Abstract
Abnormalities in cortical structure are commonly observed in children with dyslexia in key regions of the “reading network.” Whether alteration in cortical features reflects pathology inherent to dyslexia or environmental influence (e.g., impoverished reading experience) remains unclear. To address this question, we compared MRI-derived metrics of cortical thickness (CT), surface area (SA), gray matter volume (GMV), and their lateralization across three different groups of children with a historical diagnosis of dyslexia, who varied in current reading level. We compared three dyslexia subgroups with: (1) persistent reading and spelling impairment; (2) remediated reading impairment (normal reading scores), and (3) remediated reading and spelling impairments (normal reading and spelling scores); and a control group of (4) typically developing children. All groups were matched for age, gender, handedness, and IQ. We hypothesized that the dyslexia group would show cortical abnormalities in regions of the reading network relative to controls, irrespective of remediation status. Such a finding would support that cortical abnormalities are inherent to dyslexia and are not a consequence of abnormal reading experience. Results revealed increased CT of the left fusiform gyrus in the dyslexia group relative to controls. Similarly, the dyslexia group showed CT increase of the right superior temporal gyrus, extending into the planum temporale, which resulted in a rightward CT asymmetry on lateralization indices. There were no group differences in SA, GMV, or their lateralization. These findings held true regardless of remediation status. Each reading level group showed the same “double hit” of atypically increased left fusiform CT and rightward superior temporal CT asymmetry. Thus, findings provide evidence that a developmental history of dyslexia is associated with CT abnormalities, independent of remediation status.
Keywords: Dyslexia, MRI, Lateralization, Cortical thickness, Surface area, Gray matter volume
Highlights
-
•
Rightward superior temporal cortical thickness asymmetry in dyslexia
-
•
Increased left fusiform gyrus thickness in dyslexia
-
•
Primary effects present across three subgroups with differing reading levels
-
•
Cortical thickness findings were independent from volume and surface area.
-
•
“Double hit” of left fusiform anomaly and rightward temporal asymmetry in dyslexia
1. Introduction
Developmental dyslexia is a neurological condition characterized by difficulties in reading-related tasks such as word recognition and spelling in spite of normal intelligence, adequate education and motivation to read proficiently (Lyon et al., 2003). Structural MRI approaches [see Richlan et al. (2013) and Linkersdörfer et al. (2012) for meta-analyses] have identified abnormalities associated with dyslexia in regions within the reading network (Pugh et al., 2000a). Whether alteration in cortical structure reflects pathology inherent to dyslexia or environmental influence (e.g., impoverished reading experience or compensatory changes) remains unclear.
Prior studies have addressed this question using MRI measures of gray matter volume (GMV). Raschle et al. (2011) reported that pre-reading children with familial history of dyslexia have less GMV within the reading network, relative to control children without a familial history of dyslexia. This finding suggests that structural brain anomalies in dyslexia are present before reading experience rather than experience-dependent. In contrast, Krafnick et al. (2014) showed that GMV in multiple regions, including the left temporal cortex, is reduced in dyslexic children relative to age-matched controls, but not relative to reading-level-matched younger controls. The authors concluded that GMV differences in dyslexia are related to the level of current reading ability, which partially reflects the impoverished reading experience in dyslexics, rather than dyslexia per se.
Alternative measurements of cortical gray matter to GMV include cortical thickness (CT) and surface area (SA). Both CT and SA are highly heritable (Joshi et al., 2011; Panizzon et al., 2009; Rimol et al., 2010) and can delineate genetic influences on brain structure with more precision than GMV (Winkler et al., 2010). Both can be potential markers for neurodevelopmental disorders (Hazlett et al., 2011; Narr et al., 2009). In addition, CT can be affected by life experience, such as training (Engvig et al., 2010; Lazar et al., 2005). Thus far, few studies have examined CT and SA variations associated with dyslexia (Altarelli et al., 2013; Altarelli et al., 2014; Frye et al., 2010; Kushch et al., 1993). Here, we examined CT, SA and GMV to identify structural abnormalities in subgroups of dyslexia with different levels of reading ability. We used an observational design and tested remediated (i.e., normalized reading ability) and non-remediated dyslexia subgroups, as well as an age-, gender-, handedness-, and IQ-matched typically developing comparison group. If structural abnormalities are present in all subgroups with a history of dyslexia, relative to controls, this would suggest persistent cortical abnormalities that characterize dyslexia, irrespective of current reading ability. Such findings could potentially serve as early and reliable cortical markers of dyslexia in children. By contrast, abnormal CT, SA or GMV only in the non-remediated group, but not in the remediated groups would reflect the effect of current reading impairments, and thus support environmental effects (e.g., impoverished reading experience, which may be normalized in the remediated groups).
Hypothesizing that cortical abnormalities are inherent to dyslexia (Galaburda et al., 1985; Raschle et al., 2011), we predicted that altered patterns of CT, SA and/or GMV, if present, could be found across all dyslexia subgroups, regardless of remediation status. We also addressed a long-lasting question regarding the absence of a leftward structural asymmetry in the dyslexia brain (Galaburda et al., 1985; Kushch et al., 1993; Larsen et al., 1990; Leonard et al., 2001). In addition, since CT is a measure genetically and phenotypically independent from SA and GMV (Dickerson et al., 2009; Lemaitre et al., 2012; Panizzon et al., 2009; Winkler et al., 2010), we expected that CT findings would generally diverge from other measures. Finally, we evaluated whether there was an additive effect of dyslexia and remediation on gray matter structure for each surface-based metric by testing for differential effects in each remediation subgroup (i.e., whether the largest gray matter abnormalities are found in the non-remediated subgroup).
2. Material and methods
2.1. Participants
Children with a history of dyslexia (“Dys”) were identical to those published previously by Koyama et al. (2013), except for one participant excluded due to severe artifacts in the T1 image. They were native English speakers (n = 32), recruited through referrals from the clinical services at The Child Study Center at New York University Langone Medical Center and the New York International Dyslexia Association. Inclusion was based on parental report of prior diagnosis of reading disorder in accordance with DSM-IV or ICD-10, and prior written documentation. We also investigated history of previous or current DSM-IV-TR diagnoses other than dyslexia through informal interviews with parents and by reviewing prior clinical evaluations whenever available. Three out of the 32 children were diagnosed with ADHD.
Based on the current literacy competence level, measured by the Wechsler Individual Achievement Test—Second Edition (WIAT) (Wechsler, 2001), children with a history of dyslexia were sub-divided into three groups: (1) children with current deficits in both reading and spelling (“Dys-N”: Dyslexia with no remediation, n = 10), (2) children with a previous diagnosis of dyslexia but exhibiting no current reading deficit (“Dys-R”: Dyslexia with reading remediation, n = 11), and (3) children with a previous diagnosis of dyslexia but exhibiting no current deficits in either reading or spelling (“Dys-RS”: Dyslexia with reading and spelling remediation, n = 11). A reading or spelling deficit was defined as a current standard score below 85 (i.e., one standard deviation below the norm) on the WIAT Word Reading or Spelling subscales. Information from parental report (and supporting documentation when available) confirmed that none of the children in the Dys-N group had a history of targeted dyslexia intervention training prior to the current study, while all children in the Dys-R and Dys-RS groups had been in one or more targeted programs (e.g., the Orton Gillingham approach, http://www.ortonacademy.org; Wilson Language Training, http://www.wilsonlanguage.com; or various school intervention efforts). Information from prior written documentation verified a history of literacy impairment in all children in the remediation groups (standard scores lower than 85 on any type of standardized literacy test prior to remediation), and provided evidence that the majority of these children had exhibited phonological deficits.
Typically developing children (TDC, n = 32), who were native speakers of English, were selected as controls from a larger pool of children participating in ongoing studies at NYU Child Study Center. All children in the TDC group exhibited intact reading and spelling skills with both WIAT Word Reading and Spelling scores above 85. No previous or current DSM-IV-TR diagnoses were found based on the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (KSADS-PL) (Kaufman et al., 1996), which was administrated to parents and child participants separately.
The Dys and the TDC groups were group-matched on age (overall mean age = 12.1 ± 2.3 years: range = 7.7–16 years), gender, estimated full-scale IQ and handedness. Full-scale IQ was estimated with the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999); all participants had full-scale IQ above 85. Subgroups within the Dys group were also matched on the same variables. Table 1 provides demographic and cognitive measures for the Dys and the TDC groups. Table 2 provides demographic and cognitive measures for the three subgroups within the Dys group.
Table 1.
Demographic and cognitive profiles (means and standard deviations) for the Dys and the TDC groups.
| Group | Age (years) | Gender (n) | Handedness | WASI Full IQ (SS) | WIAT reading (SS) | WIAT spelling (SS) |
|---|---|---|---|---|---|---|
| Dys | 12.2 (2.3) | 17M/15F | 0L/32R | 105.7 (8.1) | 92.6 (14.3) | 86.3 (11.0) |
| TDC | 12.1 (2.2) | 16M/16F | 0L/32R | 108.9 (9.1) | 109.8 (8.8) | 111.5 (8.6) |
| t or χ2 | N.S. | N.S. | N.S. | N.S. | 5.8*** | 10.2*** |
Note. n = 32 for each group, N.S. = not significant; Dys = dyslexia, TDC = typically developing children, SS = standard score (mean = 100, standard deviation = 15), M = male, F = female, L = left, R = right, WASI = Wechsler Abbreviated Scale of Intelligence, WIAT = Wechsler Individual Achievement Test — Second Edition.
p < .001.
Table 2.
Demographic and cognitive profiles (means and standard deviations) for three subgroups within the Dys group.
| Group | Age (years) | Gender (n) | Handedness | WASI Full IQ (SS) | WIAT reading (SS) | WIAT spelling (SS) |
|---|---|---|---|---|---|---|
| Dys-N | 12.5 (2.2) | 6M/4F | 0L/10R | 108.0 (7.3) | 76.3 (9.3) | 79.2 (9.9) |
| Dys-R | 11.8 (2.2) | 5M/6F | 0L/11R | 101.3 (9.9) | 96.3 (7.3) | 82.3 (5.9) |
| Dys-RS | 12.4 (2.7) | 5M/6F | 0L/11R | 108.1 (5.1) | 103.8 (9.3) | 96.7 (7.9) |
| F or χ2 | N.S. | N.S. | N.S. | N.S. | 27.8*** | 14.6*** |
Note. N.S. = not significant; Dys-N = dyslexia with no remediation (n = 10), Dys-R = dyslexia with reading remediation (n = 11), Dys-RS = dyslexia with reading and spelling remediation (n = 11), SS = standard score (mean = 100, standard deviation = 15), M = male, F = female, L = left, R = right, WASI = Wechsler Abbreviated Scale of Intelligence, WIAT = Wechsler Individual Achievement Test — Second Edition.
p < .001.
2.2. MRI data acquisition
MRI data were collected on a Siemens Allegra 3 T scanner at the New York University Center for Brain Imaging. We acquired a high-resolution T1-weighted volume for each participant (TR = 2530 ms; TE = 3.25 ms; TI = 1100 ms; flip angle = 7°; 128 slices; field of view = 256 mm; voxel size = 1.3 × 1 × 1 mm).
2.3. Surface reconstruction and neuroanatomical measurements
FreeSurfer (5.1.0) software package (http://surfer.nmr.mgh.harvard.edu) was used to reconstruct cortical surfaces of each participant from the MRI scans. Main steps included (1) Talairach registration, (2) intensity normalization, (3) skull stripping, (4) white matter segmentation, (5) generation, refinement and tessellation of the white matter surface (i.e., the boundary between gray and white matter), (6) deformation of the white matter surface into the pial surface (i.e., the boundary between the gray matter and the cerebrospinal fluid) and (7) automatic correction of topological defects. Details of these steps are described elsewhere (Dale et al., 1999; Fischl et al., 1999, 2001). To ensure accuracy of the reconstruction, we also inspected and manually edited the reconstructed surfaces whenever necessary during the process. All inspection and editing were performed by one trained operator to avoid variability introduced by multiple raters.
CT at each vertex was measured as the average of the shortest distances from this vertex to the opposing surface, and to this vertex from the opposing surface (Fischl and Dale, 2000); SA at each vertex was measured as the average number of tessellation units surrounding it (Winkler et al., 2012). GMV at each vertex was the product of CT and SA. For group comparisons of CT, SA and GMV, cortical surfaces of each participant were registered based on folding patterns to a spherical coordinate system (Fischl et al., 1999). Individual CT, SA and GMV maps were smoothed with a Gaussian kernel (10 mm FWHM) before comparison. For group comparisons of asymmetry indices (AI), an inter-hemispheric registration procedure was adopted to register surfaces of both left and right hemispheres to a symmetrical template (Greve et al., 2013). Individual CT, SA, and GMV maps were then smoothed with a Gaussian kernel (10 mm FWHM). Asymmetry indices were constructed as (left − right) / (left + right) and calculated vertex-wise for each measure. A positive AI value indicated leftward lateralization and a negative value indicated rightward lateralization. Fig. 1 is an example of an AI map based on CT from one participant.
Fig. 1.
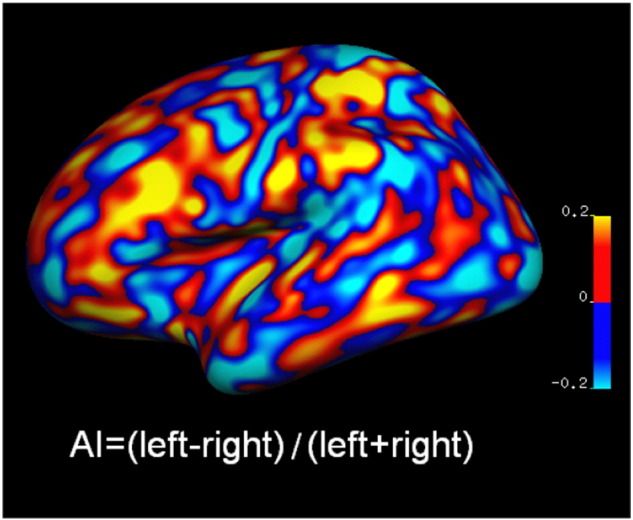
An example of an AI map based on cortical thickness (CT) from one participant. AI = asymmetry index. Positive value (red) indicated left lateralization; negative value (blue) indicated right lateralization.
2.4. Regions-of-interest (ROI) and cortical mask
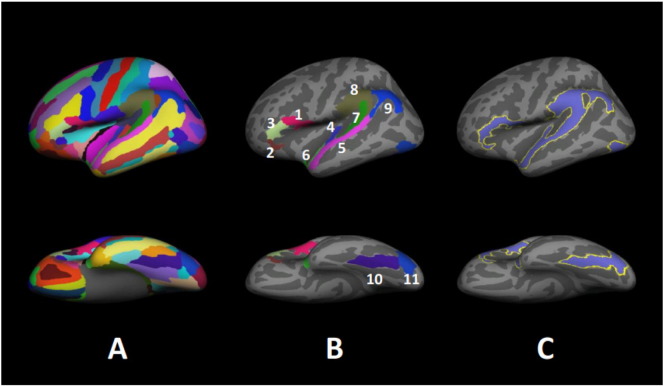
We selected 11 ROIs within cortical areas that have been reported to show gray matter abnormalities in dyslexia (Altarelli et al., 2013; Eckert, 2004; Kronbichler et al., 2008; Pernet et al., 2009). Using an anatomical parcellation atlas (Destrieux et al., 2010), we examined the following regions: (1) inferior frontal gyrus pars opercularis (IFGOp), (2) inferior frontal gyrus pars orbitalis (IFGOr), (3) inferior frontal gyrus pars triangularis (IFGTr), (4) Heschl's gyrus (HG), (5) the superior temporal gyrus (STG), (6) planum polare (PP), (7) planum temporale (PT), (8) supramarginal gyrus (SMAR), (9) angular gyrus (AG), (10) fusiform gyrus (FFG), and (11) inferior occipital gyrus (IOG). The regions were then combined into one cortical mask for each hemisphere for vertex-wise analysis. Fig. 2 shows the Destrieux atlas, the cortical ROIs, and the mask overlaid on the inflated white matter surface of the left hemisphere.
Fig. 2.
Destrieux Atlas, ROIs, and cortical mask overlaid on the inflated white matter surface of the left hemisphere. Only the left hemisphere is shown, laterally. (A) Destrieux atlas; (B) ROIs. 1)  IFGOp: inferior frontal gyrus pars opercularis, 2)
IFGOp: inferior frontal gyrus pars opercularis, 2)  IFGOr: inferior frontal gyrus pars orbitalis, 3)
IFGOr: inferior frontal gyrus pars orbitalis, 3)  IFGTr: inferior frontal gyrus pars triangularis, 4)
IFGTr: inferior frontal gyrus pars triangularis, 4)  HG: Heschl's gyrus, 5)
HG: Heschl's gyrus, 5)  STG: superior temporal gyrus, 6)
STG: superior temporal gyrus, 6)  PP: planum polare, 7)
PP: planum polare, 7)  PT: planum temporale, 8)
PT: planum temporale, 8)  SMAR: supramarginal gyrus, 9)
SMAR: supramarginal gyrus, 9)  AG: angular gyrus, 10)
AG: angular gyrus, 10)  FFG: fusiform gyrus, 11)
FFG: fusiform gyrus, 11)  IOG: inferior occipital gyrus; (C) Cortical mask derived from the ROIs
IOG: inferior occipital gyrus; (C) Cortical mask derived from the ROIs
2.5. Statistical analysis
Alterations in CT, SA, GMV, and their lateralization associated with a history of dyslexia were investigated with vertex-wise t tests between the TDC and the Dys groups within the cortical mask (vertex-wise alpha = .05). Significance maps were corrected for multiple comparisons using cluster-based Monte-Carlo simulation with 10,000 iterations of randomly generated z maps within the mask (cluster-wise alpha = .05) (Hagler et al., 2006). Group-wise comparison between each dyslexia subgroup and the TDC group was then performed in significant clusters found through this approach to further confirm our prediction that such alteration was present across all dyslexia subgroups regardless of remediation status. Possible additional effects of remediation on gray matter structures were tested by vertex-wise ANOVA between the dyslexia subgroups on each measure and its lateralization within the mask. Significant maps were corrected for multiple comparisons using the same cluster-based Monte-Carlo simulation. Finally, to consider abnormalities outside the cortical mask, we conducted vertex-wise whole-brain analyses following the same procedure for the masked analysis.
3. Results
3.1. Masked analysis results
3.1.1. Cortical thickness
Vertex-wise t tests of CT within the cortical mask revealed that the Dys group had significantly thicker cortex than the TDC group in a cluster in the left fusiform gyrus (centroid MNI: −32, −46, −20, cluster-wise p = .006) and a cluster in the right superior temporal gyrus, extending into the planum temporale (centroid MNI: 61, −28, 11, cluster-wise p = .0001).
In the left fusiform gyrus cluster, mean CT of the Dys group was significantly larger than the TDC group (Mean_Dys = 3.05 mm, Mean_TDC = 2.86 mm, t(62) = –3.654, p = .001, 95%CI = [−0.294, −0.086] mm). Group-wise comparisons of mean CT between the TDC (Mean_TDC = 2.86 mm) and the three dyslexia subgroups confirmed significant increase in CT in the Dys-R (Mean_Dys-R = 3.02 mm, t(41) = −2.13, p = .037, 95%CI = [−.30, −.01] mm) and Dys-RS (Mean_Dys-RS=3.13 mm, t(41) = −3.65, p = .001, 95%CI = [−.41, −.11] mm) groups, and marginally significant increase in the Dys-N group (Mean_Dys-N = 3.01 mm, t(40) = −1.95, p = .056, 95%CI = [−.298, 0.004] mm).
In the right superior temporal gyrus cluster, mean CT of the Dys group was significantly larger than the TDC group (Mean_Dys = 3.26 mm, Mean_TDC = 3.05 mm, t(62) = −4.025, p < .001, 95%CI = [−0.313, −0.105] mm). Group-wise comparisons of mean CT revealed CT increase in Dys-N (Mean_Dys-N = 3.31 mm, t(40) = −3.40, p = .001, 95%CI = [−.41, −.11] mm), Dys-R (Mean_Dys-R = 3.23 mm, t(41) = −2.39, p = .020, 95%CI = [−.32, −.03] mm) and Dys-RS (Mean_Dys-RS = 3.25 mm, t(41) = −2.70, p = .009, 95%CI = [−.35, −.05] mm) groups as compared to the TDC group. See Figs. 3 and 4 for a summary of findings.
Fig. 3.
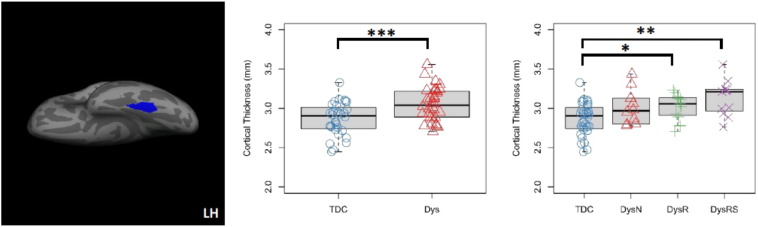
Cortical thickness (CT) increase in the left fusiform gyrus in dyslexia revealed by the masked analysis. Left: cluster in the left fusiform gyrus with significantly thicker cortex in the Dys than the TDC group (centroid MNI: −32, −46, −20, cluster-wise p = .006). Middle: boxplot and stripchart of mean CT of the significant cluster in the two group comparison. Right: boxplot and stripchart of mean CT of the significant cluster in the TDC and the three dyslexia subgroups. TDC = typically developing children, Dys = dyslexia, Dys-N = dyslexia with no remediation, Dys-R = dyslexia with reading remediation, Dys-RS = dyslexia with reading and spelling remediation, LH = left hemisphere. ***p < .001, **p < .01, *p < .05.
Fig. 4.
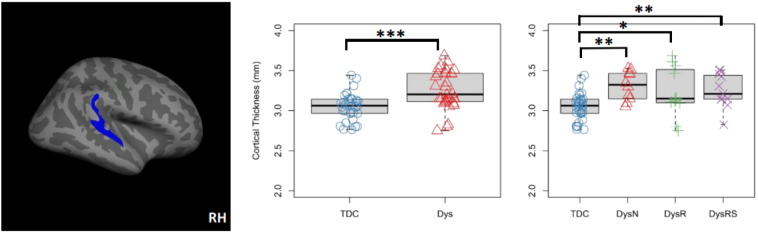
Cortical thickness (CT) increase in the right superior temporal gyrus in dyslexia revealed by the masked analysis. Left: cluster in the right superior temporal gyrus with significantly thicker cortex in the Dys than the TDC group (centroid MNI: 61, −28, 11, cluster-wise p = .0001). Middle: boxplot and stripchart of mean cortical thickness of the significant cluster in the two group comparison. Right: boxplot and stripchart of mean CT of the significant cluster in the TDC and the three dyslexia subgroups. TDC = typically developing children, Dys = dyslexia, Dys-N = dyslexia with no remediation, Dys-R = dyslexia with reading remediation, Dys-RS = dyslexia with reading and spelling remediation, RH = right hemisphere. ***p < .001, **p < .01, *p < .05.
Vertex-wise ANOVA among the dyslexia subgroups revealed no additional effect of remediation on CT.
3.1.2. Cortical thickness asymmetry
Vertex-wise t tests of AI based on CT within the cortical mask revealed a significant group difference in lateralization in the superior temporal gyrus. This cluster overlaps with the right superior temporal gyrus cluster (Fig. 4), where the Dys group had thicker cortex than the TDC group in the right hemisphere (centroid MNI: 60, −36, 9, cluster-wise p = .025). For this cluster, the Dys group was more right lateralized than the TDC group (Mean_Dys = −0.035, Mean_TDC = 0.005, t(62) = 3.717, p < .001, 95%CI = [0.188, 0.626]). Specifically, CT showed a rightward asymmetrical pattern in the Dys group (t(31) = −3.97, p < .001, 95%CI = [−.053, −.017]), while it was not significantly asymmetrical in the TDC group (t(31) = 1.241, p > .05, 95%CI = [−.008,.019]). A follow-up repeated measures ANOVA detected a significant interaction between hemisphere and group for mean CT in this cluster, F(1, 62) = 13.35, p = .001: the cortex within this cluster was significantly thicker in the Dys group (Mean_Dys = 3.18 mm) than in the TDC group (Mean_TDC = 2.96 mm) in the right hemisphere, t(62) = 3.84, p < .001, 95%CI = [−0.33, −0.11] mm. However, no significant group difference was found in the left hemisphere, Mean_Dys = 2.97 mm, Mean_TDC = 2.99 mm, t(62) = .26, p > .05, 95%CI = [−0.11, 0.14] mm. Thus, the deviation from normal lateralization pattern in the Dys group for this cluster was driven by increased CT in the right hemisphere rather than decreased CT in the left hemisphere.
Group-wise comparisons of mean AI for the superior temporal gyrus cluster between the TDC and the three dyslexia subgroups confirmed that rightward asymmetry in CT was present in the Dys-N (Mean_Dys-N = −.027, t(40) = 2.05, p = .045, 95%CI = [.0007, .064]), Dys-R (Mean_Dys-R = −.052, t(41) = 3.78, p < .001, 95%CI = [.027, .088]) and Dys-RS (Mean_Dys-RS = −.026, t(41) = 2.06, p = .044, 95%CI = [.0009, .062]) subgroups. See Fig. 5 for a summary of findings.
Fig. 5.
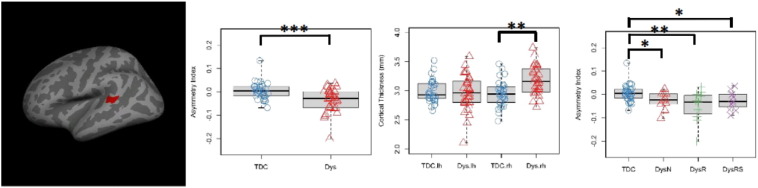
Lateralization findings in the masked analysis. Left: cluster in the superior temporal gyrus that exhibited significantly more rightward lateralization in the Dys than the TDC group (centroid MNI: 60, −36, 9, cluster-wise p = .025). Middle left: boxplot and stripchart of mean AI values for the significant cluster in the two group comparison. The TDC group showed symmetrical pattern while the Dys group was rightward asymmetrical. Middle right: boxplot and stripchart of mean cortical thickness (CT) of the significant cluster. Mean CT was comparable in the two groups in the left hemisphere, while larger in the Dys group in the right hemisphere. Right: boxplot and stripchart of mean AI of the significant cluster in the TDC and the three dyslexia subgroups. AI = asymmetry index, TDC = typically developing children, Dys = dyslexia, Dys-N = dyslexia with no remediation, Dys-R = dyslexia with reading remediation, Dys-RS = dyslexia with reading and spelling remediation. ***p < .001, **p < .01, *p < .05.
Vertex-wise ANOVA among the dyslexia subgroups revealed no additional effect of remediation on CT lateralization.
3.1.3. Surface area, gray matter volume, and lateralization indices
Vertex-wise t test within the cortical mask revealed no significant differences in either SA or GMV between the Dys and the TDC groups. Similarly, no significant group difference was observed in any lateralization index. Null findings for SA, GMV, and their lateralization were not tested across dyslexia subgroups. Vertex-wise ANOVA among dyslexia subgroups also failed to detect changes in these measures associated with remediation.
3.2. Whole-brain analysis results
Vertex-wise whole-brain analyses revealed only one significant cluster with abnormal cortical thickness increase in the right superior temporal gyrus, extending into the planum temporale, middle temporal gyrus, posterior Sylvian fissure, Heschl's gyrus and supramarginal gyrus (centroid MNI: 55.6, −26.2, 2.5, cluster-wise p = .0001). This cluster encompasses the right superior temporal gyrus cluster in the masked analysis (Fig. 4). Similar to the masked analysis results, in this cluster, mean CT of the Dys group was significantly larger than the TDC group (Mean_Dys = 3.13 mm, Mean_TDC = 2.92 mm, t(62) = −4.768, p < .001, 95%CI = [−0.29, −0.12] mm). Group-wise comparisons of mean CT revealed CT increase in Dys-N (Mean_Dys-N = 3.16 mm, t(40) = −4.58, p < .001, 95%CI = [−.34, −.13] mm), Dys-R (Mean_Dys-R = 3.10 mm, t(41) = −2.78, p = .008, 95%CI = [−.31, −.05] mm) and Dys-RS (Mean_Dys-RS = 3.12 mm, t(41) = −3.82, p < .001, 95%CI = [−.31, −.09] mm) groups as compared to the TDC group. See Fig. 6 for a summary of findings.
Fig. 6.
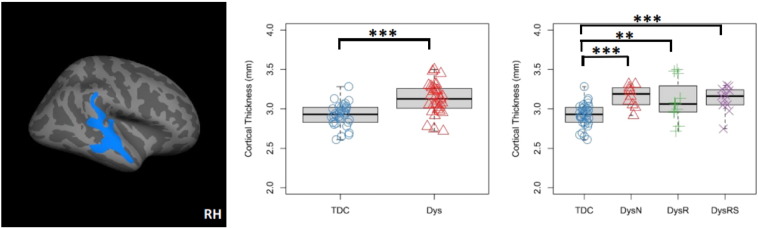
Cortical thickness (CT) increase in the right superior temporal gyrus in dyslexia revealed by whole-brain analysis. Left: cluster in the right superior temporal gyrus with significantly thicker cortex in the Dys than the TDC group (centroid MNI: 55.6, −26.2, 2.5, cluster-wise p = .0001). Middle: boxplot and stripchart of mean cortical thickness of the significant cluster in the two group comparison. Right: boxplot and stripchart of mean CT of the significant cluster in the TDC and the three dyslexia subgroups. TDC = typically developing children, Dys = dyslexia, Dys-N = dyslexia with no remediation, Dys-R = dyslexia with reading remediation, Dys-RS = dyslexia with reading and spelling remediation, RH = right hemisphere. ***p < .001, **p < .01, *p < .05.
4. Discussion
Our findings demonstrate that children with dyslexia, irrespective of remediation status, exhibit cortical thickness (CT) abnormalities in the left fusiform gyrus and the right superior temporal gyrus, extending into the right planum temporale. The CT increase in the right superior temporal gyrus contributed to a rightward asymmetry of a smaller area in the posterior superior temporal gyrus. No abnormalities were identified by either surface area (SA) or gray matter volume (GMV). This is consistent with prior studies that suggest CT as a phenotypic measure that is independent from GMV and SA (Dickerson et al., 2009; Lemaitre et al., 2012; Panizzon et al., 2009; Winkler et al., 2010). The consistency of the observed effects across three different dyslexia subgroups, irrespective of remediation status, suggests that our findings should generalize to children with a history of dyslexia diagnosis, regardless of reading level. This supports that cortical thickness abnormalities, at least in the temporal and occipitotemporal regions, are inherent to dyslexia.
4.1. Left fusiform gyrus cortical thickness increase in dyslexia
Our finding of increased CT in the left fusiform gyrus indicates persistent abnormalities in the ventral reading pathway in dyslexia. The left fusiform gyrus cluster we identified contains the Visual Word Form Area, a region critical for visual word processing (Cohen et al., 2000, 2002). This region may also play a role in object naming (McCrory et al., 2005) and phonological decoding (e.g., sounding out the spoken representation of a written word) (Desroches et al., 2010; Dietz et al., 2005). Abnormalities associated with the left fusiform gyrus in dyslexia include decreased GMV (Kronbichler et al., 2008; Raschle et al., 2011) and altered activation during reading-related tasks (Aylward et al., 2003; McCrory et al., 2005; Pugh et al., 2000a; Richlan et al., 2009, 2010). Our finding extends the range of dyslexia-associated anomalies in this region to include abnormal CT increase.
A previous study by Altarelli et al. (2013) detected dyslexia-related CT reduction in a subregion [MNI: −42, −48, −15] of the left occipitotemporal region that overlaps with our left fusiform finding. The mean age of this study sample was comparable to ours, although differences were present in girls but not boys in their study. To facilitate comparison with our findings, we performed a 2 (dyslexia, control) by 2 (girls, boys) ANOVA on mean CT of the left fusiform gyrus cluster in our sample to test for an interaction (see Supplementary Results 1). Our analysis revealed no differential effect by gender; abnormal CT increase was present in both boys and girls.
Abnormal increase in CT may result from a failure of myelination or synaptic pruning during a critical period of development. The age range of our sample (7–16) corresponds to a period of brain maturation in which wide-spread cortical thinning accompanies myelination and synaptic pruning (Sowell et al., 2004). In a sample of individuals ranging in age from 9 to 23, thinner cortex in the fusiform gyrus is associated with stronger verbal fluency (Porter et al., 2011). Similarly, in adults, thinner cortex in this region is associated with stronger performance on a phonetically irregular word reading test (Blackmon et al., 2010). Children with dyslexia may show disruption or delay of developmental pruning, potentially leading to thicker cortex. To interrogate possible age-related differences in our dyslexia sample relative to controls, we performed supplementary analyses of the interaction between age and group on CT in each significant cluster [i.e., left fusiform cluster, right superior temporal gyrus cluster, and the superior temporal gyrus cluster with significant AI difference between groups (see Supplementary Results 2)]. There were no interactions between age and group in any of these clusters. Furthermore, there was evidence for a decrease in CT as a function of age in the right superior temporal gyrus cluster in the Dys group, which is consistent with typical growth trajectories for this age range (Shaw et al., 2006, 2008; Sowell et al., 2004; Tamnes et al., 2010). Although these results should be interpreted with caution given our cross-sectional data, they rule out age-related effects as the primary factor driving the observed group differences. To scrutinize factors underlying increased left fusiform CT in dyslexia, a future study should employ a longitudinal approach to examine developmental changes in CT from the pre-reading period.
4.2. Rightward cortical thickness lateralization of the superior temporal gyrus in dyslexia
In all three dyslexia subgroups, we found increased CT in the right superior temporal gyrus, extending into the planum temporale, which resulted in deviation from normal lateralization pattern in a smaller area within this region. Whole-brain analysis revealed that abnormally increased CT extended into the surrounding areas including the posterior Sylvian fissure and perisylvian regions.
The superior temporal region is involved in auditory processing, the disruption of which is directly relevant to dyslexia (Eckert, 2004; Eckert and Leonard, 2000; Eden and Zeffiro, 1998). Increase in CT and deviation from normal lateralization pattern in this area may reflect disruption in functional networks supporting auditory processing. Functional imaging studies of dyslexia support this notion: there is hyperactivation in the right superior temporal gyrus, accompanied by underactivation in the left posterior temporal and inferior parietal regions in dyslexic children and adults (Rumsey et al., 1992; Simos et al., 2000, 2002). Similarly, a functional connectivity study showed increased connectivity with the right temporoparietal region (i.e., adjacent to our right superior temporal gyrus cluster), together with decreased connectivity with the left temporal region, during both rest and tasks (Schurz et al., 2014). Thus, our findings indicate that deviation from normal language organization in dyslexia might be reflected by increased right hemisphere CT and subsequently abnormal rightward CT lateralization in the superior temporal region.
Our study revealed CT symmetry in typically developing children and rightward asymmetry in children with dyslexia. Previous studies have reported leftward asymmetry in SA/GMV in both typically developing children and neurologically normal adults, with symmetry or rightward asymmetry in individuals with dyslexia (Altarelli et al., 2014; Galaburda et al., 1985; Kushch et al., 1993; Larsen et al., 1990). These seemingly inconsistent findings are not surprising, but rather emphasize that CT is independent from SA and GMV (Dickerson et al., 2009; Lemaitre et al., 2012; Winkler et al., 2010) with its own lateralization pattern. Further support for our results comes from studies on CT asymmetry in the general population, which have not found asymmetry in either typically developing children or healthy adults in our superior temporal gyrus cluster (Luders et al., 2006; Shaw et al., 2009; Zhou et al., 2013). Combined with previous studies, our finding indicates relative increase in the right hemisphere cortical structures as compared to the left hemisphere in dyslexia.
Previous studies suggest that activation of the right perisylvian region during language tasks is functional in dyslexia, compensating for disruptions in the left hemisphere language network (Pugh et al., 2000a,b; Rumsey et al., 1999). Right angular gyrus cerebral blood flow during single-word reading tasks is positively correlated with reading scores in dyslexia, and negatively correlated in controls (Rumsey et al., 1999). However, given the absence of remediation effects on brain structure in our study, it is not likely that findings reflect compensatory synaptogenesis and neuronal growth in the right hemisphere superior temporal gyrus. This does not mean that compensatory functional network changes are not present in this region, only that they do not appear to be accompanied by macroscopic structural changes (Eden et al., 2004; Temple et al., 2003).
That abnormalities persist regardless of remediation status indicates that deviation from normal superior temporal asymmetry in the dyslexia group is more suggestive of an anatomical anomaly associated with a history of dyslexia rather than compensatory brain growth. Similar rightward anomalies were recently found in a sample of children with dyslexia of comparable age to our sample, although results were limited to a rightward asymmetry in SA, not CT, and were found in boys but not girls (Altarelli et al., 2014). Deviation from normal planum temporale asymmetry occurs in approximately 35% of the postmortem brains of neurologically normal adults and does not identify a reading disorder in isolation (Geschwind and Levitsky, 1968). However, Galaburda et al. (1985) argued that this finding, in combination with left hemisphere cortical anomalies, might increase the likelihood of a developmental reading disorder. This “double hit” hypothesis is supported by our macro-structural MRI findings.
4.3. Normal surface area, gray matter volume and their lateralization in dyslexia
Our study detected no abnormalities in SA, GMV, or their lateralization in individuals with a history of dyslexia, although we adopted widely accepted procedures for semi-automated morphometric analysis of high resolution MRI scans (Clarkson et al., 2011; Dewey et al., 2010; Ghosh et al., 2010; Pantazis et al., 2010) and controlled for confounding variables including age, gender, handedness and IQ. This suggests that negative results should not be attributed to novel methodology or sample confounds. Also, the lack of remediation effect on these measures reduces the likelihood that variation introduced by remediation has confounded the results.
The absence of abnormalities associated with SA, GMV, and their lateralization is inconsistent with previous positive findings (Altarelli et al., 2014; Galaburda et al., 1985; Kushch et al., 1993; Larsen et al., 1990; Leonard et al., 2001; Richlan et al., 2013). However, convergence between gray matter abnormality findings has been limited as is pointed out by Richlan et al. (2013), and no net differences between dyslexia and typically developing children in GMV were detected in one study with comparable sample size to ours (Pernet et al., 2009). Similarly, normal SA and GMV lateralization in dyslexia has been reported by recent studies (Best and Demb, 1999; Eckert et al., 2003; Eckert and Leonard, 2000; Heiervang et al., 2000; Leonard et al., 1993; Leonard et al., 2001; Pernet et al., 2009; Preis et al., 1998; Robichon et al., 2000; Rumsey et al., 1997; Schultz et al., 1994). Such inconsistency across studies, along with our findings of abnormalities in CT and its lateralization associated with a history of dyslexia, supports the notion that dyslexia is associated with multiple neural risk factors (Eckert and Leonard, 2000). Our findings showing differences in dyslexia are restricted to CT. This is in accord with the notion that CT and SA are independent measures (Dickerson et al., 2009; Lemaitre et al., 2012; Winkler et al., 2010) and that GMV is influenced more by the latter than by the former (Frye et al., 2010; Kapellou et al., 2006; Winkler et al., 2010).
4.4. No effect of remediation on cortical structures in dyslexia
In our study, no changes in cortical structure associated with remediation were detected. This is in contrast to prior research showing that remediation is associated with changes in task-related brain activity (Aylward et al., 2003; Richards et al., 2000; Shaywitz et al., 2004; Simos et al., 2002, 2007; Temple et al., 2003), intrinsic functional connectivity (Koyama et al., 2013), white matter integrity (Keller and Just, 2009), and GMV (Krafnick et al., 2011). We propose several factors that might have contributed to the lack of differences in gray matter associated with remediation status in our study. First, functional and structural MRI findings do not necessarily correspond. Specifically, changes in brain functioning may not be accompanied by macroscopic changes in cortical structure (Haier et al., 2009). Second, the variety of interventions adopted by our participants, which likely affects multiple brain regions (Démonet et al., 2004), may have diluted focal effects, such as those observed in prior studies (Krafnick et al., 2011). Third, gray matter changes that are observed shortly after remediation training may be reversed when assessed after a long delay, although improvement in reading skills persists (Driemeyer et al., 2008). Finally, the smaller sample size of our remediation subgroups may have prevented more subtle effects of remediation from being detected. Our findings do not rule out remediation effect on cortical structure in dyslexia, but emphasize the presence of cortical abnormalities that persist despite remediation.
4.5. Limitations
Several limitations should be kept in mind when interpreting the findings of our study. First, we matched participant groups on age, gender, handedness and IQ to prevent the confounding effect of these factors. However, the distributions of these variables often differ between children with dyslexia and typically developing children (Eglinton and Annett, 1994; Stuebing et al., 2002); therefore, our findings should be considered generalizable to children with dyslexia who are right-handed and have an IQ within normal limits. Second, while our study reveals focal regions of abnormal CT associated with a history of dyslexia, the cellular and cytoarchitectonic nature of these abnormalities remains unknown. Prior histological studies suggest the presence of cortical ectopias and dysplasia (Galaburda et al., 1985; Humphreys et al., 1990) but these studies are few in number and require updating with more recent classification schemes for focal cortical dysplasia (Blümcke et al., 2011). Such work would provide critical information for comparing the features of cortical malformations across different neurodevelopmental disorders, such as autism and epilepsy, and would improve our knowledge of dyslexia etiology.
Furthermore, while left fusiform and right superior temporal gyrus CT abnormalities may be a persistent marker of dyslexia in childhood, this may no longer be the case in adulthood. In a sample of adults ranging in age from 20 to 42, there were no findings of CT abnormalities associated with dyslexia (Frye et al., 2010). A recent longitudinal study of CT trajectory from the autism literature may shed light on this discrepancy (Zielinski et al., 2014). In this study, abnormal CT development in autism varied with age, so that while child participants with autism had thicker cortex than typically developing participants in the occipital lobe, such a difference was not present in adolescence. Further studies that span children and adults are needed to determine whether MRI markers differ across developmental epochs.
Finally, our study design is observational and cross-sectional; therefore, we were not able to investigate within-subject changes in structure that may accompany remediation. However, our study design is comparable to prior cross-sectional studies that report structural differences pre-dating reading experience (Raschle et al., 2011) and those that report no structural differences when reading level is equated (Krafnick et al., 2014). Our results should therefore be considered an extension of this debate, with additional support for the former hypothesis of an early developmental origin for structural abnormalities (Raschle et al., 2011) rather than an acquired structural variant attributable to insufficient reading experience (Krafnick et al., 2014).
5. Conclusions
Using surface-based analysis of cortical structures, we found increased CT of the left fusiform gyrus and right superior temporal gyrus (extending into the right planum temporale) in individuals with a history of dyslexia. Thus, we detected structural abnormalities in both the ventral and dorsal processing pathways of the known reading network. These effects were irrespective of remediation status; they were present in children in two dyslexia subgroups who had remediated their reading and/or spelling performance. This suggests that structural differences are associated with early developmental factors leading to dyslexia diagnosis rather than reading level effects, as has been recently suggested (Krafnick et al., 2014). Findings of a “double hit” in the dyslexia group, characterized by cortical anomalies in the left fusiform region and abnormal rightward asymmetry of the superior temporal gyrus, supports the theory that multiple structural anomalies confer a greater risk of reading impairment (Eckert and Leonard, 2000; Galaburda et al., 1985) than an isolated structural defect.
Acknowledgments
This study was supported by grants from National Institute of Mental Health, NIMH (R01MH081218) and Stavros Niarchos Foundation and by gifts from Phyllis Green and Randolph Cowen and Finding a Cure for Epilepsy and Seizures (FACES). The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to submit for publication.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2014.11.005.
Appendix A. Supplementary data
Supplementary material.
References
- Altarelli I., Leroy F., Monzalvo K., Fluss J., Billard C., Dehaene-Lambertz G., Ramus F. Planum temporale asymmetry in developmental dyslexia: revisiting an old question. Human brain mapping. Hum. Brain Mapp. 2014 doi: 10.1002/hbm.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarelli I., Monzalvo K., Iannuzzi S., Fluss J., Billard C., Ramus F., Dehaene-Lambertz G. A functionally guided approach to the morphometry of occipitotemporal regions in developmental dyslexia: evidence for differential effects in boys and girls. J. Neurosci. 2013;33(27):11296–11301. doi: 10.1523/JNEUROSCI.5854-12.2013. 23825432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward E.H., Richards T.L., Berninger V.W., Nagy W.E., Field K.M., Grimme A.C., Cramer S.C. Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology. 2003;61(2):212–219. doi: 10.1212/01.wnl.0000068363.05974.64. 12874401 [DOI] [PubMed] [Google Scholar]
- Best M., Demb J.B. Normal planum temporale asymmetry in dyslexics with a magnocellular pathway deficit. Neuroreport. 1999;10(3):607–612. doi: 10.1097/00001756-199902250-00030. 10208598 [DOI] [PubMed] [Google Scholar]
- Blackmon K., Barr W.B., Kuzniecky R., DuBois J., Carlson C., Quinn B.T., Blumberg M. Phonetically irregular word pronunciation and cortical thickness in the adult brain. Neuroimage. 2010;51(4):1453–1458. doi: 10.1016/j.neuroimage.2010.03.028. 20302944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümcke I., Thom M., Aronica E., Armstrong D.D., Vinters H.V., Palmini A., Battaglia G. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc task Force of the ILAE diagnostic methods Commission. Epilepsia. 2011;52(1):158–174. doi: 10.1111/j.1528-1167.2010.02777.x. 21219302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson M.J., Cardoso M.J., Ridgway G.R., Modat M., Leung K.K., Rohrer J.D., Ourselin S. A comparison of voxel and surface based cortical thickness estimation methods. Neuroimage. 2011;57(3):856–865. doi: 10.1016/j.neuroimage.2011.05.053. 21640841 [DOI] [PubMed] [Google Scholar]
- Cohen L., Dehaene S., Naccache L., Lehéricy S., Dehaene-Lambertz G., Hénaff M.A., Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(2):291–307. doi: 10.1093/brain/123.2.291. 10648437 [DOI] [PubMed] [Google Scholar]
- Cohen L., Lehéricy S., Chochon F., Lemer C., Rivaud S., Dehaene S. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 2002;125(5):1054–1069. doi: 10.1093/brain/awf094. 11960895 [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. 9931268 [DOI] [PubMed] [Google Scholar]
- Démonet J.-F., Taylor M.J., Chaix Y. Developmental dyslexia. Lancet. 2004;363(9419):1451–1460. doi: 10.1016/S0140-6736(04)16106-0. [DOI] [PubMed] [Google Scholar]
- Desroches A.S., Cone N.E., Bolger D.J., Bitan T., Burman D.D., Booth J.R. Children with reading difficulties show differences in brain regions associated with orthographic processing during spoken language processing. Brain Res. 2010;1356:73–84. doi: 10.1016/j.brainres.2010.07.097. 20691675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux C., Fischl B., Dale A., Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. 20547229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey J., Hana G., Russell T., Price J., McCaffrey D., Harezlak J., Navia B. Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. Neuroimage. 2010;51(4):1334–1344. doi: 10.1016/j.neuroimage.2010.03.033. 20338250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson B.C., Feczko E., Augustinack J.C., Pacheco J., Morris J.C., Fischl B., Buckner R.L. Differential effects of aging and Alzheimer's disease on medial temporal lobe cortical thickness and surface area. Neurobiol. Aging. 2009;30(3):432–440. doi: 10.1016/j.neurobiolaging.2007.07.022. 17869384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz N.A., Jones K.M., Gareau L., Zeffiro T.A., Eden G.F. Phonological decoding involves left posterior fusiform gyrus. Hum. Brain Mapp. 2005;26(2):81–93. doi: 10.1002/hbm.20122. 15934062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driemeyer J., Boyke J., Gaser C., Büchel C., May A. Changes in gray matter induced by learning — revisited. PLoS ONE. 2008;3(7):e2669. doi: 10.1371/journal.pone.0002669. 18648501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert M. Neuroanatomical markers for dyslexia: a review of dyslexia structural imaging studies. Neuroscientist. 2004;10(4):362–371. doi: 10.1177/1073858404263596. 15271263 [DOI] [PubMed] [Google Scholar]
- Eckert M.A., Leonard C.M. Structural imaging in dyslexia: the planum temporale. Ment. Retard. Dev. Disabil. Res. Rev. 2000;6(3):198–206. doi: 10.1002/1098-2779(2000)6:3<198::AID-MRDD7>3.0.CO;2-1. 10982497 [DOI] [PubMed] [Google Scholar]
- Eckert M.A., Leonard C.M., Richards T.L., Aylward E.H., Thomson J., Berninger V.W. Anatomical correlates of dyslexia: frontal and cerebellar findings. Brain. 2003;126(2):482–494. doi: 10.1093/brain/awg026. 12538414 [DOI] [PubMed] [Google Scholar]
- Eden G.F., Jones K.M., Cappell K., Gareau L., Wood F.B., Zeffiro T.A., Flowers D.L. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44(3):411–422. doi: 10.1016/j.neuron.2004.10.019. 15504323 [DOI] [PubMed] [Google Scholar]
- Eden G.F., Zeffiro T.A. Neural systems affected in developmental dyslexia revealed by functional neuroimaging. Neuron. 1998;21(2):279–282. doi: 10.1016/s0896-6273(00)80537-1. 9728909 [DOI] [PubMed] [Google Scholar]
- Eglinton E., Annett M. Handedness and dyslexia: a meta-analysis. Percept Mot Skills. 1994;79(3f):1611–1616. doi: 10.2466/pms.1994.79.3f.1611. 7870554 [DOI] [PubMed] [Google Scholar]
- Engvig A., Fjell A.M., Westlye L.T., Moberget T., Sundseth Ø., Larsen V.A., Walhovd K.B. Effects of memory training on cortical thickness in the elderly. Neuroimage. 2010;52(4):1667–1676. doi: 10.1016/j.neuroimage.2010.05.041. 20580844 [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U.S.A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. 10984517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Liu A., Dale A.M. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. 11293693 [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Tootell R.B., Dale A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. 10619420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye R.E., Liederman J., Malmberg B., McLean J., Strickland D., Beauchamp M.S. Surface area accounts for the relation of gray matter volume to reading-related skills and history of dyslexia. Cereb. Cortex. 2010;20(11):2625–2635. doi: 10.1093/cercor/bhq010. 20154011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda A.M., Sherman G.F., Rosen G.D., Aboitiz F., Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Ann. Neurol. 1985;18(2):222–233. doi: 10.1002/ana.410180210. 4037763 [DOI] [PubMed] [Google Scholar]
- Geschwind N., Levitsky W. Human brain: left–right asymmetries in temporal speech region. Science. 1968;161(3837):186–187. doi: 10.1126/science.161.3837.186. 5657070 [DOI] [PubMed] [Google Scholar]
- Ghosh S.S., Kakunoori S., Augustinack J., Nieto-Castanon A., Kovelman I., Gaab N., Fischl B. Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11 years of age. Neuroimage. 2010;53(1):85–93. doi: 10.1016/j.neuroimage.2010.05.075. 20621657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve D.N., Van der Haegen L., Cai Q., Stufflebeam S., Sabuncu M.R., Fischl B., Brysbaert M. A surface-based analysis of language lateralization and cortical asymmetry. J. Cogn. Neurosci. 2013;25(9):1477–1492. doi: 10.1162/jocn_a_00405. 23701459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler D.J., Saygin A.P., Sereno M.I. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33(4):1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. 17011792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier R.J., Karama S., Leyba L., Jung R.E. MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual-spatial task. BMC Res Notes. 2009;2(1):174. doi: 10.1186/1756-0500-2-174. 19723307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett H.C., Poe M.D., Gerig G., Styner M., Chappell C., Smith R.G., Piven J. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch. Gen. Psychiatry. 2011;68(5):467–476. doi: 10.1001/archgenpsychiatry.2011.39. 21536976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiervang E., Hugdahl K., Steinmetz H., Inge Smievoll A., Stevenson J., Lund A., Ersland L., Lundervold A. Planum temporale, planum parietale and dichotic listening in dyslexia. Neuropsychologia. 2000;38(13):1704–1713. doi: 10.1016/s0028-3932(00)00085-3. 11099728 [DOI] [PubMed] [Google Scholar]
- Humphreys P., Kaufmann W.E., Galaburda A.M. Developmental dyslexia in women: neuropathological findings in three patients. Ann. Neurol. 1990;28(6):727–738. doi: 10.1002/ana.410280602. 2285260 [DOI] [PubMed] [Google Scholar]
- Joshi A.A., Leporé N., Joshi S.H., Lee A.D., Barysheva M., Stein J.L., Martin N.G. The contribution of genes to cortical thickness and volume. Neuroreport. 2011;22(3):101–105. doi: 10.1097/WNR.0b013e3283424c84. 21233781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapellou O., Counsell S.J., Kennea N., Dyet L., Saeed N., Stark J., Hajnal J. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3(8):e265. doi: 10.1371/journal.pmed.0030265. 16866579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, J., Birmaher, B., Brent, D., Rao, U., Ryan, N., The Schedule for Affective Disorders and Schizophrenia for School-age Children — Present and Lifetime Version (version 1.0). (1996) Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA. [DOI] [PubMed]
- Keller T.A., Just M.A. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64(5):624–631. doi: 10.1016/j.neuron.2009.10.018. 20005820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama M.S., Di Martino A., Kelly C., Jutagir D.R., Sunshine J., Schwartz S.J., Milham M.P. Cortical signatures of dyslexia and remediation: an intrinsic functional connectivity approach. PLoS ONE. 2013;8(2):e55454. doi: 10.1371/journal.pone.0055454. 23408984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafnick A.J., Flowers D.L., Luetje M.M., Napoliello E.M., Eden G.F. An investigation into the origin of anatomical differences in dyslexia. J. Neurosci. 2014;34(3):901–908. doi: 10.1523/JNEUROSCI.2092-13.2013. 24431448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafnick A.J., Flowers D.L., Napoliello E.M., Eden G.F. Gray matter volume changes following reading intervention in dyslexic children. Neuroimage. 2011;57(3):733–741. doi: 10.1016/j.neuroimage.2010.10.062. 21029785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M., Wimmer H., Staffen W., Hutzler F., Mair A., Ladurner G. Developmental dyslexia: gray matter abnormalities in the occipitotemporal cortex. Hum Brain Mapp. 2008;29(5):613–625. doi: 10.1002/hbm.20425. 17636558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushch A., Gross-Glenn K., Jallad B., Lubs H., Rabin M., Feldman E., Duara R. Temporal lobe surface area measurements on MRI in normal and dyslexic readers. Neuropsychologia. 1993;31(8):811–821. doi: 10.1016/0028-3932(93)90130-r. 8413902 [DOI] [PubMed] [Google Scholar]
- Larsen J.P., Høien T., Lundberg I., Ødegaard H. MRI evaluation of the size and symmetry of the planum temporale in adolescents with developmental dyslexia. Brain Lang. 1990;39(2):289–301. doi: 10.1016/0093-934x(90)90015-9. 2224496 [DOI] [PubMed] [Google Scholar]
- Lazar S.W., Kerr C.E., Wasserman R.H., Gray J.R., Greve D.N., Treadway M.T., Benson H. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16(17):1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. 16272874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre H., Goldman A.L., Sambataro F., Verchinski B.A., Meyer-Lindenberg A., Weinberger D.R., Mattay V.S. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol. Aging. 2012;33(3):617. doi: 10.1016/j.neurobiolaging.2010.07.013. 20739099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard C.M., Eckert M.A., Lombardino L.J., Oakland T., Kranzler J., Mohr C.M., Freeman A. Anatomical risk factors for phonological dyslexia. Cereb. Cortex. 2001;11(2):148–157. doi: 10.1093/cercor/11.2.148. 11208669 [DOI] [PubMed] [Google Scholar]
- Leonard C.M., Voeller K.K., Lombardino L.J., Morris M.K., Hynd G.W., Alexander A.W., Mao J. Anomalous cerebral structure in dyslexia revealed with magnetic resonance imaging. Arch. Neurol. 1993;50(5):461–469. doi: 10.1001/archneur.1993.00540050013008. 8489401 [DOI] [PubMed] [Google Scholar]
- Linkersdörfer J., Lonnemann J., Lindberg S., Hasselhorn M., Fiebach C.J. Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: an ALE meta-analysis. PLoS ONE. 2012;7(8):e43122. doi: 10.1371/journal.pone.0043122. 22916214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E., Narr K.L., Thompson P.M., Rex D.E., Jancke L., Toga A.W. Hemispheric asymmetries in cortical thickness. Cereb. Cortex. 2006;16(8):1232–1238. doi: 10.1093/cercor/bhj064. 16267139 [DOI] [PubMed] [Google Scholar]
- Lyon G.R., Shaywitz S., Shaywitz B. Defining dyslexia, comorbidity, teachers' knowledge of language and reading. Ann. Dyslexia. 2003;53:1–14. [Google Scholar]
- McCrory E.J., Mechelli A., Frith U., Price C.J. More than words: a common neural basis for reading and naming deficits in developmental dyslexia? Brain. 2005;128(2):261–267. doi: 10.1093/brain/awh340. 15574467 [DOI] [PubMed] [Google Scholar]
- Narr K.L., Woods R.P., Lin J., Kim J., Phillips O.R., Del’Homme M., Levitt J.G. Widespread cortical thinning is a robust anatomical marker for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(10):1014–1022. doi: 10.1097/CHI.0b013e3181b395c0. 19730275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon M.S., Fennema-Notestine C., Eyler L.T., Jernigan T.L., Prom-Wormley E., Neale M., Kremen W.S. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex. 2009;19(11):2728–2735. doi: 10.1093/cercor/bhp026. 19299253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis D., Joshi A., Jiang J., Shattuck D.W., Bernstein L.E., Damasio H., Leahy R.M. Comparison of landmark-based and automatic methods for cortical surface registration. Neuroimage. 2010;49(3):2479–2493. doi: 10.1016/j.neuroimage.2009.09.027. 19796696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet C., Andersson J., Paulesu E., Demonet J.F. When all hypotheses are right: a multifocal account of dyslexia. Hum Brain Mapp. 2009;30(7):2278–2292. doi: 10.1002/hbm.20670. 19235876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J.N., Collins P.F., Muetzel R.L., Lim K.O., Luciana M. Associations between cortical thickness and verbal fluency in childhood, adolescence, and young adulthood. Neuroimage. 2011;55(4):1865–1877. doi: 10.1016/j.neuroimage.2011.01.018. 21255662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preis S., Jäncke L., Schittler P., Huang Y., Steinmetz H. Normal intrasylvian anatomical asymmetry in children with developmental language disorder. Neuropsychologia. 1998;36(9):849–855. doi: 10.1016/s0028-3932(98)00033-5. 9740358 [DOI] [PubMed] [Google Scholar]
- Pugh K.R., Mencl W.E., Jenner A.R., Katz L., Frost S.J., Lee J.R., Shaywitz S.E. Functional neuroimaging studies of reading and reading disability(developmental dyslexia) Ment. Retard. Dev. Disabil. Res. Rev. 2000;6(3):207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. 10982498 [DOI] [PubMed] [Google Scholar]
- Pugh K.R., Mencl W.E., Shaywitz B.A., Shaywitz S.E., Fulbright R.K., Constable R.T., Gore J.C. The angular gyrus in developmental dyslexia: task-specific differences in functional connectivity within posterior cortex. Psychol Sci. 2000;11(1):51–56. doi: 10.1111/1467-9280.00214. 11228843 [DOI] [PubMed] [Google Scholar]
- Raschle N.M., Chang M., Gaab N. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage. 2011;57(3):742–749. doi: 10.1016/j.neuroimage.2010.09.055. 20884362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards T.L., Corina D., Serafini S., Steury K., Echelard D.R., Dager S.R., Berninger V.W. Effects of a phonologically driven treatment for dyslexia on lactate levels measured by proton MR spectroscopic imaging. AJNR Am. J. Neuroradiol. 2000;21(5):916–922. 10815668 [PMC free article] [PubMed] [Google Scholar]
- Richlan F., Kronbichler M., Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2009;30(10):3299–3308. doi: 10.1002/hbm.20752. 19288465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F., Kronbichler M., Wimmer H. Structural abnormalities in the dyslexic brain: a meta-analysis of voxel-based morphometry studies. Hum. Brain Mapp. 2013;34(11):3055–3065. doi: 10.1002/hbm.22127. 22711189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F., Sturm D., Schurz M., Kronbichler M., Ladurner G., Wimmer H. A common left occipito-temporal dysfunction in developmental dyslexia and acquired letter-by-letter reading? PLoS ONE. 2010;5(8):e12073. doi: 10.1371/journal.pone.0012073. 20711448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol L.M., Panizzon M.S., Fennema-Notestine C., Eyler L.T., Fischl B., Franz C.E., Pacheco J. Cortical thickness is influenced by regionally specific genetic factors. Biol. Psychiatry. 2010;67(5):493–499. doi: 10.1016/j.biopsych.2009.09.032. 19963208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichon F., Levrier O., Farnarier P., Habib M. Developmental dyslexia: atypical cortical asymmetries and functional significance. Eur. J. Neurol. 2000;7(1):35–46. doi: 10.1046/j.1468-1331.2000.00020.x. 10809913 [DOI] [PubMed] [Google Scholar]
- Rumsey J.M., Andreason P., Zametkin A.J., Aquino T., King A.C., Hamburger S.D., Cohen R.M. Failure to activate the left temporoparietal cortex in dyslexia. An oxygen 15 positron emission tomographic study. Arch. Neurol. 1992;49(5):527–534. doi: 10.1001/archneur.1992.00530290115020. 1580816 [DOI] [PubMed] [Google Scholar]
- Rumsey J.M., Donohue B.C., Brady D.R., Nace K., Giedd J.N., Andreason P. A magnetic resonance imaging study of planum temporale asymmetry in men with developmental dyslexia. Arch. Neurol. 1997;54(12):1481–1489. doi: 10.1001/archneur.1997.00550240035010. 9400357 [DOI] [PubMed] [Google Scholar]
- Rumsey J.M., Horwitz B., Donohue B.C., Nace K.L., Maisog J.M., Andreason P. A functional lesion in developmental dyslexia: left angular gyral blood flow predicts severity. Brain Lang. 1999;70(2):187–204. doi: 10.1006/brln.1999.2158. 10550226 [DOI] [PubMed] [Google Scholar]
- Schultz R.T., Cho N.K., Staib L.H., Kier L.E., Fletcher J.M., Shaywitz S.E., Duncan J.S. Brain morphology in normal and dyslexic children: the influence of sex and age. Ann. Neurol. 1994;35(6):732–742. doi: 10.1002/ana.410350615. 8210231 [DOI] [PubMed] [Google Scholar]
- Schurz M., Wimmer H., Richlan F., Ludersdorfer P., Klackl J., Kronbichler M. Resting-state and task-based functional brain connectivity in developmental dyslexia. Cereb. Cortex. 2014;184 doi: 10.1093/cercor/bhu184. 25169986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Lalonde F., Lepage C., Rabin C., Eckstrand K., Sharp W., Rapoport J. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2009;66(8):888–896. doi: 10.1001/archgenpsychiatry.2009.103. 19652128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Lerch J., Greenstein D., Sharp W., Clasen L., Evans A., Rapoport J. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2006;63(5):540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz B.A., Shaywitz S.E., Blachman B.A., Pugh K.R., Fulbright R.K., Skudlarski P., Marchione K.E. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biol. Psychiatry. 2004;55(9):926–933. doi: 10.1016/j.biopsych.2003.12.019. 15110736 [DOI] [PubMed] [Google Scholar]
- Simos P.G., Breier J.I., Fletcher J.M., Foorman B.R., Bergman E., Fishbeck K., Papanicolaou A.C. Brain activation profiles in dyslexic children during non-word reading: a magnetic source imaging study. Neurosci. Lett. 2000;290(1):61–65. doi: 10.1016/s0304-3940(00)01322-7. 10925175 [DOI] [PubMed] [Google Scholar]
- Simos P.G., Fletcher J.M., Bergman E., Breier J.I., Foorman B.R., Castillo E.M., Papanicolaou A.C. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002;58(8):1203–1213. doi: 10.1212/wnl.58.8.1203. 11971088 [DOI] [PubMed] [Google Scholar]
- Simos P.G., Fletcher J.M., Sarkari S., Billingsley R.L., Denton C., Papanicolaou A.C. Altering the brain circuits for reading through intervention: a magnetic source imaging study. Neuropsychol. 2007;21(4):485–496. doi: 10.1037/0894-4105.21.4.485. 17605581 [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Leonard C.M., Welcome S.E., Kan E., Toga A.W. Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. 15385605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebing K.K., Fletcher J.M., LeDoux J.M., Lyon G.R., Shaywitz S.E., Shaywitz B.A. Validity of IQ-discrepancy classifications of reading disabilities: a meta-analysis. Am. Educ. Res. J. 2002;39(2):469–518. [Google Scholar]
- Tamnes C.K., Østby Y., Fjell A.M., Westlye L.T., Due-Tønnessen P., Walhovd K.B. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb. Cortex. 2010;20(3):534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Temple E., Deutsch G.K., Poldrack R.A., Miller S.L., Tallal P., Merzenich M.M., Gabrieli J.D. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc. National Acad. Sci. U.S.A. 2003;100(5):2860–2865. doi: 10.1073/pnas.0030098100. 12604786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D., Wechsler Abbreviated Intelligence Scale (1999) The Psychological Corporation, San Antonio, TX.
- Wechsler D. Wechsler Individual Achievement Test-II—Abbreviated. The Psychological Corporation; San Antonio, TX.: 2001. [Google Scholar]
- Winkler A.M., Kochunov P., Blangero J., Almasy L., Zilles K., Fox P.T., Glahn D.C. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53(3):1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. 20006715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Sabuncu M.R., Yeo B.T., Fischl B., Greve D.N., Kochunov P., Glahn D.C. Measuring and comparing brain cortical surface area and other areal quantities. Neuroimage. 2012;61(4):1428–1443. doi: 10.1016/j.neuroimage.2012.03.026. 22446492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Lebel C., Evans A., Beaulieu C. Cortical thickness asymmetry from childhood to older adulthood. Neuroimage. 2013;83:66–74. doi: 10.1016/j.neuroimage.2013.06.073. 23827331 [DOI] [PubMed] [Google Scholar]
- Zielinski B.A., Prigge M.B., Nielsen J.A., Froehlich A.L., Abildskov T.J., Anderson J.S., Lange N. Longitudinal changes in cortical thickness in autism and typical development. Brain. 2014;137(6):1799–1812. doi: 10.1093/brain/awu083. 24755274 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.