary to whole brain volume differences and to be superimposed on HV differences between men and women, rendering male psychosis patients particularly vulnerable to HV loss. We furthermore observed a blunted CAR specifically in male patients and demonstrated an association between smaller HV and the lower CAR again only in male patients. Smaller left and right HVs were related to impaired global functioning and more positive symptoms in male patients only. To the best of our knowledge, the present study is the first to demonstrate a sex-specific relationship of smaller HV with both a blunted CAR and higher symptom severity in patients with a diagnosis of first-episode psychosis.
Our results confirm recent independent findings of sex differences in psychosis with regard to HV reduction (Adriano et al., 2012; Bogerts et al., 1990b; Bora et al., 2012; Exner et al., 2008; Irle et al., 2011) and HPA axis regulation (Pruessner et al., 2008; Pruessner et al., 2013). The findings are also in line with several recent studies showing a relationship between reduced hippocampal volume and more severe positive symptoms (Bodnar et al., 2010; Brambilla et al., 2013; Ebdrup et al., 2011; Kuhn et al., 2012; Watson et al., 2012). Sex differences in these studies may have been masked by the consistently larger proportion of male patients.
The observed correlation between reduced HV and a dysregulated HPA axis resembles findings in various disorders such as depression (O'rBrien et al., 1996), post-traumatic stress disorder (Yehuda, 2001), Cushing's syndrome (Starkman et al., 1992), aging (Lupien et al., 1998) and, more recently, FEP (Mondelli et al., 2010b). This association has been explained by the role of the hippocampus as a mediator of negative feedback in situations of elevated glucocorticoid levels (Jacobson and Sapolsky, 1991; Pruessner et al., 2010). Chronically elevated cortisol levels have been shown to cause atrophy of dendrites in the hippocampal CA3 region and suppression of neurogenesis of dentate gyrus granule neurons (McEwen, 1999), which can result in overall volume reduction of the structure and further dysregulation of the HPA axis. On the other hand, hippocampal integrity can already be compromised due to genetic and neurodevelopmental abnormalities and early life adversity, constituting risk factors for the development of HPA dysregulation in response to subsequent traumatic or other chronic stress situations (Buss et al., 2007; Gilbertson et al., 2002; Smith et al., 2003).
The observed relationship between HV reduction and HPA axis dysregulation and the association of both with functional and symptom outcomes are in accordance with the neural-diathesis stress model of schizophrenia (Walker and Diforio, 1997) and support the notion that both biological factors together are implicated in the disease process of psychosis. In fact, our findings suggest that the neural-diathesis stress model might be particularly relevant for male patients. The here reported sex differences in neurobiological variables could furthermore be related to other disadvantages in male compared to female patients such as a higher rate of treated incidence of psychosis (Aleman et al., 2003; Anderson et al., 2012), an earlier age of onset (Angermeyer and Kuhn, 1988), and a poorer treatment response (Angermeyer et al., 1990).
In accordance with previous studies, the left hippocampus was generally smaller than the right in both patients and healthy controls (Adriano et al., 2012) and was particularly reduced in FEP patients (Buehlmann et al., 2010; Malchow et al., 2013; Velakoulis et al., 2006). Notably smaller left HV was related to a blunted CAR, which resembles another recent study in FEP patients reporting smaller left hippocampal volume in association with higher diurnal cortisol levels (Mondelli et al., 2010b). Studies reporting smaller left HV in adults diagnosed with PTSD or dissociative identity disorder (Bremner et al., 1997; Stein et al., 1997) and FEP patients (Hoy et al., 2012) who experienced childhood trauma, suggest that stress related mechanisms are implicated in left HV loss. Similarly, another recent study demonstrated an association of small left HV with increased emotional and cortisol reactivity to stress in schizophrenia patients and healthy siblings (Collip et al., 2011).
Compromised hippocampal integrity impairs episodic, relational and spatial memory processes (Bohbot et al., 1998; Cohen et al., 1999; Eichenbaum, 1999; Squire, 1992). Indeed, some research suggests that male schizophrenia patients may be more vulnerable to cognitive deficits (Goldstein et al., 1998) and show reduced volume in brain regions implicated in verbal memory circuitry (Abbs et al., 2011). It has been suggested that the CAR occurs in response to “activation of memory representations about the self and orientation in time and space upon awakening” (Fries et al., 2009). Pathological changes to the hippocampus might compromise these cognitive representations and the associated cortisol response, thus rendering male patients more vulnerable to stress.
It has been suggested that sex and gender differences in schizophrenia are a consequence of sex differences in brain development and a higher susceptibility of male fetuses to environmental insults (Goldstein et al., 2002; Seeman, 2008). A factor that not only is crucially involved in the sexual differentiation of the brain but also affects a variety of other neuronal and behavioral processes in development and adulthood is estrogen (Abel et al., 2010; Hafner, 2003; McEwen, 2002; Seeman, 1997). Estrogen receptors have been identified in many brain structures, importantly those comprising the HPA axis, including the hippocampus (McEwen, 2002), and gonadal steroids have effects on the HPA response to stress (Handa and Weiser, 2014; Kirschbaum et al., 1999). The putative neuroprotective effect of estrogen in women and its absence in men has been suggested as an explanation for the relatively greater hippocampal volume decline over time in men (Hu et al., 2013; Lord et al., 2008; Pruessner et al., 2001; Pruessner et al., 2010). Estrogen has furthermore been shown to improve hippocampus dependent learning and memory (McEwen, 2002). In psychosis, the ‘estrogen hypothesis’ is supported by the lower prevalence rate and more favorable course of schizophrenia in women before menopause, higher rates of illness onset after menopause, and variability of psychotic symptoms over the menstrual cycle (Huber et al., 2004; Riecher-Rossler et al., 1994).
Another influential developmental factor is early life adversity, which can have important consequences for HPA axis regulation (Heim et al., 2008; Heim et al., 2009; Liu et al., 1997), hippocampal integrity (Buss et al., 2007; Driessen et al., 2000) and the development of psychosis (Fisher et al., 2013; Matheson et al., 2013; van Winkel et al., 2013; Varese et al., 2012) in adulthood. Some studies suggest that men might be particularly vulnerable to the effects of early life adversity on long-term mental health outcomes (Kivimaki et al., 2002; Pruessner et al., 2013; Shevlin et al., 2007). In support of this notion, we have recently reported an association between the blunted CAR in male FEP patients and poor self-reported parental bonding (Pruessner et al., 2013).
A limitation of the study is the cross sectional design, which prevents conclusive insights about cause and effect in the observed variables. Further limitations are the small sample size in the control group and the smaller number of female patients, which compromised the statistical power to detect significant associations in these subgroups. We have not considered several potential confounders of cortisol levels such as daylight exposure (Vreeburg et al., 2009), activity levels (Labsy et al., 2013) and oral contraceptive use (Bouma et al., 2009). The observed sex difference in HV might not be specific to psychosis, as it appears to begin in young adulthood even in a healthy population (Pruessner et al., 2001). It cannot be excluded that medication dose had an impact on hippocampal volume, although it is likely that the higher medication dose, seemingly explaining the relationship between hippocampal volume and symptoms, was a consequence of the relationship between symptom severity and medication dose in male patients. It could be considered another limitation that the CAR was the only measure of HPA axis regulation in the current study given that changes in the CAR do not necessarily correspond with diurnal measures of HPA function (Mondelli et al., 2010a).
In conclusion, our findings demonstrate sex specific reductions in hippocampal volume that are closely connected to HPA axis regulation and symptoms. We propose that these findings are likely a consequence of sex differences in neurodevelopment and a lack of the neuroprotective effects of estrogen, rendering men more vulnerable to the effects of stress and more prone to develop mental illness. Our results add to an increasing number of findings showing a disadvantage for male patients with psychosis. A better understanding of the associations between various pathophysiological mechanisms involved in the expression of sex differences in psychosis is expected to explain some of the heterogeneity in illness phenotypes, to reconcile inconsistent findings, and to open up new avenues for the development of effective interventions.
Authors' contributions
Ethical standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Conflict of interest
None of the authors declare any financial or other conflicts of interest.
Source of funding
This research was supported by a NARSAD Young Investigator Award to Dr. M. Pruessner and an operating grant (#68961) from the Canadian Institutes of Health Research (CIHR) to Drs. M. Lepage and A. Malla. Dr. M. Pruessner is a Golden Investigator (Golden Family Foundation), and Dr. Malla is funded by the Canada Research Chairs Program. The funding sources had no involvement in data collection, analysis and interpretation of the data; in the writing of the report; and in the decision to submit the article for publication.
Acknowledgements
We thank the PEPP-Montreal research staff for their help with recruitment and clinical assessments of patients. In particular, we thank Mrs. Audrey Benoit for help with recruitment of patients for MRI scanning and Mrs. Nicole Pawliuk for assistance with data management.
References
- Abbs B., Liang L., Makris N., Tsuang M., Seidman L.J., Goldstein J.M. Covariance modeling of MRI brain volumes in memory circuitry in schizophrenia: sex differences are critical. Neuroimage. 2011;56(4):1865–1874. doi: 10.1016/j.neuroimage.2011.03.079. 21497198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel K.M., Drake R., Goldstein J.M. Sex differences in schizophrenia. Int. Rev. Psychiatry. 2010;22(5):417–428. doi: 10.3109/09540261.2010.515205. 21047156 [DOI] [PubMed] [Google Scholar]
- Addington D., Addington J., Schissel B. A depression rating scale for schizophrenics. Schizophr. Res. 1990;3(4):247–251. doi: 10.1016/0920-9964(90)90005-r. 2278986 [DOI] [PubMed] [Google Scholar]
- Adriano F., Caltagirone C., Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist. 2012;18(2):180–200. doi: 10.1177/1073858410395147. 21531988 [DOI] [PubMed] [Google Scholar]
- Aleman A., Kahn R.S., Selten J.P. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch. Gen. Psychiatry. 2003;60(6):565–571. doi: 10.1001/archpsyc.60.6.565. 12796219 [DOI] [PubMed] [Google Scholar]
- Anderson K.K., Fuhrer R., Abrahamowicz M., Malla A.K. The incidence of first-episode schizophrenia-spectrum psychosis in adolescents and young adults in Montreal: an estimate from an administrative claims database. Can. J. Psychiatry. 2012;57(10):626–633. doi: 10.1177/070674371205701007. 23072954 [DOI] [PubMed] [Google Scholar]
- Andreasen N. Scale for the Assessment of Positive Symptoms (SAPS) Department of Psychiatry, College of Medicine, The University of Iowa; Iowa City: 1984. [Google Scholar]
- Andreasen N.C. The Scale for Assessment of Negative Symptoms (SANS) The University of Iowa; Iowa City: 1983. [Google Scholar]
- Angermeyer M.C., Kühn L. Gender differences in age at onset of schizophrenia. An overview. Eur. Arch. Psychiatry Neurol. Sci. 1988;237(6):351–364. doi: 10.1007/BF00380979. 3053193 [DOI] [PubMed] [Google Scholar]
- Angermeyer M.C., Kühn L., Goldstein J.M. Gender and the course of schizophrenia: differences in treated outcomes. Schizophr. Bull. 1990;16(2):293–307. doi: 10.1093/schbul/16.2.293. 2374885 [DOI] [PubMed] [Google Scholar]
- Bezchlibnik-Butler K.Z., Jeffries J.J. Clinical Handbook of Psychotropic Drugs. Hogrefe and Huber; Toronto, ON: 2006. [Google Scholar]
- Bodnar M., Malla A.K., Czechowska Y., Benoit A., Fathalli F., Joober R., Pruessner M., Pruessner J., Lepage M. Neural markers of remission in first-episode schizophrenia: a volumetric neuroimaging study of the hippocampus and amygdala. Schizophr. Res. 2010;122(1–3):72–80. doi: 10.1016/j.schres.2010.06.013. 20630708 [DOI] [PubMed] [Google Scholar]
- Bogerts B., Ashtari M., Degreef G., Alvir J.M., Bilder R.M., Lieberman J.A. Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res. 1990;35(1):1–13. doi: 10.1016/0925-4927(90)90004-p. 2367608 [DOI] [PubMed] [Google Scholar]
- Bogerts B., Falkai P., Haupts M., Greve B., Ernst S., Tapernon-Franz U., Heinzmann U. Post-mortem volume measurements of limbic system and basal ganglia structures in chronic schizophrenics. Initial results from a new brain collection. Schizophr. Res. 1990;3(5–6):295–301. doi: 10.1016/0920-9964(90)90013-w. 2282334 [DOI] [PubMed] [Google Scholar]
- Bohbot V.D., Kalina M., Stepankova K., Spackova N., Petrides M., Nadel L. Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia. 1998;36(11):1217–1238. doi: 10.1016/s0028-3932(97)00161-9. 9842767 [DOI] [PubMed] [Google Scholar]
- Bora E., Fornito A., Yücel M., Pantelis C. The effects of gender on grey matter abnormalities in major psychoses: a comparative voxelwise meta-analysis of schizophrenia and bipolar disorder. Psychol. Med. 2012;42(2):295–307. doi: 10.1017/S0033291711001450. 21835091 [DOI] [PubMed] [Google Scholar]
- Borges S., Gayer-Anderson C., Mondelli V. A systematic review of the activity of the hypothalamic-pituitary-adrenal axis in first episode psychosis. Psychoneuroendocrinology. 2013;38(5):603–611. doi: 10.1016/j.psyneuen.2012.12.025. 23369532 [DOI] [PubMed] [Google Scholar]
- Bouma E.M., Riese H., Ormel J., Verhulst F.C., Oldehinkel A.J. Adolescents' cortisol responses to awakening and social stress; effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology. 2009;34(6):884–893. doi: 10.1016/j.psyneuen.2009.01.003. 19195792 [DOI] [PubMed] [Google Scholar]
- Brambilla P., Perlini C., Rajagopalan P., Saharan P., Rambaldelli G., Bellani M., Dusi N., Cerini R., Pozzi Mucelli R., Tansella M., Thompson P.M. Schizophrenia severity, social functioning and hippocampal neuroanatomy: three-dimensional mapping study. Br. J. Psychiatry. 2013;202(1):50–55. doi: 10.1192/bjp.bp.111.105700. 23284150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Randall P., Vermetten E., Staib L., Bronen R.A., Mazure C., Capelli S., McCarthy G., Innis R.B., Charney D.S. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse — a preliminary report. Biol. Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. 8988792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant N.L., Buchanan R.W., Vladar K., Breier A., Rothman M. Gender differences in temporal lobe structures of patients with schizophrenia: a volumetric MRI study. Am. J. Psychiatry. 1999;156(4):603–609. doi: 10.1176/ajp.156.4.603. 10200741 [DOI] [PubMed] [Google Scholar]
- Buchanan T.W., Kern S., Allen J.S., Tranel D., Kirschbaum C. Circadian regulation of cortisol after hippocampal damage in humans. Biol. Psychiatry. 2004;56(9):651–656. doi: 10.1016/j.biopsych.2004.08.014. 15522248 [DOI] [PubMed] [Google Scholar]
- Buchy L., Czechowska Y., Chochol C., Malla A., Joober R., Pruessner J., Lepage M. Toward a model of cognitive insight in first-episode psychosis: verbal memory and hippocampal structure. Schizophr. Bull. 2010;36(5):1040–1049. doi: 10.1093/schbul/sbp015. 19346315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehlmann E., Berger G.E., Aston J., Gschwandtner U., Pflueger M.O., Borgwardt S.J., Radue E.W., Riecher-Rössler A. Hippocampus abnormalities in at risk mental states for psychosis? A cross-sectional high resolution region of interest magnetic resonance imaging study. J. Psychiatr. Res. 2010;44(7):447–453. doi: 10.1016/j.jpsychires.2009.10.008. 19939408 [DOI] [PubMed] [Google Scholar]
- Buss C., Lord C., Wadiwalla M., Hellhammer D.H., Lupien S.J., Meaney M.J., Pruessner J.C. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J. Neurosci. 2007;27(10):2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007. 17344396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N.J., Ryan J., Hunt C., Romine L., Wszalek T., Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9(1):83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. 10088903 [DOI] [PubMed] [Google Scholar]
- Collip D., Nicolson N.A., Lardinois M., Lataster T., van Os J., Myin-Germeys I., G.R.O.U.P Daily cortisol, stress reactivity and psychotic experiences in individuals at above average genetic risk for psychosis. Psychol. Med. 2011;41(11):2305–2315. doi: 10.1017/S0033291711000602. 21733219 [DOI] [PubMed] [Google Scholar]
- Dressendorfer R.A., Kirschbaum C., Rohde W., Stahl F., Strasburger C.J. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J. Steroid Biochem. Mol. Biol. 1992;43(7):683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Driessen M., Herrmann J., Stahl K., Zwaan M., Meier S., Hill A., Osterheider M., Petersen D. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch. Gen. Psychiatry. 2000;57(12):1115–1122. doi: 10.1001/archpsyc.57.12.1115. 11115325 [DOI] [PubMed] [Google Scholar]
- Ebdrup B.H., Skimminge A., Rasmussen H., Aggernaes B., Oranje B., Lublin H., Baaré W., Glenthøj B. Progressive striatal and hippocampal volume loss in initially antipsychotic-naive, first-episode schizophrenia patients treated with quetiapine: relationship to dose and symptoms. Int. J. Neuropsychopharmacol. 2011;14(1):69–82. doi: 10.1017/S1461145710000817. 20701823 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behav. Brain Res. 1999;103(2):123–133. doi: 10.1016/s0166-4328(99)00044-3. 10513581 [DOI] [PubMed] [Google Scholar]
- Exner C., Nehrkorn B., Martin V., Huber M., Shiratori K., Rief W. Sex-dependent hippocampal volume reductions in schizophrenia relate to episodic memory deficits. J. Neuropsychiatry Clin. Neurosci. 2008;20(2):227–230. doi: 10.1176/jnp.2008.20.2.227. 18451195 [DOI] [PubMed] [Google Scholar]
- First, M.B., Spitzer, R.L., Gibbon, M., Williams, J.B.W., 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute, New York
- Fisher H.L., Schreier A., Zammit S., Maughan B., Munafò M.R., Lewis G., Wolke D. Pathways between childhood victimization and psychosis-like symptoms in the ALSPAC birth cohort. Schizophr. Bull. 2013;39(5):1045–1055. doi: 10.1093/schbul/sbs088. 22941743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E., Dettenborn L., Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int. J. Psychophysiol. 2009;72(1):67–73. doi: 10.1016/j.ijpsycho.2008.03.014. 18854200 [DOI] [PubMed] [Google Scholar]
- Gilbertson M.W., Shenton M.E., Ciszewski A., Kasai K., Lasko N.B., Orr S.P., Pitman R.K. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 2002;5(11):1242–1247. doi: 10.1038/nn958. 12379862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.M., Seidman L.J., Goodman J.M., Koren D., Lee H., Weintraub S., Tsuang M.T. Are there sex differences in neuropsychological functions among patients with schizophrenia? Am. J. Psychiatry. 1998;155(10):1358–1364. doi: 10.1176/ajp.155.10.1358. 9766767 [DOI] [PubMed] [Google Scholar]
- Goldstein J.M., Seidman L.J., O'rBrien L.M., Horton N.J., Kennedy D.N., Makris N., Caviness V.S., Jr., Faraone S.V., Tsuang M.T. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch. Gen. Psychiatry. 2002;59(2):154–164. doi: 10.1001/archpsyc.59.2.154. 11825137 [DOI] [PubMed] [Google Scholar]
- Gunduz-Bruce H., Szeszko P.R., Gueorguieva R., Ashtari M., Robinson D.G., Kane J.M., Bilder R.M. Cortisol levels in relation to hippocampal sub-regions in subjects with first episode schizophrenia. Schizophr. Res. 2007;94(1–3):281–287. doi: 10.1016/j.schres.2007.03.025. 17490857 [DOI] [PubMed] [Google Scholar]
- Gur R.E., Turetsky B.I., Cowell P.E., Finkelman C., Maany V., Grossman R.I., Arnold S.E., Bilker W.B., Gur R.C. Temporolimbic volume reductions in schizophrenia. Arch. Gen. Psychiatry. 2000;57(8):769–775. doi: 10.1001/archpsyc.57.8.769. 10920465 [DOI] [PubMed] [Google Scholar]
- Häfner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28(Suppl. 2):17–54. doi: 10.1016/s0306-4530(02)00125-7. 12650680 [DOI] [PubMed] [Google Scholar]
- Handa R.J., Weiser M.J. Gonadal steroid hormones and the hypothalamo–pituitary–adrenal axis. Front. Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. 24246855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P.J. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl.) 2004;174(1):151–162. doi: 10.1007/s00213-003-1761-y. 15205886 [DOI] [PubMed] [Google Scholar]
- Heim C., Nater U.M., Maloney E., Boneva R., Jones J.F., Reeves W.C. Childhood trauma and risk for chronic fatigue syndrome: association with neuroendocrine dysfunction. Arch. Gen. Psychiatry. 2009;66(1):72–80. doi: 10.1001/archgenpsychiatry.2008.508. 19124690 [DOI] [PubMed] [Google Scholar]
- Heim C., Newport D.J., Mletzko T., Miller A.H., Nemeroff C.B. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. 18602762 [DOI] [PubMed] [Google Scholar]
- Hoy K., Barrett S., Shannon C., Campbell C., Watson D., Rushe T., Shevlin M., Bai F., Cooper S., Mulholland C. Childhood trauma and hippocampal and amygdalar volumes in first-episode psychosis. Schizophr. Bull. 2012;38(6):1162–1169. doi: 10.1093/schbul/sbr085. 21799213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Coupé P., Pruessner J.C., Collins D.L. Appearance-based modeling for segmentation of hippocampus and amygdala using multi-contrast MR imaging. Neuroimage. 2011;58(2):549–559. doi: 10.1016/j.neuroimage.2011.06.054. 21741485 [DOI] [PubMed] [Google Scholar]
- Hu S., Pruessner J.C., Coupé P., Collins D.L. Volumetric analysis of medial temporal lobe structures in brain development from childhood to adolescence. Neuroimage. 2013;74:276–287. doi: 10.1016/j.neuroimage.2013.02.032. 23485848 [DOI] [PubMed] [Google Scholar]
- Huber T.J., Borsutzky M., Schneider U., Emrich H.M. Psychotic disorders and gonadal function: evidence supporting the oestrogen hypothesis. Acta Psychiatr. Scand. 2004;109(4):269–274. doi: 10.1046/j.1600-0447.2003.00251.x. 15008800 [DOI] [PubMed] [Google Scholar]
- Irle E., Lange C., Ruhleder M., Exner C., Siemerkus J., Weniger G. Hippocampal size in women but not men with schizophrenia relates to disorder duration. Psychiatry Res. 2011;192(3):133–139. doi: 10.1016/j.pscychresns.2010.12.009. 21546218 [DOI] [PubMed] [Google Scholar]
- Jacobson L., Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic–pituitary–adrenocortical axis. Endocr. Rev. 1991;12(2):118–134. doi: 10.1210/edrv-12-2-118. 2070776 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Kudielka B.M., Gaab J., Schommer N.C., Hellhammer D.H. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosom. Med. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. 10204967 [DOI] [PubMed] [Google Scholar]
- Kivimäki M., Vahtera J., Elovainio M., Lillrank B., Kevin M.V. Death or illness of a family member, violence, interpersonal conflict, and financial difficulties as predictors of sickness absence: longitudinal cohort study on psychological and behavioral links. Psychosom. Med. 2002;64(5):817–825. 12271113 [PubMed] [Google Scholar]
- Kühn S., Musso F., Mobascher A., Warbrick T., Winterer G., Gallinat J. Hippocampal subfields predict positive symptoms in schizophrenia: first evidence from brain morphometry. Transl. Psychiatry. 2012;2:e127. doi: 10.1038/tp.2012.51. 22692142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labsy Z., Prieur F., Le Panse B., Do M.C., Gagey O., Lasne F., Collomp K. The diurnal patterns of cortisol and dehydroepiandrosterone in relation to intense aerobic exercise in recreationally trained soccer players. Stress. 2013;16(2):261–265. doi: 10.3109/10253890.2012.707259. 22734443 [DOI] [PubMed] [Google Scholar]
- Liu D., Diorio J., Tannenbaum B., Caldji C., Francis D., Freedman A., Sharma S., Pearson D., Plotsky P.M., Meaney M.J. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. 9287218 [DOI] [PubMed] [Google Scholar]
- Lord C., Buss C., Lupien S.J., Pruessner J.C. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: a possible window of opportunity effect. Neurobiol. Aging. 2008;29(1):95–101. doi: 10.1016/j.neurobiolaging.2006.09.001. 17030472 [DOI] [PubMed] [Google Scholar]
- Luborsky L. Clinician's judgments of mental health. Arch. Gen. Psychiatry. 1962;7:407–417. doi: 10.1001/archpsyc.1962.01720060019002. 13931376 [DOI] [PubMed] [Google Scholar]
- Lupien S.J., de Leon M., de Santi S., Convit A., Tarshish C., Nair N.P., Thakur M., McEwen B.S., Hauger R.L., Meaney M.J. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat. Neurosci. 1998;1(1):69–73. doi: 10.1038/271. 10195112 [DOI] [PubMed] [Google Scholar]
- Malchow B., Hasan A., Fusar-Poli P., Schmitt A., Falkai P., Wobrock T. Cannabis abuse and brain morphology in schizophrenia: a review of the available evidence. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263(1):3–13. doi: 10.1007/s00406-012-0346-3. 22907121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malla A., Norman R., McLean T., Scholten D., Townsend L. A Canadian programme for early intervention in non-affective psychotic disorders. Aust. N. .Z. J. Psychiatry. 2003;37(4):407–413. doi: 10.1046/j.1440-1614.2003.01194.x. 12873324 [DOI] [PubMed] [Google Scholar]
- Matheson S.L., Shepherd A.M., Pinchbeck R.M., Laurens K.R., Carr V.J. Childhood adversity in schizophrenia: a systematic meta-analysis. Psychol. Med. 2013;43(2):225–238. doi: 10.1017/S0033291712000785. 22716913 [DOI] [PubMed] [Google Scholar]
- Mazziotta J.C., Toga A.W., Evans A., Fox P., Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) Neuroimage. 1995;2(2):89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog. Horm. Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. 12017552 [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. 10202533 [DOI] [PubMed] [Google Scholar]
- Mondelli V., Dazzan P., Hepgul N., Di Forti M., Aas M., D'rAlbenzio A., Di Nicola M., Fisher H., Handley R., Marques T.R., Morgan C., Navari S., Taylor H., Papadopoulos A., Aitchison K.J., Murray R.M., Pariante C.M. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment. Schizophr. Res. 2010;116(2–3):234–242. doi: 10.1016/j.schres.2009.08.013. 19751968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli V., Pariante C.M., Navari S., Aas M., D'rAlbenzio A., Di Forti M., Handley R., Hepgul N., Marques T.R., Taylor H., Papadopoulos A.S., Aitchison K.J., Murray R.M., Dazzan P. Higher cortisol levels are associated with smaller left hippocampal volume in first-episode psychosis. Schizophr. Res. 2010;119(1–3):75–78. doi: 10.1016/j.schres.2009.12.021. 20071148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M.D., Saykin A.J., Flashman L.A., Riordan H.J. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch. Gen. Psychiatry. 1998;55(5):433–440. doi: 10.1001/archpsyc.55.5.433. 9596046 [DOI] [PubMed] [Google Scholar]
- Nopoulos P., Flaum M., Andreasen N.C. Sex differences in brain morphology in schizophrenia. Am. J. Psychiatry. 1997;154(12):1648–1654. doi: 10.1176/ajp.154.12.1648. 9396941 [DOI] [PubMed] [Google Scholar]
- O'rBrien J.T., Ames D., Schweitzer I., Colman P., Desmond P., Tress B. Clinical and magnetic resonance imaging correlates of hypothalamic–pituitary–adrenal axis function in depression and Alzheimer'rs disease. Br. J. Psychiatry. 1996;168(6):679–687. doi: 10.1192/bjp.168.6.679. 8773809 [DOI] [PubMed] [Google Scholar]
- Pantelis C., Velakoulis D., McGorry P.D., Wood S.J., Suckling J., Phillips L.J., Yung A.R., Bullmore E.T., Brewer W., Soulsby B., Desmond P., McGuire P.K. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281–288. doi: 10.1016/S0140-6736(03)12323-9. 12559861 [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Collins D.L., Pruessner M., Evans A.C. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. J. Neurosci. 2001;21(1):194–200. doi: 10.1523/JNEUROSCI.21-01-00194.2001. 11150336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner J.C., Dedovic K., Pruessner M., Lord C., Buss C., Collins L., Dagher A., Lupien S.J. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations — 2008 Curt Richter award winner. Psychoneuroendocrinology. 2010;35(1):179–191. doi: 10.1016/j.psyneuen.2009.02.016. 19362426 [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Kirschbaum C., Meinlschmid G., Hellhammer D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. 12892658 [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Li L.M., Serles W., Pruessner M., Collins D.L., Kabani N., Lupien S., Evans A.C. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb. Cortex. 2000;10(4):433–442. doi: 10.1093/cercor/10.4.433. 10769253 [DOI] [PubMed] [Google Scholar]
- Pruessner M., Boekestyn L., Béchard-Evans L., Abadi S., Vracotas N., Joober R., Pruessner J.C., Malla A.K. Sex differences in the cortisol response to awakening in recent onset psychosis. Psychoneuroendocrinology. 2008;33(8):1151–1154. doi: 10.1016/j.psyneuen.2008.04.006. 18640785 [DOI] [PubMed] [Google Scholar]
- Pruessner M., Vracotas N., Joober R., Pruessner J.C., Malla A.K. Blunted cortisol awakening response in men with first episode psychosis: relationship to parental bonding. Psychoneuroendocrinology. 2013;38(2):229–240. doi: 10.1016/j.psyneuen.2012.06.002. 22770984 [DOI] [PubMed] [Google Scholar]
- Riecher-Rössler A., Häfner H., Stumbaum M., Maurer K., Schmidt R. Can estradiol modulate schizophrenic symptomatology? Schizophr. Bull. 1994;20(1):203–214. doi: 10.1093/schbul/20.1.203. 8197416 [DOI] [PubMed] [Google Scholar]
- Ritsner M., Gibel A., Maayan R., Ratner Y., Ram E., Modai I., Weizman A. State and trait related predictors of serum cortisol to DHEA(S) molar ratios and hormone concentrations in schizophrenia patients. Eur. Neuropsychopharmacol. 2007;17(4):257–264. doi: 10.1016/j.euroneuro.2006.09.001. 17107774 [DOI] [PubMed] [Google Scholar]
- Ryan M.C., Sharifi N., Condren R., Thakore J.H. Evidence of basal pituitary–adrenal overactivity in first episode, drug naive patients with schizophrenia. Psychoneuroendocrinology. 2004;29(8):1065–1070. doi: 10.1016/j.psyneuen.2003.08.011. 15219658 [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatry. 2000;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. 11015810 [DOI] [PubMed] [Google Scholar]
- Seeman M.V. Psychopathology in women and men: focus on female hormones. Am. J. Psychiatry. 1997;154(12):1641–1647. doi: 10.1176/ajp.154.12.1641. 9396940 [DOI] [PubMed] [Google Scholar]
- Seeman M.V. Gender. In: Mueser K.T., Jeste D.V., editors. Clinical Handbook of Schizophrenia. Guilford Press; New York: 2008. pp. 575–580. [Google Scholar]
- Shevlin M., Dorahy M.J., Adamson G. Trauma and psychosis: an analysis of the National Comorbidity Survey. Am. J. Psychiatry. 2007;164(1):166–169. doi: 10.1176/ajp.2007.164.1.166. 17202562 [DOI] [PubMed] [Google Scholar]
- Smith G.N., Lang D.J., Kopala L.C., Lapointe J.S., Falkai P., Honer W.G. Developmental abnormalities of the hippocampus in first-episode schizophrenia. Biol. Psychiatry. 2003;53(7):555–561. doi: 10.1016/s0006-3223(02)01977-7. 12679232 [DOI] [PubMed] [Google Scholar]
- Squire L.R. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. 1594723 [DOI] [PubMed] [Google Scholar]
- Starkman M.N., Gebarski S.S., Berent S., Schteingart D.E. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing's syndrome. Biol. Psychiatry. 1992;32(9):756–765. doi: 10.1016/0006-3223(92)90079-f. 1450290 [DOI] [PubMed] [Google Scholar]
- Steen R.G., Mull C., McClure R., Hamer R.M., Lieberman J.A. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br. J. Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. 16738340 [DOI] [PubMed] [Google Scholar]
- Stein M.B., Koverola C., Hanna C., Torchia M.G., McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol. Med. 1997;27(4):951–959. doi: 10.1017/s0033291797005242. 9234472 [DOI] [PubMed] [Google Scholar]
- Van Venrooij J.A., Fluitman S.B., Lijmer J.G., Kavelaars A., Heijnen C.J., Westenberg H.G., Kahn R.S., Gispen-de Wied C.C. Impaired neuroendocrine and immune response to acute stress in medication-naive patients with a first episode of psychosis. Schizophr. Bull. 2012;38(2):272–279. doi: 10.1093/schbul/sbq062. 20558533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Winkel R., van Nierop M., Myin-Germeys I., van Os J. Childhood trauma as a cause of psychosis: linking genes, psychology, and biology. Can. J. Psychiatry. 2013;58(1):44–51. doi: 10.1177/070674371305800109. 23327756 [DOI] [PubMed] [Google Scholar]
- Varese F., Smeets F., Drukker M., Lieverse R., Lataster T., Viechtbauer W., Read J., van Os J., Bentall R.P. Childhood adversities increase the risk of psychosis: a meta-analysis of patient–control, prospective- and cross-sectional cohort studies. Schizophr. Bull. 2012;38(4):661–671. doi: 10.1093/schbul/sbs050. 22461484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velakoulis D., Wood S.J., Wong M.T., McGorry P.D., Yung A., Phillips L., Smith D., Brewer W., Proffitt T., Desmond P., Pantelis C. Hippocampal and amygdala Volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch. Gen. Psychiatry. 2006;63(2):139–149. doi: 10.1001/archpsyc.63.2.139. 16461856 [DOI] [PubMed] [Google Scholar]
- Vreeburg S.A., Kruijtzer B.P., van Pelt J., van Dyck R., DeRijk R.H., Hoogendijk W.J., Smit J.H., Zitman F.G., Penninx B.W. Associations between sociodemographic, sampling and health factors and various salivary cortisol indicators in a large sample without psychopathology. Psychoneuroendocrinology. 2009;34(8):1109–1120. doi: 10.1016/j.psyneuen.2009.04.024. 19515498 [DOI] [PubMed] [Google Scholar]
- Walker E.F., Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol. Rev. 1997;104(4):667–685. doi: 10.1037/0033-295x.104.4.667. 9337628 [DOI] [PubMed] [Google Scholar]
- Watson D.R., Bai F., Barrett S.L., Turkington A., Rushe T.M., Mulholland C.C., Cooper S.J. Structural changes in the hippocampus and amygdala at first episode of psychosis. Brain Imaging Behav. 2012;6(1):49–60. doi: 10.1007/s11682-011-9141-4. 22045236 [DOI] [PubMed] [Google Scholar]
- Wolf O.T., Fujiwara E., Luwinski G., Kirschbaum C., Markowitsch H.J. No morning cortisol response in patients with severe global amnesia. Psychoneuroendocrinology. 2005;30(1):101–105. doi: 10.1016/j.psyneuen.2004.05.001. 15358447 [DOI] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. J. Clin. Psychiatry. 2001;62(Suppl. 17):41–46. 11495096 [PubMed] [Google Scholar]