Abstract
Violence against women is a global public health problem. Exposure to intimate partner violence (IPV) during pregnancy has been associated with a number of adverse maternal and fetal outcomes, including delivery of a low birthweight (LBW) infant. However, there is a paucity of data from low-middle income countries (LMIC). We examined the association between antenatal IPV and subsequent LBW in a South African birth cohort. This study reports data from the Drakenstein Child Lung Health Study (DCLHS), a multidisciplinary birth cohort investigation of the influence of a number of antecedent risk factors on maternal and infant health outcomes over time. Pregnant women seeking antenatal care were recruited at two different primary care clinics in a low income, semi-rural area outside Cape Town, South Africa. Antenatal trauma exposure was assessed using the Childhood Trauma Questionnaire (CTQ) and an IPV assessment tool specifically designed for the purposes of this study. Potential confounding variables including maternal sociodemographics, pregnancy intention, partner support, biomedical and mental illness, substance use and psychosocial risk were also assessed. Bivariate and multiple regression analyses were performed to determine the association between IPV during pregnancy and delivery of an infant with LBW and/or low weight-for-age z (WAZ) scores. The final study sample comprised 263 mother-infant dyads. In multiple regression analyses, the model run was significant [r2=0.14 (adjusted r2=0.11, F(8, 212) = 4.16, p=0.0001]. Exposure to physical IPV occurring during the past year was found to be significantly associated with LBW [t=−2.04, p=0.0429] when controlling for study site (clinic), maternal height, ethnicity, socioeconomic status, substance use and childhood trauma. A significant association with decreased WAZ scores was not demonstrated. Exposure of pregnant women to IPV may impact newborn health. Further research is needed in this field to assess the relevant underlying mechanisms, to inform public health policies and to develop appropriate trauma IPV interventions for LMIC settings.
Keywords: Intimate partner violence, Trauma, Pregnancy, Low birth weight, South Africa
Introduction
The prevalence of violence against women is exceedingly high in South Africa and globally (Devries et al. 2013). Intimate partner violence (IPV) may be defined as behavior by an intimate partner that causes physical, sexual or psychological harm, including acts of physical aggression, sexual coercion, psychological abuse and controlling behaviors (WHO 2013a). In the World Health Organization’s (WHO) Multi-country Study on Women’s Health and Domestic Violence against Women, García-Moreno et al. (2005, 2006) reported that the prevalence of lifetime physical and/or sexual IPV against women aged 15–49 (n=24,097) across ten countries ranged between 15 and 71 %. Between 4 and 54 % of participants reported physical and/or sexual IPV in the past year.
A similar trend is evident in the South African context. According to a secondary analysis of data from the recent South African Stress and Health Study (SASH), a cross-sectional, nationally representative community survey of mental disorders, 31 % of the study sub-sample (n=1 229 married and cohabiting women) reported IPV exposure in their most recent intimate relationship (Gass et al. 2010). Further, in their study of female homicides, Abrahams et al. (2009) reported that 50 % of all murders committed were by current or previous intimate partners. This translates to the highest reported intimate femicide rate globally – 8.8 per 100,000 women.
In Africa, women of reproductive age constitute a particularly vulnerable sub-group, with prevalence rates of IPV during pregnancy ranging between 2 and 57 % (meta-analysis yielded an overall prevalence of 15 %) (Shamu et al. 2011). These rates are among the highest worldwide. IPV during pregnancy is of great societal and public health concern, in part because it may be associated with a range of adverse maternal and fetal sequelae. Women exposed to IPV while pregnant may experience consequent physical and mental health outcomes including pregnancy loss, inadequate weight gain, depression and posttraumatic stress disorder (Taillieu and Brownridge 2010; Martin et al. 2006; Rodriguez et al. 2008). Adverse infant outcomes may include preterm labor/delivery and low birthweight (Taillieu and Brownridge 2010; Covington et al. 2001; Murphy et al. 2004).
Low birthweight (LBW), in particular, is widespread and may be associated with a range of infant and childhood deficits (Aarnoudse-Moens et al. 2009; Harder et al. 2007). With a global LBW rate (LBWR) of approximately 15.5 % (ie. over 20 million babies born weighing less than 2,500 g worldwide) (UNICEF and WHO 2004), the vast majority of these cases occurs in low-middle income (LMIC) regions. In an early methodological assessment and meta-analysis of the determinants of LBW, Kramer (1987) reported three classes of risk factors, ie. unmodifiable factors with an established causal effect (eg. maternal reproductive history); factors with a casual effect that are modifiable in the short-term (eg. maternal nutrition, pre-pregnancy weight and substance use); and causal factors that are modifiable in the long-term (eg. maternal socioeconomic status). Further, Kramer put forward that these factors exert their effect by increasing the risk for intra-uterine growth restriction (IUGR) and/or for shortened gestational duration, both of which are mechanisms of action for LBW. In high income (HIC) countries, cigarette smoking was reported to be the major determinant of IUGR (and LBW), while poor gestational nutrition, decreased maternal anthropometry and maternal infection (malaria) were found to be most influential in LMIC regions. While pre-pregnancy weight, prior history of prematurity and cigarette smoke were reported to have well-established causal effects on decreased gestational duration, the majority of cases in HIC and LMIC countries was found to be unexplained.
In their recent systematic review and meta-analysis of studies conducted in HIC regions (United States, Norway and Australia), Murphy et al. (2004) investigated the association between IPV during pregnancy and low infant birthweight. These authors reported that women exposed to physical, sexual or emotional abuse during pregnancy were 1.4 times more likely to give birth to a newborn with LBW than those who were not abused (CI 95 % 1.1–1.8). There is some evidence that an association of similar strength may exist in LMIC countries. For example, in their survey of 110 pregnant women delivering at a public hospital in Mexico, Valdez-Santiago and Sanín-Aguirre (1996) found that those who had experienced IPV during pregnancy were approximately four times more likely to deliver LBW infants (Cl 95 % 1.3–12.3), and that the average birthweight of newborns of abused women was 540 g less (p<0.01 adjusted by age and parity) when compared to those who had not experienced such violence. Similarly, in their study of 930 pregnant teenagers from a socioeconomically disadvantaged community in Sao Paulo, Brazil, Ferri et al. (2007) found that 21.9 % of mothers reported a history of lifetime violence, with 2 % having experienced IPV during pregnancy. Further, it was found that violence during pregnancy was independently associated with LBW (prevalence ratio = 2.59, 95 % CI 1.05–6.40).
Unfortunately, despite the high burden of IPV, data from LMIC regions such as South Africa remain relatively sparse. There is a need for further work in this area to delineate the association between this form of abuse and its many potential adverse outcomes. Contextual factors particularly relevant to LMIC settings such as sociodemographic variables, childhood trauma and stress-related psychopathology may confound this association and should also be considered. The purpose of this analysis was to determine whether there is an association between exposure to physical, emotional and/or sexual IPV during pregnancy, and subsequent delivery of an infant with LBW in the context of a South African community setting, while controlling for a number of potential contextual confounders.
Methods
This study reported data from the Drakenstein Child Lung Health Study (DCLHS), a multidisciplinary birth cohort investigation of the epidemiology and etiology of childhood respiratory illness and the determinants of child health in a low socioeconomic area in South Africa. More broadly, the DCLHS encompasses the influence of a number of antecedent risk factors on maternal and infant health outcomes over time. At the time of manuscript preparation, the DCLHS was ongoing. Data collection had commenced in March 2012.
Participants
Pregnant women were recruited from two primary health care clinics—Clinics A and B (clinic names have been omitted to protect the anonymity of study participants)—in the Drakenstein sub-district of the Cape Winelands, Western Cape, South Africa, between March 2012 and June 2013. Data collection occurred at these two clinics (maternal data) as well as at a central hospital (newborn outcomes). The Drakenstein sub-district is a semi-rural, accessible and LMIC community. Participants were enrolled in the DCLHS at 20 to 24 weeks’ gestation upon presenting for antenatal booking and followed longitudinally throughout pregnancy until 2 years postnatally. Exclusion criteria were minimal in order to maximize generalizability, and focused primarily on those individuals who did not live in the region (and thus could not be readily followed up) or those who were intending to move out of the district within the following 2 years.
Ethics
The DCLHS was approved by the Faculty of Health Sciences human research ethics committees of the University of Cape Town (UCT) and Stellenbosch University in South Africa, as well as by the Western Cape Department of Health (DoH) Provincial Research Committee (endorsing all research activities carried out at clinics and hospitals in the Drakenstein sub-district). Prior to enrollment, all study participants provided written informed consent. This analysis was also approved by the institutional review board at the University of California, Los Angeles (UCLA).
Following written informed consent, those women who wished to participate were asked to complete a battery of self-report measures described below. On-site female fieldworkers administered these questionnaires to all study participants. These fieldworkers were selected based on criteria previously found to affect women’s willingness to divulge information about IPV exposure (Jansen et al. 2004), including multi-cultural and interpersonal rapport, empathy and non-judgmentalism, emotional maturity and sensitivity to the complex psychosocial issues relating to IPV. All fieldworkers had at least a Grade 12 certificate, had prior experience in collaborative psychiatric/psychological research and were fluent both in English and Afrikaans or isiXhosa. Thus, they were able to administer questionnaires in the participants’ preferred language. Further, fieldworkers received extensive in-service training on all aspects of Good Clinical Practice (GCP) (WHO 2002) prior to the commencement of data collection for the DCLHS. Those involved directly in IPV-related data collection underwent additional specialized training based on the WHO clinical and policy guidelines (WHO 2013a) and on the programme designed for the WHO Multi-Country Study on Women’s Health and Domestic Violence (Jansen et al. 2004). This training included aspects of women-centered care and first-line support for those reporting IPV (eg. gender sensitivity and help in accessing resources and mobilizing social support); as well as basic knowledge of IPV, including its causes, characteristics, impact and laws relevant to victims of abuse. Powerpoint presentations, instructional handouts and manuals, role-play sessions and directly observed interviews served as adjuncts to the basic training framework.
As participants were asked to divulge sensitive and potentially distressing information, a private consultation room was provided at each study site. Participants could choose not to answer certain questions and still remain in the study, as long as exposure and diagnostic status could be determined reasonably. They could also take breaks, if requested, to lighten the interview burden; and were free to terminate or reschedule the interview should the need arise. Refreshments (water, fruit juice and biscuits) were provided to alleviate participant fatigue during the assessment sessions. Thus, every effort was made to ensure a safe, confidential and supportive environment. Further, each participant was compensated with 100 South African rand for her time and effort, which is typical for similar South African studies.
Measures and variable calculation
The following questionnaires were administered to enrolled women at an antenatal clinic visit between 28 and 32 weeks’ gestation:
Sociodemographics
A questionnaire to assess socioeconomic status (SES) was adapted from the version used in the South African Stress and Health Study (SASH) (Myer et al. 2008) and assessed education and income; access to governmental financial assistance; household composition; and available amenities (including electricity, running water, electric stove and a functional telephone). All items are particularly relevant to the LMIC setting and may impact maternal and newborn health and well-being.
For the purposes of this study, a composite score of SES was developed. Four sociodemographic variables (as assessed by the SES questionnaire) were used to generate this score, ie. educational attainment; employment status; household income; and assets and market access. Variables were extracted as follows:
Educational attainment – participants with primary education only scored 0, those with some secondary education scored 1, those who had completed secondary education scored 2, and those with any tertiary education were assigned a score of 3.
Employment status – this variable was dichotomized, with those currently unemployed scoring 0, and those currently employed scoring 1.
Household income – total household income of less than 1,000 South African rand per month was scored 0, monthly income between 1,000 and 5,000 South African rand was scored 1, and households with an income greater than 5,000 South African rand per month were assigned a score of 3.
Assets and market access – a composite asset index was calculated as the sum of assets/infrastructure and market access. In order to assess assets/infrastructure, participants were requested to indicate the availability of the following household resources and amenities (a score of 1 was assigned for each available item): electricity, a tap or running water, a domestic servant, a flush toilet inside, a built-in kitchen sink, an electric stove or hotplate, a working telephone (including cellphone), at least 1 motor car or truck, a motorcycle or scooter, and/or a bicycle. The composite score for assets/infrastructure was then obtained by summing the individual item scores.
Similarly, the total market access score was assessed by the following questionnaire items: shopping at supermarkets, using any financial services (such as bank account, ATM card or credit card) and/or having an account at a retail store (eg. Pep). Again, each affirmative item was assigned a score of 1, and the total market access score derived by adding these individual scores.
The grand total SES score was then generated as follows:
Medical and reproductive risk
Maternal medical and reproductive health was assessed using a standardized questionnaire developed for the DCLHS. Risk profiles were quantified based on a model previously described by Collins et al. (1993), who used a similar study population and prospective cohort design as in the DCLHS; and on complementary work by Pagel et al. (1990) and Feldman et al. (2000). HIV, tuberculosis, asthma, diabetes mellitus, hypertension, cardiovascular disease, hyperemesis gravidarum, urinary tract infections and pelvic inflammatory disease are maternal biomedical conditions that may impact infant outcomes and were thus included. Similarly, reproductive risk was ascertained using an assessment of parity, prior intra-uterine death, prior LBW infant, prior premature birth, prior caesarean section, poor maternal weight gain during the current pregnancy, current multiple gestation and sex of the newborn. Further, maternal anthropometric measures taken antenatally and at regular postnatal intervals were also included. Risk factors were scored as either present (1) or absent (0), and then summed to provide a continuous composite each for medical and for reproductive risk.
Psychosocial risk
The Planning of Birth/Partner Support Questionnaire was developed for this study and adapted from questions used in the SASH (Myer et al. 2008) to assess the effect of varying degrees of social support in pregnant women. A continuous measure was generated based on a number of items which broadly assessed pregnancy intention, contraceptive use at the time of becoming pregnant, and support received from the partner. Responses were then scored as either affirmative (1), indicating a greater degree of birth planning and/or support; or negative (0). Thus, the higher the continuous score, the lower the maternal risk profile.
The Beck Depression Inventory (BDI-II), is a commonly-used and reliable screen for depressive symptoms (Beck et al. 1961, 1988, 1996a, b) which was used as the primary tool to assess for this disorder in our study population. The BDI-II has shown good validity and internal consistency, for both psychiatric and non-psychiatric subjects (Beck et al. 1988, 1996b; Sprinkle et al. 2002). Further, this tool has been validated in a low-income African American sample with similar sociodemographic characteristics as our own (Grothe et al. 2005) and has been used in a number of studies conducted in South Africa (Kagee et al. 2013; Nel and Kagee 2013; Kagee and Martin 2010). The BDI-II comprises 21 items, each assessing a symptom described in the DSM-IV criteria for major depression. All items require participants to select one of four options, scored on a severity scale from 0 (absence of symptom) to 3 (severe symptom, often with functional impairment) and pertaining to the 2 weeks prior to the assessment, including the day of questionnaire administration. Total scores are then obtained by summing individual item scores. For our purposes, BDI-II scores were taken as continuous measures (with higher scores indicating more severe depressive symptoms), as well as dichotomous variables. A cut-off score of ≥20 was used for dichotomizing participants into “probable moderate/severe clinical cases” versus “probable sub-threshold participants”, as has been described elsewhere (Lasa et al. 2000). In an early publication (Beck and Beamesdefer 1974), Beck and colleagues stated that BDI scores should not be strictly adhered to for diagnostic status. Rather, scores less than 10 should suggest the absence of a depressive disorder; those between 10 and 19, mild to moderate depressive symptoms; between 19 and 29, moderate to severe symptoms; and that scores greater than 30 should indicate severe depression. These authors also recommended that a threshold score of 12/13 would be appropriate for identifying depression in psychiatric patients, while a score of 9/10 could be used in medical patients (Beck and Beamesdefer 1974; Lasa et al. 2000).
The SRQ-20 (Harding et al. 1980; Scholte et al. 2011) was used to supplement the findings of the BDI-II in our study population. This is a WHO-endorsed measure of psychological symptoms that has been widely used in international and South African settings, and has shown good reliability and high face validity (Harpham et al. 2003; Tuan et al. 2004; Rumble et al. 1996). The SRQ-20 comprises twenty items, intended to assess for the presence of non-psychotic symptoms. These items were selected based on a number of psychiatric assessment tools used in four different LMIC countries (Mari and Williams 1986). Symptoms of depressive and anxiety disorders, as described in the DSM-IV, are included in the SRQ-20. Participants are required to respond in the affirmative (scored 1) or negative (scored 0) for each item. For our purposes, individual item scores were summed to obtain total continuous scores. Further, participants were also dichotomized as “high risk” (SRQ score >8) or “low risk” (score ≤ 8). While cut-off points vary in different cultural and geographical settings, the threshold score of 8 is widely used (Ventevogel et al. 2007; Harpham et al. 2003).
The Edinburgh Postnatal Depression Rating Scale (EPDS) (Cox et al. 1987) is a 10-item self-report measure of recent depressive symptoms, which has shown good psychometric properties in validation studies (Eberhard-Gran et al. 2001). This tool was originally developed for use in postnatal women, under the assumption that normal symptoms experienced during the perinatal period (eg. sleep and appetite changes) could be misattributed to a depressive disorder on many of the standard screening tools (including the BDI-II). The EPDS assesses for the presence of mood changes characteristic of postnatal depression, within the week preceding questionnaire completion. Each item is scored on a frequency scale, ranging from 0 to 3. As for the other measures of depression, a continuous score was obtained by summing the individual items; the higher the score, the greater the symptom severity. Further, participants were also classified as “probable cases” if they scored >13 on the EPDS. This threshold has been used in similar studies conducted in the South African LMIC context (Hartley et al. 2011).
The Modified Posttraumatic Stress Disorder Symptom Scale (MPSS) (Foa et al. 1993) is a 17-item interview that was used as a rapid screening tool for posttraumatic stress disorder (PTSD) in our study population. This tool mirrors the DSM-IV criteria for PTSD and has also shown good psychometric properties with concurrent validity. The MPSS was selected for use in this study due to its reasonably good diagnostic validity for PTSD. Participants were requested to respond to each item on a four-point frequency scale, from 0 (absence of symptom) to 3 (symptom occurs five or more times per week/very much/almost always). A final item was also included to assess for duration of symptoms, with response options including < 1 month; 1–3 months; 3 months–1 year; and > 1 year. As no MPSS cutoff score for PTSD has been clearly established (Binder et al. 2008), the DSM IV criteria were applied to the MPSS items to generate a proxy variable for PTSD diagnosis. Those items assessing the re-experiencing symptom cluster were scored as “above threshold” if their sum totaled ≥1; avoidance/emotional numbing ≥3; and increased arousal ≥2. Participants who scored above threshold across all three symptom clusters, and reported symptom duration of at least 1 month (scored ≥1 for item 18) were classified as “suspected PTSD cases”.
Substance misuse in this study sample was assessed using the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST). This tool was developed by the WHO to detect psychoactive substance use and related disorders in primary care settings. It has shown good reliability, feasibility and validity in international, multi-site studies (WHO ASSIST Working Group 2002; Humeniuk et al. 2008), and is useful for identifying substance misuse in poly-substance abusers with varying degrees of psychopathology. Seven items are included to assess alcohol and other drug use across 10 categories (ie. tobacco products; alcoholic beverages; cannabis; cocaine; amphetamine-type stimulants; inhalants, sedatives or sleeping pills; hallucinogens; opioids; and a general category entitled “other”, in which the participant is required to specify the substance used). Frequency of use is assessed for each substance, and responses scored accordingly. The ASSIST commences with a screening questionnaire to assess lifetime use of alcohol and other drugs (AOD). Individuals responding “no” to this question may then terminate the interview. For those responding “yes” to any of the substances included, the remainder of the questionnaire need only be completed in relation to those substances. Responses to questions 2–5 are scored on a five-point frequency scale, while questions 6–7 are scored on a three-point scale. Question 8 assesses a history of intravenous drug use. (A more complete description of the tool and its scoring can be found elsewhere – Newcombe et al. 2005). Total scores are then obtained for each substance by summing the individual scores for questions 2–7 (inclusive). For our purposes, only data for alcohol and tobacco use were extracted from the ASSIST, as reports of other substance use were negligible. Participants were assigned continuous scores for each of these two substances.
Exposure to trauma
The Childhood Trauma Questionnaire (CTQ) (Bernstein et al. 1994) is a 28-item inventory assessing three domains of childhood abuse (sexual, physical, and emotional), and two domains of childhood neglect (physical and emotional), occurring at or before the age of 12 years. This questionnaire has shown excellent sensitivity and specificity in the classification of childhood abuse and neglect. A 5-point frequency of occurrence scale is utilized as follows: (1) never true, (2) rarely true, (3) sometimes true, (4) often true, and (5) very often true. Thus, each subscale is scored on a spectrum from 5 (no history of abuse or neglect) to 25 (very extreme history of abuse and neglect). Three items are also included as a Minimization/Denial scale to detect potential under-reporting of abuse by participants. These items are dichotomized (ie. a response of “never” is scored 0; all others are scored 1) and added, with a sum total ≥1 indicating possible under-reporting (Bernstein and Fink 1998; Villano et al. 2004). In our sample, participants were allocated a total continuous score by summing individual items (excluding those comprising the Minimization/Denial scale). Higher scores signified greater severity of abuse. Further, participants were also dichotomized into those with a history of childhood trauma, and those without. Cut-off scores for each clinical domain as defined in the CTQ manual (Bernstein and Fink 1998) were used. Participants scoring within the “none or minimal” range were defined as below threshold for a history of childhood trauma; those in any other category (ie. “low to moderate”, “moderate to severe” or “severe to extreme”) were defined as above-threshold.
The Intimate Partner Violence (IPV) Questionnaire used in this study was adapted from the WHO multi-country study (Jewkes 2002) and the Women’s Health Study (Zimbabwe) (Shamu et al. 2011) and assessed lifetime and recent (past-year) exposure to emotional, physical and sexual abuse. Emotional abuse was assessed by the following items: being insulted or made to feel bad about oneself; being belittled or humiliated in public; being purposefully scared or intimidated; and being threatened. To assess physical abuse, women were asked about a history of having been slapped or having had something thrown at them which could hurt them; being pushed or shoved; being hit with a fist or with something else that could hurt them; being kicked, dragged, beaten, choked or burnt; and being threatened with or actually abused with a gun, knife or other weapon. Finally, sexual abuse was defined by having been physically forced to have sex when one did not want to; having sex with one’s intimate partner when one did not want to out of fear of what he might do; and/or having been forced to do something sexually that was degrading or humiliating. A 4-point frequency of occurrence scale was used: (1) never, (2) once, (3) few times, and (4) many times. Scoring guidelines were devised for the purposes of this study, and were based on prior work in similar South African studies (Dunkle et al. 2004). Participants were assessed as having had no exposure to IPV if all responses were “never” or one response was “once” (ie. isolated episode) in the questionnaire, low frequency if they answered “once” for more than one affirmative item, mid frequency if more than one response of a “few times”, but no response of “many times” was given, and high frequency if there were any responses of “many times” in the questionnaire. Participants were also allocated continuous scores by summing individual items.
The original English versions of these measures were translated into both Afrikaans and isiXhosa by professional bilingual and bicultural translators. These versions were then blindly back-translated into English by a different set of bilinguists. The two sets of English questionnaires were compared, and translation discrepancies or difficulties addressed. Original versions were modified appropriately in light of these discrepancies. All tools were pre-tested (piloted) in the community setting on a group of individuals similar to the proposed study population, thus ensuring feasibility for use.
The IPV questionnaire was designed to end all assessment sessions by a check of the participants’ post-interview state (ie. good/better, bad/worse or same/no difference). Further, participants were given the opportunity to ask questions and make closing comments. Those women with suspected IPV exposure and/or at high psychosocial risk (based on their responses to the questionnaires described above) were referred to routine service providers in the Drakenstein community. It was made clear to study participants and interviewers that the latter should not serve as counsellors; rather that they should facilitate participant referral when appropriate. This process of case detection and referral followed a standard operating procedure (SOP) document that had been designed for the purposes of this study, based on WHO guidelines (WHO 2013a). Following case identification by the fieldworkers, on-site team leaders (qualified and experienced psychiatric nurses) were immediately informed, followed by escalation to the senior study medical officer (MO). The MO then reviewed and referred participants on a case-by-case basis. Since the planning stages, the DCLHS has collaborated closely with the Drakenstein district stakeholders. Thus, investigators were able to draw on the established, reliable and accessible health infrastructure in the community. A number of mental health nurses, social workers and counsellors offering free services in the public sector were collaborators in our referral pathway. Those women requiring immediate admission and care were referred directly to the emergency department at a central hospital for further management. Thus, the DCLHS served as a mechanism for case identification and appropriate referral to participants facing IPV.
As the process of IPV data collection may be distressing and emotionally taxing for the interviewers themselves, informal debriefing sessions were held in order to support the study staff. Anecdotal evidence suggests that these sessions have been a valuable opportunity for the fieldworkers to discuss their challenges and feelings in response to the interviews.
Newborn outcomes
All babies were born at a central hospital that serves the communities of both clinics. Trained clinical staff recorded newborn birthweight at the time of delivery, using a digital scale with a precision level of 10 g.
Low birthweight (LBW) was defined as less than 2,500 g, according to the WHO parameters (WHO 2013b). However, this criterion is not corrected for gestational age. While decreased duration of pregnancy is often associated with decreased birthweight, premature infants may in fact be within the normal weight range when corrected for gestational age. Thus, for the purpose of this analysis, LBW was restricted to full-term infants (with 37 or more completed weeks’ gestation).
Low weight-for-age Z (WAZ) score (standard deviation score) is defined as a score less than or equal to 2 standard deviations below the mean weight-for-age value (WHO 1995). The Z-score classification system is advantageous, as it is sex-independent, employs a linear scale, and enables a comparison of results across age groups and indicators. For our purposes, both outcomes of interest (ie. LBW and low WAZ scores) were expressed as dichotomous and continuous variables.
Sample size
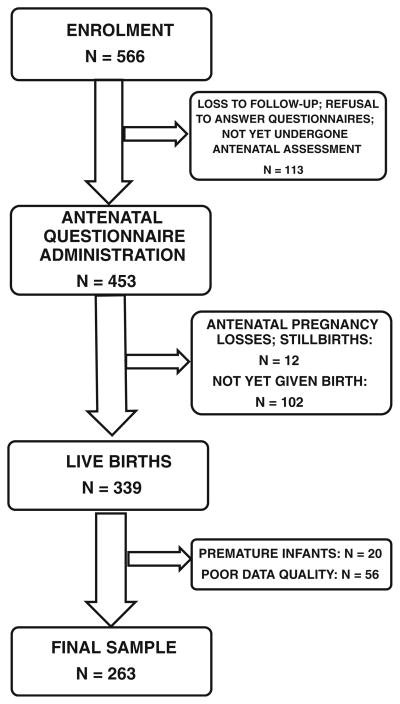
At the time of this analysis, a total of 566 women had been enrolled in the larger DCLHS. Of these, 113 had been lost to follow up, refused to participate in this sub-study or had not yet returned for questionnaire administration at the time of data collection. Thus, a total of 453 women completed the measures described above. Of this group, 12 experienced antenatal pregnancy losses or stillbirths. At the time of data collection, 339 study participants had delivered live infants. Live infants born prematurely (before 37 weeks’ completed gestation (WHO 1995)) were excluded from this analysis (n= 20). Data from a further 56 participants were omitted due to problems with quality control. Most of these quality difficulties arose in the first few months of data collection, and pertained to incorrect or incomplete questionnaires, duplicated participant identification numbers, and erroneous data capturing. Human error was noted to decrease significantly as staff training and experience improved. The final sample for this analysis comprised 263 mother-infant dyads (see Fig. 1).
Fig. 1.
Participant enrolment and sample collection
Statistical analysis
Frequency distributions were used to describe sociodemographic characteristics of interest; psychosocial risk factors (including exposure to trauma); and adverse newborn outcomes (LBW and low WAZ scores) (see under Results, below). Bivariate correlation analyses (Pearson correlation coefficients) were then applied to determine the variables to be included in the regression models. These correlation analyses included potential confounding variables such as sociodemographics of the study population; maternal relationship with partner; psychosocial stressors and psychopathology; and medical/reproductive risk.
The interaction between maternal traumatic exposure (predictor) and low infant birthweight (outcome) was then tested in the final model using multiple linear regression analyses. Based on the results of the bivariate correlation analyses, two multiple regression models were generated:
To determine the association between past-year IPV exposure and LBW
To determine the association between past-year IPV exposure and low WAZ score
Results
Maternal sociodemographics
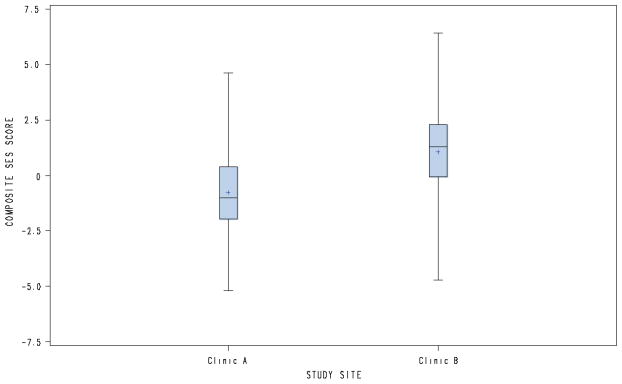
Women in this study sample ranged in age from 18 to 41 years, with a mean of 26 years. Sixty percent of women were unmarried. Although participants were recruited without regard for ethnic or racial characteristics, approximately half of our study population self-reported as Coloured and Afrikaans-speaking, with the remainder reporting as Black and isiXhosa-speaking. Most women (62 %) were born in the Drakenstein sub-district, with the majority (84 %) having received at least some high school education (Grades 8–12). Approximately a third of the sample was unemployed. Almost half (41 %) of the women received social (governmental) assistance, with all of these participants specifying that this was in the form of a childcare grant. At the time of this secondary analysis, almost two thirds (64 %) of the women lived in a house or flat, with the majority (58 %) residing in an urban setting. Once the composite continuous SES scores were calculated, participants were stratified into quartiles, ie. lowest, low-moderate, moderate-high, highest SES. While a slight preponderance (27 %) was seen in the lowest stratum, participants were fairly evenly distributed across the four categories. However, the majority of participants at Clinic A (73 %) scored within the lowest and low-moderate quartiles, compared to the minority at Clinic B (30 %). Table 1 depicts sociodemographic characteristics for the total study sample; as well as site-specific features. Figure 2 represents a comparison of the composite SES scores at each study site.
Table 1.
Sociodemographic characteristics of a subsample of women enrolled in the DCLHS, 2013 (total sample and comparison by study site)
| Variable | Number (%)a
|
||
|---|---|---|---|
| Site-specific comparison
|
Total sample | ||
| Clinic Ab | Clinic Bb | ||
| Mean age (years) | 27.02 (6.20) | 26.08 (5.58) | 26.48 (5.86) |
| Ethnicity (self-reported) | |||
| Black | 118 (99.16) | 3 (2.08) | 121 (46.01) |
| Coloured | 1 (0.84) | 141 (97.92) | 142 (53.99) |
| Home language | |||
| isiXhosa | 115 (96.64) | 2 (1.39) | 117 (44.49) |
| Afrikaans | 2 (1.68) | 138 (95.83) | 140 (53.23) |
| English | 0 | 4 (2.78) | 4 (1.52) |
| Shona | 2 (1.68) | 0 | 2 (0.76) |
| Marital status | |||
| Single | 71 (59.66) | 85 (59.03) | 156 (59.32) |
| Married/in a marriage-like relationship (eg. co-habiting; in a committed, long-term relationship) | 48 (40.34) | 59 (40.97) | 107 (40.68) |
| Place of birth | |||
| In Paarl | 39 (32.77) | 125 (86.81) | 164 (62.36) |
| Outside of Paarl, in the Western Cape | 9 (7.56) | 14 (9.72) | 23 (8.75) |
| Outside of the Western Cape, in South Africa | 69 (57.98) | 5 (3.47) | 74 (28.14) |
| Outside of South Africa | 2 (1.68) | 0 | 2 (0.76) |
| Highest level of education | |||
| Grades 1–7 | 15 (12.61) | 11 (7.64) | 26 (9.89) |
| Grades 8–12 | 98 (82.35) | 122 (84.72) | 220 (83.65) |
| Post-high school education | 6 (5.04) | 11 (7.64) | 17 (6.46) |
| Employment status | |||
| Unemployed | 12 (10.08) | 81 (56.64) | 93 (35.50) |
| Homemaker/student | 79 (66.39) | 11 (7.69) | 90 (34.35) |
| Employed | 28 (23.53) | 51 (35.66) | 79 (30.15) |
| Financial assistance (governmental grant) | |||
| No | 68 (57.14) | 85 (59.86) | 153 (58.62) |
| Yes | 51 (42.86) | 57 (40.14) | 108 (41.38) |
| Type of dwelling (current) | |||
| Shack | 66 (55.46) | 6 (4.23) | 72 (27.59) |
| Wendy house or backyward dwelling | 1 (0.84) | 22 (15.49) | 23 (8.81) |
| House or flat | 52 (43.70) | 114 (80.28) | 166 (63.60) |
| Living area (current) | |||
| Urban | 20 (17.09) | 131 (92.25) | 151 (58.30) |
| Rural | 17 (14.53) | 8 (5.63) | 25 (9.65) |
| Township | 80 (68.38) | 3 (2.11) | 83 (32.05) |
| Composite SES quartiles | |||
| Lowest | 53 (44.54) | 20 (13.61) | 73 (27.44) |
| Low-moderate | 34 (28.57) | 24 (16.33) | 58 (21.80) |
| Moderate-high | 18 (15.13) | 50 (34.01) | 68 (25.56) |
| Highest | 14 (11.76) | 53 (36.05) | 67 (25.19) |
Due to rounding, totals may not add to 100 %
Clinic names have been omitted to protect the anonymity of the study participants
Fig. 2.
Box-and-whisker comparison of composite SES scores at each study site
Biomedical/reproductive and psychosocial risk
For the vast majority of biomedical and psychosocial risk factors, prevalence rates were within the range of 0.5 to 2.5 % (see Table 2). Similarly, most participants scored low on each of the continuous measures of cumulative biomedical and reproductive risk. However, 20 % of women were HIV positive at the time of data collection (based on the results of voluntary counseling and testing). Further, most women (81 %) reported that the index pregnancy was unplanned, and almost two thirds (62 %) were found to experience insufficient weight gain during pregnancy (defined as less than 0.3 kg per week during the second and third trimesters) (Strauss and Dietz 1999).
Table 2.
Biomedical, reproductive and psychosocial risk profile of sub-sample of women enrolled in the DCLHS, 2013
| Variable | Number (%) | Mean (SD) |
|---|---|---|
| Biomedical risk factors | ||
| HIV | 53 (20.15) | |
| Asthma | 6 (2.42) | |
| Urinary tract infection | 6 (2.42) | |
| Tuberculosis | 2 (0.81) | |
| Hypertension | 2 (0.81) | |
| Hyperemesis gravidarum | 2 (0.81) | |
| Diabetes mellitus | 1 (0.40) | |
| Pelvic inflammatory disease | 1 (0.40) | |
| Cardiovascular disease | 0 | |
| Cumulative medical risk | ||
| 0 | 111 (43.36) | |
| 1 | 123 (48.05) | |
| 2 | 20 (7.81) | |
| 3 | 2 (0.78) | |
| Mean (SD) | 0.66 (0.66) | |
| Reproductive risk factors | ||
| Poor maternal weight gain during current pregnancy | 116 (62.03) | |
| Female sex of newborn | 127 (48.66) | |
| Nulliparity | 101 (38.11) | |
| Prior caesarean section | 48 (18.05) | |
| Prior LBW infant | 23 (8.68) | |
| Prior premature birth | 15 (5.66) | |
| Prior intra-uterine death | 3 (1.13) | |
| Current multiple gestation | 0 | |
| Cumulative reproductive risk | ||
| 0 | 54 (20.30) | |
| 1 | 118 (44.36) | |
| 2 | 84 (31.58) | |
| 3 | 9 (3.38) | |
| 4 | 1 (0.38) | |
| Mean (SD) | 1.19 (0.81) | |
| Psychosocial risk factors | ||
| Unplanned pregnancy | 214 (81.06) | |
| Depression | ||
| - BDI-II (dichotomous – above threshold) | 66 (27.85) | |
| - BDI-II (continuous) | 13.95 (10.10) | |
| - EPDS (dichotomous – above threshold) | 72 (30.38) | |
| - EPDS (continuous) | 9.76 (5.63) | |
| - SRQ-20 (dichotomous – above threshold) | 52 (22.22) | |
| - SRQ-20 (continuous) | 4.88 (3.96) | |
| Posttraumatic stress disorder (PTSD) | ||
| - MPSS (dichotomous – above threshold) | 77 (33.33) | |
| Substance use (continuous measures) | ||
| - Alcohol | 2.99 (7.44) | |
| - Tobacco | 7.82 (11.30) | |
The prevalence of psychopathological symptoms was also relatively high in this sample, with almost a third of participants scoring above threshold on the BDI and EPDS (measures of depression); while exactly a third screened positive for PTSD (see Measures and variable calculation, above, for standard threshold scores on these questionnaires). While the continuous measure for alcohol consumption was relatively low, tobacco use during pregnancy was common. The prevalence of substance use of participants at Clinic B was notably higher than at Clinic A. In particular, tobacco use at Clinic B was far more prevalent (mean ASSIST score of 11.85 at Clinic B, compared to 2.15 at Clinic A).
Exposure to trauma (see Table 3)
Table 3.
Childhood, lifetime and past-year trauma exposure in subsample of women enrolled in the DCLHS, 2013
| Variable | Number (%) | Mean (SD) | Interpretation |
|---|---|---|---|
| Childhood trauma | |||
| CTQ continuous | 40.59 (14.49) | ||
| CTQ dichotomous – above threshold | 113 (47.88) | ||
| - CTQ_emotional abuse (continuous) | 8.33 (3.98) | None-minimal | |
| - CTQ_physical abuse (continuous) | 7.14 (3.66) | None-minimal | |
| - CTQ_sexual abuse (continuous) | 6.48 (3.59) | Low-moderate | |
| - CTQ_emotional neglect (continuous) | 10.53 (5.04) | Low-moderate | |
| - CTQ_physical neglect (continuous) | 8.10 (3.42) | Low-moderate | |
| Intimate partner violence (adult) | |||
| Past-year IPV (dichotomous) | |||
| - Emotional IPV | 73 (31.60) | ||
| - Physical IPV | 66 (28.21) | ||
| - Sexual IPV | 32 (13.68) | ||
| Lifetime IPV | |||
| - Emotional IPV | |||
| None | 138 (58.72) | ||
| Any IPV | 97 (41.28) | ||
| Low frequency | 21 (8.94) | ||
| Mid frequency | 54 (22.98) | ||
| High frequency | 22 (9.36) | ||
| - Physical IPV | |||
| None | 149 (63.40) | ||
| Any IPV | 86 (36.59) | ||
| Low frequency | 26 (11.06) | ||
| Mid frequency | 44 (18.72) | ||
| High frequency | 16 (6.81) | ||
| - Sexual IPV | |||
| None | 207 (88.09) | ||
| Any IPV | 28 (11.92) | ||
| Low frequency | 5 (2.13) | ||
| Mid frequency | 14 (5.96) | ||
| High frequency | 9 (3.83) | ||
Almost half of the sample (48 %) scored above threshold on the CTQ (see Measures and variable calculation, above, for threshold scores) with a mean continuous score of 41 (SD 14.5). This suggests a history of some form of abuse in childhood, with a low to moderate severity index. When considering the specific sub-categories assessed on this questionnaire, it was found that the domains of sexual abuse, emotional neglect and physical neglect were scored above threshold (low-moderate severity), when compared to data in the CTQ norm group (2,200 males and females from seven heterogeneous clinical and community samples, representing diverse sociodemographic strata) (Bernstein and Fink 1998).
Using dichotomized scoring, nearly a third of women (32 %) reported a history of emotional IPV; and of physical abuse (28 %), respectively during the previous 12 months. Past-year sexual abuse was reported in 14 % of cases. Similarly, when assessing lifetime IPV experiences on the 4-point frequency scoring scale, almost half of the study sample (41 %) reported emotional abuse; 37 % reported physical abuse; and 12 % reported sexual abuse.
Newborn outcomes (see Table 4)
Table 4.
Newborn outcomes in subsample of mother-infant dyads enrolled in the DCLHS, 2013
| Variable | Site-specific comparison
|
Total sample | ||
|---|---|---|---|---|
| Clinic A | Clinic B | |||
| Birth weight in kilograms (continuous) | Median (IQR) | 3.13 (0.47) | 3.02 (0.52) | 3.07 (0.50) |
| Low birth weight (dichotomous)* | Number (%) | 9 (7.56) | 25 (17.01) | 34 (12.78) |
| WAZ score (continuous) | Median (IQR) | −0.35 (1.02) | −0.66 (1.71) | −0.52 (1.11) |
| Low WAZ score (dichotomous) | Number (%) | 8 (7.02) | 21 (14.29) | 29 (11.11) |
p<0.05
The median (IQR) for birth weight was 3.07 (0.50) kg and for low WAZ score was −0.52 (IQR=1.11). A notable clinic-specific difference was also found in infant birthweight. The median (IQR) for mothers enrolled at Clinic B [3.02 (0.52) kg] was found to be lower than that of Clinic A’s infants [3.13 (0.47) kg]. When dichotomizing birthweights at each clinic, it was found that the prevalence of LBW at Clinic B (17 %) was significantly higher than that at Clinic A (8 %, p=0.022). However, differences in dichotomized WAZ scores between sites again failed to show statistical significance.
Associations between exposure to trauma during pregnancy and newborn outcomes
Bivariate analyses were performed to determine the correlations between a number of trauma-related risk factors and subsequent newborn outcomes. Predictor variables were those pertaining to maternal sociodemographics (age, marital status, income); biomedical risk (height, and composite medical and reproductive risk); psychosocial risk (pregnancy intention, contraceptive use, degree of partner support, depression, PTSD and substance use); and trauma exposure (childhood trauma, lifetime IPV and past-year IPV). Outcome variables were LBW and low WAZ scores (expressed as continuous and dichotomous variables). A number of statistically significant correlations were found – see Table 5.
Table 5.
Bivariate analyses of the correlation between a number of trauma-related risk factors and newborn outcomes in subsample of mother-infant dyads enrolled in the DCLHS, 2013
| Pearson correlation coefficients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prob > |r| under H0: Rho=0 | ||||||||||||
| Number of Observations
| ||||||||||||
| Sociodemographics
|
Biomedical risk
|
Psychosocial risk
|
||||||||||
| Age | Marital status | Income | Height | Composite medical risk |
Composite reproductive risk |
Pregnancy intention | Contraceptive use |
Partner support | Depression
|
|||
| BDI | EPDS | SRQ | ||||||||||
|
| ||||||||||||
| Birthweight | 0.03250 | −0.03831 | −0.01912 | 0.16006 | −0.05180 | 0.01513 | −0.03944 | 0.01094 | −0.06240 | −0.06380 | −0.00570 | −0.04561 |
| 0.6179 | 0.5362 | 0.7599 | 0.0093* | 0.4092 | 0.8059 | 0.5234 | 0.8596 | 0.3515 | 0.3281 | 0.9305 | 0.4875 | |
| 238 | 263 | 258 | 263 | 256 | 266 | 264 | 264 | 225 | 237 | 237 | 234 | |
| WAZ | 0.03641 | −0.03410 | −0.02365 | 0.16884 | −0.06443 | 0.07686 | −0.04786 | 0.00367 | −0.06617 | −0.04060 | −0.00408 | −0.02266 |
| 0.5795 | 0.5857 | 0.7081 | 0.0066* | 0.3093 | 0.2158 | 0.4431 | 0.9532 | 0.3275 | 0.5383 | 0.9507 | 0.7331 | |
| 234 | 258 | 253 | 258 | 251 | 261 | 259 | 259 | 221 | 232 | 232 | 229 | |
| LBW | −0.04602 | 0.06015 | 0.01174 | −0.10513 | −0.02683 | −0.00724 | 0.04504 | 0.04969 | 0.07443 | −0.08664 | 0.02454 | −0.03689 |
| 0.4798 | 0.3312 | 0.8512 | 0.0888 | 0.6692 | 0.9065 | 0.4662 | 0.4214 | 0.2662 | 0.1837 | 0.7070 | 0.5745 | |
| 238 | 263 | 258 | 263 | 256 | 266 | 264 | 264 | 225 | 237 | 237 | 234 | |
| Low WAZ | −0.05286 | 0.01257 | −0.00436 | −0.11495 | −0.02380 | −0.02510 | 0.04732 | 0.06787 | 0.09203 | −0.07450 | 0.05233 | 0.00013 |
| 0.4209 | 0.8408 | 0.9450 | 0.0653 | 0.7075 | 0.6865 | 0.4483 | 0.2765 | 0.1728 | 0.2584 | 0.4276 | 0.9984 | |
| 234 | 258 | 253 | 258 | 251 | 261 | 259 | 259 | 221 | 232 | 232 | 229 | |
| Pearson correlation coefficients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prob > |r| under H0: Rho=0 | ||||||||||
| Number of Observations
| ||||||||||
| Psychosocial risk
|
Trauma exposure
|
|||||||||
| PTSD | Tobacco | Alcohol | Childhood trauma |
Emotional IPV (lifetime) |
Physical IPV (lifetime) |
Sexual IPV (lifetime) |
Emotional IPV (past-year) |
Physical IPV (past-year) |
Sexual IPV (past-year) |
|
|
| ||||||||||
| Birthweight | −0.00364 | −0.22291 | −0.19257 | −0.00910 | −0.09418 | −0.15920 | −0.08001 | −0.07844 | −0.14761 | −0.08612 |
| 0.9562 | 0.0006* | 0.0032* | 0.8894 | 0.1501 | 0.0146* | 0.2217 | 0.2350 | 0.0239* | 0.1893 | |
| 231 | 233 | 233 | 236 | 235 | 235 | 235 | 231 | 234 | 234 | |
| WAZ | 0.01736 | −0.23418 | −0.18142 | −0.01135 | −0.10113 | −0.15502 | −0.08901 | −0.06568 | −0.13623 | −0.09746 |
| 0.7948 | 0.0004* | 0.0060* | 0.8637 | 0.1262 | 0.0187* | 0.1786 | 0.3256 | 0.0394* | 0.1415 | |
| 227 | 228 | 228 | 231 | 230 | 230 | 230 | 226 | 229 | 229 | |
| LBW | −0.02858 | 0.19976 | 0.10378 | −0.02140 | 0.03849 | 0.12961 | 0.10801 | 0.11147 | 0.20782 | 0.08320 |
| 0.6656 | 0.0022* | 0.1141 | 0.7437 | 0.5572 | 0.0472* | 0.0986 | 0.0910 | 0.0014* | 0.2048 | |
| 231 | 233 | 233 | 236 | 235 | 235 | 235 | 231 | 234 | 234 | |
| Low WAZ | −0.04017 | 0.15135 | 0.10210 | 0.04125 | 0.07359 | 0.13511 | 0.09751 | 0.10285 | 0.18039 | 0.07484 |
| 0.5471 | 0.0223* | 0.1242 | 0.5328 | 0.2664 | 0.0406* | 0.1404 | 0.1232 | 0.0062* | 0.2594 | |
| 227 | 228 | 228 | 231 | 230 | 230 | 230 | 226 | 229 | 229 | |
p<0.05
Women with a past-year history of physical IPV exposure were more likely to deliver infants with LBW (r=0.21; p= 0.001) and with low WAZ scores (r=0.18; p=0.006). Further, a history of lifetime physical IPV exposure was found to be associated with both low infant birthweight (r=0.13; p= 0.047) and low WAZ scores (r=0.14; p=0.041). Neither sexual nor emotional IPV exposure (past-year or lifetime) yielded significant associations with the outcomes of interest. Maternal anthropometry and substance use also emerged as important risk factors for low infant birth weight. Maternal height was found to be significantly associated both with birthweight (r=0.16; p=0.009) and with WAZ scores (r= 0.17; p=0.007); as was alcohol use (association with birthweight: r=−0.19; p=0.003; association with WAZ score: r=−0.18; p=0.006). Maternal tobacco use was found to be significantly associated with all four major outcomes of interest – with birthweight (r=−0.22; p=0.0006); WAZ score (r= −0.23; p=0.0004); LBW (r=0.2; p=0.002); and low WAZ score (r=0.15; p=0.02). However, neither depression nor PTSD showed significant correlations with these outcomes.
In the final regression analyses, outcomes of interest were LBW and low WAZ scores; while the predictor variable was past-year physical IPV exposure. (As neither emotional nor sexual abuse showed significant correlations with these outcomes of interest on bivariate analyses, these sub-types were not included as predictor variables in the final regression models). Both models were controlled for study site (clinic), maternal height, ethnicity, socioeconomic status, tobacco use, alcohol use and childhood trauma, due to the effects of these variables on the outcomes of interest. The final regression model run was significant [r2=0.14 (adjusted r2=0.11, F(8, 212) = 4.16, p=0.0001]. Exposure to physical IPV occurring during the past year was found to be significantly associated with LBW [t=−2.04, p=0.0429], when controlling for these potential confounders – see Table 6. A significant association with decreased WAZ scores was not demonstrated.
Table 6.
Multiple linear regression analyses of the association between exposure to IPV during the past year and newborn outcomes in subsample of mother-infant dyads enrolled in the DCLHS, 2013
| Model 1: Multiple linear regression of the association between past-year IPV exposure and LBW
|
Model 2: multiple linear regression of the association between past-year IPV exposure and low WAZ score
|
|
|---|---|---|
| B (SE) | B (SE) | |
| Recent IPV exposure | −0.15 (0.07)* | −0.30 (0.17) |
| Study site (clinic A) | −0.49 (0.28) | −0.79 (0.63) |
| Ethnicity (Black) | 0.56 (0.28)* | 1.01 (0.63) |
| Height | 0.01 (0.004)* | 0.03 (0.01)* |
| Socioeconomic status | 0.02 (0.02) | 0.05 (0.04) |
| Tobacco | −0.006 (0.003) | −0.01 (0.008) |
| Alcohol | −0.008 (0.004) | −0.02 (0.01) |
| Childhood trauma | 0.001 (0.002) | 0.002 (0.005) |
p<0.05
Discussion
The main findings of our study were 1) a large proportion of our study sample was found to have experienced past-year IPV, with exposure to emotional IPV most frequently reported, followed by physical, and then sexual abuse; 2) a notable clinic-specific difference was found in infant birthweight. The median (IQR) for mothers enrolled at Clinic B was found to be lower than that of Clinic A’s infants. When dichotomizing birthweights at each clinic, it was found that the prevalence of LBW at Clinic B was significantly higher than that at Clinic A. However, differences in dichotomized WAZ scores between sites failed to show statistical significance; 3) in this study sample, LBW was found to be significantly predicted by past-year maternal IPV exposure, when controlling for study site (clinic), maternal height, ethnicity, socioeconomic status, substance use and childhood trauma. A significant association with decreased WAZ scores was not demonstrated.
The high prevalence of IPV in our sample is largely consistent with other studies conducted in LMIC regions. For example, in their recent critical review of studies conducted outside of Canada and the United States, Campbell et al. (2004) reported comparatively higher rates of IPV during pregnancy in LMIC regions (31.5 % in Egypt, 21 to 28 % in India, and 21 % in Saudi Arabia), when compared to HIC countries (prevalence rates ranging from 3.4 to 11.0 %). Data from the large WHO Multi-country Study on Women’s Health and Domestic Violence against Women (García-Moreno et al. 2005, 2006) have supported these findings. This study enrolled 24,000 women from 15 sites in 10 culturally diverse countries—Bangladesh, Brazil, Ethiopia, Japan, Namibia, Peru, Samoa, Serbia and Montenegro, Thailand, and the United Republic of Tanzania. The consistency of methodology used in all countries allowed a valid cross-cultural comparison and provided valuable insight into regions from which few data were previously available. A wide variation in IPV prevalence between settings was noted in this study, with comparatively lower rates reported in HIC regions such as Japan (with a 13 % lifetime prevalence of physical IPV and 6 % of sexual IPV) versus LMIC areas such as Peru (61 % of the study population reported physical IPV) and Ethiopia (59 % reported sexual IPV). Further, in countries where both large industrialized (urban) and rural regions were studied, IPV levels were consistently found to be higher in the rural populations. It seems thus that women in LMIC regions (such as South Africa) are at greater risk of IPV exposure during pregnancy than are those in HIC countries.
The varying prevalence of IPV sub-types in our study population is also noteworthy. In a prospective cohort study of 838 women post-delivery in a Chinese university teaching hospital (Leung et al. 2002), it was found that 16.6 % of women (N=139) had experienced recent abuse, with 87 of these (10.4 %) having been abused in the index pregnancy. While these prevalence rates are somewhat lower than in our study sample, most reported cases were of verbal/emotional abuse, a finding echoed in our population. This is noteworthy, as most literature to date has focused on physical and/or sexual IPV, with a relative paucity of data on the impact of verbal/emotional abuse on health outcomes.
Of the three domains of abuse, Leung et al. (2002) also reported the prevalence rate of sexual abuse (1.7 %, N=14) to be the lowest. While this is again lower than was found in our study (ie. 14 % of our participants had experienced past-year sexual abuse), it is noteworthy that sexual abuse appears to be less prevalent than either physical or emotional/verbal abuse in both study samples. One explanation may be underreporting by study participants, based either on the narrow definition of sexual abuse in the IPV questionnaire (ie. forced sexual acts) (see Silverman et al. 2007) or on the widespread stigma associated with this subtype of abuse.
To the best of our knowledge, ours is the first study to examine the prevalence of IPV during pregnancy and its association with low infant birthweight in the South African setting. In our study sample, the median newborn birthweight was found to be within the normal WHO parameters (WHO 1995, 2013b). This is in contrast to the recent WHO/UNICEF (United Nations Children’s Fund) report on country, regional and global estimates of LBW prevalence rates (UNICEF and WHO 2004), which cited the global LBW rate (LBWR) as approximately 15.5 %. In Africa, the reported prevalence is 14.3 %; and 15.4 % in South Africa (UNICEF and WHO 2004; MRC et al. 2003). However, in a recent MRC (Medical Research Council) Saving Babies Report (2003), a LBWR of 19.8 % was cited for the Western Cape. The more significant discrepancy between our findings and this provincial estimate may be attributed to a number of factors. First, the MRC data may be skewed by the much higher LBWR in rural areas in the province. Second, the majority of our study population has received at least some high school education (Grades 8–12). This is relatively high when compared to data published elsewhere. For example, in the South African Demographic and Health Survey (SADHS), a cross-sectional nationally representative study of 13,089 adult men and women (Puoane et al. 2002), it was found that only 11.8 % (N=913) of female participants had completed Grade 12 (secondary education). Third, due to the longitudinal cohort design of the larger Drakenstein Child Lung Health Study, enrolled women enter into the healthcare system early in their antenatal course, and are followed up throughout pregnancy, delivery and the postpartum period. Thus, participants have improved access to antenatal care and health education which may not have otherwise been available to them.
In our study, site-specific differences in infant birthweights were also found, with those at Clinic B lower than those at Clinic A. A number of interrelated factors may explain these differences. For example, the prevalence of substance use among pregnant women was notably higher at Clinic B than at Clinic A. In particular, tobacco use at Clinic B was far more prevalent. Further, in the final regression models, tobacco use was found to be significantly associated with LBW and with low WAZ scores. The association between smoking during pregnancy and adverse infant outcomes has been well-documented in both HIC and LMIC settings. For example, in their early cohort study of 5,166 mother-infant dyads in Pelotas, Brazil, Horta et al. (1997) reported that infants whose mothers had smoked during pregnancy were significantly more likely to have LBW than those of non-smoking mothers (OR 1.59 95 % CI 1.30–1.95). Further, it was also found that the average birthweight of these infants was 142 g lower than that of the non-smoking-exposed group. While no association between smoking and preterm delivery was reported, smoking was found to be significantly associated with intra-uterine growth restriction (OR 2.07 95 % CI 1.69–2.53). More recently, similar findings were reported by Jaddoe et al. (2008). In their nested sub-study of the Generation R Study, a population-based prospective cohort investigation of 7,098 mother-infant pairs conducted in the Netherlands, these authors found that active smoking during pregnancy was significantly associated with both LBW (adjusted OR 1.75 95 % CI 1.20, 2.56) and preterm birth (adjusted OR 1.36 95 % CI 1.04, 1.78). It was also found that active maternal smoking late in pregnancy was most strongly associated with these adverse outcomes.
A significant site-specific difference in composite SES scores was also noted. As this variable is a comprehensive indicator of education, employment status, household income and access to resources and assets, it suggests a notable contextual difference between study sites. There is some evidence that low SES may contribute to increased risk both of IPV (Cunradi et al. 2002) and of low infant birthweight (Parker et al. 1994). In their recent systematic review of 106 studies conducted in industrialized countries, Blumenshine et al. (2010) reported a significant association between low socioeconomic measures and adverse birth outcomes such as LBW and preterm delivery. Socioeconomic disadvantage and disparities were found to be consistently associated with increased risk across socioeconomic measures, birth outcomes and countries. In our study population, however, the site with higher SES had the higher prevalence of low birthweight infants. This presumably reflects the fact, noted above, that smoking is higher in the higher SES site.
In our study, maternal IPV (physical) exposure was found to be significantly associated with low infant birthweight. While there has been a paucity of data examining the association between IPV and adverse infant outcomes in LMIC regions such as South Africa, the data here are consistent with three large studies conducted in Brazil (Ferri et al. 2007), Mexico (Valdez-Santiago and Sanín-Aguirre 1996) and Uganda (Kaye et al. 2006). In the latter study, Kaye et al. (2006) conducted a prospective cohort investigation of 612 women enrolled in the second trimester of pregnancy and followed up until birth. 27.7 % (N=169) of these women reported IPV exposure during pregnancy, and delivered infants with a mean birthweight 186 g lower than that of their non-exposed counterparts. Further, these authors reported that the relative risk of a LBW delivery among IPV-exposed women was 3.78 (95 % CI 2.86–5.00). Additional obstetric complications in this sub-group included hypertension and premature rupture of membranes. While no significant association between IPV exposure and low newborn WAZ scores was demonstrated, it should be noted that LBW and WAZ scores are discrete constructs, and may thus be expected to differ slightly. Nonetheless, both outcomes reflect the adverse effect of IPV exposure during pregnancy. Thus, further work on underlying mechanisms would be useful to delineate whether the slight differences noted here are due to chance, or in fact reflect meaningful pathophysiological differences.
How then, does trauma exposure in women (either during pregnancy or across the lifespan), increase the risk of delivering a LBW infant? To date, this association has been attributed to a number of direct and indirect mechanisms. First, abdominal trauma and consequent placental damage and premature uterine contractions or rupture of membranes may explain a direct causal association (Newberger et al. 1992; Campbell et al. 1999). Further, infection or genital trauma resulting from forced sexual activity may increase the risk of adverse infant outcomes. There is also a growing body of evidence for the role of the hypothalamic-pituitary-adrenal (HPA) axis in mediating LBW delivery in abused women. It has been suggested that pregnant women exposed to psychosocial trauma experience HPA axis hyperactivity, thus resulting in increased circulating cortisol. This stress hormone then has a transplacental inhibitory effect on intrauterine fetal growth (Campbell et al. 1999; Sandman et al. 1997). Further, increased levels of CRH (corticotropin-releasing hormone, which is secreted by the hypothalamus in response to stressful stimuli) may also be associated with premature delivery (Sandman et al. 1997).
Indirect environmental mechanisms may include lack of intimate partner support, substance abuse and maternal mental illness and low socioeconomic status (Campbell et al. 1992; Murphy et al. 2004). While the degree of partner support did now show a significant correlation with low infant birthweight in our study sample; maternal substance abuse was found to be an important potential confounder in the association between IPV and LBW. Thus, both tobacco and alcohol use were controlled for in the final regression analyses. As already discussed, there is a strong body of work documenting the detrimental effect of smoking on pregnancy and newborn outcomes. Further, perinatal alcohol consumption has also been shown to increase the risk of delivering a LBW infant. For example, in their recent systematic review and meta-analysis, Patra et al. (2011) reported a dose–response relationship—heavy alcohol consumption during pregnancy was found to increase the risk of LBW, preterm delivery and a small-for-gestational-age (SGA) infant (while light to moderate alcohol consumption showed no effect).
Additional mediators of the association between IPV and LBW may include low socioeconomic status and maternal mental illness. Both factors are relevant to our economically disadvantaged study sample, in which depressive and post-traumatic symptoms are particularly prevalent. However, neither maternal depression nor PTSD showed a statistically significant correlation with our outcomes of interest in bivariate analyses. The current body of work assessing the association between maternal psychopathology and LBW also remains somewhat inconsistent.
The current study is limited by a number of methodological and analytical factors. First, this is a cross-sectional analysis, and causality is difficult to determine. Second, much of the data concerning antenatal risk factors are dependent on participant self-report. Thus, reporting bias may have led to inaccuracies in prevalence rates of trauma exposure and related variables. Third, data were collected from just one sub-district in South Africa, thus limiting national (and international) generalizability. Nonetheless, as the Drakenstein region is semi-rural, with a stable but low SES and minimal emigration/immigration patterns, it may be viewed as broadly representative of much of South Africa. Fourth, our insights may be limited by the relatively small sample size, high levels of attrition and subsequent reduced statistical power to detect small associations. Data from a notable number of participants (n=56) were omitted from analyses due to poor quality. Given the small sample size, false negative findings cannot be excluded. However, the study was adequately powered to detect clinically meaningful effect sizes, and in turn, several significant associations emerged in the analysis. Thus, our study provides a valuable complement to the small, but growing body of work on the association between IPV during pregnancy and LBW in LMIC regions. Finally, we do not measure non-IPV trauma exposure during pregnancy. While this was a purposeful attempt at a more precise and focused investigation of the sequelae of IPV, an assessment of all types of violence or trauma to which women may be exposed would be a helpful inclusion in future studies. This could potentially delineate whether exposure to any violence/trauma (as opposed to IPV specifically) during pregnancy significantly predicts delivery of a LBW infant.
Future structured longitudinal studies would also be of value in addressing the causal mechanisms between maternal exposure to abuse, and subsequent adverse infant outcomes. Further, an assessment of maternal health outcomes would be a useful addition to this body of work. Methodological adjustments to assessment tools may also be warranted. For example, standardized and cross-culturally valid definitions of IPV could improve data quality (Ballard et al. 1998; Murphy et al. 2004). Finally, a more comprehensive investigation of the role of protective environmental factors (eg. intimate partner support) should be addressed in future studies, in order to enhance our findings and the current evidence base (Ferri et al. 2007).
Such additions to the research field would help to clarify the association between antenatal IPV exposure and low infant birthweight, thus informing not only scientific knowledge but also clinical decision-making and public health policies. Many of the recommendations outlined in the WHO Multi-country Study (García-Moreno et al. 2005, 2006) hold promise. In all cases, intersectoral and multi-disciplinary collaborations seem key in translating empirical data into tangible interventions. For example, a comprehensive primary prevention programme would be integral to minimize the downstream sequelae of IPV. Strategies may include multimedia messages to increase public awareness, alter entrenched gender-related prejudices, reduce IPV-related stigma and enhance informal community support networks. The target audience of such campaigns should include both women and men, and efforts could be carried out in schools, healthcare facilities and workplaces. Primary prevention may also take the form of liaison with the police force and local government to ensure a safer physical environment for women. “High-risk” locales should be identified and resources dedicated to enhance the safety of such areas, eg. by improved lighting and/or increased police presence.
Secondary preventative strategies are also important to support and assist women exposed to IPV. As discussed by García-Moreno et al. (2005, 2006), the public health sector should be mobilized to respond to and manage the multidimensional effects of such abuse. Training of healthcare workers at all service levels should occur to ensure appropriate first-line support of women reporting IPV, as well as appropriate referral when necessary. Again, close collaboration with non-healthcare sectors (eg. the police force and/or legal services) will be essential in developing a comprehensive care network. Of particular relevance to our study, reproductive health services should also be sensitized to contribute to this network. Antenatal booking presents a unique entry point for pregnant women to access care, and for healthcare workers to provide a safe, confidential and supportive environment for women exposed to IPV during pregnancy.
More broadly, informal and formal support networks for victims of IPV should also be strengthened. Unfortunately, resource constraints (particularly in rural areas) continue to limit the availability and accessibility of this crucial level of care. In addition to governmental commitment to improving formal services (eg. social workers, psychologists, counsellors and shelters), informal sources of support such as community leaders should be mobilized. Women may well be more willing to disclose abuse in a less formal milieu, and the involvement of community members would be useful in decreasing the social stigma and shame associated with IPV.
Finally, ongoing support for IPV-related research is essential to inform these and other public health interventions. Data on the causes, prevalence and sequelae of IPV during pregnancy are needed to provide a compelling basis for action. In particular, culturally-specific modifiable risk factors for abuse that are amenable to intervention should be identified and targeted. In LMIC and resource-constrained regions, support from non-governmental and personal donors may well be necessary to supplement governmental commitment. Ultimately, studies such as ours aim to provide a deeper understanding of the magnitude, etiology and impact of IPV during pregnancy, and to contribute to improved primary and secondary prevention strategies.
Acknowledgments
The authors would like to thank colleagues for their helpful comments and contributions:
Colleen Adnams, Whitney Barnett, Leonie Coetzee, Emilee Da Costa, Kirsty Donald, Sheri Koopowitz, Dave le Roux, Crick Lund, Adele Marais, Alina Metje, Bronwyn Myers, Nyasha Nyakutira, Micky Stern, Carlijn Sturm, Mark Tomlinson, Claire van der Westhuizen, Bavanisha Vythilingum.
The entire on-site Drakenstein clinical and research team for its tireless work and commitment.
All the mothers and infants enrolled in the Drakenstein Child Lung Health Study.
Professor Stein is supported by the Medical Research Council of South Africa.
Support for this study was provided by the Bill and Melinda Gates Foundation (grant number OPP1017641), by the UCLA/South African Trauma Training Research (Phodiso) Program, Fogarty International (grant number 1 D43 TW007278) and by the National Institute of Mental Health (NIMH) Brain Disorders in the Developing World: Research Across the Lifespan program (grant number 1R21MH098662-01).
Contributor Information
Nastassja Koen, Email: nastassja.koen@gmail.com, Department of Psychiatry, Groote Schuur Hospital (J2), University of Cape Town, Cape Town, South Africa.
Gail E. Wyatt, Department of Psychiatry & Biobehavioral Sciences, UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles, CA, USA
John K. Williams, Department of Psychiatry & Biobehavioral Sciences, UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles, CA, USA
Muyu Zhang, Department of Psychiatry & Biobehavioral Sciences, UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles, CA, USA.
Landon Myer, Division of Epidemiology & Biostatistics, School of Public Health and Family Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Heather J. Zar, Department of Paediatrics and Child Health, Red Cross War Memorial Children’s Hospital, University of Cape Town, Cape Town, South Africa
Dan J. Stein, Department of Psychiatry, Groote Schuur Hospital (J2), University of Cape Town, Cape Town, South Africa. MRC Unit on Anxiety & Stress Disorders, Cape Town, South Africa
References
- Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Abrahams N, Jewkes R, Martin LJ, Mathews S, Vetten L, Lombard C. Mortality of women from intimate partner violence in South Africa: a national epidemiological study. Violence Vict. 2009;24(4):546–556. doi: 10.1891/0886-6708.24.4.546. [DOI] [PubMed] [Google Scholar]
- Ballard TJ, Saltzman LE, Gazmararian JA, Spitz AM, Lazorick S, Marks JS. Violence during pregnancy: measurement issues. Am J Public Health. 1998;88(2):274–276. doi: 10.2105/ajph.88.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Beamesdefer A. Assessment of depression: the depression inventory. Psychological measurements in psychopharmacology. Mod Probl Pharmacopsychiatry. 1974;7:151–159. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- Beck AT, Steer RA, Brown GK. [Accessed Aug 2011];Beck Depression Inventory-II (BDI-II) 1996a http://www.psychologyafrica.com/pdf/Products/Beck%20Depression%20Inventory%AE-II%20_BDI%AE-II_pdf.
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio: 1996b. [Google Scholar]
- Bernstein D, Fink L. Childhood trauma questionnaire: a retrospective self-report. The Psychological Corporation; San Antonio: 1998. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatr. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic disparities in adverse birth outcomes: a systematic review. Am J Prev Med. 2010;39(3):263–272. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Campbell JC, Poland ML, Waller JB, Ager J. Correlates of battering during pregnancy. Res Nurs Health. 1992;15(3):219–226. doi: 10.1002/nur.4770150308. [DOI] [PubMed] [Google Scholar]
- Campbell J, Torres S, Ryan J, King C, Campbell DW, Stallings RY, Fuchs SC. Physical and nonphysical partner abuse and other risk factors for low birth weight among full term and preterm babies: a multiethnic case–control study. Am J Epidemiol. 1999;150(7):714–726. doi: 10.1093/oxfordjournals.aje.a010074. [DOI] [PubMed] [Google Scholar]
- Campbell JC, García-Moreno C, Sharps P. Abuse during pregnancy in industrialized and developing countries. Violence Against Women. 2004;10(7):770–789. [Google Scholar]
- Collins NL, Dunkel-Schetter C, Lobel M, Scrimshaw SC. Social support in pregnancy: psychosocial correlates of birth outcomes and postpartum depression. J Pers Soc Psychol. 1993;65(6):1243–1258. doi: 10.1037//0022-3514.65.6.1243. [DOI] [PubMed] [Google Scholar]
- Covington DL, Hage M, Hall T, Mathis M. Pretem delivery and the severity of violence during pregnancy. J Reprod Med. 2001;46(2):1031–1039. [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychother. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Cunradi CB, Caetano R, Schafer J. Socioeconomic predictors of intimate partner violence among white, black, and Hispanic couples in the United States. J Fam Violence. 2002;17(4):377–389. [Google Scholar]
- Devries KM, Mak JYT, Garcia-Moreno C, et al. The global prevalence of intimate partner violence against women. Science. 2013;340(6140):1527–1528. doi: 10.1126/science.1240937. Published online June 20, 2013. [DOI] [PubMed] [Google Scholar]
- Dunkle KL, Jewkes RK, Brown HC, Gray GE, McIntryre JA, Harlow SD. Gender-based violence, relationship power, and risk of HIV infection in women attending antenatal clinics in South Africa. Lancet. 2004;363(9419):1415–1421. doi: 10.1016/S0140-6736(04)16098-4. [DOI] [PubMed] [Google Scholar]
- Eberhard-Gran M, Eskild A, Tambs K, Opjordsmoen S, Samuelsen SO. Review of validation studies of the Edinburgh Postnatal Depression Scale. Acta Psychiatr Scand. 2001;104(4):243–249. doi: 10.1034/j.1600-0447.2001.00187.x. [DOI] [PubMed] [Google Scholar]
- Feldman PJ, Dunkel-Schetter C, Sandman CA, Wadhwa PD. Maternal social support predicts birth weight and fetal growth in human pregnancy. Psychosom Med. 2000;62(5):715–725. doi: 10.1097/00006842-200009000-00016. [DOI] [PubMed] [Google Scholar]
- Ferri CP, Mitsuhiro SS, Barros MC, Chalem E, Guinsburg R, Patel V, Prince M, Laranjeira R. The impact of maternal experience of violence and common mental disorders on neonatal outcomes: a survey of adolescent mothers in Sao Paulo, Brazil. BMC Public Health. 2007;7:209. doi: 10.1186/1471-2458-7-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing posttraumatic stress disorder. J Trauma Stress. 1993;6(4):459–473. [Google Scholar]
- García-Moreno C, et al. WHO Multi-country Study on Women’s Health and Domestic Violence against Women: initial results on prevalence, health outcomes and women’s responses. World Health Organization; Geneva: 2005. [Google Scholar]
- Garcia-Moreno C, Jansen HAFM, Ellsberg M, Heise L, Watts CH. Prevalence of intimate partner violence: findings from the WHO multi-country study on women’s health and domestic violence. Lancet. 2006;368(9543):1260–1269. doi: 10.1016/S0140-6736(06)69523-8. [DOI] [PubMed] [Google Scholar]
- Gass JD, Stein DJ, Williams DR, Seedat S. Intimate partner violence, health behaviours, and chronic physical illness among South African women. S Afr Med J. 2010;100(9):582–585. doi: 10.7196/samj.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe KB, Dutton GR, Jones GN, Bodenlos J, Ancona M, Brantley PJ. Validation of the Beck Depression Inventory-II in a low-income African American sample of medical outpatients. Psychol Assess. 2005;17(1):110–114. doi: 10.1037/1040-3590.17.1.110. [DOI] [PubMed] [Google Scholar]
- Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol. 2007;165(8):849–857. doi: 10.1093/aje/kwk071. [DOI] [PubMed] [Google Scholar]
- Harding TW, Arango MV, Baltazar J, Climent CE, Ibrahim HHA, Ignacio LL, Murthy RS, Wig NN. Mental disorders in primary health care: a study of their frequency and diagnosis in four developing countries. Psychol Med. 1980;10:231–241. doi: 10.1017/s0033291700043993. [DOI] [PubMed] [Google Scholar]
- Harpham T, Reichenheim M, Oser R, Thomas E, Hamid N, Jaswal S, et al. Measuring mental health in a cost-effective manner. Health Policy Plan. 2003;18(3):344–349. doi: 10.1093/heapol/czg041. [DOI] [PubMed] [Google Scholar]
- Hartley M, Tomlinson M, Greco E, Comulada WS, Stewart J, le Roux I, Mbewu N, Rotheram-Borus MJ. Depressed mood in pregnancy: prevalence and correlates in two Cape Town peri-urban settlements. [Accessed Oct 2013];Reprod Health. 2011 8:9. doi: 10.1186/1742-4755-8-9. http://www.reproductive-health-journal.com/content/8/1/9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta BL, Victora CG, Menezes AM, Halpern R, Barros FC. Low birthweight, preterm births and intrauterine growth retardation in relation to maternal smoking. Paediatr Perinat Epidemiol. 1997;11(2):140–151. doi: 10.1046/j.1365-3016.1997.d01-17.x. [DOI] [PubMed] [Google Scholar]
- Humeniuk RE, Ali RA, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, Boerngen de Larcerda R, Ling W, Marsden J, Monteiro M, Nhiwhatiwa S, Pal H, Poznyak V, Simon S. Validation of the Alcohol Smoking and Substance Involvement Screening Test (ASSIST) Addiction. 2008;103(6):1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- Jaddoe VW, Troe EJ, Hofman A, Mackenbach JP, Moll HA, Steegers EA, Witteman JC. Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: the Generation R Study. Paediatr Perinat Epidemiol. 2008;22(2):162–171. doi: 10.1111/j.1365-3016.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- Jansen HAFM, Watts C, Ellsberg M, Heise L, García-Moreno C. Interviewer training in the WHO multi-country study on women’s health and domestic violence. Violence Against Women. 2004;10(7):831–849. [Google Scholar]
- Jewkes R. Intimate partner violence: causes and prevention. Lancet. 2002;359(9315):1423–1429. doi: 10.1016/S0140-6736(02)08357-5. [DOI] [PubMed] [Google Scholar]
- Kagee A, Martin L. Symptoms of depression and anxiety among a sample of South African patients living with HIV. AIDS Care. 2010;22(3):381–388. doi: 10.1080/09540120903111445. [DOI] [PubMed] [Google Scholar]
- Kagee A, Nel A, Saal W. Factor structure of the Beck Depression Inventory-II among South Africans receiving antiretroviral therapy. AIDS Care Psychol Socio-med Asp AIDS/HIV. 2013 doi: 10.1080/09540121.2013.802278. [DOI] [PubMed] [Google Scholar]
- Kaye DK, Mirembe FM, Bantebya G, Johansson A, Ekstrom AM. Domestic violence during pregnancy and risk of low birthweight and maternal complications: a prospective cohort study at Mulago Hospital, Uganda. Trop Med Int Health. 2006;11(10):1576–1584. doi: 10.1111/j.1365-3156.2006.01711.x. [DOI] [PubMed] [Google Scholar]
- Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65(5):663–737. [PMC free article] [PubMed] [Google Scholar]
- Lasa L, Ayuso-Mateos JL, Vázquez-Barquero JL, Díez-Manrique FJ, Dowrick CF. The use of the Beck Depression Inventory to screen for depression in the general population: a preliminary analysis. J Affect Disord. 2000;57(1–3):261–265. doi: 10.1016/s0165-0327(99)00088-9. [DOI] [PubMed] [Google Scholar]
- Leung WC, Kung F, Lam J, Leung TW, Ho PC. Domestic violence and postnatal depression in a Chinese community. Int J Gynaecol Obstet. 2002;79(2):159–166. doi: 10.1016/s0020-7292(02)00236-9. [DOI] [PubMed] [Google Scholar]
- Mari JJ, Williams P. A validity study of a psychiatric screening questionnaire (SRQ-20) in primary care in the city of Sao Paulo. Br J Psychiatry. 1986;148:23–26. doi: 10.1192/bjp.148.1.23. [DOI] [PubMed] [Google Scholar]
- Martin SL, Li Y, Casanueva C, Harris-Britt A, Kupper LL, Cloutier S. Intimate partner violence and women’s depression before and during pregnancy. Violence Against Women. 2006;12(3):221–239. doi: 10.1177/1077801205285106. [DOI] [PubMed] [Google Scholar]
- Medical Research Council (MRC) Research Unit for Maternal and Infant Health Care Strategies, PPIP Users, National Department of Health, Saving Babies Technical Task Team. [Accessed 5 Sept 2013];Saving Babies 2003: Fourth perinatal care survey of South Africa. 2003 http://www.ppip.co.za/wp-content/uploads/Saving-babies-2003.pdf.
- Murphy CC, Schei B, Myhr TL, Du Mont J. Abuse: a risk factor for low birth weight? A systematic review and meta-anslysis. CMAJ. 2004;164(11):1567–1572. [PMC free article] [PubMed] [Google Scholar]
- Myer L, Stein DJ, Grimsrud A, Seedat S, Williams DR. Social determinants of psychological distress in a nationally-representative sample of South African adults. Soc Sci Med. 2008;66(8):1828–1840. doi: 10.1016/j.socscimed.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A, Kagee A. The relationship between depression, anxiety and medication adherence among patients receiving antiretroviral treatment in South Africa. AIDS Care. 2013;25(8):948–955. doi: 10.1080/09540121.2012.748867. [DOI] [PubMed] [Google Scholar]
- Newberger EH, Barkan SE, Liebennan ES, et al. Abuse of pregnant women and adverse birth outcome: current knowledge and implications for practice. JAMA. 1992;267:2370–2372. [PubMed] [Google Scholar]
- Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24(3):217–226. doi: 10.1080/09595230500170266. [DOI] [PubMed] [Google Scholar]
- Pagel MD, Smilkstein G, Regen H, Montano D. Psychosocial influences on new born outcomes: a controlled prospective study. Soc Sci Med. 1990;30(5):597–604. doi: 10.1016/0277-9536(90)90158-o. [DOI] [PubMed] [Google Scholar]
- Parker JD, Schoendorf KC, Kiely JL. Associations between measures of socioeconomic status and low birth weight, small for gestational age, and premature delivery in the United States. Ann Epidemiol. 1994;4(4):271–278. doi: 10.1016/1047-2797(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Patra J, Bakker R, Irving H, Jaddoe VW, Malini S, Rehm J. Dose–response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG. 2011;118(12):1411–1421. doi: 10.1111/j.1471-0528.2011.03050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puoane T, Steyn K, Bradshaw D, Laubscher R, Fourie J, Lambert V, Mbananga N. Obesity in South Africa: the South African demographic and health survey. Obes Res. 2002;10(10):1038–1048. doi: 10.1038/oby.2002.141. [DOI] [PubMed] [Google Scholar]
- Rodriguez MA, Heilemann MV, Fielder E, Ang A, Nevarez F, Mangione CM. Intimate partner violence, depression, and PTSD among pregnant Latina women. Ann Fam Med. 2008;6(1):44–52. doi: 10.1370/afm.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumble S, Swartz L, Parry C, Zwarenstein M. Prevalence of psychiatric morbidity in the adult population of a rural South African village. Psychol Med. 1996;26:997–1007. doi: 10.1017/s0033291700035327. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-DeMet A, Dunkel-Schetter C, Porto M. Maternal stress, HPA activity, and fetal/infant outcome. Ann N Y Acad Sci. 1997;814:266–275. doi: 10.1111/j.1749-6632.1997.tb46162.x. [DOI] [PubMed] [Google Scholar]
- Scholte WF, Verduin F, van Lammeren A, Rutayisire T, Kamperman AM. Psychometric properties and longitudinal validation of the self-reporting questionnaire (SRQ-20) in a Rwandan community setting: a validation study. BMC Med Res Methodol. 2011;11:116. doi: 10.1186/1471-2288-11-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamu S, Abrahams N, Temmerman M, Musekiwa A, Zarowsky C. A systematic review of African studies on intimate partner violence against pregnant women: prevalence and risk factors. PLoS One. 2011;6(3):e17591. doi: 10.1371/journal.pone.0017591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JG, Gupta J, Decker MR, Kapur N, Raj A. Intimate partner violence and unwanted pregnancy, miscarriage, induced abortion, and stillbirth among a national sample of Bangladeshi women. BJOG. 2007;114(10):1246–1252. doi: 10.1111/j.1471-0528.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- Sprinkle SD, Lurie D, Insko SL, Atkinson G, Jones GL, Logan AR, Bissada NN. Criterion validity, severity cut scores, and test-retest reliability of the Beck Depression Inventory-II in a university counseling center sample. J Couns Psychol. 2002;49(3):381–385. [Google Scholar]
- Strauss RS, Dietz WH. Low maternal weight gain in the second or third trimester increases the risk for intrauterine growth retardation. J Nutr. 1999;129(5):988–993. doi: 10.1093/jn/129.5.988. [DOI] [PubMed] [Google Scholar]
- Taillieu TL, Brownridge DA. Violence against pregnant women: prevalence, patterns, risk factors, theories, and directions for future research. Aggress Violent Behav. 2010;15:14–35. [Google Scholar]
- The United Nations Children’s Fund and World Health Organization. [Accessed Sept 2013];Low birthweight: country, regional and global estimates. 2004 http://www.unicef.org/publications/index_24840.html.
- Tuan T, Harpham T, Huong NT. Validity and reliability of the self-reporting questionnaire 20 items in Vietnam. Hong Kong J Psychiatry. 2004;14(3):15–18. [Google Scholar]
- Valdez-Santiago R, Sanín-Aguirre LH. Domestic violence during pregnancy and its relationship with birth weight. [Article in Spanish] Salud Publica Mex. 1996;38(5):352–362. [PubMed] [Google Scholar]
- Ventevogel P, De Vries G, Scholte WF, Shinwari NR, Faiz H, Nassery R, van den Brink W, Olff M. Properties of the Hopkins Symptom Checklist-25 (HSCL-25) and the Self-Reporting Questionnaire (SRQ-20) as screening instruments used in primary care in Afghanistan. Soc Psychiatry Psychiatr Epidemiol. 2007;42(4):328–335. doi: 10.1007/s00127-007-0161-8. [DOI] [PubMed] [Google Scholar]
- Villano CL, Cleland C, Rosenblum A, Fong C, Nuttbrock L, Marthol M, Wallace J. Psychometric utility of the childhood trauma questionnaire with female street-based sex workers. J Trauma Dissociation. 2004;5(3):33–41. doi: 10.1300/J229v05n03_03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97(9):1183–1194. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- World Health Organization (WHO) [Accessed Dec 2013];Handbook for Good Clinical Research Practice (GCP) - guidance for implementation. 2002 http://apps.who.int/prequal/info_general/documents/GCP/gcp1.pdf.
- World Health Organization (WHO) [Accessed Dec 2013];Responding to intimate partner violence and sexual violence against women - WHO clinical and policy guidelines. 2013a http://apps.who.int/iris/bitstream/10665/85240/1/9789241548595_eng.pdf. [PubMed]
- World Health Organization (WHO) [Accessed Aug 2013];Health status statistics: morbidity. 2013b http://www.who.int/healthinfo/statistics/indlowbirthweight/en/