Abstract
Cerebral arteriovenous malformations (AVMs) are complex high-flow lesions that can result in devastating neurological injury when they hemorrhage. Embolization is a critical component in the management of many patients with cerebral AVMs. Embolization may be used as an independent curative therapy or more commonly in an adjuvant fashion prior to either micro- or radiosurgery. Although the treatment-related morbidity and mortality for AVMs—including that due to microsurgery, embolization, and radiosurgery—can be substantial, its natural history offers little solace. Fortunately, care by a multidisciplinary team experienced in the comprehensive management of AVMs can offer excellent results in most cases.
Keywords: aneurysm, brain arteriovenous malformation, AVM, embolization, endovascular, intracranial hemorrhage, radiosurgery, stroke, surgery

J. A. Ellis, M.D.

S. D. Lavine, M.D.
Introduction
Although the various cerebral vascular malformations had been long recognized in the literature, William McCormick in 1966 is credited for advancing the first modern pathological classification scheme for these lesions. His influence is evident in contemporary literature, which recognizes capillary telangiectasia, developmental venous anomaly, cavernous malformation, and arteriovenous malformation.1,2 For completeness, some schemes also include arteriovenous fistulae and mixed vascular malformations.3 It is the arteriovenous malformation (AVM), arguably the most complex and dangerous of the cerebral vascular lesions, that we will consider.
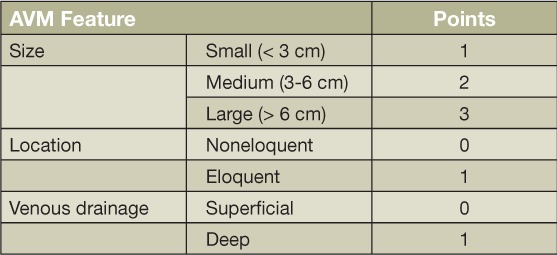
Cerebral AVMs are complex structures comprised of a network of abnormal arteries and veins that lack an intervening capillary bed, resulting in the high-flow arteriovenous (AV) shunting of blood.4,5 Although familial cases have been described, AVMs are congenital and most often occur sporadically. Various studies have reported an association with conditions such as hereditary hemorrhagic telangiectasia, Sturge-Weber disease, and Wyburn-Mason syndrome.6–9 AVMs often present with intracranial hemorrhage, but seizure, headache, and focal neurological deficits may also be seen. An annual hemorrhage risk of 2% to 4% is typically quoted and is associated with a 5% to 25% risk of death and 10% to 50% risk of neurological disability.4,8,10 Originally conceived as a surgical risk assessment tool, the Spetzler-Martin grading system that assigns points for AVM size, location, and venous drainage pattern is commonly used to describe AVMs in the clinical setting (Table 1).11
Table 1.
Spetzler-Martin AVM Grading Scale. Grade: sum of point for AVM size, location, and venous drainage. AVM: arteriovenous malformation.
Current treatment modalities for cerebral AVMs include microsurgical resection, endovascular embolization, and stereotactic radiosurgery. While these modalities may be used alone, a combination is often necessary to effect the best treatment result. Determining the type of intervention to offer or whether intervention should be offered can be difficult. Ultimately, a risk-benefit analysis should be conducted wherein the natural history of the AVM is considered against the risks associated with the proposed interventions.12,13 In the face of recent reports suggesting that medical management may be superior to interventional therapy for unruptured AVMs, at least in the short term, such analyses become all the more critical.14,15 Assembling a multidisciplinary team with expertise in the medical, surgical, endovascular, and radiation treatment of AVMs is critical for appropriately managing patients with this complex lesion.
Endovascular expertise should figure prominently in the multidisciplinary management of all AVMs. With rare exception, all AVMs should be characterized with a catheter cerebral angiogram even if conservative management is likely. Evaluation of arterial to venous flow-dynamics, the number and location of feeding vessels and draining veins, and the presence of associated aneurysms or venous outflow obstruction is critical for appropriate decision making. If endovascular intervention is elected, possible therapeutic strategies include premicrosurgical embolization, preradiosurgical embolization, curative embolization, or palliative embolization.10,16–18
Epidemiology and Natural History
Determining an estimate of the incidence and prevalence of brain AVMs has been difficult, resulting in a number of inaccurate figures being propagated in the literature.19 Rates of brain AVM detection determined by population-based studies range from 0.89 to 1.34 cases per 100,000 person-years.20–25 Similarly, variable prevalence estimates ranging from less than 0.02% to 0.2% are reported.20 The frequently cited New York Islands AVM study indicates an annual AVM detection rate of 1.34 cases per 100,000 person-years and concluded that a true prevalence may never be known due to the disease's rarity, its congenital nature, and its long asymptomatic development.22
Intracranial hemorrhage is still the most common presentation for patients harboring AVMs despite recent trends toward more frequent detection of unruptured lesions.8,26 A review of 10 large AVM series indicates that 45% to 72% (median 52%) of AVM patients present with hemorrhage.20 An influential study of 166 unoperated symptomatic brain AVM patients followed for an average of 24 years by Ondra et al. estimates a 4% annual risk of hemorrhage, a 1.7% annual risk of morbidity, and an annual mortality risk of 1%.27 A relatively large population study by ApSimon and colleagues concluded that the vast majority of brain AVMs will become symptomatic during a patient's lifetime, with the majority due to hemorrhage.24
The toll from AVM-related hemorrhage can be significant. In 2009, van Beijnum et al. reported a 40% rate of death or dependence in their population-based cohort 12 months after AVM-related intracerebral hemorrhage (ICH).28 A more optimistic report from Hartmann et al. found a 16% rate of at least moderately disabling deficit and no death after incident AVM hemorrhage in 115 patients.29 From their review of the literature, Bendok et al. assessed the risk of death after an AVM-associated hemorrhage to be from 10% to 15% while overall morbidity and mortality was estimated to be 15% per episode of hemorrhage.26
Pathophysiology
AVMs are composed of one or more feeding arteries, a nidus, and one or more draining veins (Figure 1).9,30 Small pial and perforating subcortical feeders that are not visible on angiography are also usually present. The lack of an intervening capillary bed allows for high-flow AV shunting of blood. AVMs are dynamic lesions that show evidence of active angiogenesis, inflammatory responses, and adaptive structural changes to its components over time.31 AV shunting results in feeding artery dilatation and draining vein arterialization.9,30 Histologically, the arterial elastic and muscular layers are altered. The internal elastic lamina may be reduplicated or interrupted while the media may be alternately thickened and thinned. Feeding artery aneurysms may develop in areas of thinned media while leiomyoma-like nodules can be seen in areas of thickening. Collagen deposition and luminal thrombosis leading to draining vein thickening is also observed. Hemosiderin-stained gliotic brain parenchyma often surrounds and insinuates within the nidus. Neuronal loss in the surrounding brain parenchyma due to vascular steal has also been reported.9,32
Figure 1.
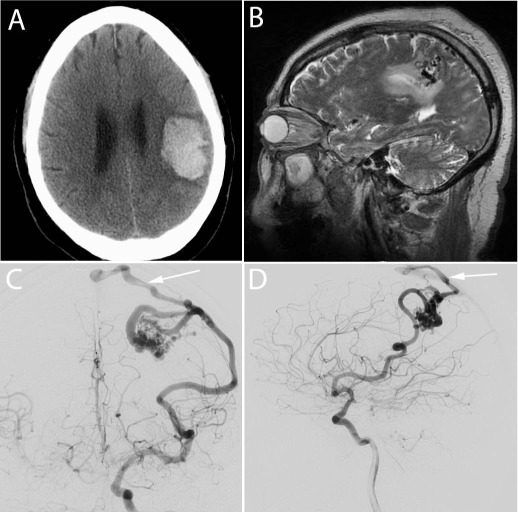
Hemorrhagic presentation of a cerebral arteriovenous malformation (AVM). This 72-year-old man presented after experiencing sudden onset right face and arm numbness associated with mild weakness. (A) Noncontrast head computerized tomography showed a left frontoparietal intracerebral hemorrhage. (B) T2-weighted brain magnetic resonance imaging showed flow voids in the left parietal lobe suggesting an underlying AVM. (C-D) Catheter cerebral angiogram confirmed the presence of a 2.5-cm AVM fed by the left middle cerebral artery with superficial venous drainage (arrow) to the sagittal sinus. Complete microsurgical resection of this AVM was performed after preoperative embolization with n-butyl cyanoacrylate.
History of Endovascular Treatment
Virchow is credited with providing the first pathological description of intracranial AVMs in 1863. In the late 1920s, early neurosurgical pioneers including Harvey Cushing and Walter Dandy attacked these lesions with great trepidation and uniformly reported poor surgical results with no successful radical resection.33 Of AVMs, Cushing is quoted as saying “It would be nothing less than foolhardy to attack one of the deep-seated racemose lesions…The surgical history of most of the reported cases shows not only the futility of an operative attack upon one of these angiomas but the extreme risk of serious cortical damage which it entails.” He was similarly pessimistic of the more superficial variety, saying “…even with this latter and surgically speaking more favorable type, there is little encouragement to be had on the side of radical treatment.”30,34 Thus these early surgical failures laid the foundation for the development of alternative or adjuvant treatment strategies such as embolization.
Although Dawbarn advocated for the embolization of head and neck cancers as early as 1904, and the first embolization of a carotid-cavernous fistula was attributed to Brooks in 1930, it would be an additional 30 years before AVM embolization was reported.35–37 Lussenhop and Spence reported the first successful embolization of a brain AVM in 1960 after introducing spherical methyl methacrylate emboli between 2.5- to 4.2-mm diameters into a surgically exposed left common carotid artery bifurcation.38 This and other flow-directed, unselective embolization techniques utilizing relatively large particles provided minimal if any long-term benefits but served as the basis for further endovascular refinements. Serbinenko subsequently reported in 1974 his development of endovascular balloon occlusion methods in the treatment of several cerebrovascular pathologies including AVMs.39 In the late 1970s, Kerber introduced more distal AVM feeding artery occlusion with liquid embolic agents by using a calibrated leak balloon microcatheter.40 Exponential advancements in endovascular technologies including microcatheter design and embolisate development was subsequently seen in the 1980s and 1990s.
Embolic Agents
A wide variety of agents have been used to embolize AVMs. Silastic spheres, gelfoam, silk suture, dehydrated ethanol, balloons, blood clots, muscle, and a number of other agents are all primarily of historical interest. At present, n-butyl cyanoacrylate, ethylene-vinyl alcohol copolymer, and, to a lesser extent, platinum coils and polyvinyl alcohol particles are used to embolize AVMs.
N-Butyl Cyanoacrylate
N-butyl cyanoacrylate (NBCA)—often referred to as simply “glue”—is a liquid embolic agent developed to replace its probably carcinogenic forerunner isobutyl cyanoacrylate. This agent polymerizes almost instantaneously when it comes in contact with ionic fluid, making the risk of microcatheter adhesion within the embolized artery high. NBCA is mixed with varying amounts of ethiodized oil or glacial acetic acid to control the rate of polymerization. Tantalum or tungsten powder may also be added to the mixture to increase radiopacity. Premature polymerization within the delivery microcatheter is prevented by rinsing it with a dextrose solution. Although NBCA is considered a permanent embolic agent that initiates a significant vascular inflammatory reaction, recanalization may occur. The risk of recanalization is especially high when NBCA is deposited in the proximal feeding artery with no penetration of the AVM nidus.
Ethylene-Vinyl Alcohol Copolymer
Ethylene-vinyl alcohol copolymer (Onyx) is a more recently developed liquid embolic agent also used for the embolization of cerebral AVMs. It is available in three premixed concentrations of increasing viscosity and composed of ethylene-vinyl alcohol copolymer and tantalum dissolved in dimethyl sulfoxide (DMSO). Onyx is thought to be a more manageable agent than NBCA as it solidifies slowly while the DMSO solvent diffuses, reducing the risk of microcatheter entrapment. Prolonged and repeated Onyx injections within the same AVM pedicle are possible and allow it to be pushed more distally toward and within the nidus. Like NBCA, Onyx is also considered a permanent embolic agent although recanalization is possible as well.
Platinum Coils and Polyvinyl Alcohol Particles
Platinum coils and polyvinyl alcohol particles (PVA) are solid embolic agents used much less frequently to embolize AVMs. Coils are not typically used to occlude AVM feeders but may be useful for slowing the flow within particular compartments to facilitate subsequent use of a liquid embolic agent. PVA particles are available in 50- to 1000-μm sizes. Vessel recanalization is expected after PVA embolization, therefore expedient postembolization surgical resection of the AVM is warranted.
Premicrosurgical Embolization
Premicrosurgical embolization is the most common role for endovascular therapy in the comprehensive multimodality management of cerebral AVMs. The general strategy for premicrosurgical embolization includes eliminating deep arterial pedicles that are encountered only during the latter stages of surgical resection and securing AVM-related aneurysms, especially if they are remote from the area of resection. Hemodynamically unrelated aneurysms may be managed conservatively. Some endovascular specialists attempt to embolize all accessible arterial pedicles, but this strategy is controversial and may be dangerous.41–43
Although it has been stated that low Spetzler-Martin grade AVMs should probably not be embolized preoperatively, it is prudent to study the diagnostic angiogram in each case and make an individual determination. In patients who require embolization of multiple pedicles, it is our opinion that a staged approach with each session separated by a week or more is safest. This allows for the gradual normalization of local and regional hemodynamics and may prevent devastating postprocedure hemorrhages secondary to rapid and widespread arterial and venous thromboses. Such an approach has been validated in the literature although controversy exists, with some practitioners favoring single-staged embolization.44,45
Preradiosurgical Embolization
The goals of preradiosurgical embolization are 2-fold: (1) to eliminate high-risk angiographic features that predispose to hemorrhage during the latency period after radiosurgical treatment, and (2) to achieve AVM volume reduction to a size amenable to radiosurgical treatment.
The treatment of aneurysms hemodynamically related to an AVM is warranted (Figure 2). This includes both proximal and pedicular aneurysms as well as intranidal aneurysms since these lesions carry a 7% to 10% yearly risk of hemorrhage.46 The use of preradiosurgical embolization to address other angioarchitectural features such as venous aneurysms or simply for flow reduction is probably not warranted.
Figure 2.
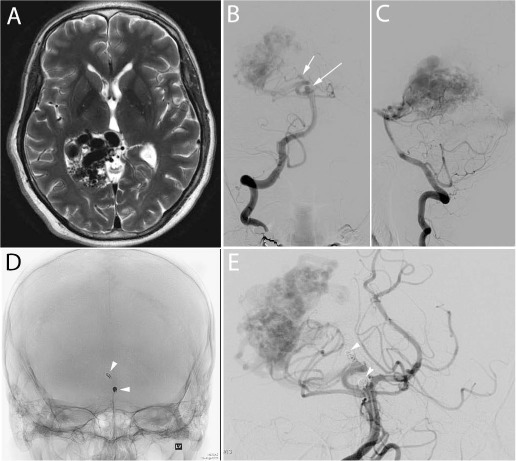
Spetzler-Martin grade IV arteriovenous malformation (AVM) with feeding artery aneurysms. This 49-year-old man presented subacutely with persistent headaches. (A) T2-weighted brain magnetic resonance imaging revealed the presence of an AVM in the posteromedial temporo-parieto-occipital lobes. A small amount of intraventricular hemorrhage was also present on imaging (not shown). (Arrows B-C) Catheter cerebral angiogram revealed the presence of two hemodynamically related aneurysms at the basilar tip and on the right posterior cerebral artery. (Arrows D-E) Both aneurysms were coiled in preparation for definitive AVM treatment with gamma knife radiosurgery.
Preradiosurgical embolization has been performed on large AVMs to reduce their volume to 10 cc or less. Obliteration rates of over 80% at 2 years have been reported for lesions that do not exceed this volume threshold.47 Gobin et al. have reported that preradiosurgical embolization reduced average AVM volumes by more than 30% in their cohort of 125 patients and allowed for a 65% rate of AVM cure in the patients rendered suitable for radiosurgery after embolization.48 Obliteration rates of 60% to 81% have been reported by other groups.41 It is noteworthy, however, that a number of reports indicate a negative association between successful AVM radiosurgical obliteration and previous embolization.49–52 For this reason, employing a strategy of preradiosurgical embolization for the purpose of AVM volume reduction is controversial at best.43
Curative Embolization
AVMs may infrequently be cured by embolization alone. To achieve such an endovascular cure, all nidal filling and early venous drainage must be eliminated (Figure 3). Patients with angiographically “cured” AVMs may still be at risk for devastating hemorrhage if unseen feeders continue to fill a partially embolized nidus. It remains a matter of debate whether permanent curative AVM embolization can in fact be achieved as recanalization through collateral channels is possible.18 After an extensive review of the literature over a 35-year period, which included 1,246 total patients, Frizzel at al. reported a 5% cure rate for AVMs treated by embolization.53 The highest rates of endovascular AVM cure are reported in series with patients carefully selected for curative embolization. Such patients typically have small AVMs with few feeding pedicles, noncompartmental fistulous (rather than plexiform) niduses, and no perinidal angiogenesis.18,41,42,48,54 Additionally, use of Onyx has been associate with higher rates of AVM cure by embolization than previously seen with other embolic agents.41
Figure 3.
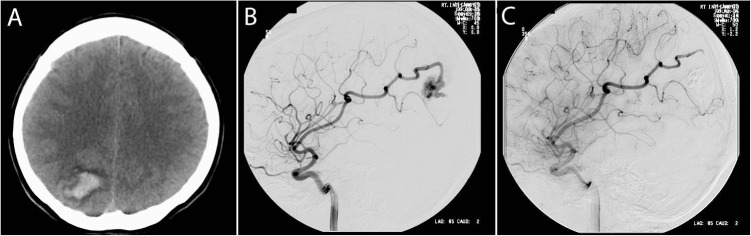
Curative arteriovenous malformation (AVM) embolization. This 35-year-old woman presented with a sudden onset of severe right-sided headache. (A) Noncontrast head computerized tomography showed an acute right parieto-occipital intracerebral hemorrhage. (B) Catheter cerebral angiogram showed a 2-cm AVM in the region of the hemorrhage supplied by a single dominant right-middle-cerebral-artery feeder. (C) Curative embolization with n-butyl cyanoacrylate was performed without complication.
Palliative Embolization
Palliative AVM embolization may be performed in patients with no hope of cure by multimodality therapy but who may derive some benefit from targeted embolization of particular angioarchitectural features. The importance of addressing aneurysms hemodynamically related to an AVM has already been mentioned (see Preradiosurgical Embolization section). Embolization to eliminate particular shunts that result in vascular steal syndromes may also be beneficial. Aside from these scenarios, partial AVM embolization is not recommended as outcomes seem to be worse than the natural history.43,55,56
Risks of Embolization
The treatment-related morbidity and mortality of AVM embolization can be substantial. Overall AVM embolization complication rates of approximately 5% to 15% have been reported.4,5 A recent meta-analysis by van Beijnum et al. found that complications leading to permanent neurological deficits or death occurred in 6.6% (range 0–18%) of patients after AVM embolization.57 Taylor et al. reported a 9% rate of permanent neurological deficits and a 2% rate of death resulting from embolization in their cohort of 201 patients undergoing preoperative embolization.58 Hartmann et al. similarly reported on 233 patients who underwent AVM embolization with a goal of either endovascular or multimodality cure. They observed a 14% rate of treatment-related neurological deficits, a 2% rate of persistent disabling deficits, and a 1% rate of death.59 Baharvahdat et al. reported an 11% procedural complication rate after 846 embolizations in 408 patients. Persistent, new disability and death attributable to hemorrhage after embolization was reported in 7.6% and 1.6% of patients, respectively.60
Conclusion
Prior to embarking on a treatment course for a cerebral AVM, it is critical that the patient understands both the natural history as well as the treatment-related risks and benefits. Safe, definitive treatment of these complex and dangerous lesions requires coordination by an experienced multidisciplinary team. The anxiety and uncertainty wrought by living with an untreated brain AVM is a significant and ubiquitous burden for both patients and their families. Indeed a substantial upfront procedural risk may be worthwhile for many patients who desire permanent AVM eradication. Embolization is a critical component of the multimodality treatment of cerebral AVMs. It is clear that many AVMs cannot be safely cured without the judicious use of this modality.
Acknowledgments
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
Funding/Support: The authors have no funding disclosures.
References
- 1.McCormick WF. The pathology of vascular (“arteriovenous”) malformations. J Neurosurg. 1966 Apr;24(4):807–16. doi: 10.3171/jns.1966.24.4.0807. [DOI] [PubMed] [Google Scholar]
- 2.McCormick WF, Nofzinger JD. “Cryptic” vascular malformations of the central nervous system. J Neurosurg. 1966 May;24(5):865–75. doi: 10.3171/jns.1966.24.5.0865. [DOI] [PubMed] [Google Scholar]
- 3.Awad IA, Robinson JR, Jr, Mohanty S, Estes ML. Mixed vascular malformations of the brain: clinical and pathogenetic considerations. Neurosurgery. 1993 Aug;33(2):179–88. doi: 10.1227/00006123-199308000-00001. discussion 88. [DOI] [PubMed] [Google Scholar]
- 4.Friedlander RM. Clinical practice. Arteriovenous malformations of the brain. New Engl J Meditor. 2007 Jun 28;356(26):2704–12. doi: 10.1056/NEJMcp067192. [DOI] [PubMed] [Google Scholar]
- 5.The Arteriovenous Malformation Study Group. Arteriovenous malformations of the brain in adults. New Engl J Meditor. 1999 Jun 10;340(23):1812–8. doi: 10.1056/NEJM199906103402307. [DOI] [PubMed] [Google Scholar]
- 6.Boyd MC, Steinbok P, Paty DW. Familial arteriovenous malformations. Report of four cases in one family. J Neurosurg. 1985 Apr;62(4):597–9. doi: 10.3171/jns.1985.62.4.0597. [DOI] [PubMed] [Google Scholar]
- 7.Larsen PD, Hellbusch LC, Lefkowitz DM, Schaefer GB. Cerebral arteriovenous malformation in three successive generations. Pediatr Neurol. 1997 Jul;17(1):74–6. doi: 10.1016/s0887-8994(97)00007-6. [DOI] [PubMed] [Google Scholar]
- 8.van Beijnum J, van der Worp HB, Schippers HM, van Nieuwenhuizen O, Kappelle LJ, Rinkel GJ et al. Familial occurrence of brain arteriovenous malformations: a systematic review. J Neurol Neurosurg Psychiatry. 2007 Nov;78(11):1213–7. doi: 10.1136/jnnp.2006.112227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moussa RF, Wong JH, Awad IA. Pathology and genetic factors. In: Stieg PE, Batjer HH, Samson D, editors. Intracranial arteriovenous malformations. New York: Informa Healthcare; 2007. pp. 21–30. p. [Google Scholar]
- 10.Ogilvy CS, Stieg PE, Awad I, Brown RD, Jr, Kondziolka D, Rosenwasser R et al. AHA Scientific Statement: Recommendations for the management of Intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke. 2001 Jun;32(6):1458–71. doi: 10.1161/01.str.32.6.1458. Special Writing Group of the Stroke Council, American Stroke Association. [DOI] [PubMed] [Google Scholar]
- 11.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986 Oct;65(4):476–83. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- 12.McInerney J, Harbaugh RE. Decision analysis for asymptomatic lesions. In: Stieg PE, Batjer HH, Samson D, editors. Intracranial arteriovenous malformations. New York: Informa Healthcare; 2007. pp. 123–34. p. [Google Scholar]
- 13.Morgan M. Therapeutic decision making. In: Winn HR, editor. Youmans neurological surgery. 6th editor. Vol 4. Philadelphia: Elsevier Saunders; 2011. pp. 4034–48. editor. p. [Google Scholar]
- 14.Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014 Feb 15;383(9917):614–21. doi: 10.1016/S0140-6736(13)62302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohr JP. Results of ARUBA are applicable to most patients with nonruptured arteriovenous malformations. Stroke. 2014 May;45(5):1541–2. doi: 10.1161/STROKEAHA.113.002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stieg PE, Janardhan V, Riina HA. Multimodality therapy: treatment algorithms. In: Stieg PE, Batjer HH, Samson D, editors. Intracranial arteriovenous malformations. New York: Informa Healthcare; 2007. pp. 135–43. p. [Google Scholar]
- 17.Starke RM, Lavine SD, Meyers PM, Connolly ES., Jr . Adjuvant endovascular management of brain arteriovenous malformations. In: Winn HR, editor. Youmans neurological surgery. 6th editon. Vol 4. Philadelphia: Elsevier Saunders; 2011. pp. 4049–64. editor. p. [Google Scholar]
- 18.Starke RM, Lavine SD, Connolly ES, Jr, Meyers PM. Endovascular management of arteriovenous malformations for cure. In: Winn HR, editor. Youmans neurological surgery. 6th editon. Vol 4. Philadelphia: Elsevier Saunders; 2011. pp. 4065–71. editor. p. [Google Scholar]
- 19.Berman MF, Sciacca RR, Pile-Spellman J, Stapf C, Connolly ES, Jr, Mohr JP et al. The epidemiology of brain arteriovenous malformations. Neurosurgery. 2000 Aug;47(2):389–96. doi: 10.1097/00006123-200008000-00023. discussion 97. [DOI] [PubMed] [Google Scholar]
- 20.Laakso A, Hernesniemi J. Arteriovenous malformations: epidemiology and clinical presentation. Neurosurg Clin N Am. 2012 Jan;23(1):1–6. doi: 10.1016/j.nec.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Stapf C, Mohr JP, Pile-Spellman J, Solomon RA, Sacco RL, Connolly ES., Jr. Epidemiology and natural history of arteriovenous malformations. Neurosurg Focus. 2001;11(5):e1. doi: 10.3171/foc.2001.11.5.2. [DOI] [PubMed] [Google Scholar]
- 22.Stapf C, Mast H, Sciacca RR, Berenstein A, Nelson PK, Gobin YP et al. The New York Islands AVM Study: design, study progress, and initial results. Stroke. 2003 May;34(5):e29–33. doi: 10.1161/01.STR.0000068784.36838.19. [DOI] [PubMed] [Google Scholar]
- 23.Hillman J. Population-based analysis of arteriovenous malformation treatment. J Neurosurg. 2001 Oct;95(4):633–7. doi: 10.3171/jns.2001.95.4.0633. [DOI] [PubMed] [Google Scholar]
- 24.ApSimon HT, Reef H, Phadke RV, Popovic EA. A population-based study of brain arteriovenous malformation: long-term treatment outcomes. Stroke. 2002 Dec;33(12):2794–800. doi: 10.1161/01.str.0000043674.99741.9b. [DOI] [PubMed] [Google Scholar]
- 25.Brown RD, Jr, Wiebers DO, Torner JC, O'Fallon WM. Incidence and prevalence of intracranial vascular malformations in Olmsted County, Minnesota, 1965 to 1992. Neurology. 1996 Apr;46(4):949–52. doi: 10.1212/wnl.46.4.949. [DOI] [PubMed] [Google Scholar]
- 26.Bendok BR, Eddleman C, Adel JG, Ali J, Batjer HH, Ondra SL. Natural History. In: Stieg PE, Batjer HH, Samson D, editors. Intracranial arteriovenous malformations. New York: Informa Healthcare; 2007. pp. 73–80. p. [Google Scholar]
- 27.Ondra SL, Troupp H, George ED, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990 Sep;73(3):387–91. doi: 10.3171/jns.1990.73.3.0387. [DOI] [PubMed] [Google Scholar]
- 28.van Beijnum J, Lovelock CE, Cordonnier C, Rothwell PM, Klijn CJ, Al-Shahi Salman R. Outcome after spontaneous and arteriovenous malformation-related intracerebral haemorrhage: population-based studies. Brain. 2009 Feb;132(Pt 2):537–43. doi: 10.1093/brain/awn318. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann A, Mast H, Mohr JP, Koennecke HC, Osipov A, Pile-Spellman J et al. Morbidity of intracranial hemorrhage in patients with cerebral arteriovenous malformation. Stroke. 1998 May;29(5):931–4. doi: 10.1161/01.str.29.5.931. [DOI] [PubMed] [Google Scholar]
- 30.Gavin CG, Kitchen ND. Pathobiology of true arteriovenous malformations. In: Winn HR, editor. Youmans neurological surgery. 6th editon. Vol 4. Philadelphia: Elsevier Saunders; 2011. pp. 4004–15. editor. p. [Google Scholar]
- 31.Kim H, Su H, Weinsheimer S, Pawlikowska L, Young WL. Brain arteriovenous malformation pathogenesis: a response-to-injury paradigm. Acta Neurochir Suppl. 2011;111:83–92. doi: 10.1007/978-3-7091-0693-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costantino A, Vinters HV. A pathologic correlate of the ‘steal’ phenomenon in a patient with cerebral arteriovenous malformation. Stroke. 1986 Jan–Feb;17(1):103–6. doi: 10.1161/01.str.17.1.103. [DOI] [PubMed] [Google Scholar]
- 33.Colby GP, Coon AL, Huang J, Tamargo RJ. Historical perspective of treatments of cranial arteriovenous malformations and dural arteriovenous fistulas. Neurosurg Clin N Am. 2012 Jan;23(1):15–25. doi: 10.1016/j.nec.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Cohen-Gadol AA, Spencer DD. The legacy of Harvey Cushing : profiles of patient care. New York: Thieme; 2007. [Google Scholar]
- 35.Vitek JJ, Smith MJ. The myth of the Brooks method of embolization: a brief history of the endovascular treatment of carotid-cavernous sinus fistula. J Neurointerv Surg. 2009 Dec;1(2):108–11. doi: 10.1136/jnis.2009.000067. [DOI] [PubMed] [Google Scholar]
- 36.Prestigiacomo CJ, Pile-Spellman P. Endovascular principles. In: Stieg PE, Batjer HH, Samson D, editors. Intracranial arteriovenous malformations. New York: Informa Healthcare; 2007. pp. 159–76. p. [Google Scholar]
- 37.Dawbarn RHM, editor. The starvation operation for malignancy in the external carotid area. J Am Med Assoc. 1904;13:792–5. editor. [Google Scholar]
- 38.Luessenhop AJ, Spence WT. Artificial embolization of cerebral arteries. Report of use in a case of arteriovenous malformation. J Am Med Assoc. 1960 Mar 12;172:1153–5. doi: 10.1001/jama.1960.63020110001009. [DOI] [PubMed] [Google Scholar]
- 39.Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974 Aug;41(2):125–45. doi: 10.3171/jns.1974.41.2.0125. [DOI] [PubMed] [Google Scholar]
- 40.Kerber CW, Bank WO, Cromwell LD. Calibrated leak balloon microcatheter: a device for arterial exploration and occlusive therapy. AJR Am J Roentgenol. 1979 Feb;132(2):207–12. doi: 10.2214/ajr.132.2.207. [DOI] [PubMed] [Google Scholar]
- 41.Crowley RW, Ducruet AF, McDougall CG, Albuquerque FC. Endovascular advances for brain arteriovenous malformations. Neurosurgery. 2014 Feb;74(Suppl 1):S74–82. doi: 10.1227/NEU.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 42.Siddiqui AH, Chen PR, Rosenwasser RH. Endovascular therapy: indications, complications, and outcome. In: Stieg PE, Batjer HH, Samson D, editors. Intracranial arteriovenous malformations. New York: Informa Healthcare; 2007. pp. 407–27. p. [Google Scholar]
- 43.Heros RC. Embolization of arteriovenous malformations. J Neurosurg. 2004 May;100(5):807–9. doi: 10.3171/jns.2004.100.5.0807. discussion 09. [DOI] [PubMed] [Google Scholar]
- 44.Spetzler RF, Martin NA, Carter LP, Flom RA, Raudzens PA, Wilkinson E. Surgical management of large AVM's by staged embolization and operative excision. J Neurosurg. 1987 Jul;67(1):17–28. doi: 10.3171/jns.1987.67.1.0017. [DOI] [PubMed] [Google Scholar]
- 45.Sahlein DH, Mora P, Becske T, Nelson PK. Nidal embolization of brain arteriovenous malformations: rates of cure, partial embolization, and clinical outcome. J Neurosurg. 2012 Jul;117(1):65–77. doi: 10.3171/2012.3.JNS111405. [DOI] [PubMed] [Google Scholar]
- 46.Bendok BR, Getch CC, Batjer HH. Associated aneurysms. In: Stieg PE, Batjer HH, Samson D, editors. Intracranial arteriovenous malformations. New York: Informa Healthcare; 2007. pp. 343–50. p. [Google Scholar]
- 47.Dion JE, Mathis JM. Cranial arteriovenous malformations. The role of embolization and stereotactic surgery. Neurosurg Clin N Am. 1994 Jul;5(3):459–74. [PubMed] [Google Scholar]
- 48.Gobin YP, Laurent A, Merienne L, Schlienger M, Aymard A, Houdart E et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 1996 Jul;85(1):19–28. doi: 10.3171/jns.1996.85.1.0019. [DOI] [PubMed] [Google Scholar]
- 49.Pollock BE, Flickinger JC, Lunsford LD, Maitz A, Kondziolka D. Factors associated with successful arteriovenous malformation radiosurgery. Neurosurgery. 1998 Jun;42(6):1239–44. doi: 10.1097/00006123-199806000-00020. discussion 44–7. [DOI] [PubMed] [Google Scholar]
- 50.Andrade-Souza YM, Ramani M, Scora D, Tsao MN, terBrugge K, Schwartz ML. Embolization before radiosurgery reduces the obliteration rate of arteriovenous malformations. Neurosurgery. 2007 Mar;60(3):443–51. doi: 10.1227/01.NEU.0000255347.25959.D0. discussion 51–2. [DOI] [PubMed] [Google Scholar]
- 51.Schwyzer L, Yen CP, Evans A, Zavoian S, Steiner L. Long-term results of gamma knife surgery for partially embolized arteriovenous malformations. Neurosurgery. 2012 Dec;71(6):1139–47. doi: 10.1227/NEU.0b013e3182720280. discussion 47–8. [DOI] [PubMed] [Google Scholar]
- 52.Kano H, Kondziolka D, Flickinger JC, Park KJ, Iyer A, Yang HC et al. Stereotactic radiosurgery for arteriovenous malformations after embolization: a case-control study. J Neurosurg. 2012 Aug;117(2):265–75. doi: 10.3171/2012.4.JNS111935. [DOI] [PubMed] [Google Scholar]
- 53.Frizzel RT, Fisher WS., 3rd. Cure, morbidity, and mortality associated with embolization of brain arteriovenous malformations: a review of 1246 patients in 32 series over a 35-year period. Neurosurgery. 1995 Dec;37(6):1031–9. doi: 10.1227/00006123-199512000-00001. discussion 39–40. [DOI] [PubMed] [Google Scholar]
- 54.Valavanis A, Yasargil MG. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg. 1998;24:131–214. doi: 10.1007/978-3-7091-6504-1_4. [DOI] [PubMed] [Google Scholar]
- 55.Han PP, Ponce FA, Spetzler RF. Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg. 2003 Jan;98(1):3–7. doi: 10.3171/jns.2003.98.1.0003. [DOI] [PubMed] [Google Scholar]
- 56.Miyamoto S, Hashimoto N, Nagata I, Nozaki K, Morimoto M, Taki W et al. Posttreatment sequelae of palliatively treated cerebral arteriovenous malformations. Neurosurgery. 2000 Mar;46(3):589–94. doi: 10.1097/00006123-200003000-00013. discussion 94–5. [DOI] [PubMed] [Google Scholar]
- 57.van Beijnum J, van der Worp HB, Buis DR, Al-Shahi Salman R, Kappelle LJ, Rinkel GJ et al. Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. JAMA. 2011 Nov 9;306(18):2011–9. doi: 10.1001/jama.2011.1632. [DOI] [PubMed] [Google Scholar]
- 58.Taylor CL, Dutton K, Rappard G, Pride GL, Replogle R, Purdy PD et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg. 2004 May;100(5):810–2. doi: 10.3171/jns.2004.100.5.0810. [DOI] [PubMed] [Google Scholar]
- 59.Hartmann A, Pile-Spellman J, Stapf C, Sciacca RR, Faulstich A, Mohr JP et al. Risk of endovascular treatment of brain arteriovenous malformations. Stroke. 2002 Jul;33(7):1816–20. doi: 10.1161/01.str.0000020123.80940.b2. [DOI] [PubMed] [Google Scholar]
- 60.Baharvahdat H, Blanc R, Termechi R, Pistocchi S, Bartolini B, Redjem H et al. Hemorrhagic complications after endovascular treatment of cerebral arteriovenous malformations. AJNR Am J Neuroradiol. 2014 May;35(5):978–83. doi: 10.3174/ajnr.A3906. [DOI] [PMC free article] [PubMed] [Google Scholar]


