Abstract
Microsurgery for brain aneurysms is a current relevant technique, as advances in endovascular and stent-assisted coiling have not solved many of the difficulties inherent in the management of complex brain aneurysms. The following review highlights the importance of microsurgical bypass techniques for the management of complex cerebrovascular aneurysms and emphasizes, through two clinical cases, the technical difficulties and indications for bypass surgery. These cases demonstrate that in selected scenarios, bypass microsurgery still offers the only viable treatment for complex aneurysms.
Keywords: intracranial aneurysm, subarachnoid hemorrhage, endovascular coiling, vascular reconstruction, extra-intra-cranial bypass

H. B. da Silva, M.D.
Introduction
Brain aneurysms are generally saccular structures that arise at the junction of intracranial arteries. They are thought to form at weak points in the arterial bifurcations due to the degeneration of the internal elastic lamina and the tunica media. The majority of brain aneurysms are acquired lesions, and while their incidence increases with age, there is a genetic predisposition that increases with a strong family history (e.g., more than one family member being affected), autosomal dominant polycystic kidney disease, and certain connective tissue disorders such as Ehlers-Danlos syndrome and fibromuscular dysplasia.1–3
The general prevalence of brain aneurysms in the U.S. population is 1% to 2%, and the annual incidence of rupture is about 10 per 100,000. Their rupture is promoted by cigarette smoking, hypertension, and hemodynamic factors that are not completely understood. In cases of rupture, the resultant brain injury leads to a 20% mortality rate4 and another 30% to 40% may suffer some form of disability and/or subsequent complications, although outcomes are better at major centers that treat more than 100 aneurysm patients per year.
The following review outlines the types and properties of complex intracranial aneurysms and highlights the benefits and risks of various treatment modalities in the surgical armamentarium, including vascular reconstruction, bypass surgery, stenting, and medical management.
Subarachnoid Hemorrhage
When aneurysms rupture, they usually cause subarachnoid hemorrhage (SAH), which is hemorrhage in the subarachnoid space around the brain, especially at the base. At times they may also rupture inside the brain, causing an intracerebral hemorrhage. Such hemorrhages may be preceded by small leaks into the subarachnoid space, by small and sudden expansions of the aneurysm wall (causing a “sentinel hemorrhage”), or by the acute onset of cranial nerve paralysis (e.g., third cranial nerve palsy). Such sentinel hemorrhages, however, are easily misdiagnosed, even in experienced emergency rooms, since the symptoms are often nonspecific. SAH is usually indicated by the acute onset of a severe headache, often referred to as “the worst headache of my life,” and other symptoms such as nausea, vomiting, loss of consciousness, and hemiparesis, depending upon the severity of the bleed.
After an acute SAH, a certain amount of brain injury occurs due to the severe brain ischemia and anoxia to brain structures; however, the aneurysm frequently stops bleeding due to the buildup of pressure outside its wall and a clot formation inside its wall. About 20% of patients with acute SAH never make it to the hospital. For those who do, and are treated expectantly, approximately 20% will rebleed within the first 2 weeks after SAH.5 Such rebleeds are often more severe than the first bleed. Therefore, in addition to stabilization, the aneurysm must be secured to prevent rebleeding. Another important problem is vasospasm, a constriction of large and small arteries in the brain thought to be caused by degradation products of oxyhemoglobin and mediated by nitric oxide receptors. If vasospasm is severe, it can cause brain ischemia, stroke, and death. Other problems after SAH include hyponatremia, hydrocephalus, and problems associated with being bedridden in an intensive care unit for more than a few days.6
Treatment of Ruptured Aneurysms
Nowadays, most ruptured aneurysms are secured within 1 or 2 days after admission to the hospital, and the modalities of treatment available consist of endovascular coiling, stent-assisted coiling, clipping, and clipping with bypass. There are varying reports on success rates of the current treatments, and methodological problems have been identified with all the major published trials, thus making it difficult for neurosurgical centers to establish strict guidelines for the treatment of ruptured aneurysms.
The first major endovascular coiling trial, the ISAT trial,7 an international randomized trial of patients with ruptured aneurysms living outside the United States, found that endovascular treatment was associated with a lower morbidity and mortality than microsurgical clipping. This trial has been criticized for several reasons: roughly 20% of ruptured aneurysm patients were eventually enrolled into the trial since only patients with a narrow aneurysm neck were eligible, and many others declined treatment; also, the quality of the endovascular treatment was exceptionally high as it was restricted to experts in the field, whereas that of the microsurgical treatment was variable and did not meet the standards typical of large cerebrovascular treatment centers in the United States.8,9 Posterior analysis revealed that patients aged 40 years and younger may benefit from clipping rather than endovascular coiling due to the low incidence of rebleeding and retreatment with clipping.10 A somewhat controversial study was performed by the Arizona-based Barrow Neurological Institute wherein patients were assigned to endovascular or microsurgical treatment on alternate days. This trial also found better results at 3 months in patients treated endovascularly.11 However, there was a very high crossover from the endovascular group into the microsurgical group due to the trial's inability to treat all patients using the endovascular technique. Also, the advantage for endovascular treatment disappeared after a year of follow-up, with both groups exhibiting similar outcomes.
Currently in major U.S. centers, endovascular coiling is the preferred treatment for patients with ruptured brain aneurysms, with the following exceptions: (1) when the aneurysm neck is not favorable for the coiling, even after a balloon is inserted inside the artery lumen to supplement standard coiling; (2) patients who are < 50 years of age; (3) patients who cannot comply with the requirement of regular follow-up to evaluate for recurrence, which is needed after coiling; and (4) patients with large or giant aneurysms who cannot successfully undergo an endovascular coiling technique.
Some aneurysms are complex in nature and have the following characteristics: size < 13 mm; dome/neck ratio < 1.5; branch vessel(s) originating from the aneurysm sac; and complicated wall structures, such as fusiform and dissecting aneurysms. In patients with complex aneurysms, intraluminal stents cannot generally be used since they require the use of dual antiplatelet agents for 3 to 6 months after shunt insertion. In some of these patients, a clip reconstruction of the neck can be performed using multiple clips, whereas other patients may require vascular bypass or reconstruction.
In 840 patients treated at our institution over a 7-year period, the chosen treatment modalities for ruptured intracranial aneurysms were clipping, coiling, clipping with bypass, and stent-assisted coiling (Figure 1). Patients treated with vascular reconstruction formed only a small subset of this group. However, the ability to treat patients with these modalities when needed has become an important requirement for major U.S. aneurysm centers.
Figure 1.
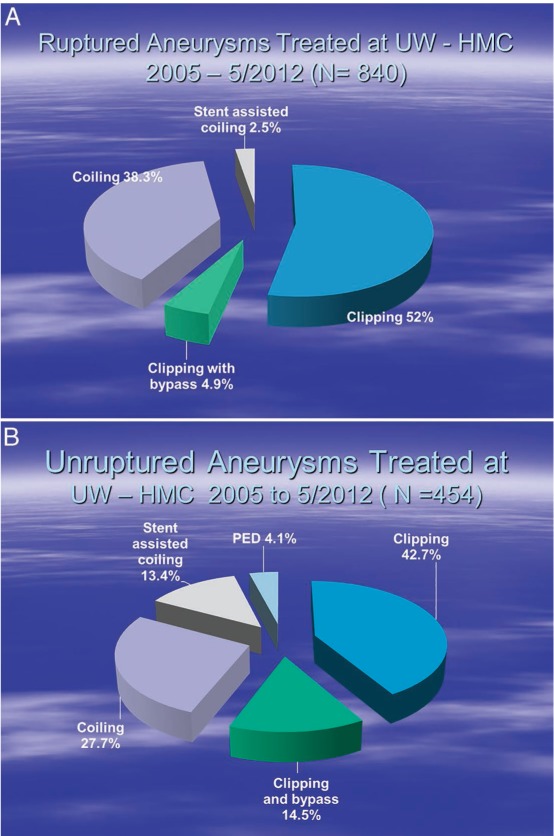
Number of patients treated at Harborview Medical Center over a 7-year period for (A) ruptured and (B) unruptured aneurysm and the chosen modality of treatment. PED: pipeline embolization device.
Unruptured Aneurysms
As stated previously, unruptured aneurysms have a large prevalence in the population. Many are discovered incidentally during a work-up for headaches, dizziness, or other unrelated neurological symptoms. Some of them may present with symptoms of recurrent headaches, cranial nerve paralysis, transient ischemic attacks, or stroke. When such aneurysms are identified, the main question is whether they should be treated or observed. This depends on an analysis of the expected rupture rates and the risks of the treatment. While no large randomized trials have been conducted to analyze unruptured aneurysms, their natural history has been studied in two pertinent trials. The ISUIA trial conducted in the United States and at international centers found that aneurysm rupture was influenced by size and location.12 Aneurysms larger than 7 mm and with posterior circulation location were more likely to rupture, as were internal carotid/posterior communicating artery aneurysms. However, anterior communicating artery (ACom) aneurysms, which show the highest propensity for rupture, were markedly underrepresented in this study. The Japanese UCAS study of unruptured cerebral aneurysms13 found that ACom and basilar tip aneurysms were more likely to rupture than middle cerebral artery (MCA) aneurysms.
The ability to use intravascular stents creates a larger range of treatment options for unruptured aneurysms. Endovascular options include simple coiling, balloon-assisted coiling, high-porosity stent-assisted coiling, and use of a low-porosity or flow-diversion stent (Pipeline®Stent Device, Covidien, Mansfield, MA). Microsurgical options include simple clipping, complex clip reconstruction, and aneurysm occlusion with the aid of a low-flow or high-flow bypass. The percentages of various aneurysm treatments in our center can be seen in Figure 1. During the last 2 years, the use of high-flow bypasses has declined somewhat due to increased use of the Pipeline device—especially for intracavernous and paraophthalmic aneurysms—as well as off-label treatments for other types of aneurysms.
Bypasses and Vascular Reconstruction
When a parent artery or one of its branches cannot be preserved during the course of an aneurysm treatment, a bypass can provide an alternate method of blood flow,14,15 thereby preventing a major or a minor stroke in the patient and thus a poor outcome. For certain types of aneurysms, this may be the only current treatment option. Traditionally, bypasses can be classified into three types: high flow, moderate flow and low flow. Tables 1 and 2 summarize the classification and indications of each of these types.
Table 1.
Classification of bypass and reconstructive procedures. STA: superficial temporal artery; MCA: middle cerebral artery; OA: occipital artery; PICA: posterior inferior cerebellar artery.

Table 2.
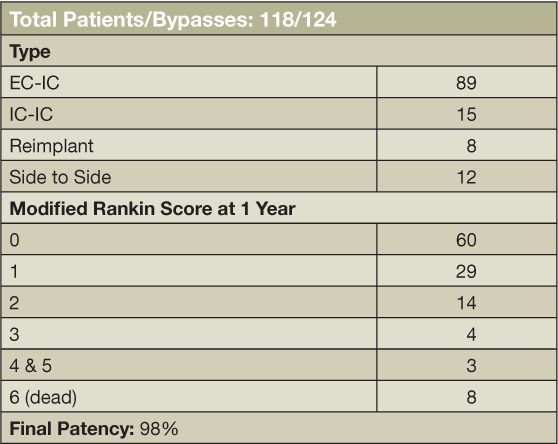
Aneurysm patients undergoing bypasses between 2005 and 2014. EC-IC: extra-intra-cranial; IC-IC: intracranial-intracranial.
Vascular reconstructions can be divided into local bypasses and extra-intra-cranial bypasses. Local bypasses include reimplantation of an artery, end-to-end anastomosis, side-to-side anastomosis, and a short interposition graft. Extra-intra-cranial (EC-IC) bypasses can be divided into low-flow bypasses (flow rate ≤ 50 ml/min), moderate-flow bypasses (flow rate between 51 to 99 mL/min.), and high-flow bypasses (flow rate ≥ 100 mL/min). Low-flow bypasses are generally constructed with a scalp artery, such as the superficial temporal artery, or the occipital artery, whereas moderate- and high-flow bypasses use a radial artery or the saphenous vein (and, on occasion, the anterior tibial artery or other veins).
The type of bypass performed primarily depends on the artery to be replaced and also, to some extent, on the available donor and graft vessels. When the intracranial artery, a dominant vertebral artery (VA), the basilar artery (BA), or the entire MCA flow needs to be replaced, then a high- or moderate-flow EC-IC bypass is usually required, involving the use of a radial artery or the saphenous vein. Smaller arteries can usually be replaced with a local bypass. On the other hand, local bypasses can be performed more easily as compared to EC-IC bypasses.
During the bypass, temporary clipping and cessation of arterial flow is required. The tolerance of different arterial segments of the brain to ischemia depends upon their location, the patient's age, and metabolic brain activity. For instance, many perforating arteries to the brain (e.g., lenticulostriate, thalamogeniculate, and mid-basilar perforating arteries) are thought to have poorer collateral circulation when compared to M2 or P2 segments of the MCA or posterior cerebral artery. However, such a tolerance can be extended by lowering the brain metabolism, which is done by placing the patient under metabolic burst suppression on the electroencephalogram, usually using propofol under anesthesia. This extends the ischemic tolerance time of arteries such as the M2 to about 60 or even 90 minutes. Also, the blood pressure is artificially raised by 20 to 30 mm Hg to improve the three collaterals, and the patient is kept well hydrated during the occlusion. The effect of the vascular occlusion can be monitored during the operation by continuous neurophysiological monitoring using somatosensory-evoked potentials and motor-evoked potentials. The patient is also usually administered intravenous heparin during the clamping to prevent clotting of the vessels.
Before deciding on which type of bypass to use, intra-arterial digital angiography is performed to image the aneurysm and surrounding vessels; the external carotid artery and its branches also should be visualized. In addition, the saphenous veins and radial arteries are imaged by duplex ultrasound to determine which graft vessels to use. We prefer a radial artery of at least 0.22 cm diameter or a saphenous vein of at least 0.3 cm diameter. The radial artery is generally preferred but may not be available, or the patient may fail the Doppler Allen's test, indicating that the palmar arch is not complete and the patient does not have adequate collaterals in the hand from the ulnar artery. The next preference is the saphenous vein followed by the anterior tibial artery. Other veins, such as the cephalic vein, may also be considered.
When the radial artery is extracted, the pressure distension technique is used to break the vasospasm. Use of this technique has greatly eliminated the occurrence of postoperative graft spasms.14,16 A slight degree of distension with heparinized saline is also important for saphenous vein grafts.
Operative Techniques
Operative approaches differ according to the particular operation performed—a local bypass or an EC-IC bypass procedure. The most widely used techniques for bypasses in cerebrovascular surgery are side-to-side anastomosis, reimplantation, and the external carotid or internal carotid high/moderate flow bypass. The following describe these techniques.
Side-to-Side Anastomosis
After placing temporary clips on two adjacent arteries, two linear or oval arteriotomies are created facing each other, and the back wall is sutured with 9/0 nylon sutures, followed by the front wall. The sutures are usually continuous; the loops are left slightly long to allow visualization of the arterial walls during suturing, and they are tightened at the end before tying the sutures.
Reimplantation
The vessel to be implanted is sectioned sharply and spatulated on one side to enlarge the orifice. The donor artery is temporarily occluded, and an oval arteriotomy is created. After attaching the two ends of the recipient artery with 10/0, 9/0, or 8/0 nylon sutures, the walls are sutured, first on the more difficult side and then the other side. When the clips are released, there may be an area of suture line leak that requires an additional suture or two.
EC-IC High/Moderate Flow Bypass
The recipient vessel is usually the M1 bifurcation of the MCA, the M2 branch of the MCA, the supraclinoid internal carotid artery (ICA), or the P2 segment of the PCA. The donor artery may be the external carotid artery, ICA (if there are good collaterals, and the artery can withstand temporary occlusion for about 30 min), the superficial temporal artery (just anterior to the zygomatic arch), the occipital artery (near the digastric groove), or the V2-V3 segment of the vertebral artery. After both the donor and recipient arteries are exposed and prepared, a tunnel is created for the passage of the graft, which may be either pre- or postauricular. The distal anastomosis is usually done first in an end-to-side fashion; the graft is led through the tunnel proximally and then attached to the donor artery using a punch to create an oval arteriotomy. The sequence may be reversed in some patients, especially with vein grafts. When the craniotomy is replaced, an opening is created in the bone for the graft. For retroauricular grafts, a groove may be created in the bone.
Postoperative Care
The patient undergoes a computed tomography (CT) scan and CT angiography immediately after the procedure, then intra-arterial digital subtraction angiography on the following day. Duplex ultrasound imaging may also be used to visualize the graft and measure the volume of flow. The patient is maintained on aspirin (325 mg) and subcutaneous heparin until discharge and remains on aspirin for about 1 year. Any problems with the graft, such as stenosis, are corrected during the operation. Follow-up protocol includes imaging the grafts through CT angiography once a year, with lengthening intervals thereafter.
Results
In the last nine years, the senior author performed 124 vascular bypasses and reconstructions for aneurysms in 118 patients (Table 2). The following two cases illustrate endovascular management with high- and moderate-flow bypass techniques.
Case 1: Giant Ruptured Fusiform Aneurysm
This 37-year-old man presented with a ruptured giant aneurysm that comprised all the M1 segment of the left MCA2 (Figure 2). He had no neurological deficit prior to the subarachnoid hemorrhage (SAH). He had arrived at the hospital with a severe headache and was noted to have deterioration in his level of consciousness, thus a right frontal external ventricular drain was placed in the emergency room. An angiogram showed a large fusiform aneurysm involving the M1 segment measuring approximately 3.7 cm x 2.6 cm (Figure 3). Due to its size and fusiform shape as well as the acute SAH, the aneurysm was impossible to treat through endovascular management or microsurgical clipping. Therefore, we proceeded with a high-flow bypass using a radial artery graft from the ECA to the M2 segment of the MCA and performed a proximal occlusion of the aneurysm. The patient underwent a frontotemporal craniotomy and orbitectomy with a preauricular scalp incision that connected the frontotemporal and upper neck incisions. The radial artery graft (RAG) was harvested from the left arm, and a left ECA to M2 bypass was made (Figure 4). Postoperative angiogram showed persistent filling of the aneurysm by retrograde flow and bypass patency (Figure 5). An increase in intracranial pressure, initially medically treated but persistent, necessitated that the patient return to the operating room for a temporal lobectomy and distal occlusion of the aneurysm. The patient made a complete recovery following the second procedure, and an angiogram performed before discharge showed the complete occlusion and confirmed the bypass patency (Figure 6).
Figure 2.
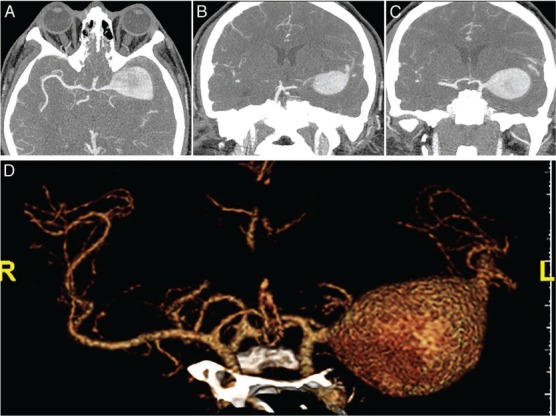
Preoperative computed tomography angiogram of the patient shows giant left M1 segment aneurysm.
Figure 3.
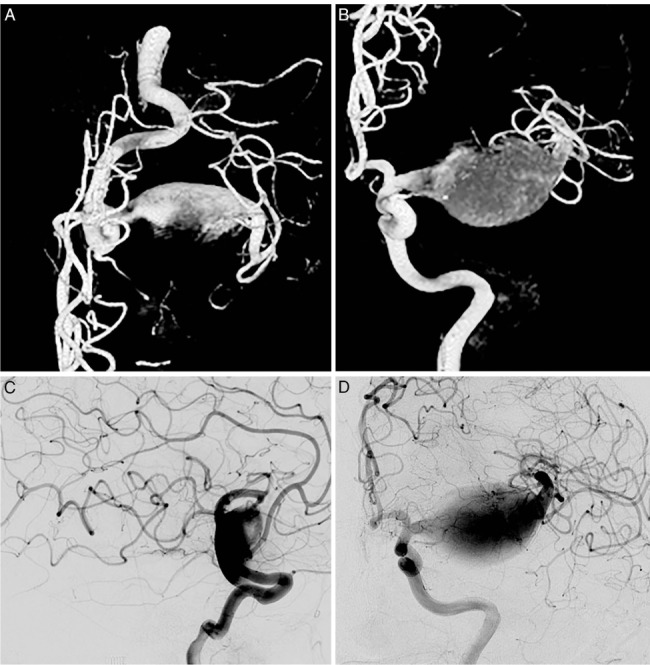
Preoperative angiogram and three-dimensional reconstructions showing the giant left M1 aneurysm.
Figure 4.
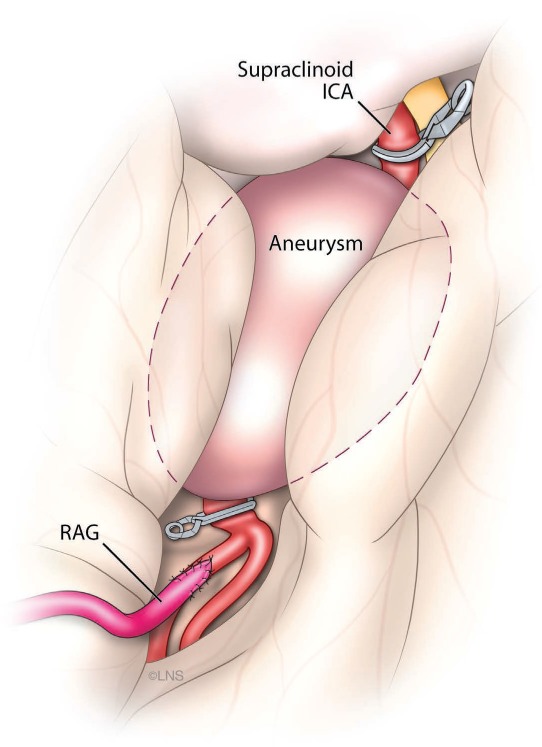
Illustration of procedure used for the patient in case 1, showing the radial artery graft to M2 bypass.
Figure 5.
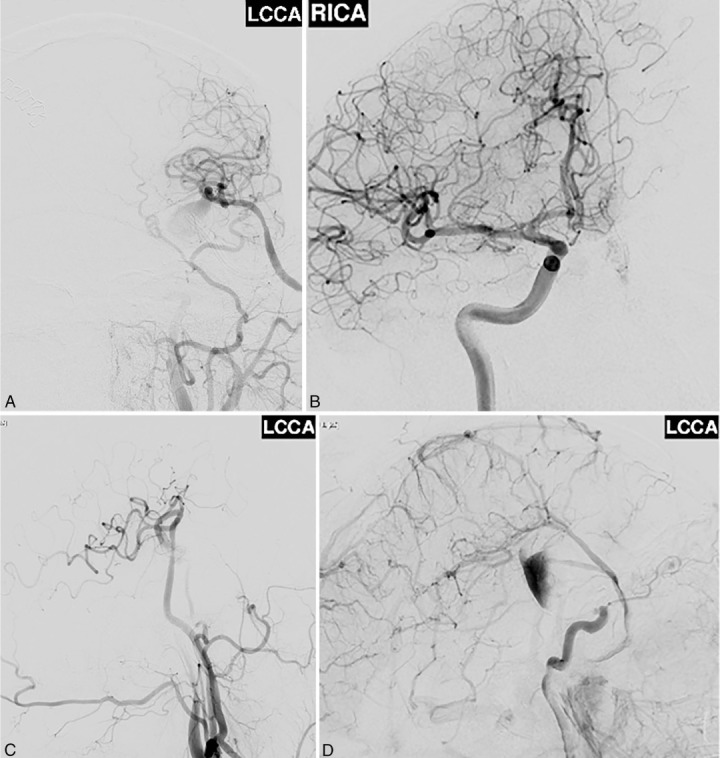
Postoperative angiogram showed persistent filling of the aneurysm by retrograde flow and the patency of the bypass after the first surgery.
Figure 6.
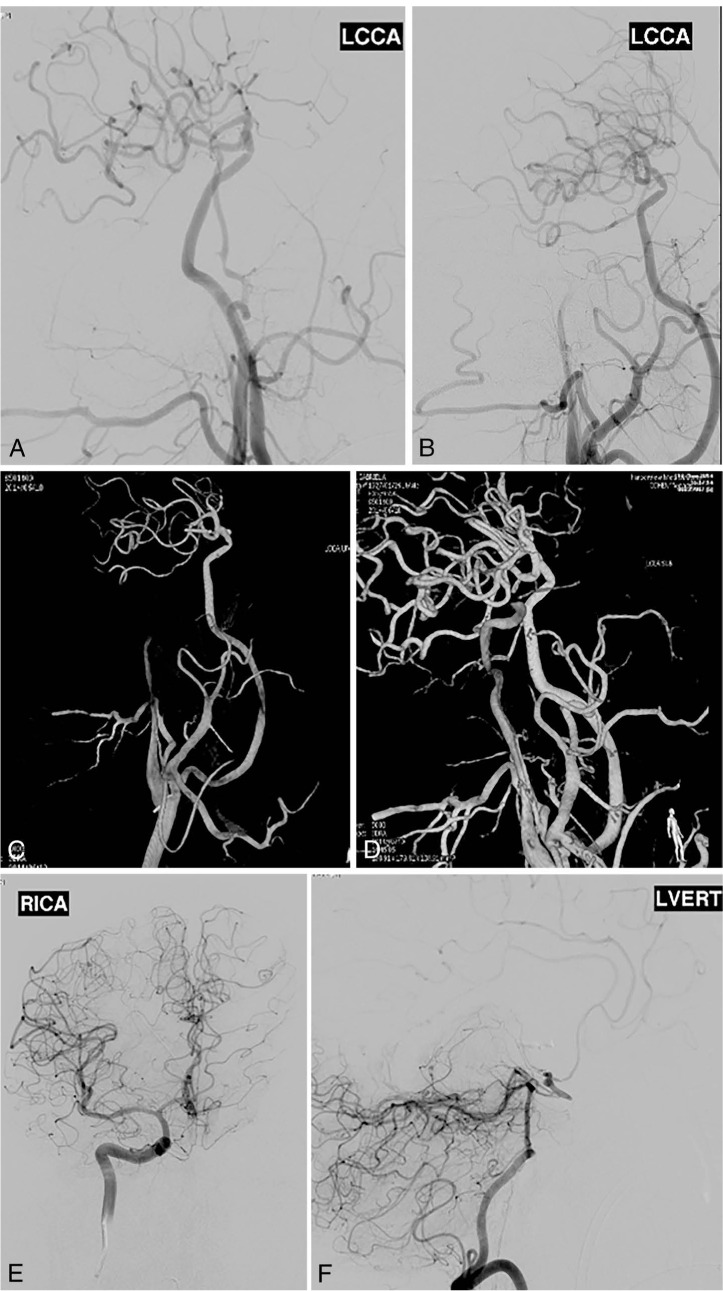
Postoperative angiogram with 3-dimensional reconstructions showing total occlusion of the aneurysm and bypass patency after the second surgery.
Case 2: Vertebral Artery Dissecting Aneurysm
This 48-year-old man had presented with a right vertebral artery dissecting aneurysm near the origin of the posterior inferior cerebellar artery (PICA) and suffered a lateral medullary infarct. He had a spontaneous onset of dysarthria and facial numbness 10 days prior to arrival and still had significant neurological deficits at presentation, including dysphagia, dysarthria, right eye ptosis, miosis, right facial numbness, left body numbness, and right upper extremity ataxia. Preoperative magnetic resonance imaging showed brain stem stroke (Figure 7). A follow-up cerebral angiogram showed that the vertebral artery was nearly occluded and the PICA itself had a 95% stenosis at the origin, putting the patient at risk of a major stroke (Figure 8). We proceeded with RAG to the PICA followed by the occlusion of the aneurysm. The patient underwent a retro sigmoid craniotomy and craniectomy, C1 laminectomy, right PICA to V3 bypass with interposition of RAG and clipping of right V4 distal to the PICA (Figure 9). His postoperative cerebral angiogram showed patency of the bypass (Figure 10). The patient recovered from the procedure but was at high risk for aspiration pneumonia due to vagal paralysis, and a PEG tube was positioned to allow swallowing to recover. The patient made an excellent recovery, with gradual improvement of his swallowing function.
Figure 7.
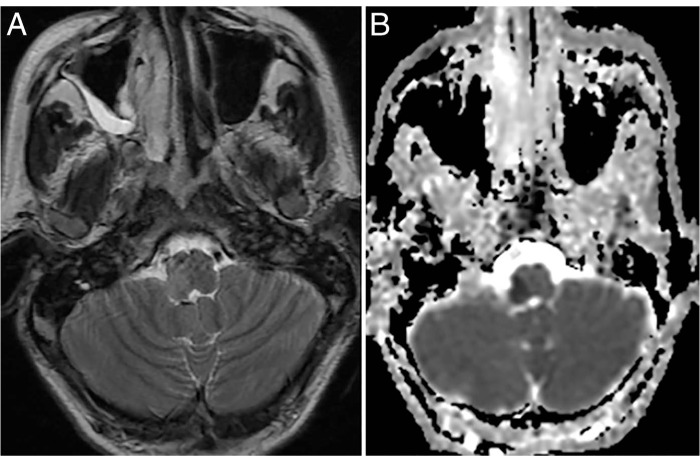
Preoperative (A) magnetic resonance imaging and (B) diffusion images showing the lateral medullary infarct.
Figure 8.
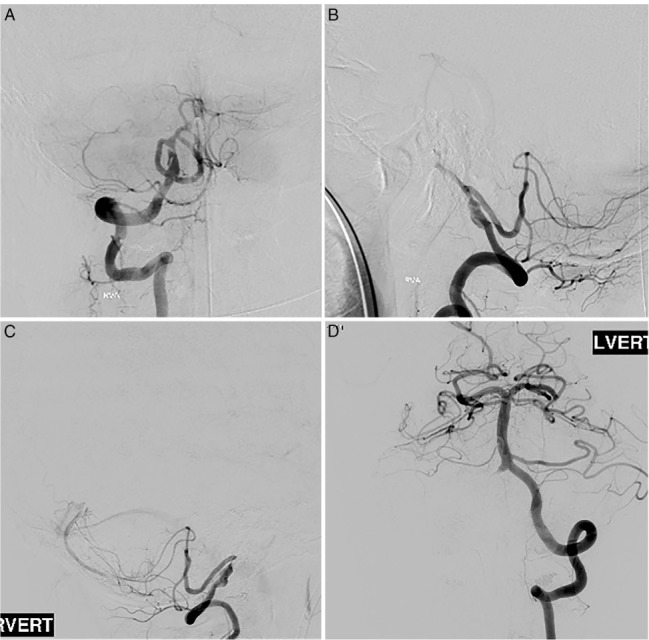
Preoperative cerebral angiogram showing the vertebral artery nearly occluded and the dominant posterior inferior cerebellar artery itself with a 95% stenosis at the origin.
Figure 9.
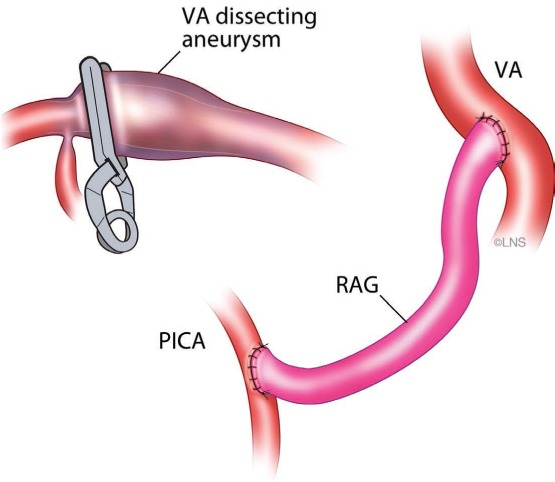
Illustration of procedure used to treat patient in case 2, showing a right posterior inferior cerebellar artery (PICA) to V3 bypass with interposition of radial artery graft and clipping of right V4 distal to PICA.
Figure 10.
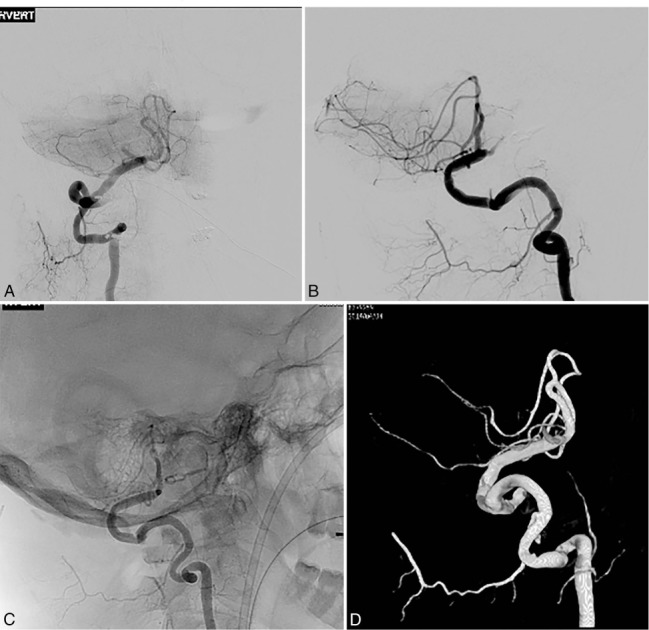
Postoperative cerebral angiogram and three-dimensional reconstruction showing patency of the bypass.
Conclusion
Vascular reconstruction and bypasses have revolutionized our treatment of complex intracranial aneurysms. While their role in the management of aneurysms has been somewhat reduced by the introduction of flow diversion stents, they still have an important role to play in the present-day surgical armamentarium. Many aneurysm cases remain challenging or technically impossible to treat with modern endovascular techniques. Therefore, it remains relevant to learn and teach bypass techniques to a younger generation of vascular neurosurgeons.
Acknowledgments
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
Funding/Support: Dr. Sekhar is a shareholder in SPI Surgical, Inc., and Viket Medical Corporation.
References
- 1.Caranci F, Briganti F, Cirillo L, Leonardi M, Muto M. Epidemiology and genetics of intracranial aneurysms. Eur J Radiol. 2013 Oct;82(10):1598–605. doi: 10.1016/j.ejrad.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 2.Alg VS, Sofat R, Houlden H, Werring DJ. Genetic risk factors for intracranial aneurysms: a meta-analysis in more than 116,000 individuals. Neurology. 2013 Jun 4;80(23):2154–65. doi: 10.1212/WNL.0b013e318295d751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke M. Systematic review of reviews of risk factors for intracranial aneurysms. Neuroradiology. 2008 Aug;50(8):653–64. doi: 10.1007/s00234-008-0411-9. [DOI] [PubMed] [Google Scholar]
- 4.Mahaney KB, Todd MM, Bayman EO, Torner JC, IHAST Investigators Acute postoperative neurological deterioration associated with surgery for ruptured intracranial aneurysm: incidence, predictors, and outcomes. J Neurosurg. 2012 Jun;116(6):1267–78. doi: 10.3171/2012.1.JNS111277. [DOI] [PubMed] [Google Scholar]
- 5.Fleming JB, Hoh BL, Simon SD, Welch BG, Mericle RA, Fargen KM et al. Rebleeding risk after treatment of ruptured intracranial aneurysms. J Neurosurg. 2011 Jun;114(6):1778–84. doi: 10.3171/2011.1.JNS101232. [DOI] [PubMed] [Google Scholar]
- 6.Mahaney KB, Todd MM, Torner JC, IHAST Investigators Variation of patient characteristics, management, and outcome with timing of surgery for aneurysmal subarachnoid hemorrhage. J Neurosurg. 2011 Apr;114(4):1045–53. doi: 10.3171/2010.11.JNS10795. [DOI] [PubMed] [Google Scholar]
- 7.Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002 Oct 26;360(9342):1267–74. doi: 10.1016/s0140-6736(02)11314-6. et al.; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. [DOI] [PubMed] [Google Scholar]
- 8.Lanzino G, Fraser K, Kanaan Y, Wagenbach A. Treatment of ruptured intracranial aneurysms since the International Subarachnoid Aneurysm Trial: practice utilizing clip ligation and coil embolization as individual or complementary therapies. J Neurosurg. 2006 Mar;104(3):344–9. doi: 10.3171/jns.2006.104.3.344. [DOI] [PubMed] [Google Scholar]
- 9.Britz GW. ISAT trial: coiling or clipping for intracranial aneurysms? Lancet. 2005 Sep 3–9;366(9488):783–5. doi: 10.1016/S0140-6736(05)67190-5. [DOI] [PubMed] [Google Scholar]
- 10.Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. 2009 May;8(5):427–33. doi: 10.1016/S1474-4422(09)70080-8. et al.; ISAT Collaborators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spetzler RF, McDougall CG, Albuquerque FC, Zabramski JM, Hills NK, Partovi S et al. The Barrow Ruptured Aneurysm Trial: 3-year results. J Neurosurg. 2013 Jul;119(1):146–57. doi: 10.3171/2013.3.JNS12683. [DOI] [PubMed] [Google Scholar]
- 12.Unruptured intracranial aneurysms–risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med. 1998 Dec 10;339(24):1725–33. doi: 10.1056/NEJM199812103392401. [DOI] [PubMed] [Google Scholar]
- 13.UCAS Japan Investigators. Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012 Jun 28;366(26):2474–82. doi: 10.1056/NEJMoa1113260. [DOI] [PubMed] [Google Scholar]
- 14.Sekhar LN, Kalavakonda C. Cerebral revascularization for aneurysms and tumors. Neurosurgery. 2002 Feb;50(2):321–31. doi: 10.1097/00006123-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Sekhar LN, Sen CN, Jho HD. Saphenous vein graft bypass of the cavernous internal carotid artery. J Neurosurg. 1990 Jan;72(1):35–41. doi: 10.3171/jns.1990.72.1.0035. [DOI] [PubMed] [Google Scholar]
- 16.Sekhar LN, Duff JM, Kalavakonda C, Olding M. Cerebral revascularization using radial artery grafts for the treatment of complex intracranial aneurysms: techniques and outcomes for 17 patients. Neurosurgery. 2001 Sep;49(3):646–58. doi: 10.1097/00006123-200109000-00023. discussion 658–9. [DOI] [PubMed] [Google Scholar]


