Abstract
There are few data on risks to biota and humans from mercury levels in saltwater fish. This paper examines mercury and selenium levels in muscle of 19 species of fish caught by recreational fisherfolk off the New Jersey shore, as a function of species of fish, size, and season, and risk of mercury to consumers. Average mercury levels ranged from 0.01 ppm (wet weight) (Menhaden Brevoortia tyrannus) to 1.83 ppm (Mako Shark Isurus oxyrinchus). There were four categories of mercury levels: very high (only Mako), high (averaging 0.3–0.5 ppm, 3 species), medium (0.14–0.20 ppm, 10 species), and low (below 0.13 ppm, 5 species). Average selenium levels for the fish species ranged from 0.18 ppm to 0.58 ppm, and had lower variability than mercury (coefficient of variation=38.3 vs 69.1%), consistent with homeostatic regulation of this essential element. The correlation between mercury and selenium was significantly positive for five and negative for two species. Mercury levels showed significant positive correlations with fish size for ten species. Size was the best predictor of mercury levels. Selenium showed no consistent relationship to fish length. Over half of the fish species had some individual fish with mercury levels over 0.3 ppm, and a third had fish with levels over 0.5 ppm, levels that pose a human health risk for high end consumers. Conversely several fish species had no individuals above 0.5 ppm, and few above 0.3 ppm, suggesting that people who eat fish frequently, can reduce their risk from mercury by selecting which species (and which size) to consume. Overall, with the exception of shark, Bluefin Tuna (Thunnus thynnus), Bluefish (Pomatomus saltatrix) and Striped Bass (Morone saxatilis), the species sampled are generally medium to low in mercury concentration. Selenium:mercury molar ratios were generally above 1:1, except for the Mako shark.
Keywords: Mercury, Selenium, Molar ratio, Fish consumption, Risk balancing
1. Introduction
For many coastal states and countries, saltwater fishing is an important commercial, recreational and subsistence activity. High fishing rates (days per year) occur in a wide range of cultures, including in rural and urban areas (Burger et al., 1999, 2001a, b; Bienenfeld et al., 2003), among Native Americans (Burger et al., 2007; Harper and Harris, 2008), and in other regions of the world (Burger et al., 2003). Fish provide fishmeal for human and aquaculture use (Brunner et al., 2009), and recreational, cultural and aesthetic pleasures (Toth and Brown, 1997; Burger, 2000, 2002). They also contain protein and valuable nutrients including polyunsaturated fatty acids and selenium.
However, levels of methylmercury (MeHg) and other contaminants in some fish are high enough to potentially cause effects on the fish themselves, on top-level predators, and on people (WHO, 1989; EPA, 1997; NRC, 2000; Consumer Reports, 2003). Consumption of mercury-contaminated fish came to attention after the outbreaks at Minamata and Niigata, Japan in the 1950s and 1960s (Harada, 1995). Fish consumption is the only significant source of methylmercury exposure for the public today (Rice et al., 2000), although historic epidemics attributed to grain seed treated with organomercurial fungicides occurred, most notably in Iraq in 1973 (Amin-zaki et al., 1978), and some mercury enters the food chain from mining (Qiu et al., 2009). Mercury occurs naturally in seawater, and coastal waters receive mercury runoff from land, input from rivers, and airborne deposition. Biomethylation of mercury occurs in sediment, allowing for food chain biomagnifications (Downs et al., 1998; Morel et al., 1998). Mercury in fish tissue may be six orders of magnitude higher than the mercury concentration in the water column (Scudder et al., 2009).
Levels of methylmercury are sufficiently high in some fish to cause adverse health effects in people consuming large quantities (Institute of Medicine, 1991, 2006; Grandjean et al., 1997; Gochfeld, 2003; Hightower and Moore, 2003; Hites et al., 2004), with neurodevelopmental effects from fetal exposure the most prominent effect (Amin-zaki et al., 1978; Crump et al., 1998; Steuerwald et al., 2000; NRC, 2000). Prenatal methylmercury has led to behavioral deficits in infants (JECFA, 2003) and to poorer cognitive test performance (Oken et al., 2008). Methylmercury can counteract the cardioprotective effects of fish consumption (Guallar et al., 2002; Rissanen et al., 2000; Salonen et al., 1995). Thus, communities that rely on fish intake for daily nutrient sustenance may be at risk from chronic, high exposure to methylmercury (Grandjean et al., 1997), as well as other persistent organic pollutants. Hughner et al. (2008) estimated that 250,000 women may be exposing their fetuses to levels of methylmercury above federal health guidelines. Similarly, high-end fish consumers, whether recreational or subsistence, are at risk from mercury exposure (Hightower and Moore, 2003; Lowenstein et al., 2010).
The U.S. Food and Drug Administration (USFDA, 2001) issued consumption advisories based on methylmercury that suggested that pregnant women and women of childbearing age who may become pregnant should limit their fish consumption, should avoid eating four types of marine fish (shark, swordfish, King Mackerel, and Tilefish), should also limit their consumption of all other fish to just 12 oz (=342 g) per week (USFDA, 2001), and there are recent warnings about canned white tuna (USFDA/USEPA, 2004a). These are all saltwater fish, while most studies of mercury levels have focused on freshwater fish (Legrand et al., 2005).
In freshwater fish, variations in water pH can account for up to 70% of the variation in mercury levels (Watras et al., 1998). Microbial methylation of mercury is favored by anaerobic conditions and low dissolved oxygen (DOC, Regnell, 1994). Much of the data dealing with the effects of fish size on mercury levels comes from freshwater fish (Simonin et al., 2008). Yet for many coastal states, consumption of saltwater fish is an important potential source of mercury exposure that has been largely ignored until recently. Fish are an important dietary item of the people living along coastal New Jersey, and recreational fishers often freeze fish for consumption at all times of the year (Pottern et al., 1989; Burger, 2005; Gobeille et al., 2005). It is therefore important to understand how to reduce the risk from mercury, and to provide the public with information on fish that are low in mercury (as well as high). Although Burger et al. (2009) examined mercury in flatfish that had relatively low levels, there is a need for a broader spectrum analysis of marine fish from one general geographical area.
Fish are an excellent, low-fat source of protein that contributes to low blood cholesterol, to positive pregnancy outcomes, and to better child cognitive test performances (Oken et al., 2008). Fish contain omega-3 (n-3) fatty acids that reduce cholesterol levels and the incidence of heart disease, stroke, and pre-term delivery (Daviglus et al., 2002; Patterson, 2002; Virtanen et al., 2008). Further, fish, particularly oceanic fish, are relatively rich in selenium, necessary for seleno-enzyme functions, and selenium has long been known to offer some protection against mercury toxicity. The public thus must choose whether to eat fish, what species to eat, as well as what size fish, what size portions, and how often. Sound choices require adequate information about a range of fish. There is some indication that the recent FDA warnings about fish consumption (USFDA, 2006), focusing on species high in mercury, have resulted in a reduction in the consumption of fish generally, and of canned fish specifically (Shimshack et al., 2007), while some authors argue that the advantages of fish consumption outweigh the mercury risk (Mozaffarian, 2009). Information on species low in mercury would be advantageous.
In this paper we examine levels of mercury in a wide range of fish species from coastal New Jersey to provide information that can be used to evaluate the potential risk to the fish themselves, to their predators, and to humans who consume them. Unlike many studies, we did not focus only on those species expected to have high levels (and thus pose the greatest risk), but examined levels in the wide range of fish caught by local recreational fishermen. Too often levels of mercury are provided for fish that people should avoid, without providing information on species that are low in mercury (and thus provide little risk). Risk balancing by the public is possible when mercury levels are available for a range of fish. The fishers requested this information after media coverage of mercury in fish, and worked with us on providing the fish samples.
Levels of selenium were analyzed because selenium offers some protection against mercury exposure (Satoh et al., 1985; Ralston, 2009; Lémire et al., 2010), lower levels of nonfatal heart attacks have been associated with higher levels of selenium (Mozaffarian, 2009), and some recent studies with animal models have suggested that some (if not most) of the adverse impacts of high methylmercury exposure occur as a result of mercury’s impairment of selenium-dependent enzyme activities (Watanabe et al., 1999a; Ralston, 2008, 2009; Ralston et al., 2008). Park and Mozaffarian (2010) reported evidence that although fish consumption substantially reduced cardiovascular risk, clinical trials demonstrated mixed and inconclusive results for cardiovascular effects of methylmercury and selenium. Ralston and others (Ralston, 2008; Peterson et al., 2009) have argued that selenium:mercury molar ratios above 1 are protective for adverse mercury affects. However, the interaction between selenium and mercury is complex and warrants continued examination. There are several issues that need further examination, but are not within the scope of this paper, including whether selenium merely chelates mercury keeping it from attacking disulfide bonds, whether mercury creates a relative selenium deficiency or inactivates essential seleno-proteins, and what other endogenous and exogenous factors influence the interaction. Ralston (2008, 2009) suggests that the molar ratio is the key value (rather than the level of methylmercury) for risk assessment.
2. Methods
Fish of 19 species were collected (2003–2008) from several sites along the New Jersey shore (Fig. 1; scientific names found in Table 1), mainly from recreational fisherfolk, who were either fishing individually or were taking part in fishing tournaments. Most of the actual sampling, however, was done by our personnel who went to local docks and fishing sites to meet fisherfolk. The 19 species are the fish most often caught by N.J. fishermen, and were selected because they are most relevant to recreational fishermen in the region. The project was a collaboration with local fishing clubs (Jersey Coast Anglers Association, Jersey Shore Shark Fishermen) and others, who greatly influenced the species collected. In many coastal regions there are a number of fishing tournaments that focus on Bluefish, Striped Bass, and Mako (all Shortfin Mako). Fish from tournaments were either taken home for consumption by the families of the fishermen, or were donated to orphanages or other facilities. We obtained either whole fish, or took an approximately 50 g sample plug biopsy from the side of the fish, over the lateral line just anterior to the tail. In addition, we obtained small individuals (below the recreational size limits) of some species (bluefish and striped bass) collected by the NJ Department of Environmental Protection trawls. Data on the entire size range are provided for comparison with other studies that concentrated on fish biology, rather than risk to fish consumers.
Fig. 1.
Map showing the locations of sampling for fish from New Jersey 2005–2008.
Table 1.
Regression models explaining variation in mercury levels in for each species from in New Jersey, 2005 and 2006. NS=not significant.
| Shortfin Mako | Atlantic Bluefin Tuna |
Striped Bass |
Bluefish | Tautog | Yellowfin Tuna |
Windowpane Flounder |
Dolphin (Mahi-mahi) |
Weakfish (Squeteague) |
Northern Kingfish |
Summer Flounder (fluke) |
Atlantic Croaker |
Scup (porgy) |
Winter Flounder | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | ||||||||||||||
| F | 2.1 | 1.9 | 12.5 | 36.4 | 6.6 | 8.6 | 15.8 | 7.1 | 6.2 | 3.7 | 4.3 | 14.7 | 0.2 | 1.1 |
| df | 4 | 4 | 8 | 9 | 6 | 4 | 6 | 4 | 7 | 6 | 7 | 5 | 4 | 4 |
| P | 0.09 | NS | <0.0001 | <0.0001 | 0 | 0 | <0.0001 | 0 | <0.0001 | 0.01 | 0 | <0.0001 | NS | NS |
| r2 | 0.16 | 0.31 | 0.43 | 0.67 | 0.56 | 0.79 | 0.7 | 0.58 | 0.53 | 0.34 | 0.11 | 0.7 | 0.05 | 0.07 |
| Factors entering F (p) | ||||||||||||||
| Season | a | b | 0.2 (NS) | 3.7 (0.03) | 7.9 (0.002) | c | 2.5 (NS) | b | 3.9 (0.03) | 2.9 (0.09) | 9.4 (0.002) | 0.4 (NS) | 0.0 (NS) | 0.3 (NS) |
| Location | 1.1 (NS) | 0.8 (NS) | 2.8 (0.06) | 2.1 (NS) | 0.1 (NS) | c | 0.1 (NS) | 0.4 (NS) | 7.9 (0.001) | 1.0 (NS) | 3.9 (0.02) | 0.0 (NS) | d | e |
| Length (cm) | 0.3 (NS) | 0.0 (NS) | 5.5 (0.02) | 0.2 (NS) | 0.3 (NS) | 16.4 (0.003) | 5.7 (0.02) | 3.3 (0.08) | 0.7 (NS) | 1.3 (NS) | 0.0 (NS) | 0.3 (NS) | 0.1 (NS) | 1.9 (NS) |
| Weight | 1.3 (NS) | 0.0 (NS) | 0.03 (NS) | 0.7 (NS) | 2.0 (NS) | 7.1 (0.03) | 0.2 (NS) | 1.0 (NS) | 0.3 (NS) | 0.4 (NS) | 9.4 (0.002) | 0.1 (NS) | 0.8 (NS) | 0.05 (NS) |
| Length×Weight | 1.3 (NS) | 0.0 (NS) | 0.0 (NS) | 7.1 (0.008) | 1.6 (NS) | 9.2 (0.01) | 0.0 (NS) | 0.6 (NS) | 2.0 (NS) | 0.7 (NS) | 9.2 (0.003) | 0.7 (NS) | 0.8 (NS) | 0.3 (NS) |
| Season×Location | a | b | 0.0 (NS) | 0.6 (NS) | 2.3 (0.07) | c | 0.4 (NS) | b | 4.9 (0.001) | 4.2 (0.005) | 7.8 (0.006) | 4.7 (0.002) | d | e |
All Shortfin Mako were collected in the spring.
All fish were collected in the summer.
91% of Yellowfin Tuna were collected in the fall from the South.
All Scup were collected from the North.
All Winter Flounder were from the North.
Fish or samples were kept in coolers and brought to the Environmental and Occupational Health Sciences Institute (EOHSI) of Rutgers University for element analysis. However, all samples were run with standard calibration curves by the same laboratory chemist to avoid any variations. All fish were analyzed individually for total mercury during the last two years of the study. At EOHSI, a 2 g (wet weight) sample of skinless fish muscle was digested in 4 ml of Fisher Scientific Trace metal grade nitric acid and 2 ml deionized water in a microwave (MD 2000 CEM), using a digestion protocol of three stages of 10 min each under 50, 100 and 150 lbs/in.2 (3.5, 7, and 10.6 kg/cm2) at 80% of total power. Digested samples were subsequently diluted to 25 ml with deionized water. The same digestion methods were used for both mercury and selenium. All laboratory equipment and containers were washed in 10% HNO3 solution and deionized water rinse prior to each use (Burger et al., 2001a).
Mercury was analyzed by the cold vapor technique using the Perkin Elmer FIMS-100 mercury analyzer, with an instrument detection level of 0.2 ng/g, and a matrix level of quantification of 0.002 μg/g. Selenium was analyzed by graphite furnace atomic absorption, with Zeeman correction. All concentrations are expressed in parts per million (ppm=μg/g) on a wet weight basis. Molar concentrations were obtained by dividing by the molecular weight (200.59 for Hg and 78.9 for Se). Since we analyzed total Hg we did not convert to MeHg (MW=215), although many studies have shown that almost all of the mercury in fish tissue is methylmercury, and 90% is a reasonable approximation of this proportion (Duffy et al., 1999), which does vary somewhat among fish types and laboratories, but not by age of the fish (Lansens et al., 1991). Similarly, Freije and Awadh (2009) reported that more than 90% of total mercury was methylmercury in marine fish. Recently, Scudder et al. (2009) suggested that about 95% of mercury present in fish is methylmercury, and that lower levels may have been biased by analytical and homogeneity variability (Bloom, 1991).
A DORM-2 Certified dogfish tissue was used as the calibration verification standard for mercury, and standard reference material (SRM) 1640, “Trace Elements in Natural Water” from the National Institute of Standards and Technology (NIST) was used for Zeeman graphite furnace atomic absorption spectroscopy (selenium) quality control evaluation. Recoveries between 90 and 110% were accepted to validate the calibration for both selenium and mercury. All specimens were run in batches that included blanks, a standard calibration curve, 2 spiked specimens, and one duplicate. The accepted recoveries for spikes ranged from 85% to 115%; 10% of samples were digested twice and analyzed as blind replicates (with agreement within 15%). For further quality control on mercury, our laboratory ran a random subset of samples in the Quebec Laboratory of Public Health; the correlation between the two laboratories was over 0.90 (P<0.0001, see Burger and Gochfeld, 2004). All results are reported as parts per million (ppm=μg/g) on wet weight basis.
Multiple regression procedures were used to determine if length or weight, location, or season contributed to explaining the variations in amount of mercury or selenium in samples (PROC GLM, SAS, 1995). Since location did not usually enter the models, it is not discussed further. The procedure adds the variable that contributes the most to the R2, then adds the next variable that increases the R2 the most, continuing until all significant variables are added. Thus variables that vary co-linearly are entered only if they add independently to explaining the variation. We used Kruskal Wallis non-parametric One-way analysis of variance (generating a χ2 statistic) to examine differences among tissues and size measurements. We also used ANOVA with Duncan Multiple Range test on log-transformed data to identify the significant differences (SAS, 1995). Kendall correlations were used to examine relationships among metals and size variables. The level for significance was designated as P<0.05. Fish species with samples below 5 individuals are shown in a footnote to Table 2, even though they are clearly not statistically valid, because they represent species that anglers caught and requested that they be tested. Correlations, and other analyses, were not conducted for species with small sample sizes. We also length normalized results for each fish, by dividing the concentration (μg/g) by length in meters, to adjust for age accumulation of mercury. This was done to minimize the effect of age and growth rate on evaluation of any relationship to environmental characteristics; mercury concentrations in fish tend to increase with fish age, and length is commonly used as a surrogate for age (Scudder et al., 2009).
Table 2.
Total mercury and selenium levels (ppm, wet weight) (μg/g) in fish species collected from New Jerseya. Given are arithmetic means±SE, standard deviation, coefficient of variation, and Kendall Tau correlation coefficients.
| Common name | Scientific name | n | Mercury | SD | CVd | Hg correlation with length |
Selenium | SD | CVe | Se correlation with length |
Hg–Se correlation | Se:Hg molar ratioc |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||||
| Mean±SE | Tau | (p) | Mean±SE | Tau | (p) | Tau | (p) | ||||||||
| Shortfin Mako | Isurus oxyrinchus | 51 | 1.83±0.17 | 1.21 | 66 | 0.29 | (0.003) | 0.26±0.014 | 0.10 | 39 | −0.08 | (NS) | 0.16 | (0.1) | 0.36 |
| Atlantic Bluefin Tuna | Thunnus thynnus | 23 | 0.52±0.03 | 0.16 | 31 | 0.05 | (NS) | 0.43±0.038 | 0.18 | 43 | 0.05 | (NS) | −0.30 | (0.02) | 2.07 |
| Striped Bass | Morone saxatilis | 178 | 0.39±0.020 | 0.27 | 68 | 0.37 | (<0.0001) | 0.30±0.010 | 0.13 | 45 | 0.13 | (0.008) | 0.09 | (0.07) | 1.91 |
| Bluefish | Pomatomus saltatrix | 206 | 0.35±0.02 | 0.30 | 86 | 0.49 | (<0.0001) | 0.37±0.009 | 0.13 | 35 | 0.04 | (NS) | 0.10 | (0.003) | 2.65 |
| Tautog | Tautoga onitis | 47 | 0.20±0.02 | 0.10 | 52 | 0.16 | (NS) | 0.19±0.013 | 0.09 | 46 | 0.14 | (NS) | 0.07 | (NS) | 2.46 |
| Yellowfin Tuna | Thunnus albacares | 45b | 0.20±0.03 | 0.17 | 85 | 0.33 | (0.1) | 0.47±0.027 | 0.18 | 38 | −0.50 | (0.01) | −0.16 | (0.1) | 6.11 |
| Windowpane Flounder | Scophthalmus aquosus | 48 | 0.18±0.02 | 0.10 | 57 | 0.60 | (<0.0001) | 0.36±0.019 | 0.13 | 37 | −0.26 | (0.01) | −0.20 | (0.03) | 4.98 |
| Dolphin (Mahi-mahi) | Coryphaena hippurus | 27 | 0.17±0.04 | 0.20 | 115 | 0.50 | (0.0003) | 0.37±0.024 | 0.12 | 33 | 0.10 | (NS) | 0.20 | (NS) | 5.52 |
| Southern Kingfish | Menticirrhus americanus | 23 | 0.17±0.02 | 0.08 | 47 | 0.58 | (0.0002) | 0.22±0.021 | 0.10 | 46 | −0.24 | (NS) | −0.03 | (NS) | 3.21 |
| Black Seabass | Centropristis striata | 19 | 0.16±0.02 | 0.07 | 45 | 0.17 | (NS) | 0.20±0.018 | 0.08 | 39 | −0.2 | (NS) | −0.20 | (NS) | 3.13 |
| Weakfish (Squeteague) | Cynoscion regalis | 60 | 0.15±0.01 | 0.11 | 71 | 0.11 | (NS) | 0.23±0.015 | 0.12 | 51 | 0.09 | (NS) | 0.29 | (0.001) | 3.77 |
| Cunner | Tautogolabrus adspersus | 7 | 0.15±0.03 | 0.08 | 54 | 0.31 | (NS) | 0.22±0.028 | 0.07 | 33 | −0.4 | (NS) | 0.20 | (NS) | 3.71 |
| Northern Kingfish | Menticirrhus saxatilis | 72 | 0.15±0.02 | 0.18 | 117 | 0.47 | (<0.0001) | 0.28±0.013 | 0.11 | 39 | −0.10 | (NS) | −0.06 | (NS) | 4.73 |
| Summer Flounder (Fluke) | Paralichthys dentatus | 260 | 0.14±0.01 | 0.08 | 58 | 0.11 | (0.008) | 0.35±0.008 | 0.13 | 37 | 0.03 | (NS) | 0.09 | (0.04) | 6.38 |
| Atlantic Croaker | Micropogonias undulatus | 63 | 0.12±0.01 | 0.07 | 61 | 0.57 | (<0.0001) | 0.48±0.023 | 0.18 | 38 | 0.10 | (NS) | 0.08 | (NS) | 10.5 |
| Scup (Porgy) | Stenotomus chrysops | 27 | 0.09±0.02 | 0.08 | 86 | 0.05 | (NS) | 0.26±0.022 | 0.11 | 45 | −0.19 | (NS) | 0.47 | (0.0005) | 7.15 |
| Winter Flounder | Pseudopleuronectes americanus | 58 | 0.06±0 | 0.03 | 53 | 0.04 | (NS) | 0.25±0.014 | 0.11 | 43 | −0.11 | (NS) | 0.02 | (NS) | 10.9 |
| Ling | Molva molva | 39 | 0.04±0.01 | 0.04 | 101 | 0.49 | (<0.0001) | 0.18±0.015 | 0.09 | 51 | 0.07 | (NS) | 0.30 | (0.01) | 12.6 |
| Atlantic Menhaden | Brevoortia tyrannus | 5 | 0.01±0.01 | 0.01 | 112 | 0.03 | (NS) | 0.24±0.03 | 0.07 | 29 | 0.10 | (NS) | 0.40 | (NS) | 61.0 |
The following species with low sample sizes had the following mercury and selenium values (ppm wet weight, means are given where n>1): Albacore (Thunnus alalunga) n=1, Hg=0.50, Se=0.53; Thresher (Alopias vulpinus) n=1 Hg=0.41, Se=0.26; Wahoo (Acanthocybium solandri) n=2 Hg=0.34, Se=0.58; Atlantic Spanish Mackerel (Scomberomorus maculatus) n=1 Hg=0.14, Se=0.39; Ocean Triggerfish (Canthidermis sufflamen) n=2 Hg=0.09, Se=0.14; Atlantic Bonito (Sarda sarda) n=3 Hg=0.05, Se=0.36.
Length was available for only 14 Yellowfin Tuna.
The Se/Hg molar ratios are calculated on unrounded mean Hg and Se values.
Average coefficient of variation across species for mercury (SD 100/mean) is 69.1.
Average coefficient of variation across species for selenium is 38.3 consistent with homeostatic regulation.
NOTE: The Se/Hg molar ratios are negatively correlated with mean mercury concentration=−0.75 (<0.0001) and not significantly correlated with Se concentration=0.06 (0.7). Kendall Tau correlation coefficients.
3. Results
3.1. Effects of different variables on mercury levels
We constructed models to examine the relative effect different variables had on explaining the intraspecific variation in mercury levels. For the significant models, the best models explained from 11 to 79% of the variability in mercury levels (Table 1). For all of the models, length (either alone or as an interaction variable with weight) explained a significant proportion of the variability. Tautog was the only species for which season (and not a measure of length or weight) was the variable that contributed the most to the variation in mercury levels, and Summer Flounder was the only species where weight alone entered as a significant factor. These data indicate that size generally contributes to explaining the variation in mercury levels in fish caught in New Jersey.
3.2. Mercury and selenium levels
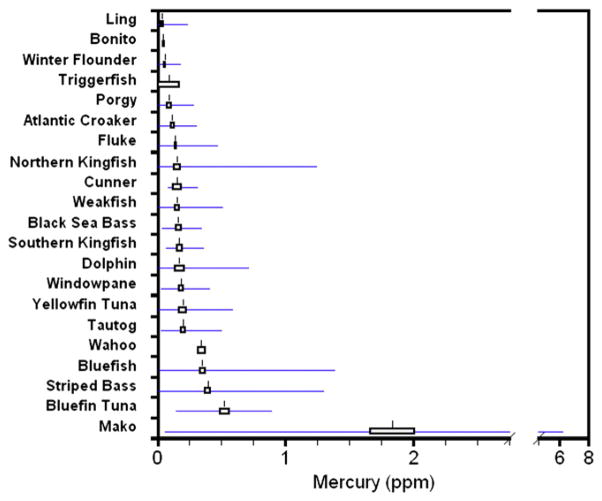
There was highly significant interspecific variation in mercury levels (coefficient of variation across species averaged 69.1), even for species with small sample sizes (Table 2, Fig. 2). Arithmetic mean mercury levels (total mercury) ranged from 0.01 ppm (menhaden) to 1.8 ppm (Mako). Based on mean mercury levels species fell into four categories: very high (1.8 ppm for Mako), high (0.3 ppm to 0.5 ppm, 3 species), medium (0.14 ppm to 0.20, 10 species), and low (0.01 ppm to 0.13 ppm, 5 species). The mostly commonly caught species (e.g. species with sample sizes over 150 fish) also ranged from relatively low (0.14 ppm for Summer Flounder), to medium (0.35 ppm and 0.39 ppm for bluefish and striped bass). Mercury levels were significantly positively correlated with fish length for half of the species, but the correlations were not high for most.
Fig. 2.
Mean (standard error, range) mercury levels (ppm, wet weight) for fish collected from coastal New Jersey 2003–2008.
Correlations may not be the best method of determining whether mercury levels increase with size of the fish, and we examined this parameter using size classes for each species (Table 3). For the 12 species where there were enough individuals in different size classes to analyze, there were significant differences in mercury levels as a function of size. These data are important because they allow future researchers, health professionals, and the public to evaluate the effect of both size and weight on mean mercury levels. Clearly, the biggest fish of each species has the highest mercury levels. Conversely, selenium did not show this correlation.
Table 3.
Arithmetic mean mercury and selenium levels by fish size (length was not obtained for all fish). For each species the Kruskal–Wallis chi square value tests the average level across size classes.
| Species | n | Mercury (ppm)
|
Selenium (ppm)
|
Weight (g)
|
|---|---|---|---|---|
| Mean±SE | Mean±SE | Mean±SE | ||
| Shortfin Mako | Size range 79 cm to 234 cm | |||
| <170 | 12 | 1.39±0.21 | 0.30±0.03 | 46,600±2810 |
| 170–185 | 13 | 1.37±0.20 | 0.23±0.03 | 60,600±1550 |
| 186–200 | 16 | 2.40±0.30 | 0.25±0.02 | 76,300±2000 |
| >200 | 9 | 2.17±0.58 | 0.27±0.03 | 100,000±4800 |
| X2(p) | 9.9 (0.02) | 2.9 (NS) | 43.2 (<0.0001) | |
| Striped Bass | Size range 12 cm to 125 cm | |||
| <70 | 46 | 0.28±0.04 | 0.30±0.03 | 2570±161 |
| 70–84 | 47 | 0.31±0.03 | 0.25±0.02 | 4780±209 |
| 85–100 | 43 | 0.42±0.03 | 0.31±0.02 | 8490±221 |
| >100 | 42 | 0.59±0.05 | 0.34±0.01 | 14,100±478 |
| X2(p) | 36.3 (<0.0001) | 13.5 (0.004) | 129 (<0.0001) | |
| Bluefish | Size range 11 cm to 86 cm | |||
| <30 | 43 | 0.15±0.02 | 0.34±0.02 | 121±18 |
| 30–45 | 63 | 0.25±0.01 | 0.39±0.02 | 464±29 |
| 46–60 | 39 | 0.26±0.02 | 0.34±0.02 | 1620±136 |
| >60 | 60 | 0.66±0.05 | 0.38±0.01 | 4080±137 |
| X2(p) | 79.3 (<0.0001) | 5.5 (NS) | 152 (<0.0001) | |
| Tautog | Size range 36 cm to 53 cm | |||
| <37 | 9 | 0.20±0.03 | 0.17±0.02 | 700±115 |
| 37–40 | 11 | 0.16±0.03 | 0.16±0.02 | 1080±46 |
| 41–44 | 12 | 0.17±0.01 | 0.19±0.03 | 1280±103 |
| >44 | 15 | 0.25±0.03 | 0.23±0.02 | 2160±142 |
| X2(p) | 4.8 (NS) | 4.3 (NS) | 28.6 (<0.0001) | |
| Windowpane Flounder | Size range 24 cm to 33 cm | |||
| <27 | 15 | 0.10±0.01 | 0.40±0.03 | 204±13 |
| 27–30 | 7 | 0.12±0.02 | 0.44±0.08 | 239±14 |
| >30 | 15 | 0.28±0.02 | 0.31±0.02 | 343±8 |
| X2(p) | 21.4 (<0.0001) | 5.3 (0.07) | 26.6 (<0.0001) | |
| Dolphin (Mahi-mahi) | Size range 52 cm to 104 cm | |||
| <60 | 7 | 0.04±0.01 | 0.33±0.04 | 1560±172 |
| 60–80 | 9 | 0.09±0.04 | 0.36±0.02 | 2660±260 |
| >80 | 10 | 0.35±0.06 | 0.38±0.04 | 5570±592 |
| X2(p) | 11.5 (0.003) | 0.65 (NS) | 20.0 (<0.0001) | |
| Weakfish (Squeteague) | Size range 30 cm to 81 cm | |||
| <36 | 12 | 0.17±0.03 | 0.19±0.04 | 345±23 |
| 36–40 | 12 | 0.14±0.03 | 0.28±0.02 | 476±26 |
| 41–50 | 10 | 0.16±0.01 | 0.28±0.03 | 791±77 |
| >50 | 15 | 0.20±0.04 | 0.21±0.03 | 2050±305 |
| X2(p) | 1.5 (NS) | 5.2 (NS) | 37 (<0.0001) | |
| Northern Kingfish | Size range 22 cm to 40 cm | |||
| <25 | 13 | 0.06±0.01 | 0.30±0.02 | 130±6 |
| 25–27 | 15 | 0.09±0.02 | 0.31±0.04 | 167±7 |
| 28–30 | 15 | 0.22±0.08 | 0.26±0.03 | 219±9 |
| >30 | 20 | 0.24±0.04 | 0.27±0.02 | 377±44 |
| X2(p) | 24.5 (<0.0001) | 3.7 (NS) | 58.6 (<0.0001) | |
| Summer Flounder | Size range 34 cm to 112 cm | |||
| <46 cm | 61 | 0.12±0.01 | 0.35±0.02 | 801±20 |
| 46–51 cm | 65 | 0.14±0.01 | 0.34±0.02 | 984±52 |
| 52–58 cm | 67 | 0.15±0.01 | 0.35±0.02 | 1450±66 |
| >58 cm | 62 | 0.16±0.01 | 0.36±0.02 | 2290±136 |
| X2(p) | 8.1 (0.04) | 0.6 (NS) | 122 (<0.0001) | |
| Atlantic Croaker | Size range 22 cm to 38 cm | |||
| <27 | 15 | 0.04±0.01 | 0.45±0.06 | 220±10 |
| 27–30 | 11 | 0.10±0.01 | 0.49±0.09 | 310±17 |
| 31–34 | 17 | 0.13±0.01 | 0.48±0.03 | 415±14 |
| >34 | 18 | 0.18±0.02 | 0.50±0.03 | 567±21 |
| X2(p) | 36.7 (<0.0001) | 3.7 (NS) | 33.2 (<0.0001) | |
| Winter Flounder | Size range 30 cm to 44 cm | |||
| <33 | 14 | 0.05±0.01 | 0.26±0.02 | 458±31 |
| 33–36 | 15 | 0.07±0.01 | 0.24±0.02 | 552±28 |
| 37–39 | 13 | 0.06±0.01 | 0.29±0.03 | 723±21 |
| >39 | 10 | 0.05±0.01 | 0.22±0.05 | 941±76 |
| X2(p) | 2.6 (NS) | 4.0 (NS) | 36 (<0.0001) | |
| Ling | Size range 16 cm to 61 cm | |||
| <18 | 7 | 0.03±0.00 | 0.24±0.02 | 40±2 |
| 18–21 | 12 | 0.02±0.00 | 0.12±0.03 | 64±3 |
| 22–32 | 9 | 0.04±0.00 | 0.21±0.02 | 115±5 |
| >32 | 10 | 0.07±0.02 | 0.22±0.02 | 478±164 |
| X2(p) | 19.4 (0.0002) | 10.7 (0.01) | 33.8 (<0.0001) | |
There was less intra- and interspecific variation in selenium levels (average coefficient of variation=38.3), consistent with some degree of homeostatic regulation (Table 2). Average selenium levels ranged from 0.18 ppm (Ling) to 0.48 ppm (Atlantic Croaker). Except for two species (Yellowfin Tuna and Windowpane), selenium levels were not correlated with size. When the relationship between length and selenium is examined by size class for 12 species (Table 3), there was a significant difference in selenium levels only for Striped Bass and Ling. These differences were not very great.
Selenium and mercury levels were significantly negatively correlated for Bluefin Tuna and Windowpane, and positively correlated for Striped Bass, Bluefish, Weakfish, Summer Flounder, Atlantic Croaker, and Ling. In all cases, the correlations were not high. Thus, if selenium moderates the toxic effects of mercury, these relationships suggest a lack of consistency both within and among species. Selenium:mercury molar ratios, however, were above 1:1, except for Mako (see Table 2).
3.3. Mercury levels with respect to various guidance levels
Another method of examining mercury levels is to compute the percentage of fish with levels above certain guidance levels (Table 4, see Discussion section). EPA has set 0.3 ppm of MeHg as its ambient water quality criterion. Some countries use 0.5 ppm as an action level, and the U.S. FDA uses 1.0 ppm as its action level. Only three species had no individuals above 0.3 ppm level. Eight species had some fish with levels well above 0.5 ppm, and only Mako Shark had many individuals above 1 ppm (Table 3).
Table 4.
Length and total mercury levels (μg/g wet weight) for all species analyzed from New Jersey anglers and trawls, 2005 through 2008. Given are arithmetic means±SE and percent above various regulatory or action levels.
| Species | Trophic levela | Total length (cm)
|
Mercury (ppm)
|
Max Hg | % above 0.30 ppm | % above 0.50 ppm | % above 1.0 ppm | |
|---|---|---|---|---|---|---|---|---|
| Mean±SE | Range | Mean±SE | ||||||
| Shortfin Mako | TP | 182±3.58 | 79–234 | 1.83±0.17 | 6.21 | 88% | 86% | 80% |
| Atlantic Bluefin Tuna | TP | 125±5.13 | 71–155 | 0.52±0.03 | 0.89 | 91% | 65% | |
| Striped Bass | TP | 83±1.55 | 12–124 | 0.39±0.02 | 1.30 | 51% | 30% | 3% |
| Bluefish | TP | 47±1.58 | 11–94 | 0.35±0.02 | 1.38 | 41% | 20% | 4% |
| Tautog | IP | 42±0.68 | 36–53 | 0.20±0.02 | 0.50 | 17% | ||
| Yellowfin Tuna | MP | 102±2.90 | 79–120 | 0.20±0.03 | 0.58 | 22% | 11% | |
| Windowpane Flounder | MP | 28±0.34 | 24–33 | 0.18±0.02 | 0.41 | 15% | ||
| Dolphin (Mahi-mahi) | MP | 73±3.21 | 52–104 | 0.17±0.04 | 0.71 | 30% | 7% | |
| Southern Kingfish | IP | 28±0.61 | 23–33 | 0.17±0.02 | 0.36 | 9% | ||
| Black Seabass | MP | 48±5.08 | 28–97 | 0.16±0.02 | 0.34 | 11% | ||
| Weakfish (Squeteague) | MP | 44±1.54 | 30–81 | 0.15±0.01 | 0.50 | 10% | 2% | |
| Cunner | MP | 7±0.41 | 6–10 | 0.15±0.03 | 0.31 | 14% | ||
| Northern Kingfish | IP | 28±0.42 | 22–40 | 0.15±0.02 | 1.24 | 8% | 4% | 1% |
| Summer Flounder (Fluke) | MP | 52±0.53 | 34–112 | 0.14±0.01 | 0.46 | 5% | ||
| Atlantic Croaker | MP | 31±0.54 | 22–38 | 0.12±0.01 | 0.31 | 2% | ||
| Scup (Porgy) | IP | 26±0.59 | 20–36 | 0.09±0.02 | 0.28 | |||
| Winter Flounder | IP | ± 0.45 | 30–44 | 0.06±0 | 0.17 | |||
| Ling | MP | 26±1.65 | 16–61 | 0.04±0.01 | 0.23 | |||
| Atlantic Menhaden | ZD | 14±0.8 | 12–15 | 0.01±0.01 | 0.01 | |||
Only 14 Yellowfin Tuna had length determinations.
TP=Top Predator (mainly fish); MP=Mixed Predator (fish & invertebrates); IP=Invertebrate Predator; ZD=Zooplankton & Detritus.
Trophic level codes are based on adult feeding. Younger smaller individuals feed at lower trophic levels than adults.
3.4. Mercury and selenium levels in different seasons
Because most fish were caught by anglers, and because of different migratory patterns, it was not possible to have equal sample sizes by season. There were significant seasonal differences in mercury levels for only 5 species (Table 5). These differences included: 1) Striped Bass levels were lower in the summer than the other two seasons, 2) Bluefish levels were over twice as high in the spring than at other times, 3) Yellowfin Tuna levels were almost twice as high in the summer as the fall, 4) Northern Kingfish levels were higher in the summer than fall, and 5) Ling levels were higher in the fall than the summer. While these conclusions are intriguing, they suggest a need for a more detailed examination of seasonal differences. There were very few seasonal differences for selenium (but sea bass and Summer Flounder, Table 6).
Table 5.
Arithmetic mean mercury levels by season with Kruskal–Wallis chi square values and p values for each species comparing among seasons.
| Species | Total mercury (ppm)
|
X2(p) | |||||
|---|---|---|---|---|---|---|---|
| n | Spring
|
n | Summer
|
n | Autumn
|
||
| Mean±SE | Mean±SE | Mean±SE | |||||
| Shortfin Mako | 52 | 1.83±0.17 | |||||
| Atlantic Bluefin Tuna | 23 | 0.52±0.03 | |||||
| Striped Bass | 51 | 0.40±0.03 | 25 | 0.23±0.03 | 102 | 0.43±0.03 | 12.7 (0.002) |
| Bluefish | 78 | 0.55±0.04 | 104 | 0.24±0.02 | 24 | 0.22±0.02 | 52.6 (<0.0001) |
| Tautog | 13 | 0.23±0.03 | 34 | 0.19±0.02 | 1.25 (NS) | ||
| Yellowfin Tuna | 12 | 0.31±0.06 | 35 | 0.16±0.03 | 7.5 (0.006) | ||
| Windowpane Flounder | 30 | 0.16±0.02 | 18 | 0.21±0.02 | 3.3 (0.07) | ||
| Dolphin (Mahi-mahi) | 26 | 0.18±0.04 | |||||
| Southern Kingfish | 23 | 0.17±0.02 | |||||
| Black Seabass | 5 | 0.13±0.03 | 13 | 0.17±0.02 | 1 | 0.20 | 0.8 (NS) |
| Weakfish (Squeteague) | 1 | 0.42 | 44 | 0.16±0.02 | 12 | 0.14±0.02 | 3.0 (NS) |
| Cunner | 6 | 0.13±0.02 | 1 | 0.31 | 2.3 (NS) | ||
| Northern Kingfish | 41 | 0.21±0.03 | 29 | 0.07±0.01 | 25.0 (<0.0001) | ||
| Summer Flounder (Fluke) | 124 | 0.13±0.01 | 134 | 0.14±0.01 | 1.2 (NS) | ||
| Atlantic Croaker | 35 | 0.11±0.01 | 28 | 0.13±0.02 | 1.1 (NS) | ||
| Scup (Porgy) | 4 | 0.13±0.03 | 23 | 0.09±0.02 | 1.05 (NS) | ||
| Winter Flounder | 30 | 0.05±0.01 | 28 | 0.06±0.01 | 0.14 (NS) | ||
| Ling | 28 | 0.03±0.00 | 11 | 0.07±0.02 | 16.4 (<0.0001) | ||
| Atlantic Menhaden | 4 | 0.12±0.01 | 1 | 0.01 | |||
Table 6.
Arithmetic mean selenium levels by season. Kruskal–Wallis chi square and p values for each species comparing across seasons.
| Species | Selenium (ppm)
|
X2(p) | |||||
|---|---|---|---|---|---|---|---|
| n | Spring
|
n | Summer
|
n | Autumn
|
||
| Mean±SE | Mean±SE | Mean±SE | |||||
| Shortfin Mako | 52 | 0.26±0.01 | |||||
| Atlantic Bluefin Tuna | 23 | 0.43±0.04 | |||||
| Striped Bass | 51 | 0.35±0.01 | 25 | 0.34±0.05 | 102 | 0.26±0.01 | 25.1 (<0.0001) |
| Bluefish | 78 | 0.37±0.01 | 104 | 0.37±0.01 | 24 | 0.35±0.02 | 1.4 (NS) |
| Tautog | 13 | 0.22±0.03 | 34 | 0.18±0.01 | 0.6 (NS) | ||
| Yellowfin Tuna | 12 | 0.48±0.06 | 35 | 0.47±0.03 | 0.08 (NS) | ||
| Windowpane Flounder | 30 | 0.38±0.03 | 18 | 0.32±0.02 | 3.8 (0.05) | ||
| Dolphin (Mahi-mahi) | 26 | 0.36±0.02 | |||||
| Southern Kingfish | 23 | 0.22±0.02 | |||||
| Black Seabass | 5 | 0.18±0.03 | 13 | 0.21±0.02 | 1 | 0.27 | 1.5 (NS) |
| Weakfish (Squeteague) | 1 | 0.37 | 44 | 0.24±0.02 | 12 | 0.18±0.02 | 4.1 (NS) |
| Cunner | 6 | 0.22±0.03 | 1 | 0.21 | 1.0 (NS) | ||
| Northern Kingfish | 41 | 0.26±0.02 | 29 | 0.31±0.02 | 2.6 (NS) | ||
| Summer Flounder (Fluke) | 124 | 0.32±0.01 | 134 | 0.38±0.01 | 11.9 (0.0006) | ||
| Atlantic Croaker | 35 | 0.54±0.04 | 28 | 0.42±0.02 | 5.7 (0.02) | ||
| Scup (Porgy) | 4 | 0.37±0.06 | 23 | 0.24±0.02 | 3.5 (0.06) | ||
| Winter Flounder | 30 | 0.25±0.02 | 28 | 0.25±0.02 | 0.03 (NS) | ||
| Ling | 28 | 0.16±0.02 | 11 | 0.23±0.03 | 3.8 (0.05) | ||
| Atlantic Menhaden | 4 | 0.23±0.04 | 1 | 0.27 | |||
4. Discussion
The present study has several advantages from the perspective of evaluating risk to both the fish themselves and to fish consumers: 1) most of the fish were caught by recreational anglers (and thus represent the fish that people eat), 2) mercury and selenium were determined in a wide range of fish, 3) mercury and selenium levels were determined from the same geographical areas over the same time period (2003–2008), and 4) mercury and selenium were each analyzed in the same laboratory, using the same methods (although the equipment used was different, the same equipment was used for each during the analysis). Many other studies examine only one or a very few fish species, and compare their results with other species from other regions (collected in other years, see Stern et al., 1996). The data indicate that mercury (and to a lesser extent, selenium), differed among different species, and these differences reflected differences in trophic level, size, season, and collection location.
4.1. Interspecific variations (trophic-level relationships)
There were interspecific differences in mercury levels in the 19 species of fish examined; mean values differed by 2 orders of magnitude. In general, species that were predators had significantly higher levels than did species that were primarily vegetarians. Fish species that are higher on the food chain generally accumulate higher levels of mercury because of bioamplification. Trophic level correlations have been reported for mercury (Wiener and Spry, 1996; Watras et al., 1998; Snodgrass et al., 2000; Burger et al., 2001b). Carnivorous species generally have higher levels than herbivores, omnivores, or planktivores, and larger predators have higher levels than smaller ones, although such differences are not always noted. Moreover, some bottom-dwelling fish can have higher levels than some predators, particularly if they ingest sediment. For example, Campbell (1994) found that a bottom dwelling Redear Sunfish (Lepomis microlophus) had higher mercury levels than bass or Bluegill Sunfish in Florida. Thus, trophic level alone is not enough to understand levels of mercury in fish.
In the present study, the top-level predator (Mako, average length of 182 cm in this study, see Table 4) had the highest level of mercury, followed by Bluefin Tuna (125 cm long), and then by Striped Bass (83 cm long) and Bluefish (47 cm long), all of which are predators. However, Yellowfin Tuna (average length of 102 cm) did not average high mercury levels (0.197 ppm), as might be expected of a top-level predator that eats primarily fish (Allain, 2005). Yellowfin, however, also eat squid and crustaceans (up to 38% of diet, Allain, 2005), while Bluefin Tuna eat mainly fish (Estrada et al., 2005). We suggest that more data are necessary for Yellowfin Tuna to understand how mercury levels vary (especially geographically). Further, at the other end of the spectrum, species with low levels of mercury were primarily vegetation or invertebrate foragers.
4.2. Mercury and selenium level, and fish size
In general, mercury levels increase with the size and age of the fish (Lange et al., 1994; Burger et al., 2001a; Pinho et al., 2002; Green and Knutzen, 2003; Simonin et al., 2008), although authors seldom examine this relationship in a range of fish. Further, it is not always the case (Stafford and Haines, 2001). At low mercury levels, the size relationships may not hold (Park and Curtis, 1997). Moreover, elimination rate is negatively correlated with size, suggesting another reason for larger fish to have significantly higher mercury levels (Trudel and Rasmussen, 1997).
Most of the studies that have examined the relationship between size and mercury levels have concentrated on freshwater fish, and few have dealt with large marine predatory fish. However, Storelli et al. (2002) reported that size and mercury levels were highly correlated for Swordfish (Xiphias gladius) and Bluefin Tuna (Thunnus thynnus) from the Mediterranean Sea, and Storelli et al. (2007) found that mercury levels increased with size for 7 species of marine fish from the Adriatic Sea. Although Yellowfin Tuna from Fiji (Thunnus albacares) showed a positive relationship between mercury and size (length and weight), Albacore Tuna (Thunnus alalunga) did not (Kumar et al., 2004). There was a positive correlation between size and mercury levels for 11 of 14 species of marine fish collected in the western Aleutians (Bering Sea/North Pacific) (Burger et al., 2007). Luten et al. (1987) found a positive correlation between size and mercury content in Atlantic Cod. Alexander et al. (1973) reported a correlation between weight and mercury concentrations for Bluefish and Striped Bass off Montauk Point on Long Island, and Burger (2009) also found mercury levels were correlated with size. Usually size is used as a surrogate for age (Boening, 2000), although Braune (1987) found that in known-aged Herring (Clupea harengus harengus), mercury level was more strongly correlated with age than with weight or length. In a study of Pacific Cod (Gadus macrocephalus), Burger and Gochfeld (2007) found that age was the variable that first entered the regression model explaining variation in mercury levels. Age was unknown for the fish in the present study.
In this study, we found that mercury levels were positively correlated with size for Mako, Striped Bass, and Bluefish, the species with the highest levels (and large sample sizes). Thus, larger fish generally had higher mercury levels. However, Bluefin Tuna showed no size relationship, indicating that the relationship of size and mercury levels, even for predators, is not always clear. Our finding, however, may partly relate to small sample sizes and a lack of great variation in the size of the tuna examined. In contrast, Storelli et al. (2002) did find a positive relationship in Bluefin Tuna from the Mediterranean.
For fish with lower mercury levels (average below 0.2 ppm), however, the relationships were less clear. For example, the correlation between mercury levels and size was high for Windowpane, Dolphin, Kingfish, Summer Flounder, Croaker, and Ling, but not for the other species. These differences were not due solely to sample size, since there were 60 Weakfish (with no correlation), and only 23 Southern Kingfish (correlation of 0.6). These data suggest that using size alone is not enough to predict low mercury levels for human populations at risk (even though for most species it is the best predictor).
Further, size (length, weight, or an interaction between the two) was the most significant factor contributing to variations in mercury levels within each species (where there were significant models), except for Weakfish and Tautog, indicating that size is generally the best indicator for the public.
Except for Striped Bass, Yellowfin Tuna, and Windowpane, there were no significant differences in selenium levels as a function of size. In bass, there was a low correlation (r=0.1) between selenium and fish size, but in the other two species the correlation was both stronger, and negative (−0.5 and −0.3 respectively). The decrease in selenium with an increase in mercury suggests that selenium would not completely moderate the effect of mercury in those fish with higher levels of mercury (Satoh et al., 1985). For most species there was no significant correlation between selenium and mercury. The correlation was high and significant (r=0.5) only in Porgy.
4.3. Mercury–selenium interaction
Ganther et al. (1972) proposed that selenium exerted some protective effect against mercury toxicity. The mechanisms contributing to a protective effect are unclear, but some of the adverse effects of mercury may be attributable to its effect on selenium-dependent enzymes, particularly interfering with anti-oxidant function (Pinheiro et al., 2009; Ralston, 2009). Thus excess selenium may chelate mercury and protect seleno-proteins, or conversely mercury may be viewed as creating a relative selenium deficiency. Mercury and methylmercury are irreversible selenoenzyme inhibitors (Watanabe et al., 1999a,b; Carvalho et al., 2008), and can indeed impair selenoprotein form and function. Ralston (2008) suggests that where the selenium:mercury molar ratio exceeds 1:1, there is adequate selenium to prevent mercury toxicity, based on rodent data. The molar ratio, however, is very sensitive to the denominator, and there was a significant inverse relationship between the ratio and the mercury concentration in the fish we studied. Some of the selenium in fish tissue may be involved directly in the seleno-proteins and is therefore available to be poisoned rather than to protect. Selenoprotein expression itself is complex with multiple levels of regulation (Rebsch et al., 2006).
The argument that selenium:mercury molar ratios greater than 1:1 are protective for mercury exposure (Kaneko and Ralston, 2007), and should therefore be incorporated in risk assessment and regulation regarding mercury and fish consumption in humans (Raymond and Ralston, 2009; Peterson et al., 2009), are intriguing, and should be examined further. It is unlikely that a single molar ratio would operate across different endpoints or effects (e.g. development, cognition, coordination, locomotion, and visual acuity) and species. Selenium levels should be presented in papers dealing with potential mercury toxicity (Peterson et al., 2009) to inform our understanding of this relationship. Further research is needed, particularly with respect to isolating, speciating and quantifying the selenium–mercury or sulphur–mercury complexes, effect of small sample sizes, and effect of low levels of mercury or selenium on the ratio.
4.4. Seasonal differences
One of the major limitations with the present study was that fish were collected by fisherfolk (an advantage in terms of relevance and importance for risk assessment), which resulted in unequal samples sizes for 1) different fish species, 2), different sized fish within a species, 3) unbalanced collections by season and location. Nonetheless, these differences reflect the fish that are actually caught by fisherfolk and are eaten by their families. Of the 19 species, we found seasonal differences in mercury levels for only five species. Even for these species there was no consistent pattern. For example, 1) Striped Bass mercury levels were lower in the summer than the other two seasons, 2) Bluefish levels were over twice as high in the spring than at other times, 3) Yellowfin Tuna levels were almost twice as high in the summer as the fall, 4) Northern Kingfish levels were higher in the summer than fall, and 5) Ling levels were higher in the fall than the summer. It is important to examine these relationships in these same species from elsewhere as they have implications for risk to people who consume them.
As might be expected, given the small coefficient of variation for selenium, there were few seasonal differences (Table 6), and those reaching statistical significance (P<0.05) were not great. Levels of selenium were significantly lower in the fall in Striped Bass, and were significantly lower in the spring for Summer Flounder. Some species, where sample sizes were large for all three seasons, showed no differences at all (e.g. Bluefish). The relatively low interspecies average coefficient of variation is consistent with the homeostatic regulation of selenium, an essential element.
4.5. Risk to the food chain
Methylmercury in fish poses a risk both to the fish themselves, and to their predators. Dietary uptake in fish probably accounts for more that 90% of the total uptake (Wiener et al., 2003), with an assimilation efficiency of methylmercury of 65–80% (Wiener and Spry, 1996). Species that are higher on the food web (e.g. predators), occupying higher trophic levels, have higher mercury levels through bioamplification. Mercury toxicity is affected by temperature, salinity, dissolved oxygen and water hardness (Boening, 2000). Methylmercury in fish accumulates in skeletal muscle, which is protective for the fish itself because the mercury exposure to the central nervous system is reduced (Wiener and Spry, 1996). In fish, methylmercury causes lack of coordination, diminished appetite, inability to feed, diminished responsiveness, lowered swimming activity, starvation, and mortality (Wiener et al., 2003).
Muscle levels of 5–20 ppm in fish are associated with apparent toxicity (Wiener et al., 2003), but few fish reach these levels (Yamashita et al., 2005). Differences in sensitivity relate to species of fish, bioavailability, and bioaccumulation rate (Niimi and Kissoon, 1994). These levels were determined for freshwater fish, and it is more likely that the toxicity levels would be higher for marine fish that have evolved with the naturally occurring mercury levels in seawater. Nonetheless, the mean levels in the species examined were all below 2 ppm, suggesting no effect on the fish themselves.
Mercury in fish is available to predators that eat fish, and it biomagnifies as it moves up the food chain (to humans or other top-level predators). The critical effects levels are 0.1 ppm for consumption by piscivorous mammals, and 0.02 for birds (Yeardley et al., 1998). The mean mercury levels in the fish from New Jersey (ranging as high as 0.52 ppm in muscle for all fish except Mako [1.83 ppm]) are clearly higher than the levels known to pose a problem for sensitive birds or mammals that scavenge them along the shore, or for sensitive marine mammals. Only four of the fish species examined had mean mercury levels below 0.1 ppm.
4.6. Risk to humans
Two sets of guidance values, tissue concentration and daily or weekly ingestion, are used for protection of human health from methylmercury in fish. Different countries and agencies have used different values. Most tissue concentration standards reference total mercury, because the added cost of speciating methylmercury is great.
The World Health Organization has set the maximum level of 0.5 ppm total mercury (Marrugo-Negrete et al., 2008). The United Kingdom and the European Union have established criteria for certain metals in fish (e.g. the level for mercury is 0.5 ppm in edible fish, with up to 1 ppm allowed for certain exempt predatory fish species). China has set standards for methylmercury in canned fish (ppm wet weight) of 0.5 ppm (except 1 ppm is allowed in shark, sailfish, tuna, pike and other high-mercury fish). The European Commission set a maximum limit for foodstuffs of 1 ppm (European Commission, 2008). The U.S. Food and Drug Administration (USFDA) has an action level for methylmercury in fish is 1.0 μg/g (ppm w/w), but this is a regulatory action level, rather than a risk level (USFDA, 2001). Originally the FDA had set 0.5 ppm as the action level, comparable to many other nations (reviewed in Burger and Gochfeld, 2004). In 1982 the European Commission set an Environmental Quality Standard of 0.3 ppm (w/w), and the US EPA (2001) promulgated 0.3 ppm as an ambient freshwater quality standard.
In the fish from New Jersey there was a wide range of mean mercury levels (refer back to Table 2), with Mako averaging 1.83 ppm, and 2 species averaging over 0.5 ppm (Bluefin Tuna and Albacore Tuna). However, another 4 species averaged above 0.3 ppm (Thresher, Striped Bass, Bluefish, and Wahoo). Thus, most of the fish in the study averaged mercury levels below 0.2 ppm, which suggests that sensitive human groups can avoid mercury exposure by carefully selecting the fish they eat. EPA (2008) also provides guidelines for the consumption of fish (4 meals/month of 8 oz (=227 g) each), suggesting that people eat only fish with values between below 0.23 ppm most fish in this study averaged within this range.
Guidance for mercury ingestion is expressed in micrograms per kilogram of body weight per day or week. The underlying assumption is that such values are protective for all individuals, for average daily consumption over a lifetime. Peak exposures are not considered. The EPA chronic oral reference dose (RfD) for methylmercury is 0.1 μg/kg/ day, based most recently on the Faroe Island studies (Grandjean et al., 1997). The U.S. Agency for Toxic Substances and Disease Research (ATSDR) has established a Minimal Risk Level of 0.3 μg/kg/day based on the Seychelles Islands study (Davidson et al., 1998). The Joint FAO/ WHO Expert Committee on Food Additives (JEFCA, 2003) established a permissible tolerable weekly intake of 1.6 μg/kg (=0.23 per day). These guidance values incorporate uncertainty factors, for protection of sensitive individuals, recognizing that many people may be able to exceed these intakes with impunity. Nonetheless they have important guidance value.
For many large fish, the species average concentration may not be the right measure for sensitive human groups (pregnant women, young children) because one large fish may be eaten for several consecutive meals, providing a series of high exposures. Ginsberg and Toal (2000) have suggested that there may be risk during pregnancy for even a single-meal exposure, particularly for fish with levels of over 2.0 ppm. We also reported the percent of fillets that were above 0.5 ppm because of the need to know the percent of times an exposure in a single meal may approach the tolerable daily intake (Berti et al., 1998). A 60 kg woman consuming an 8 oz (227 g) meal at 0.5 ppm, would consume 113 μg or 1.89 μg/kg, which is 19 times higher than the EPA’s reference dose of 0.1 μg/kg/day and 6 times higher than the ATSDR value of 0.3 μg/kg/day. Even if this were averaged out over a week, it would exceed the reference dose.
The risk from a pulsed exposure should also be examined, particularly its impact on a developing fetus at a critical developmental period. Further, this information should be provided to the public so that they can make informed decisions (see below). Providing information on risk from single-meal exposures, especially for pregnant women, is a risk communication challenge that should be considered by the FDA in addition to its existing guidance (USFDA/USEPA, 2004a). Egeland and Middaugh (1997) have called attention to the countervailing nutritional importance of fish, which increases the importance of identifying suitable local fish with low contaminant levels, especially during pregnancy. It is a matter of risk balancing (Gochfeld and Burger, 2005; NRC, 2000; IOM, 2006). In the present study, most of the fish species had some values above 0.3 ppm, and 9 species had some fish above 0.5 ppm. For some species, over 20% of single fish had mercury levels above 0.5 ppm, suggesting that a pregnant women, for example, eating that species of fish with mercury levels above 0.5 ppm occurs every fifth time she eats one of these species. With this information, pregnant women (and others) can make risk decisions based on the probability that a given fish will have mercury levels above a mercury level considered risky. Clearly more research and synthesis is required to understand the complex mechanisms of the interaction between selenium and mercury, and what bioindicators are best for assessing and managing the potential risk from mercury to fish consumers.
4.7. Risk management
The first management tool for reducing mercury in fish and shellfish is to reduce mercury in the environment (pollution prevention), which is often considered a long-term goal. In 2005, the EPA finalized the Clean Air Mercury Rule (CAMR, EPA, 2009). Although this was the first federal action to address mercury emissions directly, the rule allowed power plants to continue to emit too much mercury, the timeline for reduction was too long, and the CAMR permitted trading of mercury allowances between power plants (EPA, 2009). The Supreme Court invalidated this rule in 2009 (EPA, 2009). Thus legislation on mercury emissions is still needed in the U.S. Recent work in the Everglades of the United States (SFWMD, 2000–2005), and in Ontario, Canada (Harris et al., 2007), shows that mercury levels, particularly in freshwater fish, reflect “new” atmospheric deposition to surface waters. However, Kraepiel et al. (2003) found little change in mercury levels in tuna from Hawaii from 1971 to 1998. Presumably, management and regulation of mercury levels are changing with this recognition. It is usually the case that public political concern results in regulatory action, while concern raised only by scientists is often ignored (Lougheed, 2009).
Recent information suggests that the average American consumes about 13 g of fish and shellfish per day (or about a single three ounce meal per week, EPA, 2009). Such averages, mixing non-fish eaters with fish-eaters, obscure the many high-end fish consumption subgroups and populations. Providing people who fish and consume self-caught fish with information on mercury levels (and also on beneficial nutrients) is thus of public health importance. In this paper we provided information on mercury and selenium levels as a function of fish species, size, and season. Catching fish for tournaments is a big sport in New Jersey and elsewhere along the east coast, which results in people bringing back the largest fish they catch. Since people often bring back more fish to eat than “the big one”, a public health campaign aimed at providing information on the effect of size might be effective. This is complicated by the fact that some fish (e.g. Bluefish, Striped Bass, and other predatory fish) often travel in schools of the same age or size, and fisherfolk who come upon such a school might well catch only large or medium-sized fish. Thus, it would be possible for a pregnant woman to have several meals in a row from large-sized fish at the high end of mercury exposure. The desire to continue catching (and keeping) large fish needs to be overcome to reduce consumption of large-sized fish. Another consequence of high fish consumption by humans acts through downsizing of fish sizes due to selective harvesting of large fish (Conover et al., 2009). These conservation implications need further consideration.
In conclusion, fish consumption is a matter of risk balancing (Egeland and Middaugh, 1997; Ponce et al., 2000; Gochfeld and Burger, 2005). There are clearly both benefits and risks from fish consumption, and the public should be provided with as much information as possible to allow them to maximize the positive health benefits, while minimizing the risks from contaminants (IOM, 2006). The availability of information on how mercury varies in different fish species (as well as by size, season, and fishing location) can be valuable, especially for pregnant women and others at high risk, in reducing their mercury exposure while still consuming fish. To be effective, development of risk communication tools should involve scientists, health professionals, regulators, and the general public (Jardine et al., 2003; Knuth et al., 2003; Burger et al., 2005). But such communications must be informed by data on how mercury varies among and within each species of fish. The public can easily make use of such information, but it needs to be provided in a manner that is useful. Such information should include data on different contaminant levels (mercury and PCBs), as well as on nutrition and omega-3s.
Acknowledgments
This research was partly supported by the Jersey Coast Angler’s Association (JCAA), the Jersey Coast Shark Anglers Association (JCSA), NIEHS Center Grant (P30ES005022), the Consortium for Risk Evaluation with Stakeholder Participation (Department of Energy, # DE-FC01-06EW07053), the Wildlife Trust, and EOHSI. This research was conducted under a Rutgers University protocol, and fish samples were obtained from recreational anglers and NJ DEP trawls. We particularly thank T. Fote for advice and support throughout the study, C. Jeitner and M. Donio for field and laboratory assistance, and the many anglers in New Jersey who allowed us to collect samples from their fish, or who collected the samples for us. P. Copeland, M. Lémire, D. Mergler, N. Ralston, A. Stern and H. Zarbl provided valuable discussion on selenium and the selenium–mercury interactions. The views and conclusions expressed in this paper are solely those of the authors, and do not reflect the funding agencies.
References
- Alexander JE, Foehrenbach, Fisher S, Sullivan D. Mercury in striped bass and bluefish. NY Fish Game J. 1973;20:147–51. [Google Scholar]
- Allain V. What do tuna eat? A tuna diet study. SPC Fish Newsl. 2005;12:20–2. [Google Scholar]
- Amin-zaki L, Najeed MS, Clarkson TW, Greenwood MR. Methylmercury poisoning in Iraqi children: clinical observations over two years. Br Med J. 1978;1:163–616. doi: 10.1136/bmj.1.6113.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti PR, Receveur O, Chan HM, Kuhnlein HV. Dietary exposure to chemical contaminants from traditional food among adult Dene/Metis in the Western Northwest Territories, Canada. Environ Res. 1998;76:131–42. doi: 10.1006/enrs.1997.3797. [DOI] [PubMed] [Google Scholar]
- Bienenfeld LS, Golden AL, Garland EJ. Consumption of fish from polluted waters by WIC participants in East Harlem. J Urban Health. 2003;80:349–58. doi: 10.1093/jurban/jtg036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom NS. On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci. 1991;49:1010–7. [Google Scholar]
- Boening DW. Ecological effects, transport, and fate of mercury: a general review. Chemosphere. 2000;40:1335–51. doi: 10.1016/s0045-6535(99)00283-0. [DOI] [PubMed] [Google Scholar]
- Braune BM. Mercury accumulation in relation to size and age of Atlantic Herring (Clupea harengus harengus) from the Southwestern Bay of Fundy, Canada. Arch Environ Contam Toxicol. 1987;16:311–20. doi: 10.1007/BF01054948. [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Jones PJS, Friel S, Bartley M. Fish, human health and marine ecosystem health: policies in collision. Int J Epidemiol. 2009;38:91–100. doi: 10.1093/ije/dyn157. [DOI] [PubMed] [Google Scholar]
- Burger J. Consumption advisories and compliance: the fishing public and the deamplification of risk. J Environ Plann Manage. 2000;43:471–88. [Google Scholar]
- Burger J. Consumption patterns and why people fish. Environ Res. 2002;90:125–35. doi: 10.1006/enrs.2002.4391. [DOI] [PubMed] [Google Scholar]
- Burger J. Fishing, fish consumption, and knowledge about advisories in college students and others in central New Jersey. Environ Res. 2005;98:268–75. doi: 10.1016/j.envres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Burger J. Risk to consumers from mercury in bluefish (Pomatomus saltatrix) from New Jersey: size, season, and geographical effects. Environ Res. 2009;109:803–11. doi: 10.1016/j.envres.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Mercury in canned tuna: white versus light and temporal variation. Environ Res. 2004;96:239–49. doi: 10.1016/j.envres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Risk to consumers from mercury in Pacific cod (Gadus macrocephalus) from the Aleutians: fish age and size effects. Environ Res. 2007;105:276–84. doi: 10.1016/j.envres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Burger J, Pflugh KK, Lurig L, von Hagen LA, von Hagen SA. Fishing in urban New Jersey: ethnicity affects information sources, perception, and compliance. Risk Anal. 1999;19:217–29. doi: 10.1023/a:1006921610468. [DOI] [PubMed] [Google Scholar]
- Burger J, Gaines KF, Gochfeld M. Ethnic differences in risk from mercury among Savannah River fishermen. Risk Anal. 2001a;21:533–44. doi: 10.1111/0272-4332.213130. [DOI] [PubMed] [Google Scholar]
- Burger J, Gaines KF, Boring CS, Stephens WL, Jr, Snodgrass J, Gochfeld M. Mercury and selenium in fish from the Savannah River: species, trophic level, and locational differences. Environ Res. 2001b;87:108–18. doi: 10.1006/enrs.2001.4294. [DOI] [PubMed] [Google Scholar]
- Burger J, Fleischer J, Gochfeld M. Fish, shellfish, and meat meals of the public in Singapore. Environ Res. 2003;93:254–61. doi: 10.1016/s0013-9351(03)00015-x. [DOI] [PubMed] [Google Scholar]
- Burger J, Stern AH, Gochfeld M. Mercury in commercial fish: optimizing individual choices to reduce risk. Environ Health Perspect. 2005;113:266–71. doi: 10.1289/ehp.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Jeitner C, Burke S, Stamm T, Snigaroff R, et al. Mercury levels and potential risk from subsistene foods from the Aleutians. Sci Total Environ. 2007;384:93–105. doi: 10.1016/j.scitotenv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Burger J, Jeitner C, Donio M, Shukla S, Gochfeld M. Factors affecting mercury and selenium levels in New Jersey flatfish: low risk to human consumers. J Toxicol Environ Health. 2009;72:853–60. doi: 10.1080/15287390902953485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KR. Concentrations of heavy metals associated with urban runoff in fish living in stormwater treatment ponds. Arch Environ Contam Toxicol. 1994;27:352–6. [Google Scholar]
- Carvalho CML, Chew E-H, Hashemy LI, Lu J, Holmgren A. Inhibition of the human thioredoxin system: a molecular mechanism of mercury toxicity. J Biol Chem. 2008;283:11913–23. doi: 10.1074/jbc.M710133200. [DOI] [PubMed] [Google Scholar]
- Commission European. Commission regulation No 629/2008/setting maximum levels for contaminants in foodstuffs. Off J Eur Union. 2008;51:4–10. [Google Scholar]
- Conover DO, Munch SB, Arnott SA. Reversal of evolutionary downsizing caused by selective harvest of large fish. Proc R Soc. 2009;276:2015–20. doi: 10.1098/rspb.2009.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consumer Reports. American’s fish: fair or foul? New York, NY: Consumers Union; 2003. [accessed Dec. 3, 2010]. Available: http://www.mindfully.org/Food/American-Fish.htm. [Google Scholar]
- Crump KS, Kjellstrom T, Shipp AM, Silvers A, Stewart A. Influence of prenatal mercury exposure upon scholastic and psychological test performance: benchmark analysis of a New Zealand cohort. Risk Anal. 1998;18:701–13. doi: 10.1023/b:rian.0000005917.52151.e6. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA. 1998;280:701–7. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- Daviglus M, Sheeshka J, Murkin E. Health benefits from eating fish. Comments Toxicol. 2002;8:345–74. [Google Scholar]
- de Pinho AP, Guimaraes JRD, Marins AS, Costa PAS, Olavo G, Valentin J. Total mercury in muscle tissue of five shark species from Brazilian offshore waters: effects of feeding habit, sex, and length. Environ Res. 2002;89:250–8. doi: 10.1006/enrs.2002.4365. [DOI] [PubMed] [Google Scholar]
- Downs SG, Macleod CL, Lester JM. Mercury in precipitation and its relation to bioaccumulation in fish: a literature review. Water Air Soil Pollut. 1998;108:149–87. [Google Scholar]
- Duffy LK, Scofield E, Rodgers T, Patton M, Bowyer RT. Comparative baseline levels of mercury, HSP 70 and HSP 60 in subsistence fish from the Y-K Delta region of Alaska. Comp Biochem Physiol. 1999;124C:181–6. doi: 10.1016/s0742-8413(99)00055-9. [DOI] [PubMed] [Google Scholar]
- Egeland GM, Middaugh JP. Balancing fish consumption benefits with mercury exposure. Science. 1997;278:904–1906. doi: 10.1126/science.278.5345.1904. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency (EPA) Mercury study report to Congress. Washington, D.C: U.S. Environmental Protection Agency; 1997. (EPA-452/R-97-004) [Google Scholar]
- Environmental Protection Agency (EPA) [Accessed December 2, 2010];Freshwater criterion for fish. 2001 http://www.epa.gov/fedrgstr/EPA-WATER/2001/January/Day-08/w217.htm.
- Environmental Protection Agency (EPA) EPA’s 2008 report on the environment (final report) Washington, D.C: Environmental Protection Agency; 2008. (EPA/600/ R-07/045 F) [Google Scholar]
- Environmental Protection Agency (EPA) [Accessed December 2, 2010];Clean air mercury rule. 2009 http://www.epa.gov/mercuryrule/
- Estrada JA, Lutcavage M, Thorrold SR. Diet and trophic position of Atlantic bluefin tuna (Thunnus thynnus) inferred from stable carbon and nitrogen isotope analysis. Mar Biol. 2005;147:37–45. [Google Scholar]
- Freije A, Awadh M. Total and methylmercury intake associated with fish consumption in Bahrain. Water Environ J. 2009;23:155–64. [Google Scholar]
- Ganther HE, Goudie C, Sunde ML, Kopecky MJ, Wagner R, Sang-Hwang OH, et al. Selenium relation to decreased toxicity of methylmercury added to diets containing tuna. Science. 1972;72:1122–4. doi: 10.1126/science.175.4026.1122. [DOI] [PubMed] [Google Scholar]
- Ginsberg GL, Toal BF. Development of a single-meal fish consumption advisory for methylmercury. Risk Anal. 2000;20:41–7. doi: 10.1111/0272-4332.00004. [DOI] [PubMed] [Google Scholar]
- Gobeille AK, Morland KV, Bopp RF, Godbold JH, Landrigan PJ. Body burdens of mercury in lower Hudson River area anglers. Environ Res. 2005;101:205–12. doi: 10.1016/j.envres.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Gochfeld M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol Environ Saf. 2003;56:174–9. doi: 10.1016/s0147-6513(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Gochfeld M, Burger J. Good fish/bad fish: a composite benefit–risk by dose curve. Neurotoxicology. 2005;26:511–20. doi: 10.1016/j.neuro.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:418–28. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Green NW, Knutzen J. Organohalogens and metals in marine fish and mussels and some relationships to biological variables at reference localities in Norway. Mar Pollut Bull. 2003;46:362–77. doi: 10.1016/S0025-326X(02)00515-5. [DOI] [PubMed] [Google Scholar]
- Guallar E, Sanz-Gallardo MI, van’t Veer P, Bode P, Aro A, Gomez-Aracena J. Heavy metals and Myocardial Infarction Study Group: mercury, fish oils, and the risk of myocardial infarction. N Engl J Med. 2002;347:1747–54. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- Harper BL, Harris SG. A possible approach for setting a mercury risk-based action level based on tribal fish ingestion rates. Environ Res. 2008;107:60–8. doi: 10.1016/j.envres.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Harris RC, Rudd JWM, Amyot M, Babiarz CL, beaty KG, Blanchfield PJ, et al. Whole-ecosystem study shows rapid fish-mercury response to changes in mercury deposition. Proc Natl Acad Sci. 2007;104:16586–91. doi: 10.1073/pnas.0704186104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower JM, Moore D. Mercury levels in high-end consumers of fish. Environ Health Perspect. 2003;111:604–8. doi: 10.1289/ehp.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites RA, Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. Global assessment of organic contaminants in farmed salmon. Science. 2004;303:226–9. doi: 10.1126/science.1091447. [DOI] [PubMed] [Google Scholar]
- Hughner RS, Maher JK, Childs NM. Review of food policy and consumer issues of mercury in fish. J Am Coll Nutritionists. 2008;27:185–94. doi: 10.1080/07315724.2008.10719690. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (IOM) Seafood safety. Washington, DC: National Academy Press; 1991. [Google Scholar]
- Institute of Medicine (IOM) Seafood choices: balancing benefits and risks. Washington, DC: National Academy Press; 2006. [Google Scholar]
- Jardine CG, Hrudey SE, Shortreed JH, Craig L, Krewski D, Furgal C, et al. Risk management frameworks for human health and environmental risks. J Toxicol Environ Health B. 2003;6:569–641. doi: 10.1080/10937400390208608. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives). Sixty-first meeting; Rome. 10–19 June 2003; [Accessed November 27, 2010]. summary and conclusions; 2003 http://www.who.int/ipcs/food/jecfa/summaries/en/summary_61.pdf. [Google Scholar]
- Kaneko JJ, Ralston NV. Selenium and mercury in pelagic fish in the central north Pacific near Hawaii. Biol Trace Elem Res. 2007;119:242–54. doi: 10.1007/s12011-007-8004-8. [DOI] [PubMed] [Google Scholar]
- Knuth B, Connelly NA, Sheeshka J, Patterson J. Weighing health benefits and health risk information when consuming sport-caught fish. Risk Anal. 2003;23:1185–97. doi: 10.1111/j.0272-4332.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- Kraepiel AM, Keller K, Chin HB, Malcolm EG, Morel FM. Sources and variations of mercury in tuna. Environ Sci Technol. 2003;37:5551–8. doi: 10.1021/es0340679. [DOI] [PubMed] [Google Scholar]
- Kumar M, Aalbersberg B, Mosley L, Kumar M, Aalbersberg B, Mosley L. Mercury levels in Fijian seafoods and potential health implications. [accessed Dec 4, 2010];Report for WHO. IAS Technical Report No. 2004/03; 2004. 2004 :35. http://www.wpro.who.int/internet/resources.ashx/FOS/docs/mercury_level_fijian_seafoods.pdf.
- Lange TR, Royals HE, Connor LL. Mercury accumulation in largemouth bass (Micropterus salmoides) in a Florida lake. Arch Environ Contam Toxicol. 1994;27:466–71. doi: 10.1007/BF00214837. [DOI] [PubMed] [Google Scholar]
- Lansens P, Leermakers M, Vaeyens W. Determination of methylmercury in fish by headspace-gas chromatography with microwave-induced-plasma detection. Water Air Soil Pollut. 1991;56:103–15. [Google Scholar]
- Legrand M, Arp P, Ritchie C, Chan HM. Mercury exposure in two coastal communities of the Bay of Funday, Canada. Environ Res. 2005;98:14–21. doi: 10.1016/j.envres.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Lémire M, Fillion M, Frenette B, Mayer A, Philibert A, Passos CJS, Guimarães JRD, Barbosa F, Jr, Mergler D. Selenium and mercury in the Brazilian Amazon: opposing influences on age-related cataracts. Environmental Health Perspectives. 2010 doi: 10.1289/ehp.0901284. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lougheed T. Outside looking in. Environ Health Perspect. 2009;117:A105–10. doi: 10.1289/ehp.117-a104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein JH, Burger J, Jeitner CW, Amato G, Kolokotronis SO, Gochfeld M. DNA barcodes reveal species-specific mercury levels in tuna sushi that pose a health risk to consumers. Biol Lett. 2010;21:2010. doi: 10.1098/rsbl.2010.0156. Epub Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luten JB, Bouquet W, Riekwel-Booy G, Rauchbaar AB, Scholte MWM. Mercury in flounder, Platichtys flesus, cod, Gadus morhua, and perch, Perca fluviatilis, in relation to their length and environment. Bull Environ Contam Toxicol. 1987;38:318–23. doi: 10.1007/BF01606681. [DOI] [PubMed] [Google Scholar]
- Marrugo-Negrete J, Verbel JO, Ceballos EL, Benitez LN. Total mercury and methylmercury concentrations in fish from the Mojana region of Colombia. Environ Geochem Health. 2008;30:21–30. doi: 10.1007/s10653-007-9104-2. [DOI] [PubMed] [Google Scholar]
- Morel FM, Kraepiel MAML, Amyot M. The chemical cycle and bioaccumulation of mercury. Annu Rev Ecol Syst. 1998;29:543–66. [Google Scholar]
- Mozaffarian D. Fish, mercury, selenium and cardiovascular risk: current evidence and unanswered questions. Int J Environ Res Public Health. 2009;6:1894–916. doi: 10.3390/ijerph6061894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (NRC) Toxicological effects of methylmercury. Washington DC: National Academy Press; 2000. [Google Scholar]
- Niimi AJ, Kissoon GP. Evaluation of the critical body burden concept based on inorganic and organic mercury toxicity to rainbow trout (Oncorhynchus mykiss) Arch Environ Contam Toxicol. 1994;26:169–78. doi: 10.1007/BF00224801. [DOI] [PubMed] [Google Scholar]
- Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, et al. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am J Epidemiol. 2008;167:1171–81. doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JG, Curtis LR. Mercury distribution in sediments and bioaccumulation by fish in two Oregon reservoirs: point-source and nonpoint source impacted systems. Arch Environ Contam Toxicol. 1997;33:423–9. doi: 10.1007/s002449900272. [DOI] [PubMed] [Google Scholar]
- Park K, Mozaffarian D. Omega-3 fatty acids, mercury, and selenium in fish and the risk of cardiovascular disease. Curr Artherosclerosis Rep. 2010;12:1–9. doi: 10.1007/s11883-010-0138-z. [DOI] [PubMed] [Google Scholar]
- Patterson J. Introduction—comparative dietary risk: balance the risks and benefits of fish consumption. Comments Toxicol. 2002;8:337–44. [Google Scholar]
- Peterson SA, Ralston NVC, Whanger PD, Oldfield JE, Mosher WD. Selenium and mercury interactions with emphasis on fish tissue. Environ Bioindic. 2009;4:318–34. [Google Scholar]
- Pinheiro MCN, de Nascimento JLM, Silveira LCL, daRocha JBT, Aschner M. Mercury and selenium — a review on aspects related to the health of human populations in the Amazon. Environ Bioindic. 2009;4:222–45. doi: 10.1080/15555270903143440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce RA, Bartell SM, Wong EY, LaFlamme D, Carrington C, Lee RC, et al. Use of quality-adjusted life year weights with dose–response models for public health decisions: a case study of the risks and benefits of fish consumption. Risk Anal. 2000;20:529–42. doi: 10.1111/0272-4332.204050. [DOI] [PubMed] [Google Scholar]
- Pottern GB, Huish MT, Kerby JH. Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (mid-Atlantic): bluefish. US Fish Wildl Serv Biol Rep. 1989;82(11.94):1–21. [Google Scholar]
- Qiu G, Feng X, Wang S, Fu X, Shang L. Mercury distribution and speciation in water and fish from abandoned Hg mines Wanshan, Guizhou province, China. Sci Total Environ. 2009;407:5162–8. doi: 10.1016/j.scitotenv.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Ralston NVC. Selenium health benefit values as seafood safety criteria. Eco Health. 2008;5:442–55. doi: 10.1007/s10393-008-0202-0. [DOI] [PubMed] [Google Scholar]
- Ralston NVC. Introduction to 2nd issue on special topic: selenium and mercury as interactive environmental indicators. Environ Bioindic. 2009;4:286–90. [Google Scholar]
- Ralston NVC, Ralston CR, Blackwell JL, III, Raymond LJ. Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology. 2008;29:802–11. doi: 10.1016/j.neuro.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Raymond LJ, Ralston NVC. Selenium’s importance in regulatory issues regarding mercury. Fuel Process Technol. 2009;90:1333–8. [Google Scholar]
- Rebsch CM, Penna FJ, III, Copeland PR. Selenoprotein expression is regulated at multiple levels in prostate cells. Cell Res. 2006;16:940–8. doi: 10.1038/sj.cr.7310117. [DOI] [PubMed] [Google Scholar]
- Regnell O. The effect of pH and dissolved oxygen levels on methylation and partitioning of mercury in freshwater model systems. Environ Pollut. 1994;84:7–13. doi: 10.1016/0269-7491(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Rice G, Swartout J, Mahaffey K, Schoeny R. Derivation of U.S. EPS’s oral Reference Dose (RfD) for methylmercury. Drug Chem Toxicol. 2000;23:41–54. doi: 10.1081/dct-100100101. and Toxicology 35, 335–344. [DOI] [PubMed] [Google Scholar]
- Rissanen T, Voutilainen S, Nyyssonen K, Lakka TA, Salonen JT. Fish oil-derived fatty acids, docosahexaenoic acid and docosaphentaenoic acid, and the risk of acute coronary events: the Kuopio ischaemic heart disease risk factor study. Circulation. 2000;102:2677–9. doi: 10.1161/01.cir.102.22.2677. [DOI] [PubMed] [Google Scholar]
- Salonen JT, Seppanen K, Nyyssonen K, Korpela H, Kauhanen J, Kantola M, et al. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation. 1995;91:645–55. doi: 10.1161/01.cir.91.3.645. [DOI] [PubMed] [Google Scholar]
- Satoh H, Yasuda N, Shimai S. Development of reflexes in neonatal mice prenatally exposed to methylmercury and selenite. Toxicol Lett. 1985;25:199–203. doi: 10.1016/0378-4274(85)90082-7. [DOI] [PubMed] [Google Scholar]
- Scudder BC, Chaser LC, Wentz DA, Bauch NJ, Brigham ME, Moran PW, et al. US Dept of Interior, Report 2009–5109. Reston, Virginia: 2009. Mercury in fish, bed sediment, and water from streams across the United States, 1998–2005; p. 74. [Google Scholar]
- Shimshack JP, Ward MB, Beatty KM. Mercury advisories: information, education, and fish consumption. J Environ Econ Manage. 2007;53:158–79. [Google Scholar]
- Simonin HA, Loukmas JJ, Skinner LC, Roy KM. Lake variability: key factors controlling mercury concentrations in New York state fish. Environ Pollut. 2008;154:107–15. doi: 10.1016/j.envpol.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Snodgrass JW, Jagoe CH, Bryan AL, Jr, Burger J. Effects of trophic status, and wetland morphology, hydroperiod and water chemistry on mercury concentrations in fish. Can J Fish Aquat Sci. 2000;57:171–80. [Google Scholar]
- South Florida Water Management District. Consolidated report of the South Florida Water Management District. West Palm Beach, Florida: SFWMD; 2000–2005. (a series of yearly reports) [Google Scholar]
- Stafford CP, Haines TA. Mercury contamination and growth rate in two piscivore populations. Environ Toxicol Chem. 2001;20:2099–101. [PubMed] [Google Scholar]
- Statistical Analysis System (SAS) SAS users’ guide. Cary, NC: Statistical Institute, Inc; 1995. [Google Scholar]
- Stern AH, Korn LR, Ruppell BE. Estimation of fish consumption and methylmercury intake in the New Jersey population. J Expo Anal Environ Epidemiol. 1996;6:503–27. [PubMed] [Google Scholar]
- Steuerwald U, Weihe P, Jorgansen PJ, Bjerve K, Brock J, Heinzow B, et al. Maternal seafood diet, methylmercury exposure, and neonatal neurological function. J Pediatr. 2000;136:599–605. doi: 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- Storelli MM, Stuffler RG, Marcotrigiano GO. Total and methylmercury residues in tunafish from the Mediterranean Sea. Food Addit Contam. 2002;19:715–20. doi: 10.1080/02652030210153569. [DOI] [PubMed] [Google Scholar]
- Storelli M, Barone G, Piscitelli G, Marcotrigiano GO. Mercury in fish: concentrations vs fish size and estimates of mercury intake. Food Addit Contam. 2007;24:1353–7. doi: 10.1080/02652030701387197. [DOI] [PubMed] [Google Scholar]
- Toth JF, Jr, Brown RB. Racial and gender meanings of why people participate in recreational fishing. Leis Sci. 1997;19:129–46. [Google Scholar]
- Trudel M, Rasmussen JB. Modeling the elimination of mercury by fish. Environ Sci Technol. 1997;31:1716–22. [Google Scholar]
- US Food and Drug Administration (U.S. FDA) FDA consumer advisory. Washington, DC: U.S. Food and Drug Administration; 2001. [Google Scholar]
- US Food and Drug Administration (U.S. FDA) Mercury levels in commercial fish and shellfish. Washington, D.C: U.S. Food and Drug Administration; 2006. [accessed Dec. 4, 2010]. http://www.fda.gov/food/foodsafety/product-specificinformation/seafood/foodbornepathogenscontaminants/methylmercury/ucm115644.htm. [Google Scholar]
- USFDA/USEPA. [accessed Nov 27, 2010];What you need to know about mercury in fish and shellfish advice for: women who might become pregnant, women who are pregnant, nursing mothers, young children. 2004a http://www.fda.gov/food/resourcesforyou/consumers/ucm110591.htm.
- USFDA/USEPA Joint Advisory. U.S. Food and Drug Administration/U.S. Environmental Protection Agency. [accessed Nov 14, 2010];2004b EPA-823-R-04-005. http://www.fda.gov/food/foodsafety/product-specificinformation/seafood/foodbornepathogenscontaminants/methylmercury/ucm115662.htm.
- Virtanen JK, Mozaffarian D, Chiuve SE, Rimm EB. Fish consumption and risk of major chronic disease in men. Am J Clin Nutr. 2008;88:1618–25. doi: 10.3945/ajcn.2007.25816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe C, Yin K, Kasanuma Y, Satoh H. In utero exposure to methylmercury and Se deficiency converge on the neurobehavioral outcome in mice. Neurotoxicol Teratol. 1999a;21:83–8. doi: 10.1016/s0892-0362(98)00036-1. [DOI] [PubMed] [Google Scholar]
- Watanabe C, Yoshida K, Kasanuma Y, Satoh H. In utero methylmercury exposure differentially affects the activities of selenoenzymes in the fetal mouse brain. Environ Res. 1999b;80:208–14. doi: 10.1006/enrs.1998.3889. [DOI] [PubMed] [Google Scholar]
- Watras CJ, Back RC, Halvorsen S, Hudson RJM, Morrison KA, Wente SP. Bioaccumulation of mercury in pelagic freshwater food webs. Sci Total Environ. 1998;219:183–208. doi: 10.1016/s0048-9697(98)00228-9. [DOI] [PubMed] [Google Scholar]
- Wiener JG, Spry DJ. Toxicological significance of mercury in freshwater fish. In: Beyer WN, Heins GH, Redmon-Norwood AW, editors. Environmental contaminants in wildlife. Boca Raton FL: Lewis Publ; 1996. pp. 297–339. [Google Scholar]
- Wiener JG, Krabbenhoft DP, Heinz GH, Scheuhammer M. Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr, editors. Handbook of ecotoxicology. Boca Raton FL: Lewis Publ; 2003. pp. 409–63. [Google Scholar]
- World Health Organization (WHO) Mercury-environmental aspects. Geneva, Switzerland: WHO; 1989. [Google Scholar]
- Yamashita Y, Omura Y, Okazaki E. Total mercury and methylmercury in commercially important fishes of Japan. Fish Sci. 2005;71:1029–35. [Google Scholar]
- Yeardley RB, Jr, Lazorchak JM, Paulsen SG. Elemental fish tissue concentration in northeastern U.S. Lakes: evaluation of an approach to regional assessment. Environ Toxicol Chem. 1998;17:1875–84. [Google Scholar]