Abstract
Experience shapes the central nervous system throughout life. Structural and functional plasticity confers a remarkable ability on the brain, allowing neural circuits to adequately adapt to dynamic environments. This process can require selective adjustment of many excitatory and inhibitory synapses in an organized manner, in such a way as to enhance representations of behaviorally important sensory stimuli while preserving overall network excitability. The rules and mechanisms that orchestrated these changes across different synapses and throughout neuronal ensembles are beginning to be understood. Here, we review the evidence connecting synaptic plasticity to functional plasticity and perceptual learning, focusing on the roles of various neuromodulatory systems in enabling plasticity of adult neural circuits. However, the challenge remains to appropriately leverage these systems and forms of plasticity to persistently improve perceptual abilities and behavioral performance.
Keywords: neuromodulation, sensory cortex, synaptic plasticity, excitatory–inhibitory balance, perception
1 INTRODUCTION
Phylogenetic evolution ensures that sensory perception matches the type of environment experienced by an organism, largely by applying genetically encoded filters at the level of sensory organs (e.g., audible and visible spectra) (Darwin, 1859). In addition, throughout ontogenetic development, organisms refine and optimize their sensory acuity by experience-dependent mechanisms. In mammals, these mechanisms can be rapid and long-lasting, are more pronounced during development, and rely primarily on structural and functional plasticity of neocortical circuits (de Villers-Sidani and Merzenich, 2011; de Villers-Sidani et al., 2007; Hensch, 2005).
Unlike the developing neocortex and the adult association areas, primary sensory and motor cortices become resistant to activity-induced synaptic modifications after maturation (Hensch, 2005). This might explain the maintenance of stable perceptual and motor representations throughout life, a requirement for easily and successfully navigating familiar environments. However, demanding or novel behavioral tasks can release sensory cortices from an implastic state in order to allow adaptive modifications to internal representations (Bao et al., 2004; Dahmen and King, 2007; Elbert et al., 2002; Flor et al., 1995; Pantev et al., 2001; Polley et al., 2006; Sterr et al., 1998). A growing number of studies have indicated that information about behavioral state is conveyed to the sensory cortex by a diverse array of neuromodulators (Lee and Dan, 2012). Different neuromodulators have been shown to initiate and control plasticity in the adult brain by coordinating modifications at selected sets of neuronal synapses. The precise set of changes that occur to synaptic transmission and network function depends on which neuromodulatory system or systems are activated and on the extent to which intracellular Ca2+ signaling and NMDA receptor activation are engaged by different stimulus patterns.
Understanding the synaptic mechanisms by which experience shapes functional sensory circuits during development and adulthood is crucial for designing therapeutic interventions to correct inherited and acquired defects in sensory perception. Here, we will summarize recent research describing circuit mechanisms by which the adult neocortex encodes salient and relevant properties of the environment, how neuromodulators control these mechanisms, and how they contribute to perceptual learning. We will emphasize remaining questions that concern the contribution of different neuromodulators to cortical plasticity and the distinctions between adult and developmental plasticity. Although we will focus on the auditory cortex and acoustic behavioral tasks, references to other sensory cortical areas will be made.
2 ENCODING SENSORY ENVIRONMENTS IN CORTICAL NEURAL CIRCUITS
Brains are plastic throughout life but are particularly sensitive to external input during development (Shatz and Stryker, 1978; Wiesel and Hubel, 1963a, b). At this time, axons and dendrites in the neocortex arborize profusely and establish synaptic connections (Benson et al., 2001). Neurons in sensory cortices develop stable receptive fields when the complement of excitatory and inhibitory synaptic inputs matures, making them differentially sensitive to one or more attributes of the environment (Dorrn et al., 2010; Hensch and Stryker, 2004; Hensch et al., 1998; Sun et al., 2010). In vivo intracellular electrophysiological recordings during the presentation of well-defined stimulus sets can estimate the relative contribution of excitatory and inhibitory inputs to the synaptic receptive field of a neuron (Fig. 1A). Excitatory inputs are isolated by clamping the membrane potential of the patched cortical neuron at −70 mV, the reversal potential for chloride, whereas inhibitory inputs are measured by clamping the cell at the reversal potential for sodium (~0 mV). The ratio of excitatory to inhibitory currents is then calculated for each stimulus presented.
FIGURE 1.
Information encoding in the primary auditory cortex. (A) Excitatory and inhibitory synaptic tuning curves of an example neuron; triangle indicates the best frequency of this neuron (reproduced from Froemke et al., 2007). (B) Example spiking tuning profile (reproduced from Froemke et al., 2013). (C) Tonotopic map in the primary auditory cortex based on characteristic frequency (reproduced from Kenet et al., 2007).
During development, activity in sensory circuits gradually increases the correlation of cortical excitatory and inhibitory receptive fields with respect to the stimulus set. For example, in the developing auditory cortex, evoked excitation and inhibition are unbalanced at the time hearing begins (around postnatal day 10 in rodents) but become progressively balanced over the course of development, reaching a relatively high degree of cotuning in adulthood (Dorrn et al., 2010). The correlation between excitatory and inhibitory inputs may dictate the stability of synaptic receptive fields. Unbalanced excitation in the developing auditory cortex allows for rapid activity-induced retuning of synaptic inputs. In young (between postnatal days 12 and 21) but not adult rats, patterned presentation of a pure tone with known frequency and intensity rapidly strengthened the excitatory and inhibitory synaptic currents evoked by the patterned tone, thus retuning the synaptic neuronal profile. In addition, patterned stimulation increased the correlation between excitatory and inhibitory inputs nonspecifically, improving the overall balance in the developing auditory cortex and thus imposing a refractory period for additional activity-induced synaptic modifications (Dorrn et al., 2010).
Developmental adjustments in the ratio of excitatory to inhibitory inputs appear to contribute to the maturation of other sensory cortices like the somatosensory and visual cortex (Hensch and Stryker, 2004). In the somatosensory cortex, for example, Chittajallu and Isaac (2010) showed that the thalamic drive of feedforward inhibition strengthens progressively during the first eleven postnatal days, leading to a doubling of the average ratio between evoked GABA and AMPA currents on layer 4 stellate cells. This results in a decrease of the integration window measured as the half-width of postsynaptic potentials, which may slow down plasticity. Normal recruitment of parvalbumin neurons in this microcircuit is experience-dependent and is impaired after whisker trimming (Chittajallu and Isaac, 2010; Daw et al., 2007).
The relationship between the strength and timing of excitatory and inhibitory currents controls input integration and determines what stimuli evoke suprathreshold responses and therefore the spiking tuning profile of a neuron (Fig. 1B). The most widely studied types of receptive fields in the auditory cortex are in the frequency and intensity domains. Neurons in the primary auditory cortex can spike with short latency (5–10 ms) after the presentation of pure tones of different frequencies. The frequency that evokes the strongest response is known as the best frequency of the neuron. Generally, but not always, neurons in the auditory cortex have a single best frequency, and this also represents their characteristic frequency, meaning the frequency that activates them when played at threshold amplitude. In the intensity domain, the majority of neurons in the rat primary auditory cortex and some but not all of the other auditory fields respond monotonically to increases in sound amplitude (Polley et al., 2007).
At the neuronal population level, the development of synaptic and spiking receptive fields leads to the formation of sensory maps—for example, tonotopic map in the auditory cortex, retinotopy, ocular dominance, and orientation columns in the visual cortex and the whisker barrel fields in the rodent somatosensory cortex (Hubel and Wiesel, 1963; Merzenich et al., 1973; Woolsey and Van der Loos, 1970) (Fig. 1C). Alterations in synaptic maturation can explain changes in sensory maps induced by developmental exposure to different environments (Chang and Merzenich, 2003; Sengpiel et al., 1999; Zhang et al., 2001). A large body of literature documents this dependency of sensory maps on experience, especially during particularly sensitive developmental windows, called critical periods. The existence of critical periods is a phenomenon characterized in most sensory cortices and even in structures thought to be important for emotional learning, such as the amygdala (Gogolla et al., 2009). Interestingly, distinct critical windows have been identified not only for different modalities but also for different functions of the same sensory system. These staggered critical periods explain the systematic and somewhat stereotyped (from simple to complex) accumulation of perceptual abilities, as is the case in language acquisition (Insanally et al., 2009; Sanes and Woolley, 2011). Can these highly plastic conditions be recapitulated in the mature brain to allow rapid and lasting enhancement of perceptual prowess?
The relationship between synaptic plasticity and excitatory–inhibitory balance is consistent in the adult sensory cortex in the sense that correlated synaptic inputs are resistant to activity-induced modifications. However, when the correlation breaks down mostly due to neuromodulatory forces and excitation becomes unbalanced by inhibition, sensory circuits become plastic and synaptic weights adapt to best represent environmental attributes (Bakin and Weinberger, 1996; Froemke et al., 2007, 2013; Kilgard and Merzenich, 1998a, b; Letzkus et al., 2011). In the next section, we will review recent data indicating that activation of various modulatory systems can alter cortical excitatory–inhibitory balance and thus enable experience-dependent synaptic modifications. The dynamics of these effects can differ substantially from one neuromodulatory system to another, a difference that would be expected to have a major impact on functional maps and on sensory performance.
3 EFFECTS OF NEUROMODULATION ON PLASTICITY IN ADULT SENSORY CORTICES
Different behavioral states can be visualized in a multidimensional space, where each dimension represents a distinct psychological variable (attention, aversion, attraction, motivation, or empathy) or, correspondingly, neuromodulatory system (e.g., acetylcholine, norepinephrine, dopamine, serotonin, or peptide modulators such as oxytocin) (Fig. 2). Different combinations of behavioral states and thus of their biological correlates are called into action during danger, food seeking, sexual behavior, and maternal behavior. For example, during classical fear-learning paradigms in rodents, a sensory stimulus is paired with a foot shock. This shock has been found to induce increased firing of noradrenergic neurons in the locus coeruleus, dopaminergic neurons in the ventral tegmental area, and most likely cholinergic neurons in the basal forebrain (Brischoux et al., 2009; Chen and Sara, 2007). Moreover, many neuromodulatory centers in the brainstem, basal forebrain, and the hypothalamus are directly connected. For example, locus coeruleus neurons receive dopaminergic input from the ventral tegmental area and the substantia nigra pars compacta and serotoninergic input from dorsal raphe neurons. In turn, noradrenergic fibers from the locus coeruleus synapse on ventral tegmental area, substantia nigra, and basal forebrain cells. Basal forebrain neurons also receive dopaminergic and serotoninergic inputs (Eggermann et al., 2001; Sara and Bouret, 2012).
FIGURE 2.
Multidimensionality of behavioral states. Multiple neuromodulatory systems contribute in various combinations to creating different behavioral states. To allow visualization, only three dimensions and three behavioral states are shown here.
Neuromodulators act on distributed neural circuits to generate and store specific patterns of activity within neuronal ensembles important for behavioral performance. Blocking the activity of these neuromodulators systemically or specifically in their sensory cortex terminal fields can prevent associative and perceptual learning (Fletcher and Wilson, 2002; Kroon and Carobrez, 2009; Letzkus et al., 2011). Recent in vitro data on the dynamics of adult sensory cortex plasticity uncovered both shared and specific modes of action for different neuromodulators (Kruglikov and Rudy, 2008). Can different neuromodulator combinations orchestrate adaptive synaptic plasticity in sensory cortices? In answering this question further, it is important to (1) study the effects of different neuromodulatory systems separately and at different naturalistic activation frequencies and intensities and (2) uncover the combinatorial actions of these neuromodulators in ratios that would be relevant for behavioral states (Fig. 2). An example of cooperative effects of two neuromodulators was described in the visual cortex, where integrity of both noradrenergic and cholinergic fibers is required for proper development of the ocular dominance columns (Bear and Singer, 1986). In this example, the two neuromodulators have a compensatory type of interaction, but in other structures or under different conditions, they might act synergistically or additively.
Next, we will discuss recent in vivo data on the role of the basal forebrain and other neuromodulatory systems in inducing synaptic plasticity of sensory cortices.
3.1 Basal forebrain
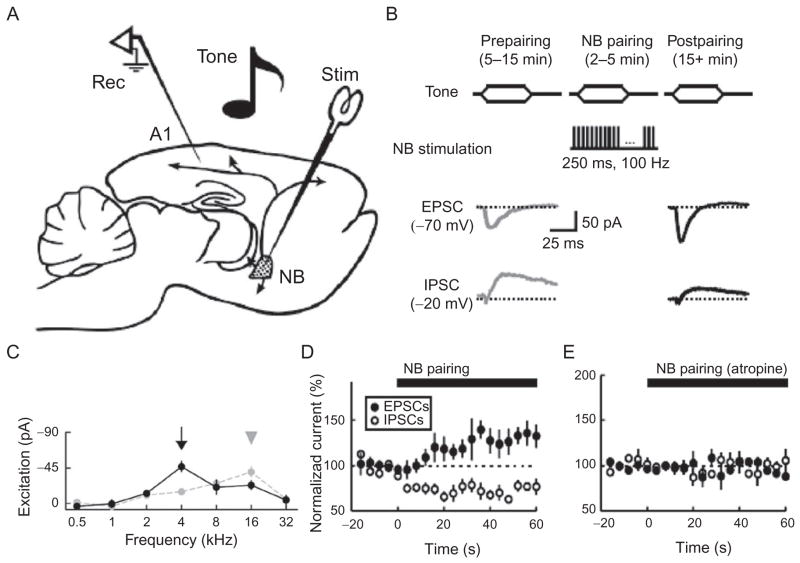
The basal forebrain is a collection of cholinergic, GABAergic, and glutamatergic projection neurons and local GABAergic interneurons, distributed in three main complexes: medial septum/ventral diagonal band complex projecting to the hippocampus, horizontal limb of the diagonal band projecting to the olfactory bulb and the piriform cortex, and nucleus basalis magnocellularis and substantia innominata complex projecting uniformly to the neocortex (Gritti et al., 2003, 2006; Manns et al., 2001; Mesulam, 2004; Zaborszky, 2002; Zaborszky et al., 1999, 2005). Nucleus basalis neurons have elevated spiking and bursting activity during wakefulness and REM sleep and can also discharge in response to behaviorally relevant, novel, or recent sensory stimuli (Cape and Jones, 2000; Duque et al., 2007; Kanai and Szerb, 1965; Manns et al., 2000a, b; Szymusiak et al., 2000). Nucleus basalis neurons can be electrically stimulated to induce acetylcholine release in their terminal fields, which leads to desynchronization of cortical electrical activity and enhanced cortical responsiveness to sensory stimuli (Metherate and Ashe, 1991, 1993). Pairing of nucleus basalis stimulation with the presentation of a sensory stimulus results in long-lasting cortical plasticity (Bakin and Weinberger, 1996; Froemke et al., 2007; Kilgard and Merzenich, 1998b) (Fig. 3A–C). However, electrical stimulation is not specific and can simultaneously activate other neuronal populations in the nucleus basalis. Recently, specific stimulation of cholinergic neurons has been achieved using optogenetics in mice and rats expressing channelrhodopsin-2 in neurons positive for choline acetyltransferase, an enzyme necessary for the biosynthesis of acetylcholine (Kalmbach et al., 2012; Witten et al., 2010). Future studies using this approach are necessary to parse out the contribution of identified cell types in the basal forebrain to cortical activation, cortical plasticity, and behavior.
FIGURE 3.
Synaptic modifications induced by nucleus basalis pairing. (A) Sagittal drawing through the rodent brain shows the position of the stimulating electrode in the nucleus basalis (NB) and the recording pipette in the primary auditory cortex (A1). The extensive cortical projections of cholinergic neurons are shown. (B) The pairing procedure: pure tones of various frequencies are played prepairing in a pseudorandomized order, and then one frequency is paired with nucleus basalis stimulation and then the full set of tones is again played postpairing. Representative excitatory and inhibitory synaptic currents evoked by the paired tone were recorded pre- and postpairing in the same cell. (C) Retuning of the excitatory synaptic curve: the response to the paired stimulus increases and the response to the original best stimulus decreases. (D) Fast decrease in inhibition and slower increase in excitation evoked by the paired stimulus during pairing. (E) Blocking muscarinic receptors in the auditory cortex prevents the effects of pairing. Artwork in (A) by Jana Pivkova.
(Reproduced from Froemke et al. (2007) and Froemke et al. (2013)).
Acetylcholine release in the cortex can induce activation of pyramidal neurons (Detari, 2000; Detari et al., 1999; Dringenberg and Vanderwolf, 1997; Linster and Hasselmo, 2001). In the auditory cortex, two disinhibitory network mechanisms have been described in vivo. One of them depends on activation of muscarinic receptors in mid- and deep cortical layers, which results in a rapid depression of stimulus-evoked inhibitory inputs on pyramidal neurons (Froemke et al., 2007; Metherate and Ashe, 1993). Most likely, this effect depends on decreased release of GABA from fast-spiking interneurons that express presynaptic muscarinic M2 receptors (Kruglikov and Rudy, 2008). In the adult rat primary auditory cortex, repeatedly pairing nucleus basalis activation with the presentation of a pure tone of defined frequency and intensity leads to a decrease in the inhibition evoked by the paired stimulus and therefore to a spectrally restricted increase in the excitatory–inhibitory ratio. At the same time, activation of muscarinic receptors may extend the integration window for excitatory inputs as is the case in the somatosensory cortex (Kruglikov and Rudy, 2008). Together, these muscarinic actions seem to permit Hebbian plasticity of excitatory inputs at the paired stimulus and a gradual retuning of the synaptic and spiking receptive fields (Bakin and Weinberger, 1996; Froemke et al., 2007) (Fig. 3D and E). At the population level, these modifications induce an overrepresentation of the paired stimulus in the tonotopic map (Kilgard and Merzenich, 1998b).
The other mechanism by which cholinergic inputs trigger disinhibition in the auditory cortex has been characterized in upper cortical layers by Letzkus et al. (2011). Activation of nicotinic receptors on layer 1 inhibitory neurons leads to their robust spiking, which results in the inhibition of their postsynaptic partners—layer 2–3 parvalbumin-positive interneurons. The decreased firing of parvalbumin-positive interneurons diminishes their inhibitory control over upper-layer pyramidal neurons. When this disinhibitory event coincides in time with a sensory stimulus, the evoked response in pyramidal neurons increases dramatically. It is unclear whether this mechanism can lead to long-lasting retuning in the auditory cortex or in structures downstream, but it is required for learning stimulus–fear associations (Letzkus et al., 2011). Therefore, although generated by different cholinergic receptor activities and different microcircuits, a similar Hebbian form of plasticity would act in superficial and deep layers of the sensory cortex to enlarge the representation of the behaviorally relevant stimuli. Interestingly, the opposing effects of acetylcholine on two types of inhibitory transmission have also been described in the visual cortex in slices, indicating a conserved mode of cholinergic action on microcircuits across different sensory cortical areas (Xiang et al., 1998).
What would be the advantage of having two (or more) seemingly independent yet correlated mechanisms by which acetylcholine enhances stimulus representation in deep and superficial cortical layers? This might allow for projection-specific fine-tuning of evoked responses in the sensory cortex. The vast majority of upper-layer pyramidal neurons project cortically, whereas deep-layer neurons can project either cortically or subcortically (Koester and O’Leary, 1994). It is possible that for certain behavioral tasks, it would be advantageous to cotune cortical circuits with different projection patterns, whereas for other behaviors, it would be more beneficial if these different circuits exhibited distinct sensitivities to sensory inputs or to neuromodulatory and behavioral control (Chen et al., 2013).
The effects of cholinergic modulation in the auditory cortex are dynamic and remarkably specific to subsets of synapses. During the first 30 min after pairing an auditory stimulus with electrical stimulation of the nucleus basalis, the strength of the paired excitatory input increases and becomes the new peak of the tuning profile. At the level of inhibitory inputs, after the immediate disinhibitory episode observed during the pairing procedure, inhibition gradually and slowly retunes to balance changes in excitation. Excitatory and inhibitory corticocortical connections appear to undergo most of the modifications observed, whereas thalamocortical synapses are resistant to the long-lasting effects of cholinergic modulation (Froemke et al., 2007). An extraordinary level of specificity is observed in the dynamics of excitatory tuning profile of pyramidal neurons. As the paired input increases in strength, the original best stimulus evokes less and less excitation. The excitatory currents corresponding to the rest of the stimulus set remain unchanged. This indicates that network excitability is strictly conserved. How do neurons specifically and uniquely depress inputs corresponding to the original best stimulus of each cell? This remarkable level of control depends on the original best stimulus being present a sufficient number of times after the pairing procedure ends. If this stimulus is not played postpairing, another presented strong input will be depressed in order to conserve excitability in the network. These experiments show that the precisely coordinated changes in synaptic function are activity- and history-dependent (Froemke et al., 2013). We also found that these long-term synaptic modifications induced by acetylcholine require cortical muscarinic and NMDA receptor activity (Froemke et al., 2013).
Although most of the discussed physiological effects of acetylcholine on cortical circuits have been described in the rodent auditory cortex, similar findings have been reported in the other primary sensory cortices. We will enumerate in the succeeding text additional recent findings in the somatosensory, visual, and piriform cortices.
Nucleus basalis activation results in muscarinic receptor-dependent decorrelation of activity in the rodent visual cortex during the visualization of natural scenes. This phenomenon described by Goard and Dan (2009) is believed to increase the perceptual capacity of the network. The authors also showed increased reliability of unitary responses in the lateral geniculate nucleus and the visual cortex following nucleus basalis stimulation.
In the somatosensory cortex, an additional interesting mechanism by which cholinergic innervation could induce plasticity has been reported by Takata, Mishima, and colleagues. In an elegant study, they showed that acetylcholine released during nucleus basalis stimulation activates muscarinic receptors on astrocytes, which induces calcium waves in these cells and the subsequent release of D-serine. In turn, D-serine binds neuronal NMDA receptors and, when this coincides with stimulus-evoked activity, a long-lasting potentiation at the paired stimulus ensues (Takata et al., 2011). Importantly, a similar role for astrocytes in cholinergic-mediated cortical plasticity was later described in the visual cortex, indicating that this may be a general mechanism to modify cortical synapses (Chen et al., 2012).
In the piriform cortex, acetylcholine released by neurons in the horizontal limb of the diagonal band can also have immediate and long-lasting effects. Specifically, acetylcholine depolarizes both pyramidal and inhibitory neurons, increasing their spontaneous and stimulus-evoked spiking (Barkai and Hasselmo, 1994; Tseng and Haberly, 1989a, b). Stimulation of basal forebrain projections initially suppresses inputs from association fibers but subsequently enhances these inputs, a phenomenon thought to contribute to odor discrimination (Hasselmo and Barkai, 1995; Linster and Hasselmo, 2001; Wilson, 2001). In the piriform cortex, an additional level of regulation has been reported, where acetylcholine decreases both the adaptation of pyramidal cell spiking to current injections and the afterhyperpolarization, thus permitting Hebbian plasticity (Barkai and Hasselmo, 1994; Constanti and Sim, 1987; Saar et al., 2001).
Due to its possible role in the progression of Alzheimer’s disease, the basal fore-brain generated a large interest in the scientific community and therefore provides a model for exploring how neuromodulation and brain state affect processing and plasticity in cortical circuits. We will next summarize some of the recent insights into other neuromodulatory systems.
3.2 Locus coeruleus
Noradrenergic fibers originating in the locus coeruleus uniformly innervate the entire forebrain, including sensory cortices. Changes in the tonic firing rate of locus coeruleus neurons and thus in noradrenaline release play an essential role in regulating the sleep–wake cycle and cortical arousal (Aston-Jones and Bloom, 1981a, b; Berridge et al., 1993; Constantinople and Bruno, 2011; Hobson et al., 1975; Roussel et al., 1967). Locus coeruleus neurons discharge phasically in response to novel sensory stimuli in all modalities (Aston-Jones and Bloom, 1981a, b; Foote et al., 1980; Herve-Minvielle and Sara, 1995; Rasmussen et al., 1986; Sara et al., 1994). If the sensory stimulus is associated with either reward or punishment, the evoked responses of locus coeruleus neurons are maintained and even enhanced (Aston-Jones et al., 1994; Bouret and Richmond, 2009; Bouret and Sara, 2004; Jacobs et al., 1991; Sara and Segal, 1991).
Several studies reported the acute effects of locus coeruleus activation on sensory-evoked responses. Tonic and phasic stimulations can enhance stimulus-evoked responses in initially responsive cells of the barrel and piriform cortex and can induce responses in previously silent cells (Bouret and Sara, 2002; Devilbiss and Waterhouse, 2004, 2011; Lecas, 2004; McLean and Waterhouse, 1994). However, some cells in the barrel cortex can also be suppressed by tonic activation of noradrenergic fibers (Devilbiss and Waterhouse, 2004). In the auditory cortex of guinea pigs, ionophoretic application of noradrenaline has been reported to suppress evoked responses (Manunta and Edeline, 1999). The circuits and synaptic mechanisms by which noradrenaline released from the locus coeruleus controls cortical responses to sensory stimuli remain to be fully understood. Also, despite the fact that locus coeruleus activity has been repeatedly linked to the learning process, so far, few studies addressed the long-term effects of pairing a sensory stimulus with the activation of noradrenergic neurons on cortical stimulus representations. Therefore, a unified theory for how locus coeruleus might modulate synaptic activity in the neocortex to recruit neuronal ensembles that aptly represent behaviorally relevant stimuli is still missing.
3.3 Ventral tegmental area
In the mammalian forebrain, dopamine is released from dopaminergic fibers originating in the ventral tegmental area or substantia nigra. These fibers abundantly innervate the striatum and regions of the prefrontal cortex but are relatively sparse in the rest of the neocortex and thalamus, including sensory areas (Dahlstrom and Fuxe, 1964; Haber et al., 2000; Lindvall and Bjorklund, 1974; Williams and Goldman-Rakic, 1998). However, repeatedly pairing the presentation of an auditory stimulus with phasic stimulation of the ventral tegmental area has been shown to result in an expansion of the paired tone representation in primary and secondary auditory cortex (Bao et al., 2001). These effects could be supported by the few dopaminergic projections in the auditory cortex or could be generated by feedback from prefrontal areas. Various studies in the striatum and prefrontal cortex documented the effects of D1- versus D2-dopamine receptor activation on synaptic plasticity, but to our knowledge, they have not been replicated in primary sensory areas. Importantly, D2-dopmaine receptors have been implicated in the generation of perceptual psychotic episodes in schizophrenia and other psychiatric disorders (Seeman, 2013). It is therefore crucial to understand how dopamine controls processing and plasticity in sensory circuits.
3.4 Raphé nuclei
Neurons in the dorsal and medial raphe nuclei of the midbrain secrete serotonin and project abundantly to the entire forebrain via the medial frontal bundle (Calizo et al., 2011; Dahlstrom and Fuxe, 1964; Hornung et al., 1990). As is the case with most neuromodulatory systems, additional cell types reside in these nuclei (GABAergic, glutamatergic, and peptidergic) (Calizo et al., 2011; Waselus et al., 2006).
As with dopamine, serotonin and its receptors appear to play an important role in the generation of perceptual psychotic episodes in schizophrenia and following the use of psychedelic drugs (Gonzalez-Maeso et al., 2008; Lesch and Waider, 2012). Therefore, it is of crucial importance to understand the mechanisms by which serotonin impacts immediate and long-lasting activity in neocortical circuits. Some of the molecular mechanisms supporting serotonin actions started being elucidated. Both pyramidal and inhibitory neurons in the cortex express multiple types of metabotropic serotoninergic receptors. These receptors can regulate the activity of potassium channels and the levels of intracellular calcium and can interact directly or indirectly with AMPA, NMDA, and metabotropic glutamate receptors, thus controlling synaptic transmission, neuron excitability, and network excitatory to inhibitory balance (Bockaert et al., 2010; Moreau et al., 2010; Ogren et al., 2008; Yuen et al., 2005, 2008).
An interesting role for serotonin in cross-modal communication has been recently reported by Jitsuki and colleagues (Jitsuki et al., 2011). The study showed that visual deprivation in rats increased serotonin in the barrel cortex, where it promoted the phosphorylation and synaptic delivery of GluR1 glutamate receptor subunit, thus facilitating synaptic transmission at the layer 4 to layer 2/3 synapses and the sharpening of receptive fields in layer 2/3 neurons. Given the reported association between serotonin and perceptual synesthesia, more cross-modal and multimodal studies need to be conducted (Brang and Ramachandran, 2008).
3.5 Oxytocinergic system in the paraventricular and supraoptic nuclei of the hypothalamus
Oxytocin is a representative peptidergic neuromodulator synthesized in the paraventricular and supraoptic nuclei of the hypothalamus. It can be released from magno-cellular neurons in the bloodstream via the hypothalamic–hypophyseal axis, but it can also be released by parvocellular fibers in a more targeted manner to cortical and subcortical brain regions (Brownstein et al., 1980). In the mouse cortex, some of these fibers innervate the temporal areas, including the auditory cortex; the insular, cingulate, and entorhinal cortex; and the frontal association areas (Knobloch et al., 2012). Oxytocin is strongly linked to maternal behavior, being heavily released during parturition and lactation. Therefore, a large body of research focuses on the role of oxytocin in modulating maternal care. There is evidence that mother and virgin mice show differential cortical responses to sensory cues, particularly to pup ultrasound vocalizations (Cohen et al., 2011; Liu et al., 2006). Whether oxytocin can induce this differential representation of sounds and the circuit mechanisms by which it does so remains to be elucidated. An interesting circuit mechanism was described in the central amygdala, where oxytocin release in the lateral central amygdala activates a subset of interneurons that then inhibit pyramidal output neurons in the medial part of the central amygdala (Knobloch et al., 2012). This circuit mediates the anxiolytic effects of oxytocin (Knobloch et al., 2012; Viviani et al., 2011). It is important to determine if similar actions underlie the tuning of responses to pup calls in maternal auditory cortex.
4 COMMONALITIES AND DIFFERENCES BETWEEN DEVELOPMENTAL AND ADULT PLASTICITY IN SENSORY CORTICES
To what extent do the described modifications induced by neuromodulatory systems, in particular the basal forebrain, in adult circuits recapitulate the developmental critical windows? Both cases rely on somewhat transient excitatory to inhibitory imbalances, and we hypothesize that this condition favors induction of NMDA receptor-dependent forms of long-term synaptic modification. However, beyond this basic feature, it is likely that developmental and adult cortical plasticity are substantially different in other ways. During development, inhibitory elements are progressively integrated into the network and this process dictates the closure time and duration of staggered critical periods. In the adult brain, restricted sets of inhibitory inputs have to be actively and specifically suppressed. Changes in sensory representations induced by early life exposure to altered environments are very long-lasting and in many cases permanent (Hensch, 2005). On the contrary, plasticity in the adult sensory cortex is often transient. Following nucleus basalis pairing, synaptic and spiking profiles of neurons return to their original tuning in time and, as a consequence, sensory maps recover their initial representation (Kilgard, 2012; Reed et al., 2011). Although consecutive pairing episodes likely generate savings in the circuit and cumulate their effects on cortical plasticity, this is not sufficient to permanently alter adult map representations. This observation raises two important questions. First, how can good perceptual performance be maintained in the absence of relevant stimulus overrepresentation in the primary sensory cortex? Secondly, how do neuronal circuits revert to their original organization?
With regard to the first question, one possible answer is that the representation of the learned stimulus increases in multiple brain structures connected in series. In this model, the expansion of stimulus representation in the primary sensory cortex following learning or nucleus basalis pairing improves performance during the initial behavioral stages but becomes less marked and less important in time. This would allow the sensory cortex to recover a mostly unbiased representation of the sensory space. On the contrary, stimulus representations in structures downstream of the primary sensory cortex become progressively more defined and more important for behavioral performance. Recent findings support this model (Quirk et al., 1997; Sacco and Sacchetti, 2010; Znamenskiy and Zador, 2013). For example, Sacco and colleagues showed that the retrieval of 1-month-old but not new sensory fear associations requires intact secondary sensory cortices (Sacco and Sacchetti, 2010). However, the exact mechanisms by which the secondary sensory areas and other structures like the striatum, the amygdala, and the prefrontal cortex are integrated in the circuit remain to be determined.
Could sensory cortical plasticity contribute to this proposed progressive engagement of downstream elements in the network? One possibility is that the convergent synchronized activity of a larger pool of sensory cortical cells on a downstream post-synaptic neuron will push the membrane potential of this neuron above the spike threshold, thus generating a postsynaptic spike. Therefore, either classical long-term potentiation or spike timing-dependent potentiation of synapses in structures post-synaptically connected to sensory cortices becomes possible. As the cortical map recovers normal representation in time, synapses of neurons that continue to respond to the paired stimulus maintain strong conductances with the postsynaptic cell, whereas synapses of neurons that recover their original best stimulus will be depressed (e.g., through spike timing-dependent depression) (Fig. 4).
FIGURE 4.
Proposed model for how plasticity in the auditory cortex contributes to constructing memory traces in downstream structures. Prepairing or prelearning, connections between presynaptic neurons in the auditory cortex and postsynaptic downstream “effector” cells are weak (small hexagons). Postpairing, the synchronous discharge of auditory neurons induced spiking of the “effector” cell (purple color) and potentiation of synapses (large hexagons). Later on, after auditory neurons recover their original tuning, most of the synapses are depressed but some are still strong and capable to drive firing of the postsynaptic neuron.
Two nonexclusive mechanisms are proposed here to answer the second question—how do adult sensory circuits recover their original tuning following plasticity? As discussed earlier, nucleus basalis pairing modifies corticocortical inputs but spares the thalamocortical ones (Froemke et al., 2007). It is possible that these spared inputs will slowly drive the network back to its original state. Alternatively or concomitantly, synchronized spontaneous activity in cortical neurons can recalibrate the network. In the auditory and visual cortices, neurons selectively responding to the same stimulus feature have a higher probability to synchronize their spontaneous activity or have a higher connectivity probability (Bao et al., 2003; Ko et al., 2011). In their remarkable study, Ko, Hofer, and colleagues imaged calcium spikes in the mouse visual cortex to identify neuronal ensembles responding to different orientations and directions of the visual stimulus. They then prepared acute slices from the imaged brains, recovered the recorded neurons, and used intracellular recordings to measure the connectivity probability between pairs of cells. Neurons had significantly higher probability of being connected to other neurons with similar orientation and direction selectivity than to neurons with distinct selectivities. If these properties are unaffected by nucleus basalis pairing, they can possibly drive the sensory network back to its basal state.
Although we did not address here the role of neuromodulators during development, this is an important issue for timing corrective or enhancing manipulations of neuromodulatory centers. The majority of neuromodulators and neuromodulatory receptors are abundantly expressed during development, including in primordial sensory systems, when they can play important roles in cell fate determination, cell migration, axon growth and guidance, dendrite growth, and synapse formation (Erzurumlu and Gaspar, 2012). Neuromodulation is also required for normal circuit assembly and plasticity in developmental sensory cortices. For example, excess serotonin resulting from knockout of the serotonin transporter in the mouse alters the normal development of thalamocortical fibers in the somatosensory cortex and thus prevents the formation of barrel fields (Persico et al., 2001). In the visual cortex, nor-adrenergic and cholinergic fibers are required for normal ocular dominance plasticity following developmental monocular deprivation (Bear and Singer, 1986).
5 IMPROVING PERCEPTION BY MANIPULATING NEUROMODULATION
We discussed in previous sections different mechanisms by which neuromodulators can promote plasticity in sensory cortices by generating excitatory to inhibitory synaptic imbalances. This indicates at least two important sites in the circuit where manipulations could allow increased plasticity and behavioral improvement: cortical inhibitory interneurons and neuromodulatory centers. Recently, multiple research programs capitalized on the advent of optogenetic and pharmacogenetic tools and embarked on a series of studies where different types of interneurons or of neuromodulatory neurons can be specifically activated or inhibited with different patterns and under different timescales. Some of the published work showed control over cognitive and emotional behavioral performance using these approaches (Brown et al., 2012; Chaudhury et al., 2013; Kvitsiani et al., 2013; Sohal et al., 2009; Witten et al., 2011).
In an elegant study, Lee and colleagues showed that similar level of control could be achieved in the perceptual domain (Lee and Dan, 2012). They showed that driving the activity of parvalbumin interneurons optogenetically sharpened the tuning of orientation-selective cortical neurons in the visual cortex. At the behavioral level, this approach improved stimulus discrimination as determined by significant increases in the discriminability index. However, it is unclear whether these improvements in behavior endure over time. Based on the electrophysiological data presented here, we believe that control over the neuromodulatory systems can generate robust and long-lasting improvement in behavioral performance. Next, we will describe supporting evidence that temporally discrete manipulations of the cholinergic system can improve different dimensions of auditory perception.
The functions of the auditory system are to detect, identify, and localize stimuli that might be behaviorally relevant. These capacities are acquired during development as a result of synaptic maturation in the sensory circuit and can be improved by training in adulthood (Sanes and Woolley, 2011). Lesions of cortical cholinergic fibers can result in decreased detection, identification, and localization of stimuli and impaired learning, storage, and flexible retrieval of sensory associations (Berger-Sweeney et al., 2000; Butt et al., 2002; Cabrera et al., 2006; Leach et al., 2013; Vale-Martinez et al., 2002).
Sensory detection refers to the capacity to perceive the presence of a signal. As a general rule, detection degrades with age due to alterations at the level of sensory organs. In human auditory perception, the cumulative effect of age on hearing is called presbycusis. It becomes manifest around age 18, when detection of high-frequency sounds (>16 kHz) becomes greatly reduced. In time, most frequencies, including those in the speech range (~300–3400 Hz), will be perceived only at high intensities (Liu and Yan, 2007). Pathological conditions unrelated to normal aging can negatively impact the detection ability of sensory systems. When developed during childhood, such pathological conditions can interfere with the normal maturation of neuronal circuits and thus can have long-lasting effects on sensory perception. It is therefore important to design interventions for correcting and enhancing auditory perception.
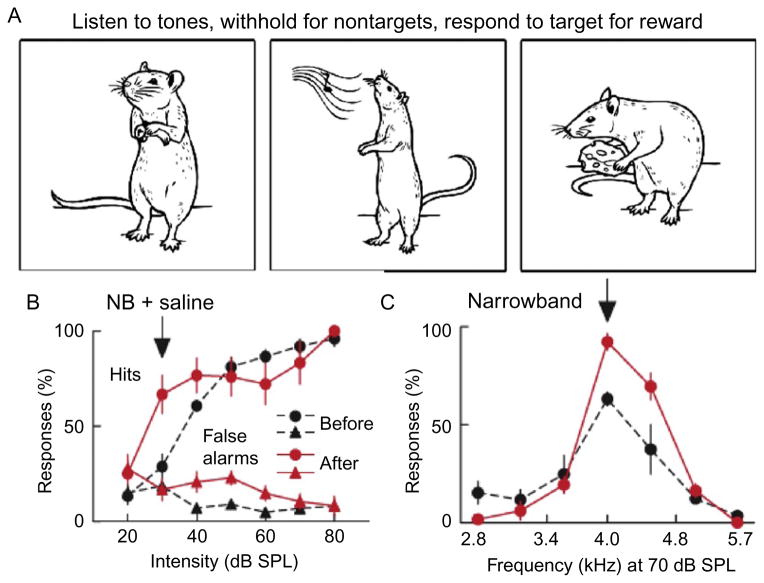
In our studies of rodent auditory perception, we trained rats to make a nose poke in response to a particular tone frequency (target tone, 4 kHz) and to withhold from nose poking in response to other frequencies (Fig. 5A). During training, the target tone was played at high amplitude, 70 dB SPL. After the animal learned the association, we varied the amplitude of the tones from soft (20 dB SPL) to loud (80 dB SPL). Unsurprisingly, the hit rate increased with the amplitude of the target tone, such that low-amplitude sounds were hardly perceptible under normal conditions. When we paired the presentation of the target tone at low amplitudes (30–40 dB SPL) with the stimulation of the basal forebrain for 5 min, the hit rate postpairing increased for the paired amplitude, indicating increased detection (Fig. 5B). This improvement was not observed when muscarinic and NMDA receptors in the auditory cortex were blocked during the pairing procedure. Thus, engaging the cholinergic system can lead to improved auditory detection in adults. Importantly, this occurs even when the pairing is done outside of the behavioral context or in anesthetized rats, indicating that plasticity in the auditory system alone is sufficient to improve detection and that modifications at sensory–motor circuits are not required for achieving this (Froemke et al., 2013; Reed et al., 2011).
FIGURE 5.
Nucleus basalis pairing can improve behavioral performance on perceptual tasks. (A) The go–no go operant conditioning task: rats learn to nose poke in response to 4 kHz tones and to withhold from poking after other frequencies. (B) Before pairing (black lines), rats do not detect low-intensity targets, but after pairing (red lines) a 4 kHz tone played at 30 dB SPL with nucleus basalis pairing, detection at the paired intensity increases significantly. (C) Pairing of the target tone with nucleus basalis stimulation improves recognition of the target tone from foil tones that are at a small perceptual distance from each other (same color code as in b).
Reproduced from Froemke et al. (2013).
Perceptual identification or recognition can refer to two related concepts. First, it refers to ability of a sensory system to identify a stimulus as a specific sensory object or as part of such object. Secondly, it refers to the ability to discriminate or separate a stimulus from background noise or from other coincidental stimuli. In the adult rat primary auditory cortex, we and others found that changing synaptic weights by pairing the presentation of the target tone with basal forebrain stimulation led to retuning of spiking receptive fields and therefore to increased representation of the target stimulus to the disadvantage of foil stimuli (Detari et al., 1999; Froemke et al., 2013). At the behavioral level, the pairing resulted in improved recognition of the target tone from foil tones even when these were at short perceptual distance from each other (one-sixth of an octave) and therefore hard to discriminate under normal conditions (Fig. 5C).
In the future, optogenetic and pharmacogenetic control of cholinergic and other neuromodulatory fibers will offer a more specific control of cortical circuits and will likely result in enduring enhancement of perception.
6 CONCLUSIONS
We summarized here recent data showing that experience, via the activation of neuromodulatory systems, can modify cortical neural circuits to improve perception. We stress the following points:
Multiple neuromodulatory systems respond during a particular behavioral task. The effects of neuromodulators on plasticity should be studied not only in isolation but also in behaviorally relevant combinations as well.
Neuromodulators can modify connections in several different microcircuits within the same cortical column independently. This could indicate a projection-specific modulation of synaptic inputs under various behavioral tasks.
Unlike developmental critical periods, plasticity in the adult sensory cortex results from active and selective decorrelation of excitatory and inhibitory inputs.
Whereas experience-induced synaptic and map modifications during development can be extremely long lasting, plasticity in adult sensory cortices is generally transient. Although the time constant of circuit modifications facilitated by neuromodulation and experience could vary substantially in the adult, one hypothesis is that longer-lasting synaptic changes are more capable of sustaining perceptual learning and require fewer repetitions to accumulate and to construct the memory in downstream structures.
The function of neuromodulation can differ substantially between adult and developing brains; therefore, corrective manipulations must be adjusted to the age of the subject.
Stimulating neuromodulatory centers can enable enduring perceptual improvements and behavioral modifications on several dimensions, even when manipulations are done off-line.
Optogenetic and pharmacogenetic tools promise a more specific way to manipulate brain circuits in order to correct and enhance sensory perception.
References
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981a;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981b;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci U S A. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan V, Merzenich M. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–162. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bao S, Chang EF, Davis JD, Gobeske KT, Merzenich MM. Progressive degradation and subsequent refinement of acoustic representations in the adult auditory cortex. J Neurosci. 2003;23:10765–10775. doi: 10.1523/JNEUROSCI.23-34-10765.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci. 2004;7:974–981. doi: 10.1038/nn1293. [DOI] [PubMed] [Google Scholar]
- Barkai E, Hasselmo ME. Modulation of the input/output function of rat piriform cortex pyramidal cells. J Neurophysiol. 1994;72:644–658. doi: 10.1152/jn.1994.72.2.644. [DOI] [PubMed] [Google Scholar]
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- Benson DL, Colman DR, Huntley GW. Molecules, maps and synapse specificity. Nat Rev Neurosci. 2001;2:899–909. doi: 10.1038/35104078. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Stearns NA, Frick KM, Beard B, Baxter MG. Cholinergic basal forebrain is critical for social transmission of food preferences. Hippocampus. 2000;10:729–738. doi: 10.1002/1098-1063(2000)10:6<729::AID-HIPO1010>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55:381–393. doi: 10.1016/0306-4522(93)90507-c. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Perroy J, Becamel C, Marin P, Fagni L. GPCR interacting proteins (GIPs) in the nervous system: roles in physiology and pathologies. Annu Rev Pharmacol Toxicol. 2010;50:89–109. doi: 10.1146/annurev.pharmtox.010909.105705. [DOI] [PubMed] [Google Scholar]
- Bouret S, Richmond BJ. Relation of locus coeruleus neurons in monkeys to Pavlovian and operant behaviors. J Neurophysiol. 2009;101:898–911. doi: 10.1152/jn.91048.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Locus coeruleus activation modulates firing rate and temporal organization of odour-induced single-cell responses in rat piriform cortex. Eur J Neurosci. 2002;16:2371–2382. doi: 10.1046/j.1460-9568.2002.02413.x. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. Eur J Neurosci. 2004;20:791–802. doi: 10.1111/j.1460-9568.2004.03526.x. [DOI] [PubMed] [Google Scholar]
- Brang D, Ramachandran VS. Psychopharmacology of synesthesia; the role of serotonin S2a receptor activation. Med Hypotheses. 2008;70:903–904. doi: 10.1016/j.mehy.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Tan KR, O’Connor EC, Nikonenko I, Muller D, Luscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–456. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;207:373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Butt AE, Noble MM, Rogers JL, Rea TE. Impairments in negative patterning, but not simple discrimination learning, in rats with 192 IgG-saporin lesions of the nucleus basalis magnocellularis. Behav Neurosci. 2002;116:241–255. doi: 10.1037//0735-7044.116.2.241. [DOI] [PubMed] [Google Scholar]
- Cabrera SM, Chavez CM, Corley SR, Kitto MR, Butt AE. Selective lesions of the nucleus basalis magnocellularis impair cognitive flexibility. Behav Neurosci. 2006;120:298–306. doi: 10.1037/0735-7044.120.2.298. [DOI] [PubMed] [Google Scholar]
- Calizo LH, Akanwa A, Ma X, Pan YZ, Lemos JC, Craige C, Heemstra LA, Beck SG. Raphe serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology. 2011;61:524–543. doi: 10.1016/j.neuropharm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cape EG, Jones BE. Effects of glutamate agonist versus procaine microinjections into the basal forebrain cholinergic cell area upon gamma and theta EEG activity and sleep-wake state. Eur J Neurosci. 2000;12:2166–2184. doi: 10.1046/j.1460-9568.2000.00099.x. [DOI] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FJ, Sara SJ. Locus coeruleus activation by foot shock or electrical stimulation inhibits amygdala neurons. Neuroscience. 2007;144:472–481. doi: 10.1016/j.neuroscience.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, Le C, Sur M. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc Natl Acad Sci U S A. 2012;109:E2832–2841. doi: 10.1073/pnas.1206557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Carta S, Soldado-Magraner J, Schneider BL, Helmchen F. Behaviour-dependent recruitment of long-range projection neurons in somatosensory cortex. Nature. 2013;499:336–340. doi: 10.1038/nature12236. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Isaac JT. Emergence of cortical inhibition by coordinated sensory-driven plasticity at distinct synaptic loci. Nat Neurosci. 2010;13:1240–1248. doi: 10.1038/nn.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Rothschild G, Mizrahi A. Multisensory integration of natural odors and sounds in the auditory cortex. Neuron. 2011;72:357–369. doi: 10.1016/j.neuron.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Constanti A, Sim JA. Muscarinic receptors mediating suppression of the M-current in guinea-pig olfactory cortex neurones may be of the M2-subtype. Br J Pharmacol. 1987;90:3–5. doi: 10.1111/j.1476-5381.1987.tb16818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron. 2011;69:1061–1068. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Dahmen JC, King AJ. Learning to hear: plasticity of auditory cortical processing. Curr Opin Neurobiol. 2007;17:456–464. doi: 10.1016/j.conb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Darwin C. On the Origin of Species by Means of Natural Selection. J. Murray; London: 1859. [Google Scholar]
- Daw MI, Ashby MC, Isaac JT. Coordinated developmental recruitment of latent fast spiking interneurons in layer IV barrel cortex. Nat Neurosci. 2007;10:453–461. doi: 10.1038/nn1866. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Merzenich MM. Lifelong plasticity in the rat auditory cortex: basic mechanisms and role of sensory experience. Prog Brain Res. 2011;191:119–131. doi: 10.1016/B978-0-444-53752-2.00009-6. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27:180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detari L. Tonic and phasic influence of basal forebrain unit activity on the cortical EEG. Behav Brain Res. 2000;115:159–170. doi: 10.1016/s0166-4328(00)00256-4. [DOI] [PubMed] [Google Scholar]
- Detari L, Rasmusson DD, Semba K. The role of basal forebrain neurons in tonic and phasic activation of the cerebral cortex. Prog Neurobiol. 1999;58:249–277. doi: 10.1016/s0301-0082(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J Neurosci. 2004;24:10773–10785. doi: 10.1523/JNEUROSCI.1573-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. Phasic and tonic patterns of locus coeruleus output differentially modulate sensory network function in the awake rat. J Neurophysiol. 2011;105:69–87. doi: 10.1152/jn.00445.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nature. 2010;465:932–936. doi: 10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Neocortical activation: modulation by multiple pathways acting on central cholinergic and serotonergic systems. Exp Brain Res. 1997;116:160–174. doi: 10.1007/pl00005736. [DOI] [PubMed] [Google Scholar]
- Duque A, Tepper JM, Detari L, Ascoli GA, Zaborszky L. Morphological characterization of electrophysiologically and immunohistochemically identified basal fore-brain cholinergic and neuropeptide Y-containing neurons. Brain Struct Funct. 2007;212:55–73. doi: 10.1007/s00429-007-0143-3. [DOI] [PubMed] [Google Scholar]
- Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, Muhlethaler M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- Elbert T, Sterr A, Rockstroh B, Pantev C, Muller MM, Taub E. Expansion of the tonotopic area in the auditory cortex of the blind. J Neurosci. 2002;22:9941–9944. doi: 10.1523/JNEUROSCI.22-22-09941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex. Eur J Neurosci. 2012;35:1540–1553. doi: 10.1111/j.1460-9568.2012.08075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher ML, Wilson DA. Experience modifies olfactory acuity: acetylcholine-dependent learning decreases behavioral generalization between similar odorants. J Neurosci. 2002;22:RC201. doi: 10.1523/JNEUROSCI.22-02-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci U S A. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke R, Merzenich M, Schreiner C. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–434. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Carcea I, Barker AJ, Yuan K, Seybold BA, Martins AR, Zaika N, Bernstein H, Wachs M, Levis PA, et al. Long-term modification of cortical synapses improves sensory perception. Nat Neurosci. 2013;16:79–88. doi: 10.1038/nn.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, et al. Identification of a serotonin/ glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti I, Manns ID, Mainville L, Jones BE. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J Comp Neurol. 2003;458:11–31. doi: 10.1002/cne.10505. [DOI] [PubMed] [Google Scholar]
- Gritti I, Henny P, Galloni F, Mainville L, Mariotti M, Jones BE. Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience. 2006;143:1051–1064. doi: 10.1016/j.neuroscience.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Barkai E. Cholinergic modulation of activity-dependent synaptic plasticity in the piriform cortex and associative memory function in a network biophysical simulation. J Neurosci. 1995;15:6592–6604. doi: 10.1523/JNEUROSCI.15-10-06592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Stryker MP. Columnar architecture sculpted by GABA circuits in developing cat visual cortex. Science. 2004;303:1678–1681. doi: 10.1126/science.1091031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve-Minvielle A, Sara SJ. Rapid habituation of auditory responses of locus coeruleus cells in anaesthetized and awake rats. Neuroreport. 1995;6:1363–1368. doi: 10.1097/00001756-199507100-00001. [DOI] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- Hornung JP, Fritschy JM, Tork I. Distribution of two morphologically distinct subsets of serotoninergic axons in the cerebral cortex of the marmoset. J Comp Neurol. 1990;297:165–181. doi: 10.1002/cne.902970202. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Shape and arrangement of columns in cat’s striate cortex. J Physiol. 1963;165:559–568. doi: 10.1113/jphysiol.1963.sp007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insanally MN, Kover H, Kim H, Bao S. Feature-dependent sensitive periods in the development of complex sound representation. J Neurosci. 2009;29:5456–5462. doi: 10.1523/JNEUROSCI.5311-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Abercrombie ED, Fornal CA, Levine ES, Morilak DA, Stafford IL. Single-unit and physiological analyses of brain norepinephrine function in behaving animals. Prog Brain Res. 1991;88:159–165. doi: 10.1016/s0079-6123(08)63805-4. [DOI] [PubMed] [Google Scholar]
- Jitsuki S, Takemoto K, Kawasaki T, Tada H, Takahashi A, Becamel C, Sano A, Yuzaki M, Zukin RS, Ziff EB, et al. Serotonin mediates cross-modal reorganization of cortical circuits. Neuron. 2011;69:780–792. doi: 10.1016/j.neuron.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach A, Hedrick T, Waters J. Selective optogenetic stimulation of cholinergic axons in neocortex. J Neurophysiol. 2012;107:2008–2019. doi: 10.1152/jn.00870.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai T, Szerb JC. Mesencephalic reticular activating system and cortical acetylcholine output. Nature. 1965;205:80–82. doi: 10.1038/205080b0. [DOI] [PubMed] [Google Scholar]
- Kenet T, Froemke RC, Schreiner CE, Pessah IN, Merzenich MM. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proc Natl Acad Sci U S A. 2007;104:7646–7651. doi: 10.1073/pnas.0701944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP. Harnessing plasticity to understand learning and treat disease. Trends Neurosci. 2012;35:715–722. doi: 10.1016/j.tins.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998a;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998b;1:727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Ko H, Hofer SB, Pichler B, Buchanan KA, Sjostrom PJ, Mrsic-Flogel TD. Functional specificity of local synaptic connections in neocortical networks. Nature. 2011;473:87–91. doi: 10.1038/nature09880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester SE, O’Leary DD. Development of projection neurons of the mammalian cerebral cortex. Prog Brain Res. 1994;102:207–215. doi: 10.1016/S0079-6123(08)60541-5. [DOI] [PubMed] [Google Scholar]
- Kroon JA, Carobrez AP. Olfactory fear conditioning paradigm in rats: effects of midazolam, propranolol or scopolamine. Neurobiol Learn Mem. 2009;91:32–40. doi: 10.1016/j.nlm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–924. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013;498:363–366. doi: 10.1038/nature12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach ND, Nodal FR, Cordery PM, King AJ, Bajo VM. Cortical cholinergic input is required for normal auditory perception and experience-dependent plasticity in adult ferrets. J Neurosci. 2013;33:6659–6671. doi: 10.1523/JNEUROSCI.5039-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecas JC. Locus coeruleus activation shortens synaptic drive while decreasing spike latency and jitter in sensorimotor cortex. Implications for neuronal integration. Eur J Neurosci. 2004;19:2519–2530. doi: 10.1111/j.0953-816X.2004.03341.x. [DOI] [PubMed] [Google Scholar]
- Lee SH, Dan Y. Neuromodulation of brain states. Neuron. 2012;76:209–222. doi: 10.1016/j.neuron.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Waider J. Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron. 2012;76:175–191. doi: 10.1016/j.neuron.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Luthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl. 1974;412:1–48. [PubMed] [Google Scholar]
- Linster C, Hasselmo ME. Neuromodulation and the functional dynamics of piriform cortex. Chem Senses. 2001;26:585–594. doi: 10.1093/chemse/26.5.585. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Yan D. Ageing and hearing loss. J Pathol. 2007;211:188–197. doi: 10.1002/path.2102. [DOI] [PubMed] [Google Scholar]
- Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur J Neurosci. 2006;23:3087–3097. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge profiles of juxtacellularly labeled and immunohistochemically identified GABAergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000a;20:9252–9263. doi: 10.1523/JNEUROSCI.20-24-09252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge properties of juxtacellularly labeled and immunohistochemically identified cholinergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000b;20:1505–1518. doi: 10.1523/JNEUROSCI.20-04-01505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Mainville L, Jones BE. Evidence for glutamate, in addition to acetylcholine and GABA, neurotransmitter synthesis in basal forebrain neurons projecting to the entorhinal cortex. Neuroscience. 2001;107:249–263. doi: 10.1016/s0306-4522(01)00302-5. [DOI] [PubMed] [Google Scholar]
- Manunta Y, Edeline JM. Effects of noradrenaline on frequency tuning of auditory cortex neurons during wakefulness and slow-wave sleep. Eur J Neurosci. 1999;11:2134–2150. doi: 10.1046/j.1460-9568.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- McLean J, Waterhouse BD. Noradrenergic modulation of cat area 17 neuronal responses to moving visual stimuli. Brain Res. 1994;667:83–97. doi: 10.1016/0006-8993(94)91716-7. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Knight PL, Roth GL. Cochleotopic organization of primary auditory cortex in the cat. Brain Res. 1973;63:343–346. doi: 10.1016/0006-8993(73)90101-7. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. The cholinergic innervation of the human cerebral cortex. Prog Brain Res. 2004;145:67–78. doi: 10.1016/S0079-6123(03)45004-8. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Basal forebrain stimulation modifies auditory cortex responsiveness by an action at muscarinic receptors. Brain Res. 1991;559:163–167. doi: 10.1016/0006-8993(91)90301-b. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14:132–143. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Moreau AW, Amar M, Le Roux N, Morel N, Fossier P. Serotoninergic fine-tuning of the excitation-inhibition balance in rat visual cortical networks. Cereb Cortex. 2010;20:456–467. doi: 10.1093/cercor/bhp114. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Eriksson TM, Elvander-Tottie E, D’Addario C, Ekstrom JC, Svenningsson P, Meister B, Kehr J, Stiedl O. The role of 5-HT(1A) receptors in learning and memory. Behav Brain Res. 2008;195:54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Pantev C, Engelien A, Candia V, Elbert T. Representational cortex in musicians. Plastic alterations in response to musical practice. Ann N Y Acad Sci. 2001;930:300–314. [PubMed] [Google Scholar]
- Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, et al. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci. 2001;21:6862–6873. doi: 10.1523/JNEUROSCI.21-17-06862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Morilak DA, Jacobs BL. Single unit activity of locus coeruleus neurons in the freely moving cat. I During naturalistic behaviors and in response to simple and complex stimuli. Brain Res. 1986;371:324–334. doi: 10.1016/0006-8993(86)90370-7. [DOI] [PubMed] [Google Scholar]
- Reed A, Riley J, Carraway R, Carrasco A, Perez C, Jakkamsetti V, Kilgard MP. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron. 2011;70:121–131. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Roussel B, Buguet A, Bobillier P, Jouvet M. Locus ceruleus, paradoxal sleep, and cerebral noradrenaline. C R Seances Soc Biol Fil. 1967;161:2537–2541. [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Long-lasting cholinergic modulation underlies rule learning in rats. J Neurosci. 2001;21:1385–1392. doi: 10.1523/JNEUROSCI.21-04-01385.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco T, Sacchetti B. Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science. 2010;329:649–656. doi: 10.1126/science.1183165. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Woolley SM. A behavioral framework to guide research on central auditory development and plasticity. Neuron. 2011;72:912–929. doi: 10.1016/j.neuron.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ, Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76:130–141. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Segal M. Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog Brain Res. 1991;88:571–585. doi: 10.1016/s0079-6123(08)63835-2. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Vankov A, Herve A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res Bull. 1994;35:457–465. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Seeman P. Schizophrenia and dopamine receptors. Eur Neuropsychopharmacol. 2013;23(9):999–1009. doi: 10.1016/j.euroneuro.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Sengpiel F, Stawinski P, Bonhoeffer T. Influence of experience on orientation maps in cat visual cortex. Nat Neurosci. 1999;2:727–732. doi: 10.1038/11192. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Ocular dominance in layer IV of the cat’s visual cortex and the effects of monocular deprivation. J Physiol. 1978;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterr A, Muller MM, Elbert T, Rockstroh B, Pantev C, Taub E. Changed perceptions in Braille readers. Nature. 1998;391:134–135. doi: 10.1038/34322. [DOI] [PubMed] [Google Scholar]
- Sun YJ, Wu GK, Liu BH, Li P, Zhou M, Xiao Z, Tao HW, Zhang LI. Fine-tuning of pre-balanced excitation and inhibition during auditory cortical development. Nature. 2010;465:927–931. doi: 10.1038/nature09079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymusiak R, Alam N, McGinty D. Discharge patterns of neurons in cholinergic regions of the basal forebrain during waking and sleep. Behav Brain Res. 2000;115:171–182. doi: 10.1016/s0166-4328(00)00257-6. [DOI] [PubMed] [Google Scholar]
- Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, Hirase H. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci. 2011;31:18155–18165. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng GF, Haberly LB. Deep neurons in piriform cortex. I Morphology and synaptically evoked responses including a unique high-amplitude paired shock facilitation. J Neurophysiol. 1989a;62:369–385. doi: 10.1152/jn.1989.62.2.369. [DOI] [PubMed] [Google Scholar]
- Tseng GF, Haberly LB. Deep neurons in piriform cortex. II Membrane properties that underlie unusual synaptic responses. J Neurophysiol. 1989b;62:386–400. doi: 10.1152/jn.1989.62.2.386. [DOI] [PubMed] [Google Scholar]
- Vale-Martinez A, Baxter MG, Eichenbaum H. Selective lesions of basal forebrain cholinergic neurons produce anterograde and retrograde deficits in a social transmission of food preference task in rats. Eur J Neurosci. 2002;16:983–998. doi: 10.1046/j.1460-9568.2002.02153.x. [DOI] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- Waselus M, Galvez JP, Valentino RJ, Van Bockstaele EJ. Differential projections of dorsal raphe nucleus neurons to the lateral septum and striatum. J Chem Neuroanat. 2006;31:233–242. doi: 10.1016/j.jchemneu.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J Neurophysiol. 1963a;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963b;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Scopolamine enhances generalization between odor representations in rat olfactory cortex. Learn Mem. 2001;8:279–285. doi: 10.1101/lm.42601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somato-sensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005;25:5488–5501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Feng J, Yan Z. Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of n-methyl-D-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J Biol Chem. 2008;283:17194–17204. doi: 10.1074/jbc.M801713200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L. The modular organization of brain systems. Basal forebrain: the last frontier. Prog Brain Res. 2002;136:359–372. doi: 10.1016/s0079-6123(02)36030-8. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I. The basal forebrain corticopetal system revisited. Ann N Y Acad Sci. 1999;877:339–367. doi: 10.1111/j.1749-6632.1999.tb09276.x. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Buhl DL, Pobalashingham S, Bjaalie JG, Nadasdy Z. Three-dimensional chemoarchitecture of the basal forebrain: spatially specific association of cholinergic and calcium binding protein-containing neurons. Neuroscience. 2005;136:697–713. doi: 10.1016/j.neuroscience.2005.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001;4:1123–1130. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
- Znamenskiy P, Zador AM. Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature. 2013;497:482–485. doi: 10.1038/nature12077. [DOI] [PMC free article] [PubMed] [Google Scholar]