Abstract
Hereditary Multiple Exostoses (HME) is an autosomal-dominant disorder characterized by benign cartilage tumors (exostoses) forming near the growth plates, leading to severe health problems. EXT1 and EXT2 are the two genes known to harbor heterozygous loss-of-function mutations that account for the vast majority of the primary genetic component of HME. However, patients present with wide clinical heterogeneity, suggesting that modifier genes play a role in determining severity. Our previous work has pointed to an imbalance of β-catenin signaling being involved in the pathogenesis of osteochondroma formation. TCF7L2 is one of the key ‘gate-keeper’ TCF family members for Wnt/β-catenin signaling pathway, and TCF7L2 and EXT2 are among the earliest associated loci reported in genome wide appraisals of type 2 diabetes (T2D). Thus we investigated if the key T allele of single nucleotide polymorphism (SNP) rs7903146 within the TCF7L2 locus, which is strongly over-represented among T2D cases, was also associated with HME. We leveraged genotype data available from ongoing GWAS efforts from genomics and orthopaedic centers in the US, Canada and Italy. Collectively 213 cases and 1,890 controls were analyzed and, surprisingly, the T allele was in fact significantly under-represented in the HME patient group [P=0.009; odds ratio=0.737 (95% C.I. 0.587 - 0.926)]; in addition, the direction of effect was consistent within each individual cohort. Immunohistochemical analyses revealed that TCF7L2 is differentially expressed and distributed in normal human growth plate zones, and exhibits substantial variability in human exostoses in terms of staining intensity and distribution. In summary, the data indicate that there is a putative genetic connection between TCF7L2 and EXT in the context of HME. Given this observation, we suggest that these loci could possibly modulate shared pathways, in particular with respect to β-catenin, and their respective variants interplay to influence HME pathogenesis as well as T2D.
Keywords: Transcription factor 7 like 2 (TCF7L2), Hereditary Multiple Exostoses (HME), osteochondroma, Exostosin (EXT), type 2 diabetes (T2D)
Introduction
Several genome wide association studies (GWAS) have been conducted on type 2 diabetes (T2D) and revealed TCF7L2 (transcription factor 7–like 2) - a member of the TCF family, a group of transcription factors involved in Wnt/β-catenin pathway - and EXT2 (Exostosin-2) –an already established causative gene of Hereditary Multiple Exostoses (HME) - as both being among the strongest loci involved in the disease, showing up as early as the very first such report in 2007 [1].
HME is an autosomal-dominant disorder, characterized by the presence of benign exostoses (known also as osteochondromas) that are cartilage-capped outgrowths forming next to the growth plates of long bones and other skeletal elements [2, 3]. The exostoses can interfere with growth plate function and skeletal development, and children presenting with HME typically display growth retardation and skeletal deformities, potentially having additional clinical problems. In about 2 to 5% of the patients, the exostoses progress to malignant chondrosarcomas and become life threatening [4]. The vast majority (∼80-90%) of HME patients carry heterozygous loss-of-function mutations in the genes encoding Exostosin-1 (EXT1) or EXT2 [5, 6]. EXT1 and EXT2 are Golgi-associated glycosyltranferases enzymes, responsible for heparan sulfate (HS) synthesis, and their inactivation results in HS deficiency throughout the body tissues [7, 8]. So far, over 650 unique mutations have been described in the two causative genes, most of which are nonsense, frame shift, or splice-site mutations, distributed across the EXT1 and EXT2 genes [9]. HME is characterized by a wide clinical heterogeneity, both within the same family and among unrelated patients bearing identical mutations. Thus there is still need to explain both the remaining primary genetic component to the trait and to characterize the genetic influences that drive the large range in severity seen among patients. The presence of modulating genes that contribute to the broad clinical spectrum of HME phenotype, that directly influence EXT gene regulation and HS functions or act via other biological pathways, has been speculated for some time.
We have previously shown that lower quantities of β-catenin are present in the cartilage caps of osteochondroma specimens and that β-catenin deficient mouse model has some features in common with the HME mouse model, including exostoses formation [10]. Interactions between the Wnt signaling pathway and heparan sulfate proteoglycans had been previously described in Drosophila and vertebrates, and previous studies have indicated that heparan sulfate proteoglycans stabilize Wnt proteins and modulate Wnt signaling activities either negatively or positively [11]. Taking our previous findings together, our evidence suggests a possible role of Wnt signaling in HME etiology. Activation of canonical Wnt signaling pathway results in β-catenin translocation into the nucleus, where it forms a transcriptional complex with a member of the TCF family [12].
Given the results from T2D GWAS plus the Wnt signaling pathway observations, we performed association analyses on the TCF7L2 genotype data available from genomics and orthopedic centers in the US, Canada and Italy. In addition, we carried out immunohistochemical analysis of TCF7L2 expression in the exostoses and growth plate.
Materials and Methods
Research subjects for genetic analysis
Philadelphia
All subjects were consecutively recruited from the Greater Philadelphia area from 2006 to 2012 at the Children's Hospital of Philadelphia (CHOP). Our study cohort consisted of 52 pediatric HME cases of European ancestry and 1,707 population based controls derived from the same collection. The study was approved by the Institutional Review Board of CHOP. Parental informed consent was given for each study participant for both the blood collection and subsequent genotyping.
Italy
Our study cohort consisted of 86 Italian HME cases, distributed all over Italian regions, recruited at the Day Clinic of the Genetic Department of Rizzoli Orthopaedic Institute between 2004 and 2011. The patients were compared against two groups of controls, one derived from the 1000 Genomes TSI (‘Tuscans from Italy’) sample set, and the other including subjects sampled from six villages in north-east of Italy and one in the south. The sampling was made randomly and on voluntary basis.
Houston
Our study cohort consisted of 75 HME cases of European ancestry, which were compared against controls derived from the 1000 Genomes CEU (‘Northern Europeans from Utah’) sample set. The subjects were recruited and were ascertained from orthopedic centers in the US and Canada. This study was approved by the University of Texas Committee for the Protection of Human Subjects.
Genotyping
Illumina Infinium™ assay
We derived the genotypes for rs7903146 leveraging the data derived from high throughput genome-wide SNP genotyping, using the Illumina Infinium™ II HumanHap550 or Human 610 BeadChip technology (Illumina, San Diego), at one of the three participating sites. Samples were drawn from genome wide datasets that yielded SNPs with call rates <95%, minor allele frequency <1%, missing rate per person <2% and Hardy-Weinberg equilibrium P < 10-5 in order to assess most accurately the role of rs7903146
Genetic Analysis
HME is a very rare disorder so this is the largest number of HME cases assembled with genotype data. Given that the TCF7L2 locus confers an odds ratio of approximately 1.4 in the context of T2D and has been reported in other cohorts of T2D of a similar size to what we have here, we know that we had over 90% power to observe an association if it existed.
All patients were clinically diagnosed according to previously described criteria [9]. Age of patients participating to the study ranged between 4 and 72 years, with a strong prevalence of pediatric subjects and age of onset; 55% of them were males and 45% were females. We selected controls that were geographically matched to represent population frequencies of the allele we were studying, without any inclusion or exclusion criteria for given traits. As such, this was the most conservative way to ascertain if there is an over or under representation of a variant in a given disease cohort against the general population. The two Italian control sets were representative of two very different settings. As pointed out above, one set was derived from the 1000 Genomes TSI (‘Tuscans from Italy’) project. The second and larger set derived from several Italian villages with a high degree of consanguinity [13]. Because of the latter, there was concern about using this set as a main control set; however, we do mention in a footnote to Table 1 that this set also supported our observations.
Table 1. Frequency of the T allele of rs7903146 within the TCF7L2 locus in HME cases versus population-based controls in three different populations.
| Allele Frequency | |||||
|---|---|---|---|---|---|
| Philadelphia | Cases (n=52) | Controls (n=1,707) | OR | 95% CI | P |
| 0.231 | 0.313 | 0.659 | 0.415-1.046 | 0.075 | |
| Italy | Cases (n=86) | Controls (n=98)a | OR | 95% CI | P |
| 0.302 | 0.398 | 0.656 | 0.425-1.011 | 0.056 | |
| Houston | Cases (n=75) | Controls (n=85)b | OR | 95% CI | P |
| 0.220 | 0.329 | 0.574 | 0.348-0.948 | 0.029 | |
| Cases (n=213) | Controls (n=1890) | OR | 95% CI | P | |
| COMBINED | 0.256 | 0.318 | 0.737 | 0.587-0.926 | 0.009 |
P-values were derived by chi-square test; OR – odds ratio; 95% CI – 95 percent confidence interval for the odds ratio.
Controls derived from the 1000 Genomes TSI (‘Tuscans from Italy’) sample set. When considering an additional control set derived from several Italian villages (see Methods for further description) from both north and south of the country (n=2,482; rs7903146 T allele frequency = 0.327), the direction of the effect was consistent (OR= 0.894) but no longer close to significance (P = 0.50)
Controls derived from 1000 Genomes CEU (‘Northern Europeans from Utah’) sample set
Association was assessed using chi-square to calculate two-sided P-values on allele count differences between cases and controls, either in the separate cohorts and/or when combined. Odds ratios and the corresponding 95% confidence intervals were also calculated for the association analyses.
Human tissues for immunohistochemistry
Paraffin sections of exostosis (n=14) surgically removed from consented Hereditary Multiple Exostosis (HME) patients as well as rib cartilages (n=5) from control patients removed during autopsy were provided from CHOP's Pathology Core. The slides we used in this study had been made for clinical diagnosis, but were no longer needed and completely de-identified before provided.
Immunohistochemistry
Paraffin sections were treated with 1 mM EDTA solution (pH 8.0) for 20 min at 95C after de-paraffinization, treated with protease K (20 μg/ml) for 10 min at 37C and incubated with anti-TCF7L2 or TCF7L1 polyclonal antibodies (LifeSpan BioSciences, Inc., Seattle, WA) or non-immune rabbit IgG (5μg/ml, Vector Laboratories, Burlingame, CA). Antibody binding was visualized using the NovaRED Peroxidase substrate kit (Vector Laboratories) according to the manufacture protocol and followed by counterstaining with methyl green. Staining results were observed by two independent researchers and evaluated as 0 for negative, 1 for marginal, 2 for positive and 3 for strong positive (more than 50% positive cells of the total) staining. Collagen 10 staining has been performed as previously reported [10].
Results
Association of rs7903146 within the TCF7L2 locus with HME
We compared the allele frequency of the minor T allele of rs7903146 within TCF7L2, which is already well established to be over-represented among T2D cases, between HME cases and population matched controls (Table 1). In all cohorts, we observed an under-representation of this allele among cases when compared to controls. In Philadelphia, an odds ratio (OR) of 0.659 was observed, yielding a borderline significant P-value of 0.075. Similarly in Italy, an OR of 0.656 was observed when comparing cases against the allele frequency for Tuscans in the 1000 Genomes database, yielding a borderline significant P-value of 0.056. More strikingly, in Houston an OR of 0.574 was observed when comparing cases against the allele frequency for Northern Europeans from Utah in the 1000 Genomes database, yielding a significant P-value of 0.029. Overall, when these datasets were combined, an OR of 0.659 was observed, yielding a significant P-value = 0.009.
Expression of TCF7L2 in exostoses and growth plate
Given that the T allele of rs7903146 within TCF7L2 was significantly under-represented so consistently across all three populations of HME patients, we queried how this locus might be relevant to the pathogenesis of exostosis formation.
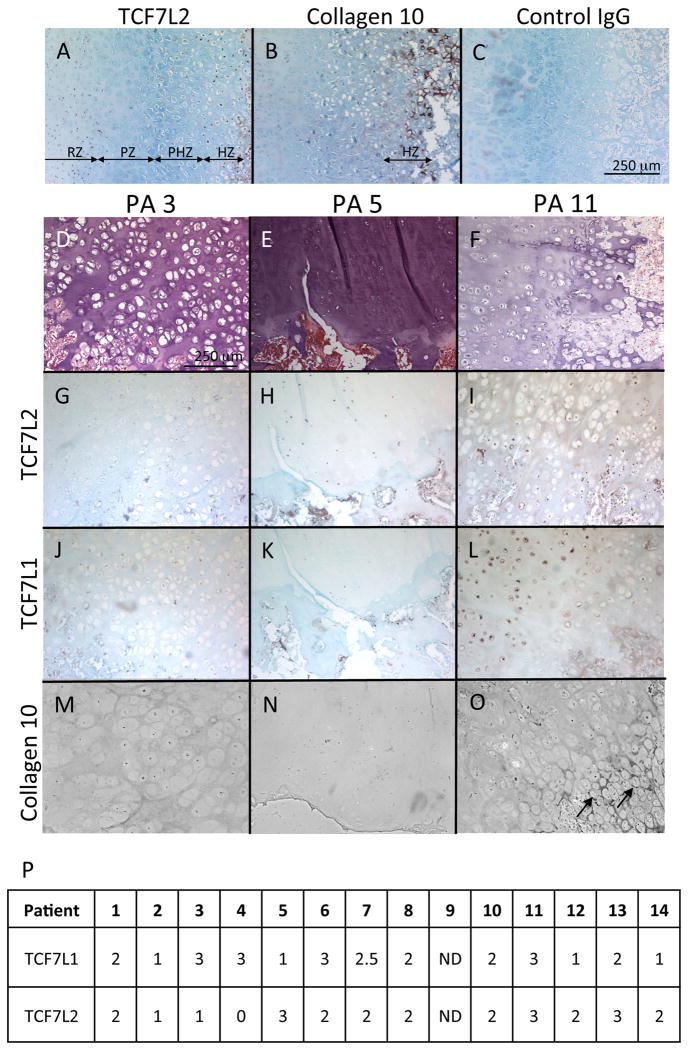
As an initial step to understanding the role of TCF7L2 in HME, we asked whether TCF7L2 is expressed in exostoses and if its expression is altered compared to control cartilage. Longitudinal sections of control ribs were prepared and stained with anti-TCF7L2 antibody that recognizes the N-terminal domain of TCF7L2. As shown in Fig. 1A, the TCF7L2 was detected in two zones of growth plate, the resting zone and hypertrophic zone where collagen 10 is expressed (Fig. 1B), but hardly found in the proliferating zone and prehypertrophic zone. The negative control antibodies did not yield any significant staining (Fig. 1C). Such restricted regulation of TCF7L2 expression indicates that TCF7L2 may play an important role as a component of Wnt/β-catenin signaling pathway in the growth plate, as previously reported [14].
Figure 1.
Immunolocalization of TCF7L2 in exostoses and rib growth plates.
A-O, Sections of rib growth plate obtained from autopsy (A-C) and exostoses obtained from HME patients (PA 1-14) (D-O) were subjected to immunochemical staining forTCF7L2 (A. G-I), collagen 10 (B, M-O), TCF7L1 (J-L) or control IgG (C). D-F are the images of hematoxylin and eosin staining corresponding to G-I, respectively. The bar represents 250 mm for A-O. Note that collagen 10 was only detected in the limited region (O, arrows). P, TCF7L1 and TCF7L2 staining results were scored as 1-3 (0,negative; 1, marginal; 2, positive; and 3, strong positive). ND, not determined.
Exostosis specimens were obtained from 14 patients at surgery and subjected to immunohistochemical analysis for TCF7L2. Most of the samples showed immunoreactivity with the anti-TCF7L2 antibody (Fig. 1P). However, there was a significant variation in the expression pattern and intensity of the staining among the specimens (Figs. 1G-1I). Although some exostosis cartilage tissues organize growth plate like structures, they did not exhibit the specific expression pattern of TCF7L2 observed in normal growth plate (Fig. 1A vs Figs. 1D-1F). To confirm that variation in TCF7L2 staining was not due to the quality of the specimens, we performed immunostaining for TCF7L1, another member of the LEF/TCF family. TCF7L1 expression also showed large variation between the exostosis samples independently from TCF7L2 staining (Figs 1J-1L, 1P). The non-immune rabbit IgG incubation did not show positive staining (data not shown). Collagen 10 was absent in most of cells in the exostosis, but limitedly detected in the region close to the boundary to the bone in some cases (Figs. 1M-1O). These findings indicate that TCF7L2 expression is deregulated in exostosis cartilage.
Discussion
This is the first report of a possible association between the established T2D-associated SNP, rs7903146, within the TCF7L2 locus and HME; furthermore, unlike T2D where the T allele is in excess among patients, this same variant is surprisingly under-represented among HME cases. Therefore, TCF7L2 may be a modulating gene that contributes to the broad clinical spectrum of HME phenotype and influences insufficiency of EXT genes and HS functions leading to alterations of molecular and cellular actions.
In addition to its obvious association with T2D, there is also a very strong connection between TCF7L2 and cancer. The key 8q24 locus found to be the most strongly associated genomic region with a number of cancers through GWAS contributes to the disease pathogenesis through mutation of an upstream TCF7L2-binding element driving the transcription of the MYC gene [15]. Indeed, it is has been known for some years that TCF7L2 (formerly TCF4) harbors specific mutations that strongly influence colorectal cancer risk [16]. Given this knowledge, one could have hypothesized a possible increase in the allele frequency of rs7903146 in HME, as this disease is characterized by multifocal ostechondroma formation; however, in light of the findings we report here, plus our previous studies indicating that HME is correlated with Wnt signaling [10], it is likely that there is a decrease in the activity of the Wnt/β-catenin signaling pathway in this disease context, with TCF7L2 being one of the key mediators in this process.
How variants in TCF7L2 influence risk in the context of T2D is unknown and the primary site(s) of action for the gene product in the context of the disease is still unclear; however, with the correlation with HME, perhaps more light can be shed on its precise role in disease pathogenesis. When first implicated in T2D, it was speculated that TCF7L2 influenced blood glucose homeostasis via alteration of levels of glucagon-like peptide 1 in the gut [17]. However, other studies have shown that the TCF7L2 variant is associated with increased TCF7L2 expression and decreased insulin secretion, possibly implicating the pancreatic β-cell, although adipose and liver have also been implicated [18].
According to the fact that this locus also appears to play a role in HME, one is also obligated to note that TCF7L2 is also expressed in developing embryonic cartilage [14] and in adult cartilage [19], plus exerts a role in regulation of endochondral ossification [14] and articular cartilage homeostasis [19]. Deregulation of TCF7L2 in exostosis could reflect a disorder of molecular signaling in the lesion, since TCF7L2 can be a stimulator and suppressor of the Wnt/β-catenin signaling pathway [12, 20] and a downstream molecule for further signaling [19]. Furthermore, this might be a result of irregular crosstalk in the affected lesion of the Wnt/β-catenin signaling with other signaling pathways, such as BMP and hedgehog [21].
It should be noted that all the HME cases analyzed in this study were derived from a pediatric cohort so these children are not in the age group that typically present with T2D. As such, we were not in a position to analyze any possible correlation between T2D and HME in this context.
In conclusion, the data in this study indicate that there is a genetic connection between TCF7L2 and related factors with HME. We therefore suggest that genetic variation in this context possibly modulates shared pathways, in particular with respect to b-catenin, leading to a potential interplay that influences HME pathogenesis as well as that of T2D.
Acknowledgments
We would like to thank the MHE Research Foundation for all their dedication and work on behalf of patients and families affected by MHE and Ms. L. Cantley for technical assistance. This publication was made possible by Grants AR058382, AR061758, and a Foerderer Award (FY2012) from the Children's Hospital of Philadelphia.
Footnotes
Disclosures: All authors state that they have no conflicts of interest on this study.
Author's roles: Study design, FS, MP, ME-I and SFAG; Study conduct, AG and SFAG; Data/sample collection, EP, JPB, JPD, TB, HH, JTH, LS and SFAG; Data analysis, FS, EP, PD, JPB, HH, JTH, LS, ME-I, and SFAG; Data interpretation, FS, MP, ME-I and SFAG; Drafting manuscript, FS, MP, ME-I and SFAG.
References
- 1.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–5. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 2.Schmale GA, Conrad EU, 3rd, Raskind WH. The natural history of hereditary multiple exostoses. J Bone Joint Surg Am. 1994;76:986–92. doi: 10.2106/00004623-199407000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Stieber JR, Dormans JP. Manifestations of hereditary multiple exostoses. J Am Acad Orthop Surg. 2005;13:110–20. doi: 10.5435/00124635-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Porter DE, Simpson AH. The neoplastic pathogenesis of solitary and multiple osteochondromas. J Pathol. 1999;188:119–25. doi: 10.1002/(SICI)1096-9896(199906)188:2<119::AID-PATH321>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 5.Ahn J, Ludecke HJ, Lindow S, Horton WA, Lee B, Wagner MJ, Horsthemke B, Wells DE. Cloning of the putative tumour suppressor gene for hereditary multiple exostoses (EXT1) Nat Genet. 1995;11:137–43. doi: 10.1038/ng1095-137. [DOI] [PubMed] [Google Scholar]
- 6.Stickens D, Zak BM, Rougier N, Esko JD, Werb Z. Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development. 2005;132:5055–68. doi: 10.1242/dev.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hecht JT, Hayes E, Haynes R, Cole WG, Long RJ, Farach-Carson MC, Carson DD. Differentiation-induced loss of heparan sulfate in human exostosis derived chondrocytes. Differentiation. 2005;73:212–21. doi: 10.1111/j.1432-0436.2005.00025.x. [DOI] [PubMed] [Google Scholar]
- 8.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–7. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 9.Pedrini E, Jennes I, Tremosini M, Milanesi A, Mordenti M, Parra A, Sgariglia F, Zuntini M, Campanacci L, Fabbri N, Pignotti E, Wuyts W, Sangiorgi L. Genotype-phenotype correlation study in 529 patients with multiple hereditary exostoses: identification of “protective” and “risk” factors. J Bone Joint Surg Am. 2011;93:2294–302. doi: 10.2106/JBJS.J.00949. [DOI] [PubMed] [Google Scholar]
- 10.Cantley L, Saunders C, Guttenberg M, Candela ME, Ohta Y, Yasuhara R, Kondo N, Sgariglia F, Asai S, Zhang X, Qin L, Hecht JT, Chen D, Yamamoto M, Toyosawa S, Dormans JP, Esko JD, Yamaguchi Y, Iwamoto M, Pacifici M, Enomoto-Iwamoto M. Loss of beta-catenin induces multifocal periosteal chondroma-like masses in mice. Am J Pathol. 2013;182:917–27. doi: 10.1016/j.ajpath.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–21. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- 12.Cadigan KM, Waterman ML. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esko T, Mezzavilla M, Nelis M, Borel C, Debniak T, Jakkula E, Julia A, Karachanak S, Khrunin A, Kisfali P, Krulisova V, Ausrele Kucinskiene Z, Rehnstrom K, Traglia M, Nikitina-Zake L, Zimprich F, Antonarakis SE, Estivill X, Glavac D, Gut I, Klovins J, Krawczak M, Kucinskas V, Lathrop M, Macek M, Marsal S, Meitinger T, Melegh B, Limborska S, Lubinski J, Paolotie A, Schreiber S, Toncheva D, Toniolo D, Wichmann HE, Zimprich A, Metspalu M, Gasparini P, Metspalu A, D'Adamo P. Genetic characterization of northeastern Italian population isolates in the context of broader European genetic diversity. Eur J Hum Genet. 2013;21:659–65. doi: 10.1038/ejhg.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikasa M, Rokutanda S, Komori H, Ito K, Tsang YS, Date Y, Yoshida CA, Komori T. Regulation of Tcf7 by Runx2 in chondrocyte maturation and proliferation. J Bone Miner Metab. 2011;29:291–9. doi: 10.1007/s00774-010-0222-z. [DOI] [PubMed] [Google Scholar]
- 15.Sur IK, Hallikas O, Vaharautio A, Yan J, Turunen M, Enge M, Taipale M, Karhu A, Aaltonen LA, Taipale J. Mice Lacking a Myc Enhancer That Includes Human SNP rs6983267 Are Resistant to Intestinal Tumors. Science. 2012 doi: 10.1126/science.1228606. [DOI] [PubMed] [Google Scholar]
- 16.Duval A, Rolland S, Tubacher E, Bui H, Thomas G, Hamelin R. The human T-cell transcription factor-4 gene: structure, extensive characterization of alternative splicings, and mutational analysis in colorectal cancer cell lines. Cancer Res. 2000;60:3872–9. [PubMed] [Google Scholar]
- 17.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–3. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 18.Hansson O, Zhou Y, Renstrom E, Osmark P. Molecular function of TCF7L2: Consequences of TCF7L2 splicing for molecular function and risk for type 2 diabetes. Curr Diab Rep. 2010;10:444–51. doi: 10.1007/s11892-010-0149-8. [DOI] [PubMed] [Google Scholar]
- 19.Ma B, Zhong L, van Blitterswijk CA, Post JN, Karperien M. T cell factor 4 is a pro-catabolic and apoptotic factor in human articular chondrocytes by potentiating nuclear factor kappaB signaling. J Biol Chem. 2013;288:17552–8. doi: 10.1074/jbc.M113.453985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoppler S, Kavanagh CL. Wnt signalling: variety at the core. J Cell Sci. 2007;120:385–93. doi: 10.1242/jcs.03363. [DOI] [PubMed] [Google Scholar]
- 21.Huegel J, Sgariglia F, Enomoto-Iwamoto M, Koyama E, Dormans JP, Pacifici M. Heparan sulfate in skeletal development, growth, and pathology: the case of hereditary multiple exostoses. Dev Dyn. 2013;242:1021–32. doi: 10.1002/dvdy.24010. [DOI] [PMC free article] [PubMed] [Google Scholar]