Abstract
Transforming growth factor-β1 (TGF-β1) upregulation occurs in virtually all chronic kidney diseases and is associated with podocyte injury and proteinuria; however, the mechanisms contributing to this in vivo are ambiguous. In vitro, incubation of podocytes with TGF-β1 induced Wnt1 expression, β-catenin activation, and stimulated the expression of Wnt/β-catenin downstream target genes. Ectopic expression of Wnt1 or β-catenin mimicked TGF-β1, induced Snail1, and suppressed nephrin expression. The Wnt antagonist, Dickkopf-1, blocked TGF-β1-induced β-catenin activation, Snail1 induction, and nephrin suppression. In vivo, ectopic expression of TGF-β1 induced Wnt1 expression, activated β-catenin, and upregulated Wnt target genes such as Snail1, MMP-7, MMP-9, desmin, Fsp1, and PAI-1 in mouse glomeruli, leading to podocyte injury and albuminuria. Consistently, concomitant expression of Dickkopf-1 gene abolished β-catenin activation, inhibited TGF-β1-triggered Wnt target gene expression, and mitigated albuminuria. Thus, canonical Wnt/β-catenin signaling mediates TGF-β1-driven podocyte injury and proteinuria. These studies suggest that Wnt/β-catenin signaling may be exploited as a therapeutic target for the treatment of proteinuric kidney diseases.
Keywords: β-catenin, podocyte, proteinuria, TGF-β1, Wnt
Podocyte injury is one of the major culprits resulting in an impaired glomerular filtration, which is manifested by proteinuria in the clinical setting.1 Characterized as terminally differentiated glomerular visceral epithelial cells with highly sophisticated foot processes, podocytes possess little proliferative capacity in adult kidneys and are susceptible to various metabolic, hemodynamic, immune, and toxic injuries.2,3 Transforming growth factor-β1 (TGF-β1), a potent fibrogenic cytokine that is upregulated in virtually all kinds of chronic kidney diseases,4,5 is a well-characterized pathogenic factor that regulates podocyte structure and function in a hostile way. In vitro, TGF-β1 has been shown to induce podocytes to undergo epithelial–mesenchymal transition (EMT),6 and at a high concentration it triggers podocyte apoptosis.7 Aberrant regulation of TGF-β1 expression is clearly associated with podocyte dysfunction and proteinuria in patients with various chronic kidney disorders. Transgenic mice overexpressing TGF-β1 under albumin promoter spontaneously develop proteinuria and progressive kidney disease.8,9 These studies have clearly established an intimate linkage of hyperactive TGF-β1 to the pathogenesis of podocyte injury and proteinuria. However, exactly how TGF-β1 induces podocyte injury in vivo remains poorly understood.
Wnt/β-catenin signaling is an evolutionarily conserved, outside-in signal pathway that has a fundamental role in controlling many biological processes such as embryonic development and tissue homeostasis.10,11 At the cellular level, this signaling regulates cell morphology, proliferation, motility, and cell fate. At least two Wnt ligands, Wnt4 and Wnt9b, are clearly involved in the epithelial differentiation of metanephric mesenchymal progenitors during nephron formation,12,13 whereas they are considered to be silent in adult kidney. Inappropriate expression of Wnts and reactivation of β-catenin are common findings in a wide variety of kidney diseases in animal models and in humans, including obstructive nephropathy, adriamycin nephropathy, ischemia/reperfusion-induced acute kidney injury, polycystic kidney diseases, chronic allograft nephropathy, and diabetic nephropathy.14–17
Depending on different receptors and cellular content, Wnt proteins can activate and transduce their intracellular signaling through both canonical (β-catenin-dependent) and non-canonical (β-catenin-independent) pathways.10,11 Earlier studies have shown that the canonical Wnt/β-catenin signaling is decisively implicated in the pathogenesis of podocyte dysfunction and proteinuria, as genetic ablation of β-catenin in a podocyte-specific manner protects mice against development of proteinuria after adriamycin injury.15,18 Pharmacological activation of β-catenin by lithium chloride causes proteinuria in mice,15 whereas inhibition of Wnt/β-catenin by paricalcitol mitigates an established proteinuria in adriamycin nephropathy.19 These findings led us to hypothesize that canonical Wnt/β-catenin signaling might have a role in mediating TGF-β1-driven podocyte injury and proteinuria.
In this study, we demonstrate that hyperactive TGF-β1 alone is sufficient to induce podocyte injury and proteinuria in healthy mice, which is accompanied by Wnt1 induction, β-catenin activation, and induction of numerous Wnt target genes in the glomeruli. Consistently, inhibition of Wnt signaling by its antagonist ameliorates TGF-β1-triggered podocyte injury in vivo, suggesting a crucial role for the canonical Wnt/β-catenin signaling in mediating this process. Therefore, targeting this signaling could offer renal protection against the development and progression of proteinuric kidney diseases.
RESULTS
Induction of Wnt expression by TGF-β1 in podocytes
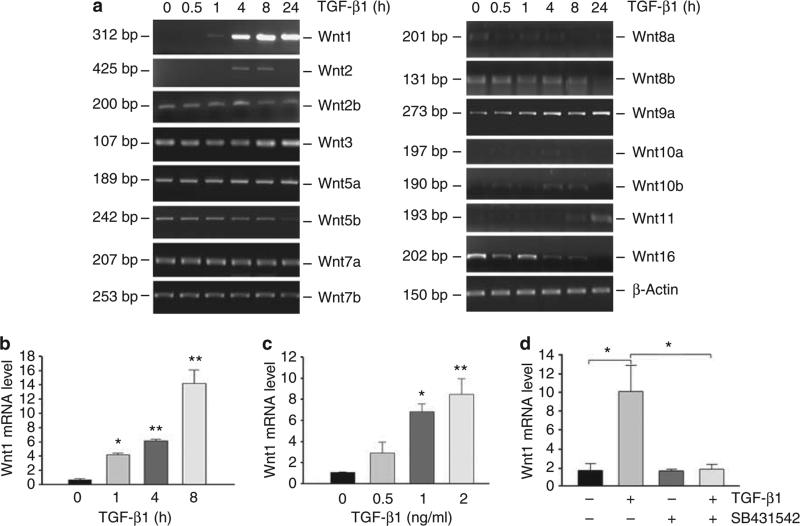
We first performed a systematic analysis of the mRNA expression of all Wnt genes in cultured mouse podocytes after incubation with TGF-β1 by reverse transcription-PCR (RT-PCR) approach. As shown in Figure 1a, a comprehensive survey of all 19 Wnts revealed a marked induction of Wnt1 mRNA expression in podocytes after TGF-β1 treatment. Several other Wnts, including Wnt2, Wnt9a, Wnt10b, and Wnt11, was also induced at different time points, whereas a slight inhibition of Wnt5b and Wnt16 was evident, particularly at later time points. Notably, the expression of Wnt3a, Wnt4, and Wnt9b was undetectable in cultured podocytes throughout the experiments (data not shown). Among all Wnts examined, the induction of Wnt1 mRNA by TGF-β1 was most predominant. Quantitative real-time RT-PCR results revealed that Wnt1 mRNA expression was induced by ~14-fold at 8 h after TGF-β1 treatment in mouse podocytes (Figure 1b). The induction of Wnt1 mRNA expression also occurred in a dose-dependent manner, and TGF-β1 induced its mRNA level at the concentration as low as 0.5 ng/ml, which reached the peak at 2 ng/ml (Figure 1c). Notably, induction of Wnt1 was dependent on TGF-β receptor signaling, as SB431542, an inhibitor of TGF-β type I receptor, completely abolished Wnt1 induction by TGF-β1 in podocytes (Figure 1d).
Figure 1. Expression of Wnt genes is induced by transforming growth factor-β1 (TGF-β1) in podocytes.
(a) Reverse transcription-PCR (RT-PCR) demonstrates an altered expression of various Wnts mRNA in cultured mouse podocytes after treatment with TGF-β1 at 2 ng/ml for various periods of time as indicated. (b, c) Quantitative real-time RT-PCR reveals that TGF-β1 induced Wnt1 mRNA expression in a time-and dose-dependent manner. Wnt1 mRNA levels were assessed by real-time RT-PCR in mouse podocytes after treatment with a fixed amount of TGF-β1 (2 ng/ml) for various periods of time as indicated (b) or with various concentrations of TGF-β1 for 24 h (c). *P<0.05; **P<0.01 versus controls (n = 3). (d) Induction of Wnt1 by TGF-β1 is dependent on TGF-β receptor signaling. Mouse podocytes were pretreated with TGF-β type I receptor inhibitor SB431542 at 10 μmol/l for 1 h, and then treated with TGF-β1 at 2 ng/ml for 8 h. Wnt1 mRNA was assessed by real-time RT-PCR. *P<0.05 (n = 3).
TGF-β1 activates β-catenin and induces its target gene expression
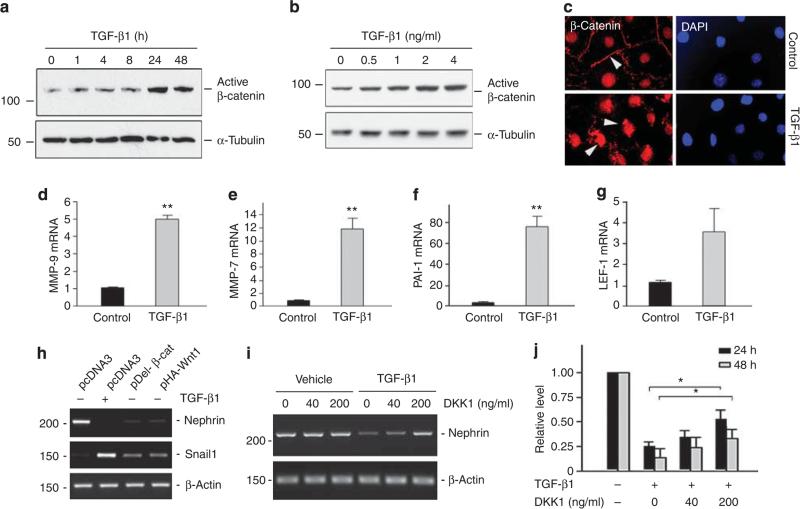
To examine the biological consequence of Wnt induction in podocytes, we next investigated the activation of β-catenin, the principal downstream mediator of canonical Wnt signaling, in podocytes after TGF-β1 treatment. As shown in Figure 2a, active, dephosphorylated form of β-catenin protein was induced in cultured mouse podocytes at 24 h after incubation with TGF-β1, which sustained to 48 h. This activation of β-catenin significantly lagged behind the Wnt1 mRNA induction by TGF-β1. The induction of active β-catenin protein was also dose dependent, and TGF-β1 induced active β-catenin at a concentration as low as 0.5 ng/ml, which reached the peak at 2 ng/ml (Figure 2b). We further examined β-catenin activation and its subcellular distribution in podocytes after incubation with TGF-β1 for 24 h. As illustrated in Figure 2c, β-catenin predominantly displayed a plasma membrane-associated staining pattern in the resting, control podocytes (Figure 2c, arrowhead). However, upon stimulation by TGF-β1, β-catenin underwent nuclear translocation, with disappearance of the plasma membrane-associated staining and concomitant emergence of nuclear β-catenin (Figure 2c, arrowheads). This nuclear translocation of β-catenin, together with the western blot results (Figure 2a and b), clearly indicates the activation of canonical Wnt/β-catenin signaling in podocytes after TGF-β1 treatment.
Figure 2. Transforming growth factor-β1 (TGF-β1) activates β-catenin and induces its target gene expression in podocytes.
(a, b) Western blot analyses demonstrate that TGF-β1 induced active β-catenin protein expression in a time- and dose-dependent manner. Podocytes were treated with a fixed amount of TGF-β1 (2 ng/ml) for various periods of time as indicated (a) or with various concentrations of TGF-β1 for 48 h (b). Total cell lysates were immunoblotted with specific antibodies against active β-catenin and α-tubulin, respectively. (c) Immunofluorescence staining demonstrates the activation of β-catenin and nuclear translocation in mouse podocytes after treatment with TGF-β1 (2 ng/ml) for 24 h. Arrowheads indicate β-catenin staining. (d–g) TGF-β1 induces the expression of Wnt/β-catenin target genes in podocytes. Mouse podocytes were incubated with TGF-β1 (2 ng/ml) for 24 h. The expression of Wnt/β-catenin target genes MMP-9 (d), MMP-7 (e), PAI-1 (f), and LEF-1 (g) was assessed by real-time reverse transcription-PCR (RT-PCR). **P<0.01 (n = 3). (h) TGF-β1/Wnt1/β-catenin cascade is sufficient for triggering nephrin suppression and Snail1 induction in podocytes. Mouse podocytes were incubated with TGF-β1 at 2 ng/ml for 48 h, or transiently transfected with expression vector pHA-Wnt1, pDel-β-cat, or pcDNA, respectively. Representative RT-PCR results demonstrated the mRNA expression of nephrin and Snail1. (i, j) Treatment of mouse podocytes with Dickkopf-1 (DKK1) preserves nephrin mRNA expression repressed by TGF-β1. Podocytes were pretreated with recombinant protein DKK1 at 40 or 200 ng/ml for 1 h, followed by the treatment with TGF-β1 at 2 ng/ml. Representative RT-PCR results (i) and quantitative data (j) demonstrate the mRNA expression of nephrin. *P<0.05 versus group treated with TGF-β1 alone (n = 3). DAPI, 4′,6-diamidino-2-phenylindole.
We examined the expression of several putative target genes of Wnt/β-catenin after TGF-β1 stimulation in podocytes. As shown in Figure 2d–g, MMP-9, MMP-7, PAI-1, and LEF-1 were induced by TGF-β1 in podocytes, as demonstrated by quantitative real-time RT-PCR analyses. We also assessed the expression of nephrin and Snail1, two genes that are directly relevant to podocyte dysfunction. As shown in Figure 2h, TGF-β1 induced Snail1 and suppressed nephrin expression in podocytes, as previously reported.6 Transient transfection with either Flag-tagged N-terminal truncated, stabilized β-catenin expression vector (pDel-β-cat) or HA-tagged Wnt1 expression vector (pHA-Wnt1) mimicked the action of TGF-β1. Furthermore, preincubation with recombinant Dickkopf-1 (DKK1), a secreted Wnt antagonist that specifically blocks the canonical pathway of Wnt signaling,20 was able to preserve, at least partially, nephrin expression in a dose-dependent manner (Figure 2i and j). These results suggest an important role for Wnt/β-catenin in mediating the pathogenic action of TGF-β1 in podocytes.
Ectopic expression of TGF-β1 targets glomerular podocytes in vivo
To investigate the potential role of TGF-β1/Wnt1/β-catenin signaling in podocyte injury in vivo, we sought to express bioactive, exogenous TGF-β1 by use of a hydrodynamic-based gene delivery approach.21–23 As shown in Figure 3a, several mammalian expression plasmid vectors were constructed, which contained the coding region of mouse wild-type TGF-β1 (pWT-TGF-β1), C-terminus mature TGF-β1 (pCT-TGF-β1), and constitutively active TGF-β1 (pCA-TGF-β1), respectively. The pCA-TGF-β1 vector contained two cysteine to serine mutations at the positions 223 and 225 (C223S/C225S), which produces bioactive TGF-β1 that does not require acid activation,24 rendering it constitutively active. To test the bioactivity of these TGF-β1 expression vectors, we examined their ability to induce the expression of PAI-1, a sensitive TGF-β1 target gene.25 As shown in Figure 3b, little PAI-1 induction was observed in podocytes after transient transfection with either pWT-TGF-β1 or pCT-TGF-β1, compared with empty vector pcDNA3. However, transfection with pCA-TGF-β1 significantly induced PAI-1 expression, corroborating the bioactivity of this mutant, constitutively active TGF-β1 (Figure 3b). Furthermore, transient transfection of mouse podocytes with pCA-TGF-β1 induced β-catenin activation and Snail1 expression, which was largely abrogated by coexpression of DKK1 gene (Figure 3c and d). It is noteworthy that transfection DKK1 gene in podocytes did not affect the expression of TGF-β1 transgene (Supplementary Figure S1 online).
Figure 3. Ectopic expression of transforming growth factor-β1 (TGF-β1) in podocytes activates β-catenin in a Wnt-dependent manner.
(a) Diagram depicts the construction of wild-type TGF-β1 (pWT-TGF-β1), C-terminus mature TGF-β1 (pCT-TGF-β1), and constitutively active TGF-β1 (pCA-TGF-β1) expression vectors. (b) Expression vector pCA-TGF-β1 markedly induces PAI-1 gene expression in podocytes. Mouse podocytes were transiently transfected with expression vector pWT-TGF-β1, pCT-TGF-β1, pCA-TGF-β1 and pcDNA3 for 48 h, respectively. Total cell lysates were immunoblotted with specific antibodies against PAI-1 and actin, respectively. (c) Ectopic expression of TGF-β1 activates β-catenin in podocytes, which is blocked by Wnt antagonist Dickkopf-1 (DKK1). Podocytes were transfected with constitutively active TGF-β1 expression vector (pCA-TGF-β1), DKK1 expression vector (pFlag-DKK1), or empty vector pcDNA3, as indicated, for 48 h. Total cell lysates were immunoblotted with specific antibodies against active β-catenin and α-tubulin, respectively. (d) DKK1 reduces TGF-β1-mediated Snail1 induction. Mouse podocytes were transfected with various plasmids as indicated for 48 h. Snail1 mRNA expression was assessed by real-time reverse transcription-PCR. *P<0.05; **P<0.01 (n = 3).
We next administrated the pCA-TGF-β1 expression plasmid into mice by a single intravenous injection. As shown in Figure 4a and b, single administration of pCA-TGF-β1 plasmid markedly increased TGF-β1 protein levels in the blood circulation and in the glomeruli, as detected by a specific enzyme-linked immunosorbent assay (ELISA) at 18 h after plasmid injection. Figure 4c shows that Smad3 phosphorylation, a surrogate marker for TGF-β receptor activation, was observed in the glomerular lysates of the kidneys at 1 or 2 days after injection. Double immunofluorescence staining for both phosphorylated Smad3 (red) and nephrin (green) revealed that podocytes in the glomeruli were preferentially responsive to TGF-β1, with a positive staining for phosphorylated Smad3 after TGF-β1 gene delivery (Figure 4d, arrowheads), although Samd3 activation in tubular cells was also evident. Weak staining for phosphorylated Smad3 was detectable in the mesangial area in normal control kidneys, but it did not significantly increase after pCA-TGF-β1 injection.
Figure 4. Ectopic expression of transforming growth factor-β1 (TGF-β1) targets glomerular podocytes in vivo.
(a, b) Delivery of constitutively active TGF-β1 gene in vivo induces TGF-β1 protein in the circulation and in the glomeruli. Mice were injected intravenously with pCA-TGF-β1 or pcDNA3, respectively. Plasma (a) and glomerular TGF-β1 (b) were assayed by a specific enzyme-linked immunosorbent assay at 18 h after plasmid injection. **P<0.01 (n = 6). (c, d) Delivery of constitutively active TGF-β1 gene induces Smad3 phosphorylation in glomerular podocytes in vivo. At 1 or 2 days after a single intravenous injection of either TGF-β1-expressing plasmid (pCA-TGF-β1) or empty vector (pcDNA3), glomerular lysates were prepared and immunoblotted with anti-phospho-Smad3 and anti-Smad1/2/3 antibodies (c). Mouse kidney sections at 2 days after plasmid injection were immunostained with antibodies against phospho-specific Smad3 (red) and nephrin (green) (d). Arrowheads indicate phospho-Smad3-positive podocytes.
TGF-β1 activates Wnt/β-catenin signaling in podocytes in vivo
We examined the effects of TGF-β1 on Wnt/β-catenin signaling in podocyte in vivo. Quantitative real-time RT-PCR results revealed an increased expression of Wnt1 gene in the glomeruli, which was detected at day 1 and sustained at day 2 after TGF-β1 plasmid injection (Figure 5a). Immunostaining also demonstrated an increased Wnt1 protein in mouse glomeruli after pCA-TGF-β1 injection (Figure 5b, arrowheads). Subcellular fractionation of the isolated glomeruli, followed by western blot analyses, indicated that β-catenin in both cytoplasmic and nuclear compartments was markedly induced after TGF-β1 gene delivery in vivo (Figure 5c). Compared with pcDNA3 controls, ectopic expression of TGF-β1 clearly promoted β-catenin to undergo nuclear translocation (Figure 5c), suggesting the activation of canonical Wnt signaling.
Figure 5. Ectopic expression transforming growth factor-β1 (TGF-β1) activates Wnt/β-catenin signaling and induces its downstream target gene expression in vivo.
(a) Quantitative real-time reverse transcription-PCR (RT-PCR) reveals that TGF-β1 induced Wnt1 mRNA expression in mouse glomeruli. (b) TGF-β1 induces Wnt1 protein expression in mouse glomeruli. Kidney cryosections were prepared from mouse kidneys at 2 days after injection of TGF-β1 expression plasmid (pCA-TGF-β1) or empty vector (pcDNA3), and then stained with antibodies against Wnt1 (red) or nephrin (green), respectively. Arrowheads indicate positive Wnt1 staining in the glomeruli. (c) TGF-β1 induces cytoplasmic β-catenin accumulation and its nuclear translocation in mouse glomeruli. Glomeruli were isolated from mouse kidneys at 1 day after injection of TGF-β1 expression plasmid (pCA-TGF-β1) or empty vector (pcDNA3). Nuclear and cytoplasmic proteins from the isolated glomeruli were prepared and immunoblotted with antibodies against β-catenin, histone H3, or glyceraldehyde 3-phosphate dehydrogenase (GAPDH), respectively. The ratios of β-catenin per control proteins (histone H3 for nuclear protein and GAPDH for cytoplasmic protein) are shown. (d–i) Quantitative real-time RT-PCR reveals that TGF-β1 induces the mRNA expression of Wnt/β-catenin downstream genes such as Snail1 (d), MMP-9 (e), MMP-7 (f), desmin (g), Fsp-1 (h), and PAI-1 (i). Various mRNA levels were assessed by quantitative real-time RT-PCR in the isolated glomeruli in different groups as indicated. Data are presented as mean±s.e.m. of three glomerular preparations; each of them contains a pool of the glomeruli from two animals. *P<0.05; **P<0.01 versus pcDNA3 control group.
We further studied the expression of several putative Wnt/β-catenin target genes in the glomeruli after TGF-β1 gene delivery. As shown in Figure 5d–i, numerous well-characterized Wnt/β-catenin target genes, including Snail1, MMP-9, MMP-7, desmin, fibroblast-specific protein (Fsp1)/S100A4, and PAI-1, were upregulated in mouse glomeruli after ectopic expression of exogenous TGF-β1.
Ectopic expression of TGF-β1 induces podocyte injury and proteinuria
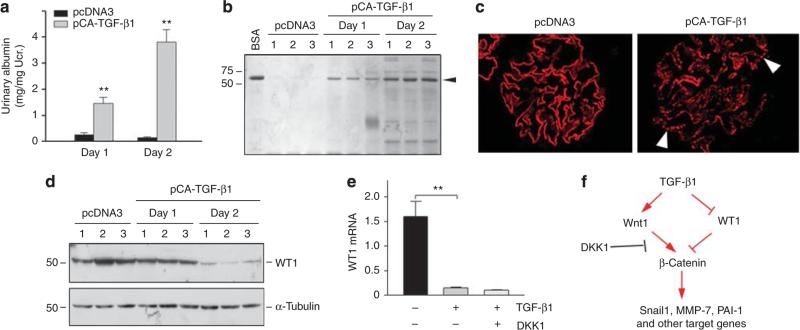
We next investigated the functional consequence of Wnt/β-catenin activation in podocytes by TGF-β1 in vivo. Analysis of urine samples revealed that urinary albumin levels were substantially increased in mice that received TGF-β1 plasmid injection, compared with pcDNA3 controls (Figure 6a). SDS-polyacrylamide gel electrophoresis analysis demonstrated that albumin was the major constituent of urine proteins in these mice (Figure 6b), suggesting defective glomerular filtration. Consistently, exogenous TGF-β1 induced an altered nephrin distribution, from a linear staining along glomerular basement membrane to granular pattern, as illustrated by immunofluorescence staining, although it did not significantly affect nephrin abundance (Figure 6c).
Figure 6. Ectopic expression of transforming growth factor-β1 (TGF-β1) results in proteinuria and podocyte injury.
(a) Urinary albumin levels in mice after ectopic expression of exogenous TGF-β1. Mice were injected intravenously with TGF-β1-expression plasmid (pCA-TGF-β1) or empty vector (pcDNA3). Urinary albumin was determined at 1 or 2 days after injection, and expressed as mg/mg creatinine. **P<0.01 versus pcDNA3 control (n = 6). (b) Representative SDS-polyacrylamide gel electrophoresis shows the urinary proteins at day 1 and day 2 after pCA-TGF-β1 plasmid injection. Arrowhead denotes albumin. (c) Immunofluorescence staining shows the expression and distribution pattern of nephrin in pcDNA3 and pCA-TGF-β1 groups at 2 days after plasmid injection. Arrowheads indicate a dot-like pattern of nephrin after TGF-β1 plasmid injection. (d) Western blot analysis demonstrates the suppression of Wilms tumor 1 (WT1) by TGF-β1 in vivo. Whole glomerular lysates were immunoblotted with anti-WT1 and α-tubulin. (e) TGF-β1 suppresses WT1 expression by a Wnt-independent pathway in vitro. Mouse podocytes were incubated with TGF-β1 (2 ng/ml) in the absence or presence of Dickkopf-1 (DKK1; 200 ng/ml) for 24 h. WT1 mRNA expression was assessed by real-time RT-PCR. **P<0.01 (n = 3). (f) Diagram shows the TGF-β1 signaling leading to podocyte injury. TGF-β1 induces Wnt1 and activates β-catenin, which can be blocked by DKK1. TGF-β1 also inhibits WT1, leading to derepression of β-catenin. Ucr., urine creatinine.
The expression of Wilms tumor 1 (WT1), an important transcription factor that is exclusively expressed in podocytes in adult kidney, was significantly suppressed after TGF-β1 plasmid injection (Figure 6d). The inhibition of WT1 expression by TGF-β1 was confirmed in vitro (Figure 6e). However, coincubation with DKK1 and TGF-β1 did not restore WT1 expression (Figure 6e), suggesting that it is independent of Wnt signaling. A diagram showing the TGF-β1 signaling leading to podocyte injury is presented in Figure 6f.
Ectopic expression of DKK1 gene blocks Wnt/β-catenin signaling and reduces proteinuria
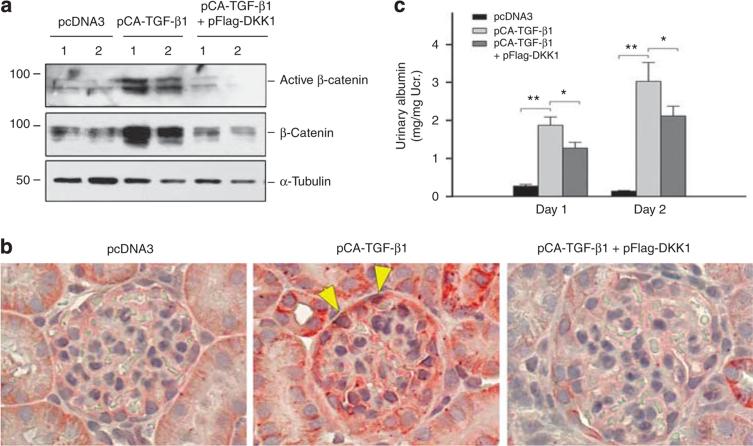
To validate a role of Wnt/β-catenin signaling in mediating TGF-β1-induced podocyte injury and proteinuria, we sought to block Wnt signaling in vivo by its natural antagonist DKK1. To this end, mice were injected concurrently with TGF-β1 expression vector (pCA-TGF-β1) in the presence or absence of DKK1 expression vector (pFlag-DKK1). Renal expression of exogenous DKK1 by this approach was reported previously.14 As shown in Figure 7a, ectopic expression of constitutively active TGF-β1 induced both active and total β-catenin expression in the glomeruli, as demonstrated by western blot analyses of glomerular lysates. However, concomitant expression of DKK1 gene abolished β-catenin induction and activation. Immunohistochemical staining produced similar results. As shown in Figure 7b, renal β-catenin protein was increased in glomerular podocytes and tubular cells after ectopic expression of TGF-β1 (Figure 7b, arrowheads). The positive β-catenin staining localized in the cytoplasmic and nuclear compartments was significantly diminished when DKK1 gene was concurrently administrated. Interestingly, delivery of DKK1 gene significantly mitigated albuminuria induced by TGF-β1 (Figure 7c), indicating an important role for Wnt/β-catenin signaling in mediating TGF-β1-induced podocyte injury and proteinuria.
Figure 7. Ectopic expression of Dickkopf-1 (DKK1) blocks β-catenin activation and reduces albuminuria induced by transforming growth factor-β1 (TGF-β1) in vivo.
(a) Western blot analyses demonstrate that DKK1 blocks the TGF-β1-induced active and total β-catenin expression in mouse glomeruli. Whole glomerular lysates were immunoblotted with anti-active β-catenin, β-catenin, and α-tubulin antibodies, at day 2 after intravenous plasmid injection. (b) Representative micrographs demonstrate β-catenin expression and localization in different groups at day 2. Mouse kidney sections were immunostained with antibody against β-catenin. Arrowheads indicate β-catenin-positive podocytes. (c) Urinary albumin levels in different group as indicated. *P<0.05; **P<0.01 (n = 6).
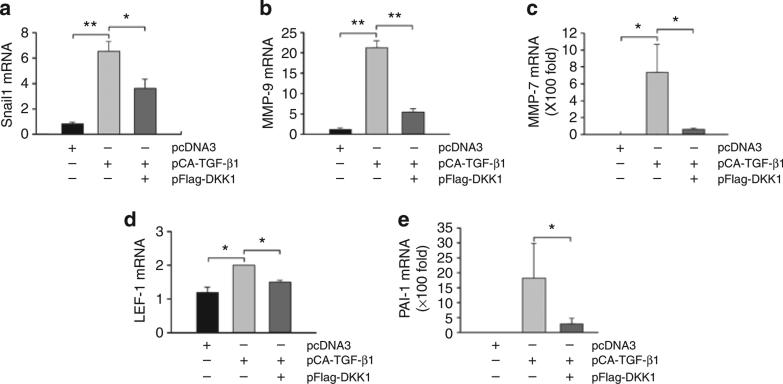
We next examined the expression of Wnt/β-catenin downstream target genes in the glomeruli of these mice. As shown in Figure 8a–e, administration of DKK1 gene resulted in significant inhibition of the Wnt/β-catenin target genes. Quantitative RT-PCR revealed that DKK1 significantly inhibited the mRNA expression of Snail1, MMP-9, MMP-7, LEF-1, and PAI-1 genes, which were induced in mouse glomeruli after TGF-β1 expression (Figure 8).
Figure 8. Exogenous Dickkopf-1 (DKK1) blocks Wnt/β-catenin target gene expression induced by transforming growth factor-β1 (TGF-β1) in vivo.
Quantitative real-time reverse transcription-PCR reveals that exogenous DKK1 inhibits TGF-β1-induced mRNA expression of Wnt/β-catenin target genes, including Snail1 (a), MMP-9 (b), MMP-7 (c), LEF-1 (d), and PAI-1 (e). Various mRNA levels were assessed in the isolated glomeruli from mice injected with different expression vectors as indicated at day 2. Data are presented as mean±s.e.m. of three glomerular preparations, each of them containing a pool of the glomeruli from two animals. *P<0.05; **P<0.01 (n = 3).
DISCUSSION
The results presented here clearly illustrate a critical role for Wnt/β-catenin signaling in meditating the pathogenesis of podocyte injury and proteinuria in vivo after ectopic expression of TGF-β1. We demonstrate that Wnt1 is upregulated and β-catenin is activated in the glomeruli after ectopic expression of TGF-β1 through a hydrodynamic-based gene delivery approach, which leads to the overexpression of several key Wnt target genes including Snail1, resulting in podocyte injury and onset of proteinuria. Moreover, Wnt antagonist DKK1 is effective in blocking TGF-β1-mediated β-catenin activation in vivo and in vitro, leading to significant inhibition of Wnt target genes and mitigation of albuminuria in mice. These studies establish a simple mouse model in which ectopic expression of TGF-β1, per se, is sufficient to cause podocyte injury in vivo and result in proteinuric kidney disease. Our data also underscore that targeting Wnt/β-catenin could be a promising novel therapeutic strategy for the treatment of a variety of proteinuric kidney disease in humans.
TGF-β1, as a fibrogenic factor, is upregulated in the injured kidneys in experimental animal models and in humans of virtually every type of chronic kidney diseases.4,5 Although hyperactive TGF-β signaling is considered to be a major profibrotic stimulus in mesangial injury and expansion, little is known about its pathological action in podocytes in vivo. Earlier studies on transgenic mice overexpressing TGF-β1 under albumin promoter (Alb/TGF-β1 mice) have pointed toward a causative role of TGF-β1 in the pathogenesis of nephropathies, as these mice spontaneously develop protein-uria, progressive glomerulosclerosis, and interstitial fibrosis, with advanced kidney failure leading to ~25% animal death at 5–12 weeks of age.8,9 However, perhaps owing to tremendous heterogeneity in renal pathology and the difficulty to maintain the colony, these mice have not been widely used as an animal model of chronic kidney diseases. In this study, by a single injection of naked plasmid vector encoding constitutively active TGF-β1, we have established a simple, quick, reproducible, and accessible mouse model of podocyte injury and proteinuria (Figure 6). As TGF-β1 is the sole culprit of kidney injury in this model, these mice could also serve as an in vivo model to dissect the cellular and molecular mechanism of TGF-β1 action in the kidneys. Interestingly, of the three major cell types in the glomeruli, podocytes seem to be the most susceptible cells that are readily responsive to TGF-β1 stimulation in vivo, as illustrated by Smad3 phosphorylation (Figure 4). Such an activation of TGF-β1 leads to induction of numerous Wnt genes, particularly and predominantly Wnt1 (Figures 1 and 5), which results in the stabilization and accumulation of β-catenin, as well as its nuclear translocation. In addition, TGF-β1 and Wnts may work in concert to regulate gene expression, as previous studies suggest that β-catenin can interact and cross-talk with Smads.26 In short, TGF-β1 is categorically linked to the activation of Wnt/β-catenin, a developmental signaling that is recently implicated in podocyte dysfunction and proteinuria.15
How activation of Wnt/β-catenin signaling causes podocyte injury remains elusive, but it could be related to its ability to regulate podocyte dedifferentiation and EMT, a cell phenotypic alteration process taking place in embryonic development, cancer metastasis, and tissue fibrosis.27–29 Previous studies demonstrate that podocytes are able to undergo EMT in vitro after TGF-β1 stimulation, and this process could be a potential pathway leading to podocyte dysfunction and proteinuria under pathological conditions.6 In agreement with this notion, EMT has been proposed as a potential explanation for podocyte dysfunction, detachment, and depletion in human diabetic nephropathy.30 It is interesting to point out that Snail1, a key transcription factor controlling EMT,31–33 is a downstream target gene of Wnt/β-catenin signaling.34 Snail1, in turn, could directly suppress nephrin expression in podocytes and induces the expression of other mesenchymal markers.15,35 Indeed, either incubation with TGF-β1 or overexpression of Wnt1 or stabilized β-catenin is sufficient to induce Snail1 expression and suppress nephrin expression in podocytes (Figure 2). However, ectopic expression of TGF-β1 in vivo does not suppress nephrin expression (Figures 4 and 5), although TGF-β1/Wnt1/β-catenin cascade induces Snail1 both in vitro and in vivo. The reason behind this discrepancy between in vitro and in vivo nephrin regulation by TGF-β1 is unknown. It is possible that TGF-β1 in vivo, a much more complex setting, is able to cross-talk and interact with other signal routes, resulting in divergent regulation of nephrin expression. This issue clearly needs more studies.
It should be noted that TGF-β1 not only induces Wnt1 but also represses WT1 via a mechanism independent of Wnt1. As WT1 is a known negative regulator of β-catenin signaling,36,37 and loss of WT1 would lead to derepression of β-catenin activity, this suggests that TGF-β1 may activate β-catenin through both Wnt1-dependent and WT1-dependent pathways (Figure 6f), which could lead to an exaggerated expression of its downstream target genes. Besides Snail1, numerous β-catenin target genes are identified to be activated in the glomeruli upon TGF-β1 expression, such as MMP-9, MMP-7, LEF-1, Fsp1, and PAI-1. Among these genes, induction of LEF-1, a DNA-binding transcription factor that interacts with β-catenin by forming a trans-activation protein complex,38 clearly favors a positive feedback loop that re-enforces Wnt signaling. MMPs are a family of extracellular proteases that are responsible for the degradation of the extracellular matrix and other substrates during tissue remodeling.39 Although the exact action of MMPs in podocyte injury is unclear, MMP-mediated extracellular domain shedding of slit diaphragm-associated proteins could have a role.40 PAI-1, a well-characterized fibrogenic factor, is another direct downstream target of Wnt/β-catenin signaling,25 and its induction may also contribute to the development of nephropathy in mice after TGF-β1 expression.41 It is conceivable that each of these target genes activated by Wnt/β-catenin could promote podocyte injury and proteinuria in their own ways. However, it is interesting to note that many of these genes, including Snail1, MMPs, Fsp1, and PAI-1, share the same property by promoting cell migration.41–43 An increased podocyte migration could render them from stationary to motile state, which could reduce their adhesions to glomerular basement membrane and promote detachment. This view is in line with previous observation that EMT leads to an increased podocyte detachment and depletion in diabetic nephropathy.30
Our present study also indicates that targeting Wnt/bcatenin signaling might be an effective strategy to mitigate podocyte injury and proteinuria. DKK1, a secreted Wnt antagonist, specifically binds to the Wnt coreceptors LRP-5/6, leading to blockade of the canonical Wnt signaling.20,44 Indeed, DKK1 gene therapy blocks β-catenin activation, suppresses Wnt/β-catenin target gene expression, and mitigates albuminuria. Notably, perhaps because DKK1 merely blocks Wnt1-dependent, but not WT1-dependent, β-catenin signaling (Figure 6f), it displays a partial amelioration of proteinuria after TGF-β1 injection (Figure 7c). This suggests that direct inhibition of β-catenin could be a better strategy for renal protection, although not tested yet. As multiple strategies to target Wnt/β-catenin are currently available, more studies are warranted in the future to evaluate their therapeutic efficacy in the preclinical setting.
MATERIALS AND METHODS
Cell culture and treatment
The conditionally immortalized mouse podocyte cell line was kindly provided by Dr Peter Mundel and maintained as described previously.6 To propagate podocytes, cells were cultured at 33 °C in RPMI-1640 medium supplemented with 10% fetal bovine serum and recombinant interferon-γ (Invitrogen, CarIsbad, CA). To induce differentiation, podocytes were grown under nonpermissive conditions at 37 °C in the absence of interferon-γ and in the presence of retinoic acid (10−6 mol/l) and 1,25-dihydroxyvitamin D (10−7 mol/l).45 Podocytes were treated with recombinant TGF-β1 (R&D Systems, Minneapolis, MN) at the concentration of 2 ng/ml, unless otherwise indicated. Recombinant mouse DKK1 (R&D Systems) was used for pretreatment at 40 or 200 ng/ml for 1 h, followed by incubation with TGF-β1 for 24 h. For inhibiting TGF-β receptor signaling, podocytes were pretreated with SB431542 (Tocris Bioscience, Ellisville, MO) at 10 μmol/l for 1 h, followed by incubation with TGF-β1. For some studies, podocytes were transiently transfected with either HA-tagged Wnt1 expression vector (pHA-Wnt1; Upstate Biotechnology, Lake Placid, NY), Flag-tagged N-terminal truncated, stabilized β-catenin expression vector (pDel-β-cat), constitutively active TGF-β1 expression vector (pCA-TGF-β1; see below), or Flagged-tagged DKK1 expression vector (pFlag-DKK1; provided by Dr Xi He, Harvard Medical School, Boston, MA) by using Lipofectamine 2000 reagent (Invitrogen), as described previously.15
Construction of pCA-TGF-β1 vector
The expression plasmid vectors containing mouse wild-type TGF-β1 (pWT-TGF-β1), C-terminus mature TGF-β1 (pCT-TGF-β1), and constitutively active TGF-β1 (pCA-TGF-β1) were constructed by using standard molecular cloning techniques. The pCA-TGF-β1 vector contained two cysteine to serine mutations at the positions 223 and 225 (C223S/C225S). This expression vector was constructed by using the QuikChange II XL site-directed mutagenesis kit, and it produces bioactive TGF-β1 that does not require acid activation, as reported previously.24 The correct sequences of different expression vectors were confirmed by sequencing at the DNA Sequencing Core Facility of the University of Pittsburgh.
Animal models
Male BALB/c mice that weighed ~20–22 g were purchased from Harlan Sprague Dawley (Indianapolis, IN). For delivery of mouse TGF-β1 gene, constitutively active TGF-β1 expression plasmid (pCA-TGF-β1) was injected intravenously at 0.3 mg/kg body weight by using a hydrodynamics-based in vivo gene transfer approach, as described previously.21,22 For delivery of human DKK1 gene, naked DKK1 expression plasmid (pFlag-DKK1) was injected intravenously at 2 mg/kg body weight by using the same approach. Control mice were administered an injection of empty vector pcDNA3 plasmid in an identical manner. Mice were killed at day 1 and day 2 after injection, and kidney tissues were collected for various analyses. Animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Isolation of glomeruli
Glomeruli were isolated by differential sieving technique according to the method described elsewhere.46 Briefly, the kidneys were excised and pressed with a spatula through a stainless steel screen of #100 mesh and rinsed with 1% albumin in cold phosphate-buffered saline (PBS-A) through successive screens of #200 mesh and #282 mesh, respectively. The glomeruli were collected on the #282-mesh screen and suspended in PBS-A. After centrifugation at 200 g for 10 min, glomeruli were collected for subsequent analyses.
Urinary albumin and creatinine assay
Urinary albumin level was measured by using a mouse Albumin ELISA Quantification kit, according to the manufacturer's protocol (Bethyl Laboratories, Montgomery, TX). Urine creatinine was determined by a routine procedure as described previously.47
RT-PCR and real-time PCR
Total RNA was extracted using the TRIzol RNA isolation system (Invitrogen). The first strand of complementary DNA was synthesized using 2 μg of RNA in 20 μl of reaction buffer by reverse transcription using AMV-RT (Promega, Madison, WI) and random primers at 42 °C for 30 min. PCR was carried out using a standard PCR protocol with 1 μl aliquot of complementary DNA, HotStarTaq polymerase (Qiagen, Valencia, CA), and specific primer pairs. The sequences of the primer pairs were given in Supplementary Table S1 online, whereas the primer pairs of 19 Wnt genes were described previously.14 For quantitative determination of mRNA levels, a real-time RT-PCR was performed on an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA), as described previously.48 The PCR reaction mixture in a 25 μl volume contained 12.5 μl of 2 × SYBR Green PCR Master Mix (Applied Biosystems), 10 μl of diluted reverse transcription product (1:10), and 0.5 μmol/l sense and antisense primer sets. PCR reaction was run by using standard conditions.48 After sequential incubations at 50 °C for 2 min and 95 °C for 10 min, the amplification protocol consisted of 40 cycles of denaturation at 95 °C for 15 s, and annealing and extension at 60 °C for 60 s. The mRNA levels of various genes were calculated after normalizing with β-actin.
Western blot analysis
Cultured mouse podocytes were lysed in SDS sample buffer. The isolated glomeruli were pooled and lysed with radioimmunoprecipitation assay buffer containing 1% NP-40, 0.1% SDS, 100 μg/ml phenylmethylsulfonyl fluoride, 1% protease inhibitor cocktail, and 1% phosphatase I and II inhibitor cocktail (Sigma, St Louis, MO) in PBS on ice. The supernatants were collected after centrifugation at 13,000 g at 4 °C for 20 min. Protein expression was analyzed by western blot analysis as described previously.6 The primary antibodies used were as follows: anti-β-catenin (no. 6101541; BD Transduction, San Jose, CA), anti-dephosphorylated, active β-catenin (no. 05-665; Millipore, Billerica, MA), anti-phospho-Smad3 (Ser423/425; no. 9514; Cell Signaling, Boston, MA), anti-Smad1/2/3 (sc-7960; Santa Cruz Biotechnology, Santa Cruz, CA), anti-PAI-1 (sc-5297; Santa Cruz Biotechnology), anti-WT1 (ab15249; Abcam, Cambridge, MA) anti-Histone H3 (ab1791; Abcam), anti-actin (sc-1616; Santa Cruz Biotechnology), anti-glyceraldehyde 3-phosphate dehydrogenase (no. 4300; Ambion, Austin, TX), and anti-α-tubulin (T9026; Sigma).
Determination of TGF-β1 levels by ELISA
Plasma and glomerular TGF-β1 levels were determined by using a commercial Quantikine TGF-β1 ELISA kit in accordance with the protocol specified by the manufacturer (R&D Systems).23 Briefly, at 18 h after plasmid injection, mouse plasma was collected and glomeruli were isolated by differential sieving technique. Glomeruli were homogenized in the buffer containing 20 mmol/l Tris-HCl, pH 7.5, 2 mol/l NaCl, 0.1% Tween-80, 1 mmol/l EDTA, and 1 mmol/l PMSF, as described previously.22 After centrifugation at 19,000 g for 20 min at 4 °C, the supernatant was recovered for determination of TGF-β1 by ELISA. Plasma and glomerular TGF-β1 levels were expressed as ng/ml and pg/mg protein, respectively.
Nuclear and cytoplasmic fractionation
For preparation of nuclear protein, isolated mouse glomeruli were collected and washed twice with cold PBS. Nuclear protein was prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents, according to the protocols specified by the manufacturer (Thermo Scientific, Rockford, IL), as described previously.49
Immunofluorescent and Immunohistochemical staining
Kidney cryosections at 4-mm thickness were fixed for 15 min in 4% paraformaldehyde, followed by permeabilization with 0.2%. Triton X-100 in PBS for 10 min at room temperature. After blocking with 10% donkey serum for 60 min, slides were immunostained with primary antibodies against nephrin (Fitzgerald Industries International, Concord, MA), Wnt1 (ab15251; Abcam), or phosphorylated Smad3 (Ser423/425) (cat. no. 9514, Cell Signaling). To visualize the primary antibodies, slides were stained with Cy2- or Cy3-conjugated secondary antibodies (Sigma). The slides were viewed under Olympus FluoView 500 confocal microscope (Olympus, Center Valley, PA). Immunofluorecence staining of cultured podocytes and immunohistochemical staining of kidney sections was performed by using an established protocol.47 Cell culture coverslips and paraffin-embedded sections were stained with polyclonal rabbit anti-β-catenin antibody (ab-15180; Abcam, Cambridge, MA). Slides were viewed with a Nikon Eclipse E600 microscope equipped with a digital camera (Melville, NY).
Statistical analysis
All data examined were expressed as mean±s.e.m. Statistical analysis was performed using SigmaStat software (Jandel Scientific Software, San Rafael, CA). Comparison between groups was made using one-way analysis of variance, followed by the Student– Newman–Keuls test. A P-value of <0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health Grants DK064005 and DK071040.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Patrakka J, Tryggvason K. New insights into the role of podocytes in proteinuria. Nat Rev Nephrol. 2009;5:463–468. doi: 10.1038/nrneph.2009.108. [DOI] [PubMed] [Google Scholar]
- 2.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 3.Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 4.Schnaper HW, Jandeska S, Runyan CE, et al. TGF-beta signal transduction in chronic kidney disease. Front Biosci. 2009;14:2448–2465. doi: 10.2741/3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13:2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Kang YS, Dai C, et al. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172:299–308. doi: 10.2353/ajpath.2008.070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu DT, Bitzer M, Ju W, et al. TGF-beta concentration specifies differential signaling profiles of growth arrest/differentiation and apoptosis in podocytes. J Am Soc Nephrol. 2005;16:3211–3221. doi: 10.1681/ASN.2004121055. [DOI] [PubMed] [Google Scholar]
- 8.Kopp JB, Factor VM, Mozes M, et al. Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Lab Invest. 1996;74:991–1003. [PubMed] [Google Scholar]
- 9.Schiffer M, Bitzer M, Roberts IS, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt-Ott KM, Barasch J. WNT/beta-catenin signaling in nephron progenitors and their epithelial progeny. Kidney Int. 2008;74:1004–1008. doi: 10.1038/ki.2008.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll TJ, Park JS, Hayashi S, et al. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 14.He W, Dai C, Li Y, et al. Wnt/β-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai C, Stolz DB, Kiss LP, et al. Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20:1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Toerne C, Schmidt C, Adams J, et al. Wnt pathway regulation in chronic renal allograft damage. Am J Transplant. 2009;9:2223–2239. doi: 10.1111/j.1600-6143.2009.02762.x. [DOI] [PubMed] [Google Scholar]
- 17.Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol. 2005;16:2373–2384. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 18.Heikkila E, Juhila J, Lassila M, et al. β-Catenin mediates adriamycin-induced albuminuria and podocyte injury in the adult mouse kidneys. Nephrol Dial Transplant. 2010;25:2437–2446. doi: 10.1093/ndt/gfq076. [DOI] [PubMed] [Google Scholar]
- 19.He W, Kang YS, Dai C, et al. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semenov MV, Tamai K, Brott BK, et al. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 21.Dai C, Yang J, Liu Y. Single injection of naked plasmid encoding hepatocyte growth factor prevents cell death and ameliorates acute renal failure in mice. J Am Soc Nephrol. 2002;13:411–422. doi: 10.1681/ASN.V132411. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Chen S, Huang L, et al. Sustained expression of naked plasmid DNA encoding hepatocyte growth factor in mice promotes liver and overall body growth. Hepatology. 2001;33:848–859. doi: 10.1053/jhep.2001.23438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai C, Yang J, Bastacky S, et al. Intravenous administration of hepatocyte growth factor gene ameliorates diabetic nephropathy in mice. J Am Soc Nephrol. 2004;15:2637–2647. doi: 10.1097/01.ASN.0000139479.09658.EE. [DOI] [PubMed] [Google Scholar]
- 24.Brunner AM, Marquardt H, Malacko AR, et al. Site-directed mutagenesis of cysteine residues in the pro region of the transforming growth factor beta 1 precursor. Expression and characterization of mutant proteins. J Biol Chem. 1989;264:13660–13664. [PubMed] [Google Scholar]
- 25.He W, Tan R, Dai C, et al. Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/β-catenin signaling. J Biol Chem. 2010;285:24665–24675. doi: 10.1074/jbc.M109.091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirota M, Watanabe K, Hamada S, et al. Smad2 functions as a co-activator of canonical Wnt/beta-catenin signaling pathway independent of Smad4 through histone acetyltransferase activity of p300. Cell Signal. 2008;20:1632–1641. doi: 10.1016/j.cellsig.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol. 2010;21:212–222. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi Y, Iwano M, Toyoda M, et al. Epithelial-mesenchymal transition as an explanation for podocyte depletion in diabetic nephropathy. Am J Kidney Dis. 2009;54:653–664. doi: 10.1053/j.ajkd.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Boutet A, De Frutos CA, Maxwell PH, et al. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 2006;25:5603–5613. doi: 10.1038/sj.emboj.7601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 33.Vincent T, Neve EP, Johnson JR, et al. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009;11:943–950. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ten Berge D, Koole W, Fuerer C, et al. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsui I, Ito T, Kurihara H, et al. Snail, a transcriptional regulator, represses nephrin expression in glomerular epithelial cells of nephrotic rats. Lab Invest. 2007;87:273–283. doi: 10.1038/labinvest.3700518. [DOI] [PubMed] [Google Scholar]
- 36.Chang H, Gao F, Guillou F, et al. Wt1 negatively regulates beta-catenin signaling during testis development. Development. 2008;135:1875–1885. doi: 10.1242/dev.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim MS, Yoon SK, Bollig F, et al. A novel Wilms tumor 1 (WT1) target gene negatively regulates the WNT signaling pathway. J Biol Chem. 2010;285:14585–14593. doi: 10.1074/jbc.M109.094334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hovanes K, Li TW, Munguia JE, et al. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28:53–57. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- 39.Brabletz T, Jung A, Dag S, et al. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999;155:1033–1038. doi: 10.1016/s0002-9440(10)65204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerke P, Sellin L, Kretz O, et al. NEPH2 is located at the glomerular slit diaphragm, interacts with nephrin and is cleaved from podocytes by metalloproteinases. J Am Soc Nephrol. 2005;16:1693–1702. doi: 10.1681/ASN.2004060439. [DOI] [PubMed] [Google Scholar]
- 41.Eddy AA, Fogo AB. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol. 2006;17:2999–3012. doi: 10.1681/ASN.2006050503. [DOI] [PubMed] [Google Scholar]
- 42.Stein U, Arlt F, Walther W, et al. The metastasis-associated gene S100A4 is a novel target of beta-catenin/T-cell factor signaling in colon cancer. Gastroenterology. 2006;131:1486–1500. doi: 10.1053/j.gastro.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 43.Okada H, Danoff TM, Kalluri R, et al. Early role of Fsp1 in epithelialmesenchymal transformation. Am J Physiol. 1997;273:F563–F574. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- 44.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 45.Takano Y, Yamauchi K, Hiramatsu N, et al. Recovery and maintenance of nephrin expression in cultured podocytes and identification of HGF as a repressor of nephrin. Am J Physiol Renal Physiol. 2007;292:F1573–F1582. doi: 10.1152/ajprenal.00423.2006. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Tolbert EM, Sun AM, et al. Primary structure of rat HGF receptor and induced expression in glomerular mesangial cells. Am J Physiol. 1996;271:F679–F688. doi: 10.1152/ajprenal.1996.271.3.F679. [DOI] [PubMed] [Google Scholar]
- 47.Dai C, Saleem MA, Holzman LB, et al. Hepatocyte growth factor signaling ameliorates podocyte injury and proteinuria. Kidney Int. 2010;77:962–973. doi: 10.1038/ki.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Tan X, Dai C, et al. Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:1907–1918. doi: 10.1681/ASN.2008090930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D, Li Y, Wu C, et al. PINCH1 is transcriptional regulator in podocytes that interacts with WT1 and represses podocalyxin expression. PLoS ONE. 2011;6:e17048. doi: 10.1371/journal.pone.0017048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.