Abstract
Background
Altered basal ganglia function has been implicated in the pathophysiology of youth Major Depressive Disorder (MDD). Studies have generally focused on characterizing abnormalities in ventral “affective” corticostriatal loops supporting emotional processes. Recent evidence however, has implicated alterations in functional connectivity of dorsal “cognitive” corticostriatal loops in youth MDD. The contribution of dorsal versus ventral corticostriatal alterations to the pathophysiology of youth MDD remains unclear.
Methods
Twenty-one medication-free patients with moderate-to-severe MDD between the ages of 15 and 24 years old were matched with 21 healthy control participants. Using resting-state functional connectivity magnetic resonance imaging we systematically investigated connectivity of eight dorsal and ventral subdivisions of the striatum. Voxelwise statistical maps of each subregion's connectivity with other brain areas were compared between the depressed and control groups.
Results
Depressed youths showed alterations in functional connectivity that were confined to the dorsal corticostriatal circuit. Compared to controls, depressed patients showed increased connectivity between the dorsal caudate nucleus and ventrolateral prefrontal cortex bilaterally. Increased depression severity correlated with the magnitude of dorsal caudate connectivity with the right dorsolateral prefrontal cortex. There were no significant between-group differences in connectivity of ventral striatal regions.
Conclusions
The results provide evidence that alterations in corticostriatal connectivity are evident at the early stages of the illness and are not a result of antidepressant treatment. Increased connectivity between the dorsal caudate, which is usually associated with cognitive processes, and the more affectively related ventrolateral prefrontal cortex may reflect a compensatory mechanism for dysfunctional cognitive-emotional processing in youth depression.
Keywords: Major Depressive Disorder, Youth, Functional magnetic resonance imaging, Striatum, Functional connectivity
Highlights
-
•
We systematically examine dorsal and ventral striatal connectivity in youth MDD.
-
•
Alterations in functional connectivity in youth MDD are confined to the dorsal circuit.
-
•
Youths with MDD show increased dorsal caudate connectivity with the VLPFC.
-
•
Altered dorsal caudate–VLPFC connectivity in MDD may reflect a compensatory response.
1. Introduction
Molecular, structural and functional alterations of the basal ganglia are consistently described pathophysiological features of Major Depressive Disorder (MDD) and remain central to current neurobiological models (Price and Drevets, 2010, 2012). Neuroimaging studies have reported reduced caudate and putamen volumes in MDD patients (Bora et al., 2012; Krishnan et al., 1992; Matsuo et al., 2008) as well as reduced activation of basal ganglia regions during reward based learning and stimulus provocation (Pizzagalli et al., 2009; Smoski et al., 2009; Steele et al., 2007; Stoy et al., 2012). Regarding the latter, functional imaging studies primarily implicate alterations of ventral striatal–prefrontal circuitry as underlying core symptoms of the disorder including low mood, anhedonia and psychomotor retardation (Price and Drevets, 2010, 2012). These findings have been interpreted in line with the classical neuroanatomical schema of topologically organized ventral (“affective”) versus dorsal (“cognitive”) corticostriatal circuits (Alexander et al., 1986; Haber, 2003; Postuma and Dagher, 2006).
As highlighted in a recent review of functional magnetic resonance imaging (fMRI) studies (Kerestes et al., 2014), there is an emerging and specific research focus on the neurobiological correlates of adolescent and young adulthood-onset depression; the developmental period that coincides with the peak age of onset of the disorder (Blazer et al., 1994). Task-based fMRI studies of such populations suggest that there may be especially pronounced changes in ventral striatal function during the early stages of the disorder (Chantiluke et al., 2012; Forbes et al., 2006; Forbes et al., 2009; Shad et al., 2011). Specifically, young depressed patients have demonstrated blunted ventral caudate/nucleus accumbens and putamen activation in the context of reward-based learning together with predominantly increased activation of ventral medial prefrontal, anterior cingulate and orbitofrontal cortical regions — all major components of the so-called “ventral-affective” corticostriatal circuit. Pre-treatment ventral striatal and medial prefrontal cortex activation have been found to predict treatment response to psychotherapy and medication in this population (Forbes et al., 2010). Taken together these studies imply system-wide disturbances in the integrated function (“functional connectivity”) of ventral corticostriatal regions in youth onset depression, although few studies to date have confirmed this notion directly.
Resting-state fMRI has emerged over the past decade as a powerful tool for examining the large-scale organization and functional connectivity of brain networks in healthy and clinical populations (Fornito and Bullmore, 2010). It has been successfully used to map brain corticostriatal circuits in vivo, providing clear and compelling evidence for the existence of functionally organized dorsal and ventral circuits as well as specific alterations across diverse neuropsychiatric disorders including obsessive–compulsive disorder (Harrison et al., 2009; Harrison et al., 2013), psychosis (Dandash et al., 2014), autism (Di Martino et al., 2011) and depression (Furman et al., 2011). Perhaps surprisingly, evidence has emerged in support of primary functional connectivity alterations involving dorsal as opposed to ventral corticostriatal circuits in adults (Furman et al., 2011) and young (Gabbay et al., 2013) depressed populations. Specifically, Furman and colleagues (2011) reported decreased functional connectivity between the ventral striatum and subgenual anterior cingulate cortex in adult depressed patients, but increased connectivity between the dorsal caudate and dorsolateral prefrontal cortex. By contrast, Gabbay and colleagues (2013) reported increased connectivity between dorsal and ventral striatal regions and the dorsomedial prefrontal cortex in medication-free adolescents (12–19 years) with depression. Collectively, these findings challenge the notion of ventral regions being the primary site of basal ganglia alterations in MDD (Cullen et al., 2009; Davey et al., 2012a; Jiao et al., 2011; Shad et al., 2011; Zhu et al., 2012). However, considering the non-overlap of findings between these two existing studies, and the inconsistent evidence for the nature of striatal connectivity abnormalities in young depressed patients, further examination appears warranted.
The aim of the current study was therefore to systematically examine corticostriatal network alterations in a well characterized sample of young people with moderate-to-severe MDD. Clinically milder cases were excluded from this analysis as we suspect that the broader range of illness severity represented in past studies may have contributed to the inconsistency of results. To achieve a more homogenous sample, all patients were medication-free at the time of the imaging assessment similar to Gabbay et al. (2013), but distinct to Furman et al. (2011) who examined medicated patients. As indicated above, our primary intention was to address the nature of dorsal versus ventral corticostriatal alterations and the prevailing hypothesis that depression is primarily associated with changes in ventral-affective corticostriatal circuits.
2. Methods and materials
2.1. Participants
Twenty-one depressed patients (mean age 19.3, S.D. ±2.5) and 21 age, gender, education and estimated IQ-matched healthy subjects (mean age 19.2, S.D. ±2.3) were recruited from an ongoing larger study (Table 1). Each participant's MRI data included here satisfied our imaging quality control criteria (see below). Patients were recruited from three specialized youth mental health clinics in Melbourne, Australia. All patients were between the age of 15 and 24 years old, in accordance with the youth focus of these mental health services. Patients had a primary diagnosis of Major Depressive Disorder determined by the Structured Clinical Interview for DSM-IV (SCID; First et al., 2002). Exclusion criteria included current or past diagnosis of a psychotic disorder, bipolar disorder, substance dependence disorder, pervasive developmental disorder or intellectual disability. Depression severity was assessed with the Montgomery–Åsberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979), where a score ≥20 was required for study inclusion (see Supplementary Table 1 for further information). Healthy subjects were recruited through primarily online advertisements and were confirmed to be without current or past diagnosis of an Axis I psychiatric or neurological disorder. Participants were excluded if they were being treated with psychoactive medication or were pregnant, which was confirmed with a urine pregnancy test. All participants (and their parents if <18 years of age) provided written informed consent to complete this study after a complete description of its protocol, which was approved by the Melbourne Health Human Research Ethics Committee.
Table 1.
Participant demographics and clinical variables.
| MDD |
HC |
Statistics |
p value (two-tailed) |
|
|---|---|---|---|---|
| (n = 21) | (n = 21) | |||
| Age (years) (S.D.) | 19.3 (2.5) | 19.2 (2.3) | t(40) = 0.1 | 0.9 |
| Female, % (n) | 52 (11) | 52 (11) | χ2(1) = 1.0 | 1 |
| Education, mean years (S.D.) | 12.1 (1.9) | 13 (1.6) | t(40) = 1.5 | 0.4 |
| WTAR, mean (S.D.) | 104.7 (8.2) | 107.8 (7.5) | t(40) = 1.2 | 0.6 |
| MADRS score, mean (S.D.) | 33.7 | – | ||
| First episode of depression, % (n) | 33 (7) | – | ||
| Median length of episode (weeks) | 20 | – | ||
| Co-morbid anxiety disorder, % (n) | 71 (15) | – |
MDD, Major Depressive Disorder; HC, healthy controls; WTAR, Wechsler Test of Adult Reading; MADRS, Montgomery–Åsberg Depression Rating Scale; S.D., standard deviation.
2.2. Image acquisition
A 3T Signa Excite system (General Electric) equipped with an 8-channel phased-array head coil was used in combination with ASSET parallel imaging (Sunshine Hospital, Western Health, Melbourne). The functional sequence consisted of a Single shot gradient recalled EPI sequence with a parallel imaging (“ASSET”) in the steady state (repetition time, 2000 ms; echo time, 35 ms; and pulse angle, 90°) in a 23-cm field of view, with a 64 × 64-pixel matrix and a slice thickness of 3.5 mm (no gap). 36 interleaved slices were acquired parallel to the anterior–posterior commissure line with a 20° anterior tilt to achieve more optimal coverage of ventral brain regions. The total sequence time was 8 min, corresponding to 240 whole brain echo-planar imaging volumes. For this sequence, participants were instructed to simply relax, stay awake and to lie still without moving, while keeping their eyes closed throughout. A high-resolution T1-weighted anatomical image was also acquired for each subject to assist with functional time-series co-registration using the following 3D BRAVO sequence: 140 contiguous slices; repetition time, 7900 ms; echo time, 3000 ms; flip angle, 13°; in a 25.6-cm field of view, with a 256 × 256 pixel matrix and a slice thickness of 1 mm (no gap). To assist with noise reduction all participants used foam insert earplugs. To assist with head immobility, foam-padding inserts were placed around the participants head inside the coil.
2.3. Image pre-processing
Imaging data were transferred and processed on a Linux platform running MATLAB version 8.2 (The MathWorks Inc, Natick, Mass). Preprocessing was performed with Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging, UK). Motion correction was performed by aligning each participant's time series to the first image using least squares minimization and a six-parameter (rigid body) spatial transformation. Participants' data were excluded if movement in the translational or rotational planes exceeded 2 mm or 2°, respectively. These realigned functional images were then co-registered to each participant's respective T1 anatomical scan, which were segmented and spatially normalized to the International Consortium for Brain Mapping template using the unified segmentation approach (re-sliced to 2 mm isotropic resolution). Functional images were smoothed with a 6-mm (full-width, half maximum) Gaussian filter.
2.4. Functional connectivity analysis
Resting-state striatal functional connectivity was assessed using a multiple region of interest (“seed-based ROI”) method adapted from Di Martino et al. (2008), and replicated by our group (Dandash et al., 2014; Fornito et al., 2013; Harrison et al., 2009; Harrison et al., 2013). Briefly, 4 striatal seed ROIs were defined in both hemispheres as 3.5 mm radial spheres at the following stereotaxic coordinates: dorsal caudate (x = ±13, y = 15, z = 9); ventral striatum/nucleus accumbens (z = ±9, y = 9, z = –8); dorsal caudal putamen (x = ±28, y = 1, z = 3) and the ventral rostral putamen (x = ±25, y = 8, z = 6). To reproduce the work of Di Martino et al. (2008) showing segregated dorsal and ventral striatal functional connectivity maps, two additional seeds (of no interest) were placed in the ventral caudate superior (x = ±10, y = 15, z = 0) and dorsal rostral putamen (x = ±25, y = 8, z = 6). In addition to our signals of interest, we derived estimates of white matter, CSF and global signal fluctuations for each subject by segmenting each subjects' anatomical scan. Each tissue type was thresholded at 50% tissue probability and binarized to create three nuisance variable masks. Although it has been suggested that the removal of the global signal can induce negative or anti-correlated relationships between brain regions (Murphy et al., 2009), there is good evidence that the removal of the global signal significantly improves the specificity of resting-state fMRI results, particularly for positive correlations (see Van Dijk et al., 2010 for a detailed analysis and an extensive list of supporting evidence). Signals were then extracted for each seed region and nuisance mask by calculating the mean ROI value across the times series for each participant.
Functional connectivity maps were estimated for each striatal region for each participant by including the striatal region time series and nuisance signals as predictors of interest and no interest, respectively, in whole brain, linear regression analyses in SPM8, conduced separately for each hemisphere. A high-pass filter of 128 s was used to remove low frequency drifts. Each of the 3 nuisance covariates were fully orthogonalized (using an iterative Gram-Schmidt method) and then removed from each seed's time-series along with 6 head-motion parameters (3 translation, 3 rotation estimates) by linear regression, resulting in a general linear model that comprised 6 “noise-cleaned” seeds and 9 nuisance variables (Harrison et al., 2009). Contrast images were generated for each participant by estimating the regression coefficient between all brain voxels and each regions' time-series, respectively.
For second-level, between-group analyses, a random-effect 2 × 2 [Group (control, MDD)] × [hemisphere (left seed, right seed)] factorial design was used in SPM8. Within-group statistical maps were thresholded at a false discovery rate (FDR) of p < .05 for the whole brain (Fig. 1). As an additional control for residual head motion influences, the mean inter-scan motion (calculated as the mean displacement between volumes across the scan) for each subject was included as a covariate in the second-level analysis as per Van Dijk et al. (2010) and extensively detailed in Pujol et al. (2014). Each 2nd level model was also covaried for age and gender. Between-group effects (main effects of group and group × hemisphere interactions) were performed by implicitly masking t-contrasts with a global conjunction mask that consisted of the within-subjects effect calculated for both groups for the combined left and right hemisphere maps for each of the four seeds, respectively. Between-group contrasts were thresholded using a p < .05 (FWE) cluster-wise corrected threshold determined using a permutation-based framework (1000 permutations with a cluster-forming threshold of p < .001) as implemented in the REST toolbox (Song et al., 2011). Using this method, AlphaSim simulates the data based on the smoothness of the data and assigns a cluster-extent value above which results are considered significant and not due to chance. The size of the cluster reflects the extent to which each of the striatal subregions are functionally and anatomically connected, and therefore, larger ROIs will yield a larger connectivity profile. The minimum estimated cluster-sizes for significant connectivity with each striatal seed were: dorsal caudate, 30 voxels; ventral caudate/nucleus accumbens, 12 voxels; dorsal (caudal) putamen, 15 voxels; and ventral (rostral) putamen, 6 voxels.
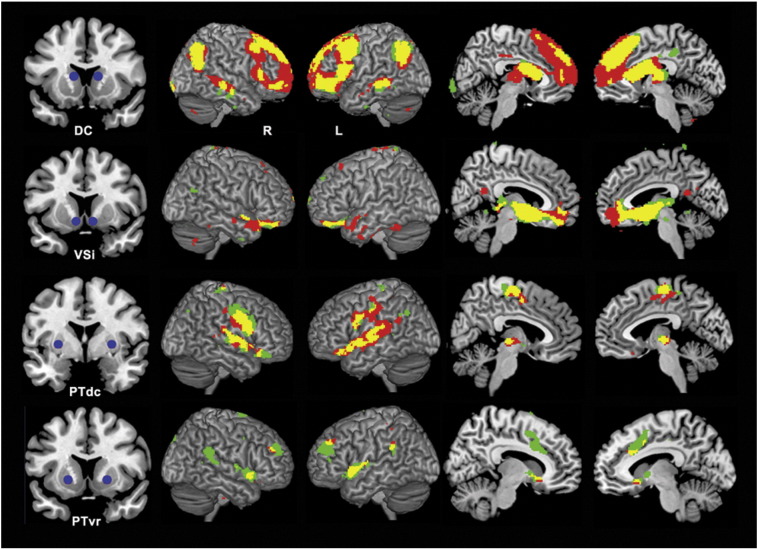
Fig. 1.
Significant within-group (seed effect) functional connectivity maps of the dorsal caudate (DC), ventral striatum (VSi), dorsal caudal putamen (PTdc) and ventral rostral putamen (PTvr). Green indicates control subjects; red, patients with MDD and yellow represents the overlap. R, Right hemisphere; L, left hemisphere. Sagittal slices are displayed at ±6 (DC), +6 and −5 (VSi), ±5 (PTdc) and ±9 (PTvr).
To summarize, the following procedures were adopted to correct for the confounding effects of motion on functional connectivity measures: i) exclusion of participants with excessive movement, determined as greater than 2 mm or 2° in the translational or rotational planes respectively, ii) inclusion of motion parameter estimates in the first-level models, and iii) correction of mean inter-scan head motion in the second-level between-group analysis.
2.5. Relationships with clinical variables
To examine the relationship between striatal functional connectivity and clinical measures of depression severity in patients, we performed a multiple linear regression analysis between total MADRS score and connectivity of the four seed region connectivity maps. The correlations with each of the seeds were masked by each respective within-group connectivity map. All results were displayed at p < .05 cluster-wise corrected using a permutation-based framework (1000 permutations with a cluster-forming threshold of p < .01) as implemented in the REST toolbox (Song et al., 2011). The minimum estimated cluster-sizes were: dorsal caudate, 181 voxels; ventral caudate/nucleus accumbens, 116 voxels; dorsal (caudal) putamen, 130 voxels; and ventral (rostral) putamen, 82 voxels.
3. Results
3.1. Within-group
Across the four striatal regions, both patients and controls showed dorsal and ventral striatal functional connectivity patterns that were broadly consistent with previous studies (Dandash et al., 2014; Di Martino et al., 2008; Harrison et al., 2009) recapitulating known patterns of anatomical connectivity (Alexander et al., 1986; Haber, 2003) (see Fig. 1).
For the dorsal caudate, both groups showed connectivity with dorsal prefrontal and parietal lobe regions including the dorsal medial frontal gyrus, lateral and inferior frontal cortex, and parietal cortex. There was also connectivity with primary and secondary motor cortices including pre-motor cortex and supplementary motor area. Overall the pattern of dorsal caudate connectivity overlapped in both groups, although connectivity with frontal regions was clearly more diffuse in patients. For the ventral caudate/nucleus accumbens, both groups showed connectivity with the medial and lateral orbitofrontal cortex, anterior cingulate cortex, globus pallidus and thalamus. For the dorsal putamen, both groups showed connectivity with primary and secondary motor cortices including premotor cortex and supplementary motor area as well as the precentral and postcentral gyrus, the insula and brainstem regions including the thalamus. The ventral putamen was associated with connectivity in the dorsal anterior cingulate, the inferior parietal cortex and superior temporal gyrus in both groups.
3.2. Between-group
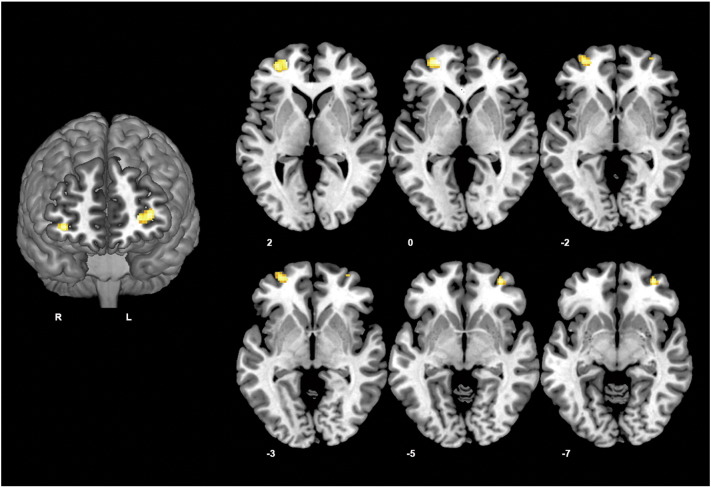
Comparison of the depressed group and healthy control group revealed significant differences in functional connectivity with respect to the dorsal caudate seed only. Specifically, depressed patients showed a diffuse pattern of increased connectivity between the dorsal caudate and ventrolateral prefrontal cortex bilaterally (left cluster peak coordinates x = −24, y = 54, z = 0; cluster size = 105 voxels; T(1,78) = 4.03; right cluster peak coordinates x = 34, y = 52, z = –6; cluster size = 32 voxels; T(1,78) = 3.82) (Fig. 2). There were no significant between-group differences for the ventral striatum, dorsal or ventral putamen seeds, nor were there any group × hemisphere interactions.
Fig. 2.
Z-score statistical maps showing significant between-group differences in corticostriatal functional connectivity of the DC seed. Left: rendered coronal image displaying the region of bilateral ventrolateral prefrontal cortex that had increased connectivity with the DC in MDD vs. controls. Right: axial slices showing the full extent of both the left and right ventrolateral clusters. R, Right hemisphere; L, left hemisphere. Coronal slice displayed at y= 53. Results are displayed at p < .001, uncorrected.
3.3. Brain–behaviour correlations
A significant positive correlation was found between dorsal caudate functional connectivity and overall depression severity (total MADRS score). Specifically, greater functional connectivity between the dorsal caudate and a region of the right dorsolateral prefrontal cortex predicted greater severity (peak coordinates x = 22 y= 46; z = 36; cluster size = 185 voxels; T = 3.8; Fig. 3).
Fig. 3.
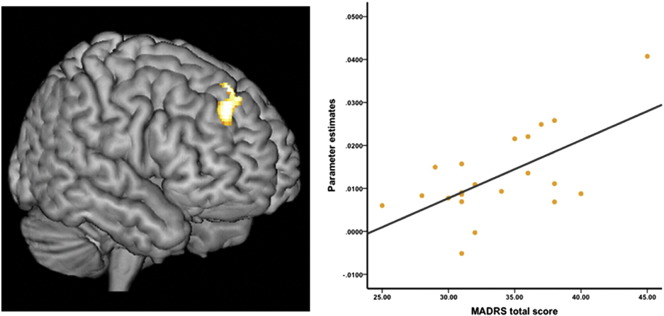
Relationship between depression severity and DC connectivity in depressed youths. Left: rendered sagittal image showing the cluster in right dorsolateral prefrontal cortex that was associated with illness severity, Ke = 185, p < .05 (FWE) cluster-wise corrected. Right: scatter-plot showing the significant association between MADRS total scores and connectivity between the dorsal caudate and right dorsolateral prefrontal cortex, R = .63, p = .001.
4. Discussion
It remains unclear whether alterations in dorsal or ventral corticostriatal circuits are the primary site of basal ganglia functional alterations in depression, particularly in its early stages. We have identified specific alterations implicating the dorsal “cognitive” corticostriatal circuit, with young depressed people exhibiting increased functional connectivity between the dorsal caudate nucleus and ventrolateral prefrontal cortex bilaterally. Increased depression severity was associated with the magnitude of dorsal caudate functional connectivity with right dorsolateral prefrontal cortex, although this region was not significantly altered in the patient group.
The results of this study provide further evidence for a role of the striatum in the pathophysiology of depression (Chantiluke et al., 2012; Davey et al., 2012b; Forbes et al., 2006; Forbes et al., 2009; Shad et al., 2011). Our findings also add to the emerging literature in youth depressed populations by showing that alterations in dorsal striatal connectivity are evident at the early stages of illness and are not a result of antidepressant treatment. Our results are partially consistent with the findings from an adult MDD study (Furman et al., 2011), which showed dysconnectivity between the dorsal caudate and lateral prefrontal cortex in MDD patients. However in contrast to Gabbay et al. (2013), who reported increased connectivity between dorsal and ventral striatal regions with dorsomedial PFC in an adolescent MDD sample, we did not find any alterations with the dorsomedial PFC, nor did we find any differences in ventral corticostriatal circuits. In addition, Gabbay et al. (2013) reported correlations between illness severity and connectivity between predominantly ventral striatum with midline regions. In contrast we found illness severity to be associated specifically with dorsal caudate–dorsolateral prefrontal connectivity. However the observed correlation in our study needs to be interpreted cautiously as this region was not identified as being significantly altered in our depressed group.
As stated above, patients showed diffuse frontal connectivity with the dorsal caudate compared to controls. This pattern of connectivity was much more expansive in patients, particularly in the right hemisphere. We did not see this pattern of connectivity in controls; in contrast, connectivity was confined to more dorsal prefrontal regions in controls. These findings are in line with studies of healthy populations using the same method that have shown dorsal caudate connectivity with more dorsal prefrontal and anterior cingulate regions (Dandash et al., 2014; Di Martino et al., 2008; Fornito et al., 2013; Harrison et al., 2009; Harrison et al., 2013). Lateral regions of the prefrontal cortex are generally considered as playing an integral role in higher-order executive functions including working memory and the regulation of emotion through cognitive control processes including cognitive reappraisal (Buhle et al., 2014; Dolcos and McCarthy, 2006; Vincent et al., 2008). The ventrolateral prefrontal cortex in particular, is thought to mediate ‘affective’ regulation processes at the interface of cognition and emotion, such as inhibiting irrelevant emotional distracter stimuli that compete for cognitive resources (Anticevic et al., 2010; Dolcos and McCarthy, 2006). The ventrolateral prefrontal cortex plays an integral role in the cognitive reappraisal of emotion, a process that a) involves modifying appraisal strategies in working memory (McRae et al., 2010) and b) is disturbed in depression (Beauregard et al., 2006; Fladung et al., 2010; Johnstone et al., 2007). Thus taken together, one tentative hypothesis is that our findings reflect a compensatory response to reappraise emotional material and filter negative information in working memory in young people with depression.
The fact that perturbations in functional connectivity of the ventrolateral prefrontal cortex are evident in youth depression may also reflect altered neurodevelopment of the prefrontal cortex. Adolescence is characterized by rapid cortical maturation (increased synaptic pruning, increased myelination and neuronal plasticity) (Gogtay et al., 2004; Rubia, 2013; Sowell et al., 2004) of neural regions implicated in reward processing and emotion regulation including both dorsal and ventral divisions of the lateral prefrontal cortex (Rubia, 2013). A more diffuse and less localized pattern of neuronal connectivity between the dorsal caudate, which is usually associated with cognitive processes, and the more affectively related ventrolateral prefrontal cortex may reflect altered development of these circuits or a compensatory response for dysfunctional cognitive-emotional processing in youth depression. It is also plausible that the increased dorsal caudate connectivity with the ventrolateral prefrontal cortex that we observed in patients reflects a ventral system alteration. While we interpret these differences as reflecting a primary dorsal network alteration, directionality cannot be inferred from these analyses alone. Indeed, it could be argued that ventrolateral prefrontal cortical changes themselves represent a primary ventral network alteration being driven by ventral corticostriatal loops. Taking this view, our results demonstrate some consistency with previous reports of ventral corticostriatal changes in studies of youth MDD (Chantiluke et al., 2012; Forbes et al., 2006; Forbes et al., 2009; Gabbay et al., 2013; Shad et al., 2011) and extend upon them by showing direct alterations in corticostriatal connectivity.
An important issue that this study did not address is the effects of developmental stage on brain connectivity. Although we chose to include patients from 15–24 years old to encompass early adolescence to early adulthood, as stated above, this age range does encompass a time of rapid neurodevelopment (Gogtay et al., 2004; Rubia, 2013; Sowell et al., 2004). Although we did covary for age in our analyses, it will be important for future studies to address the effects of cortical maturation and puberty on brain connectivity in adolescents at-risk for MDD. In addition, we did not include a urine toxicology screen to assess for active substance use, and therefore we cannot rule out potential drug effects on our functional connectivity results. Finally, we did not include a specific measure of anhedonia. Anhedonia is a core symptom of youth MDD (Lewinsohn et al., 2003) and has been found to predict later MDD (Pine et al., 1999). Correlations between anhedonia and striatal activity and connectivity have been reported in both adult (Keedwell et al., 2005) and adolescent (Gabbay et al., 2013) MDD. Given the complex interplay between anhedonia, decision-making about reward, and the corticostriatal loops mediating reward processes (Lewis et al., 2004; McNab and Klingberg, 2008), future studies examining corticostriatal circuits connectivity and associations with anhedonia will be important.
In summary, our systematic examination of dorsal and ventral corticostriatal connectivity in youth MDD shows evidence of abnormalities that are confined to the dorsal corticostriatal circuit. The increased connectivity between the dorsal caudate and bilateral frontal regions implicated in emotional regulation suggests a compensatory mechanism at the early stage of the illness, or perhaps altered neurodevelopment of the ventrolateral prefrontal cortex. Future studies examining how the development of frontostriatal systems contributes to depression vulnerability will help elucidate biomarkers of the illness and inform neurobiological models of the disorder.
The following are the supplementary data related to this article.
Clinical characteristics of MDD patients.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2014.12.017.
Role of funding source
This study was funded by a National Health and Medical Research Council of Australia (NHMRC) Project Grant to BJH (ID: 1064643). BJH was supported by an NHMRC Clinical Career Development fellowship (ID: 628509). SW was supported by an NHMRC Career Development fellowship (ID: 1007716). CGD was supported by an NHMRC Clinical Career Development fellowship (ID: 1061757).
Acknowledgements
We thank the staff from the Medical Imaging Department, Western Health, Sunshine Hospital (St. Albans, Melbourne) for their support and contributions to this work.
Contributor Information
Rebecca Kerestes, Email: rebecca.kerestes@unimelb.edu.au.
Ben J. Harrison, Email: habj@unimelb.edu.au.
Orwa Dandash, Email: orwa.dandash@unimelb.edu.au.
Katerina Stephanou, Email: kstep@unimelb.edu.au.
Sarah Whittle, Email: swhittle@unimelb.edu.au.
Jesus Pujol, Email: jpujol@crccorp.es.
Christopher G. Davey, Email: c.davey@unimelb.edu.au.
References
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. 3085570 [DOI] [PubMed] [Google Scholar]
- Anticevic A., Repovs G., Barch D.M. Resisting emotional interference: brain regions facilitating working memory performance during negative distraction. Cogn. Affect. Behav. Neurosci. 2010;10(2):159–173. doi: 10.3758/CABN.10.2.159. 20498341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M., Paquette V., Lévesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17(8):843–846. doi: 10.1097/01.wnr.0000220132.32091.9f. 16708026 [DOI] [PubMed] [Google Scholar]
- Blazer D.G., Kessler R.C., McGonagle K.A., Swartz M.S. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am. J. Psychiatry. 1994;151(7):979–986. doi: 10.1176/ajp.151.7.979. 8010383 [DOI] [PubMed] [Google Scholar]
- Bora E., Harrison B.J., Davey C.G., Yücel M., Pantelis C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol. Med. 2012;42(4):671–681. doi: 10.1017/S0033291711001668. 21910935 [DOI] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., Lopez R., Onyemekwu C., Kober H., Weber J., Ochsner K.N. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. 23765157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantiluke K., Halari R., Simic M., Pariante C.M., Papadopoulos A., Giampietro V., Rubia K. Fronto-striato-cerebellar dysregulation in adolescents with depression during motivated attention. Biol. Psychiatry. 2012;71(1):59–67. doi: 10.1016/j.biopsych.2011.09.005. 22015111 [DOI] [PubMed] [Google Scholar]
- Cullen K.R., Gee D.G., Klimes-Dougan B., Gabbay V., Hulvershorn L., Mueller B.A., Camchong J., Bell C.J., Houri A., Kumra S., Lim K.O., Castellanos F.X., Milham M.P. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci. Lett. 2009;460(3):227–231. doi: 10.1016/j.neulet.2009.05.022. 19446602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandash O., Fornito A., Lee J., Keefe R.S., Chee M.W., Adcock R.A., Pantelis C., Wood S.J., Harrison B.J. Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr. Bull. 2014;40:904–913. doi: 10.1093/schbul/sbt093. 23861539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey C.G., Harrison B.J., Yücel M., Allen N.B. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol. Med. 2012;42(10):2071–2081. doi: 10.1017/S0033291712000323. [DOI] [PubMed] [Google Scholar]
- Davey C.G., Yücel M., Allen N.B., Harrison B.J. Task-related deactivation and functional connectivity of the subgenual cingulate cortex in major depressive disorder. Front. Psychiatry. 2012;3:14. doi: 10.3389/fpsyt.2012.00014. 22403553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Kelly C., Grzadzinski R., Zuo X.N., Mennes M., Mairena M.A., Lord C., Castellanos F.X., Milham M.P. Aberrant striatal functional connectivity in children with autism. Biol. Psychiatry. 2011;69(9):847–856. doi: 10.1016/j.biopsych.2010.10.029. 21195388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Scheres A., Margulies D.S., Kelly A.M., Uddin L.Q., Shehzad Z., Biswal B., Walters J.R., Castellanos F.X., Milham M.P. Functional connectivity of human striatum: a resting state FMRI study. Cereb. Cortex. 2008;18(12):2735–2747. doi: 10.1093/cercor/bhn041. 18400794 [DOI] [PubMed] [Google Scholar]
- Dolcos F., McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J. Neurosci. 2006;26(7):2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. 16481440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M., Spitzer R., Gibbon M., Williams J.B.W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. research version, patient edition (SCID-I/P) Biometrics Research, NY State Psychiatric Institute; New York: 2002. [Google Scholar]
- Fladung A.K., Baron U., Gunst I., Kiefer M. Cognitive reappraisal modulates performance following negative feedback in patients with major depressive disorder. Psychol. Med. 2010;40(10):1703–1710. doi: 10.1017/S0033291709992170. 20047704 [DOI] [PubMed] [Google Scholar]
- Forbes E.E., Hariri A.R., Martin S.L., Silk J.S., Moyles D.L., Fisher P.M., Brown S.M., Ryan N.D., Birmaher B., Axelson D.A., Dahl R.E. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am. J. Psychiatry. 2009;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336. 19047324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., Olino T.M., Ryan N.D., Birmaher B., Axelson D., Moyles D.L., Dahl R.E. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cogn. Affect. Behav. Neurosci. 2010;10(1):107–118. doi: 10.3758/CABN.10.1.107. 20233959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., Christopher May J., Siegle G.J., Ladouceur G.J., Ryan C.D., Carter C.S., Birmaher B., Axelson D.A., Dahl R.E. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J. Child Psychol. Psychiatry. 2006;47(10):1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. 17073982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Bullmore E.T. What can spontaneous fluctuations of the blood oxygenation-level-dependent signal tell us about psychiatric disorders? Curr. Opin. Psychiatry. 2010;23(3):239–249. doi: 10.1097/YCO.0b013e328337d78d. 20216219 [DOI] [PubMed] [Google Scholar]
- Fornito A., Harrison B.J., Goodby E., Dean A., Ooi C., Nathan P.J., Lennox B.R., Jones P.B., Suckling J., Bullmore E.T. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 2013;70(11):1143–1151. doi: 10.1001/jamapsychiatry.2013.1976. 24005188 [DOI] [PubMed] [Google Scholar]
- Furman D.J., Hamilton J.P., Gotlib I.H. Frontostriatal functional connectivity in major depressive disorder. Biol. Mood Anxiety Disord. 2011;1(1):11. doi: 10.1186/2045-5380-1-11. 22737995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V., Ely B.A., Li Q., Bangaru S.D., Panzer A.M., Alonso C.M., Castellanos F.X., Milham M.P. Striatum-based circuitry of adolescent depression and anhedonia. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52(6):628–641. doi: 10.1016/j.jaac.2013.04.003. 23702452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., 3rd, Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. 15148381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. 14729134 [DOI] [PubMed] [Google Scholar]
- Harrison B.J., Pujol J., Cardoner N., Deus J., Alonso P., López-Solà M., Contreras-Rodríguez O., Real E., Segalàs C., Blanco-Hinojo L., Menchon J.M., Soriano-Mas C. Brain corticostriatal systems and the major clinical symptom dimensions of obsessive–compulsive disorder. Biol. Psychiatry. 2013;73(4):321–328. doi: 10.1016/j.biopsych.2012.10.006. 23200527 [DOI] [PubMed] [Google Scholar]
- Harrison B.J., Soriano-Mas C., Pujol J., Ortiz H., López-Solà M., Hernández-Ribas R., Deus J., Alonso P., Yücel M., Pantelis C., Menchon J.M., Cardoner N. Altered corticostriatal functional connectivity in obsessive–compulsive disorder. Arch. Gen. Psychiatry. 2009;66(11):1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. 19884607 [DOI] [PubMed] [Google Scholar]
- Jiao Q., Ding J., Lu G., Su L., Zhang Z., Wang Z., Zhong Y., Li K., Ding M., Liu Y. Increased activity imbalance in fronto-subcortical circuits in adolescents with major depression. PLOS One. 2011;6(9):e25159. doi: 10.1371/journal.pone.0025159. 21949877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J. Neurosci. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. 17699669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell P.A., Andrew C., Williams S.C., Brammer M.J., Phillips M.L. The neural correlates of anhedonia in major depressive disorder. Biol. Psychiatry. 2005;58(11):843–853. doi: 10.1016/j.biopsych.2005.05.019. 16043128 [DOI] [PubMed] [Google Scholar]
- Kerestes R., Davey C.G., Stephanou K., Whittle S., Harrison B.J. Functional brain imaging studies of youth depression: a systematic review. Neuroimage Clin. 2014;4:209–231. doi: 10.1016/j.nicl.2013.11.009. 24455472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K.R., McDonald W.M., Escalona P.R., Doraiswamy P.M., Na C., Husain M.M., Figiel G.S., Boyko O.B., Ellinwood E.H., Nemeroff C.B. Magnetic resonance imaging of the caudate nuclei in depression. preliminary observations. Arch. Gen. Psychiatry. 1992;49(7):553–557. doi: 10.1001/archpsyc.1992.01820070047007. 1627046 [DOI] [PubMed] [Google Scholar]
- Lewinsohn P.M., Pettit J.W., Joiner T.E., Jr., Seeley J.R. The symptomatic expression of major depressive disorder in adolescents and young adults. J. Abnorm. Psychol. 2003;112(2):244–252. doi: 10.1037/0021-843x.112.2.244. 12784834 [DOI] [PubMed] [Google Scholar]
- Lewis S.J., Dove A., Robbins T.W., Barker R.A., Owen A.M. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. Eur. J. Neurosci. 2004;19(3):755–760. doi: 10.1111/j.1460-9568.2004.03108.x. 14984425 [DOI] [PubMed] [Google Scholar]
- Matsuo K., Rosenberg D.R., Easter P.C., MacMaster F.P., Chen H.H., Nicoletti M., Caetano S.C., Hatch J.P., Soares J.C. Striatal volume abnormalities in treatment-naive patients diagnosed with pediatric major depressive disorder. J. Child Adolesc. Psychopharmacol. 2008;18(2):121–131. doi: 10.1089/cap.2007.0026. 18439110 [DOI] [PubMed] [Google Scholar]
- McNab F., Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat. Neurosci. 2008;11(1):103–107. doi: 10.1038/nn2024. 18066057 [DOI] [PubMed] [Google Scholar]
- McRae K., Hughes B., Chopra S., Gabrieli J.D., Gross J.J., Ochsner K.N. The neural bases of distraction and reappraisal. J. Cogn. Neurosci. 2010;22(2):248–262. doi: 10.1162/jocn.2009.21243. 19400679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. 444788 [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. 18976716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine D.S., Cohen E., Cohen P., Brook J. Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder? Am. J. Psychiatry. 1999;156(1):133–135. doi: 10.1176/ajp.156.1.133. 9892310 [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A., Holmes A.J., Dillon D.G., Goetz E.L., Birk J.L., Bogdan R., Dougherty D.D., Iosifescu D.V., Rauch S.L., Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. 19411368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma R.B., Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb. Cortex. 2006;16(10):1508–1521. doi: 10.1093/cercor/bhj088. 16373457 [DOI] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. 19693001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. 22197477 [DOI] [PubMed] [Google Scholar]
- Pujol J., Macià D., Blanco-Hinojo L., Martínez-Vilavella G., Sunyer J., de la Torre R., Caixàs A., Martín-Santos R., Deus J., Harrison B.J. Does motion-related brain functional connectivity reflect both artifacts and genuine neural activity? NeuroImage. 2014;101:87–95. doi: 10.1016/j.neuroimage.2014.06.065. 24999036 [DOI] [PubMed] [Google Scholar]
- Rubia K. Functional brain imaging across development. Eur. Child Adolesc. Psychiatry. 2013;22:719–731. doi: 10.1007/s00787-012-0291-8. 22729957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shad M.U., Bidesi A.P., Chen L.A., Ernst M., Rao U. Neurobiology of decision making in depressed adolescents: a functional magnetic resonance imaging study. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50(6):612–621. doi: 10.1016/j.jaac.2011.03.011. 21621145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski M.J., Felder J., Bizzell J., Green S.R., Ernst M., Lynch T.R., Dichter G.S. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J. Affect. Disord. 2009;118(1–3):69–78. doi: 10.1016/j.jad.2009.01.034. 19261334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.W., Dong Z.Y., Long X.Y., Li S.F., Zuo X.N., Zhu C.Z., He Y., Yan C.G., Zang Y.F. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLOS One. 2011;6(9):e25031. doi: 10.1371/journal.pone.0025031. 21949842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Toga A.W. Mapping changes in the human cortex throughout the span of life. Neuroscientist Rev. J. Bringing Neurobiol. Neurol. Psychiatry. 2004;10(4):372–392. doi: 10.1177/1073858404263960. 15271264 [DOI] [PubMed] [Google Scholar]
- Steele J.D., Kumar P., Ebmeier K.P. Blunted response to feedback information in depressive illness. Brain J. Neurol. 2007;130(9):2367–2374. doi: 10.1093/brain/awm150. 17586866 [DOI] [PubMed] [Google Scholar]
- Stoy M., Schlagenhauf F., Sterzer P., Bermpohl F., Hägele C., Suchotzki K., Schmack K., Wrase J., Ricken R., Knutson B., Adli M., Bauer M., Heinz A., Ströhle A. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J. Psychopharmacol. 2012;26(5):677–688. doi: 10.1177/0269881111416686. 21926423 [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R., Hedden T., Venkataraman A., Evans K.C., Lazar S.W., Buckner R.L. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. 19889849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J.L., Kahn I., Snyder A.Z., Raichle M.E., Buckner R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. 18799601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Wang X., Xiao J., Liao J., Zhong M., Wang W., Yao S. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol. Psychiatry. 2012;71(7):611–617. doi: 10.1016/j.biopsych.2011.10.035. 22177602 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical characteristics of MDD patients.