Targeted cancer therapeutics administered alone or in combination with conventional chemotherapies represent a milestone in cancer treatment.1 However, benefit from these approaches is muted by the intrinsic and acquired resistance of cancer cells, suggesting new generation targeted therapeutics and a better understanding of the molecular basis of cancer cell resistance to current therapies is warranted to improve cancer patient outcomes. One promising targeted therapeutic is the tumor necrosis factor superfamily death ligand TRAIL, which selectively kills diseased cells (e.g., cancerous, transformed or virally infected) without harming healthy cells and can repress tumor metastasis.2–4
TRAIL triggers the extrinsic pathway of apoptosis by binding to DR4 and DR5, which directs recruitment of the adaptor FADD and the initiator caspase, procaspase-8, to assemble a death-inducing signaling complex (DISC). Two types of cellular signaling pathways emanate from the DISC to execute the apoptotic program (Fig. 1). In type I cells, the DISC/caspase-8 signal is sufficiently robust to directly activate executioner caspases, including caspase-3. Type II cells, which represent most cell types, require an amplification step to activate executioner caspases. This signal is mediated by the proapoptotic Bcl-2 family protein Bid, which links the extrinsic apoptotic pathway to the intrinsic pathway by triggering mitochondria membrane permeabilization and caspase-9 activation, thereby amplifying caspase-3 activation (reviewed in ref. 5).
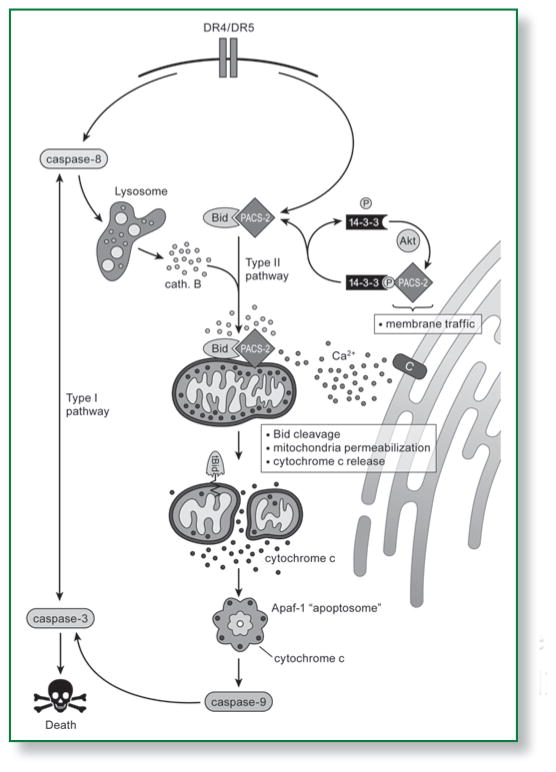
Figure 1.
A model summarizing how PACS-2 may integrate membrane traffic with TRAIL-induced apoptosis. Apoptosis can be triggered by intracellular stress or by death ligands. In the Type I pathway, initiator caspase activation can directly lead to the activation of executioner caspases. In the Type II pathway, death signals are amplified by apoptotic Bcl-2 proteins such as Bid and released ER calcium, which converge on mitochondria and collaborate with released lysosomal cathepsins to trigger membrane permeabilization and cytochrome c release. Cytosolic cytochrome c then binds Apaf-1 to trigger the caspase-9-dependent activation of caspase-3 leading to cell death. TRAIL-induced apoptosis requires the multi-functional sorting protein PACS-2. In healthy cells PACS-2 mediates secretory pathway traffic, including localization of the calcium release channel TRPP2 (labeled C) to the ER. Binding of TRAIL to the DR4/5 death receptors triggers PACS-2 to promote apoptosis by releasing TRPP2 from the ER and by mediating translocation of Bid to mitochondria. Akt and 14-3-3 combine to regulate the ability of PACS-2 to switch between its roles in secretory pathway trafficking with mitochondria permeabilization. In healthy or non-apoptotic cells, Akt phosphorylates PACS-2 at Ser437, which binds 14-3-3 proteins and is required for PACS-2 to mediate secretory pathway traffic. TRAIL triggers release of 14-3-3 from PACS-2 and dephosphorylation of Ser437, which enables PACS-2 to mediate Bid translocation to mitochondria and prevents PACS-2-mediated secretory pathway traffic, causing TRPP2 to release from the ER. This results in an elevation of ER calcium that further promotes mitochondria membrane permeabilization.
Unfortunately, many tumor cells are resistant to TRAIL, suggesting that the underlying regulation of TRAIL-induced apoptosis is complex.6 Understanding the molecular basis of TRAIL-induced apoptosis is therefore key to developing this therapeutic strategy. Regulators of TRAIL action include a collection of death-inducing and decoy receptors, and several signaling molecules, such as Akt, c-myc and NFκB proteins. Akt combines with 14-3-3 proteins to repress the action of several proapoptotic proteins, including Bad, Bim and the proapoptotic FOXO transcriptional regulators.5 The 14-3-3 proteins bind more than 200 ‘client’ phosphoproteins in vivo to mediate cell survival as well as cell cycle division events. 14-3-3 proteins typically bind and inactivate clients at specific phosphorylation sites. This includes Bad, which is phosphorylated by Akt at Ser136 to establish a 14-3-3 binding site. Upon loss of Akt survival signals or apoptotic activation of PP2A, Bad is dephosphorylated and releases bound 14-3-3 proteins.
Unlike Bad and Bax, Bid neither binds 14-3-3 proteins nor is it a known Akt substrate. Yet elevated expression of Akt promotes TRAIL resistance in many tumors at the level of Bid cleavage.7 These findings suggest that Akt/14-3-3 may target a Bid partner required for TRAIL-induced apoptosis of diseased cells. One Bid partner is the multi-functional sorting protein PACS-2.8 In healthy or non-apoptotic cells PACS-2 integrates secretory pathway trafficking with ER-mitochondria communication. In response to apoptotic inducers, PACS-2 becomes an apoptotic mediator in diseased cells that has two roles—promoting apoptotic calcium signaling and the translocation of Bid to mitochondria to facilitate activation of downstream executioner caspases (reviewed in ref. 9).
We determined that PACS-2 is an essential TRAIL effector, required for killing colon cancer cells in vitro and virally infected hepatocytes in vivo.10 Consistent with earlier findings that the PACS-2 locus at chromosome 14q32.33—a hot-spot of chromosome instability in solid tumors11—is lost in colorectal cancer, we found that PACS-2 protein expression is frequently absent in patients suffering from invasive colorectal cancer. Together, these findings suggest that loss of PACS-2 expression may contribute to the colorectal cancer sequence and may be a biomarker for TRAIL-resistant cancers.
In agreement with earlier studies, biochemical analyses showed PACS-2 was required for TRAIL-induced apoptosis of transformed cells at the level of Bid activation. Interestingly, side-by-side experiments revealed PACS-2 was required for the ability of TNFα and FasL to kill diseased but not healthy cells in vivo or in vitro. These findings suggest that signaling pathways leading from death receptor activation to Bid cleavage may fundamentally differ between healthy and diseased cells. These findings further suggest that the type I/II descriptors warrant additional consideration of healthy versus diseased phenotypes, as diseased cells generally have increased antiapoptotic and survival signaling that may require the action of homeostatic regulators such as PACS-2 to enhance apoptosis through the type II pathway.
We identified the biochemical basis for the ability of TRAIL to switch PACS-2 from a membrane traffic regulator to an apoptotic effector that mediates Bid action. In healthy cells, Akt1 phosphorylates PACS-2 at Ser437. Phosphorylated Ser437 binds 14-3-3 proteins with high affinity, which is required for PACS-2 to mediate ER localization of the antiapoptotic calcium release channel TRPP2 and to repress PACS-2 apoptotic activity. TRAIL triggers dephosphorylation of Ser437 and release of bound 14-3-3, reprogramming PACS-2 to redistribute TRPP2 from the ER and promote Bid activation and mitochondria membrane permeabilization. Together, our studies identify the phosphorylation state of PACS-2 Ser437 as a molecular switch that integrates cellular homeostasis with TRAIL-induced apoptosis.
The ability of dephosphorylated PACS-2 to promote release of TRPP2 from the ER and mediate Bid translocation to mitochondria may reflect a larger role for PACS-2 in TRAIL action (Fig. 1). Similar to TRAIL, addition of ceramide or siRNA knockdown of PACS-2 releases antiapoptotic TRPP2 from the ER, increasing the amount of lumenal ER calcium sufficient to trigger mitochondria membrane permeabilization.8,12,13 Together with the findings by Wegierski et al.12, these results raise the possibility that dephosphorylated PACS-2 may contribute to the increased apoptotic destruction observed in autosomal dominant polycystic kidney disease (ADPKD). But whether dephosphorylated PACS-2 promotes apoptotic ER calcium signaling in cancer cells by regulating the trafficking of TRPP2 or perhaps another antiapoptotic calcium release channel such as TRPM8, which contains a candidate PACS-2 binding site and manifests apoptotic resistance in breast, colon, lung and prostate cancers remians to be determined.14 Nonetheless, these findings raise the intriguing possibility that apoptotic signaling triggers PACS-2 Ser437 dephosphorylation to orchestrate Bid translocation with the elevation of ER calcium sufficient to trigger mitochondria membrane permeabilization. Moreover, recent findings that TRAIL action also requires communication between the endosomal/lysosomal and mitochondria and that PACS-2 mediates ER as well as endosomal trafficking,5,9 raise the possibility that dephosphorylated PACS-2 may integrate multiple membrane trafficking steps to drive TRAIL-induced apoptosis in diseased cells.
Acknowledgments
G.T. is supported by National Institutes of Health grants DK37274 and P50CA97186 and OCTRI RR0241.
References
- 1.Kabbinavar FF, et al. Oncologist. 2008;13:1021–9. doi: 10.1634/theoncologist.2008-0003. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi A, et al. J Clin Oncol. 2008;26:3621–30. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 3.Grosse-Wilde A, et al. J Clin Invest. 2008;118:100–10. doi: 10.1172/JCI33061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mundt B, et al. Gut. 2005;54:1590–6. doi: 10.1136/gut.2004.056929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aslan JE, et al. Traffic. 2009 doi: 10.1111/j.1600-0854.2009.00951.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kruyt FA. Cancer Lett. 2008;263:14–25. doi: 10.1016/j.canlet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Nesterov A, et al. J Biol Chem. 2001;276:10767–74. doi: 10.1074/jbc.M005196200. [DOI] [PubMed] [Google Scholar]
- 8.Simmen T, et al. EMBO J. 2005;24:717–29. doi: 10.1038/sj.emboj.7600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youker RT, et al. Biochem J. 2009;421:1–15. doi: 10.1042/BJ20081016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aslan JE, et al. Mol Cell. 2009;34:497–509. doi: 10.1016/j.molcel.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson GR, et al. Cancer Res. 2001;61:8274–83. [PubMed] [Google Scholar]
- 12.Wegierski T, et al. EMBO J. 2009;28:490–9. doi: 10.1038/emboj.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kottgen M, et al. EMBO J. 2005;24:705–16. doi: 10.1038/sj.emboj.7600566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monteith GR, et al. Nat Rev Cancer. 2007;7:519–30. doi: 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]