Abstract
We analyzed arsenic, cadmium, chromium, lead, manganese, mercury, and selenium in the feathers of common eiders (Somateria mollissima) and tufted puffins (Fratercula cirrhata) from Amchitka and Kiska islands (Aleutians). Between species, puffins had 10 times higher chromium (arithmetic mean = 1820 ppb), 7.5 times higher selenium (mean = 6600 ppb), and 3 times higher mercury (mean = 2540 ppb) than eiders. Eiders had significantly higher levels of manganese than puffins. Puffins are higher on the food chain than eiders, which is reflected in their generally higher levels of metals in their feathers. Interisland differences were generally small, and there were few significant differences as a function of the three nuclear test locations on Amchitka. The only sex-related difference was that female puffins had higher mercury than males (arithmetic mean of 3060 ppb vs. 2270 ppb). Mean levels of metals in the feathers of puffins and eiders from the Aleutians were low compared with comparable studies elsewhere, and the relatively low levels of metals do not indicate the potential for adverse behavioral or reproductive effects in the birds themselves, nor pose concern for other consumers, including subsistence hunters.
Governmental agencies, Tribal Nations, policy-makers, and the general public are concerned about the health of species and their environments, and they require indicators that assess levels of contaminants and their possible effects on the individuals themselves as well as consumers of these organisms. Levels of chemicals are elevated in marine and coastal ecosystems because of the influx from rivers, runoff, point-source pollution, and atmospheric transport and deposition (Burger and Gochfeld 2001; Furness and Rainbow 1990). Many chemicals, such as mercury, are transported all over the world, including to relatively isolated lakes, marshes, and oceanic environments (Fitzgerald 1989; Hammerschmidt et al. 2006; Houghton et al 1992). In some studies, metal levels are higher at higher latitudes and in the northern versus the southern hemisphere (Savinov et al 2003). Marine contamination by metals and other pollutants is a source of concern, and species that forage in aquatic environments are particularly vulnerable because of the potential for rapid movement of contaminants in aquatic food chains, compared to movement in terrestrial environments, and because chemicals in estuarine and shallow marine waters can be stored in bottom sediments (including the intertidal), providing a mobilizable pool for years to come.
Marine birds are useful as bioindicators of pollution (Furness 1993; Furness and Camphuysen 1997; Gochfeld 1980; Peakall 1992; Walsh 1990) because they are exposed to a wide range of chemicals and occupy a wide range of trophic levels. Some species are at the top of the food chain, making them susceptible to bioaccumulation of pollutants (Burger and Gochfeld 2002; Lewis and Furness 1991; Nygard et al 2001). Feathers are useful indicators of metal contamination because (1) they are easy to collect noninvasively and to store for decades or longer, (2) birds sequester metals in their feathers, (3) the proportion of body burden that is in feathers is relatively constant for each metal, (4) a relatively high proportion of the body burden of mercury is stored in the feathers (Burger 1993) due to their affinity for the sulfhydryl-rich melanin pigments, and (5) there is a high correlation between levels of contaminants in the diet of seabirds and levels in their feathers (Burger 1993; Monteiro and Furness 1995). Breast feathers are the best indicator of whole-body burdens (Furness et al. 1986).
Many oceanic islands are far from the main anthropogenic sources of contamination found in temperate regions, such as urban, industrial and agricultural emissions, effluents, and runoff (Mailman 1980), yet even some polar regions have been found to contain pollutants (Metcheva et al 2006; Nygard et al 2001). Oceanic regions are extremely important to the fishing industry. The Bering Sea ecosystem, for example, provides a large percentage of the fish and shellfish for commercial sale in the United States (AFSC 2006). Dutch Harbor in the Aleutians, the port for commercial fish in the Bering Sea, had the highest tonnage of fish landings in the world in 2003 and provides 17% of Alaska’s $811 million fish landings (2.3 million metric tons of fish; NOAA 2004). Understanding contaminant levels in oceanic ecosystems and having bioindicators for these systems are very important. Moreover, in many remote areas, subsistence hunters and Native Americans use wild-caught foods. In the Aleutians, the local Aleut population eats invertebrates, shellfish, fish and birds, including eiders and puffins (Patrick 2002).
In this article we examine the concentrations of arsenic, cadmium, chromium, lead, manganese, mercury, and selenium in the feathers of common eiders (Somateria mollissima) and tufted puffins (Fratercula cirrhata) collected from breeding colonies on Amchitka and Kiska islands in the Aleutian Chain of Alaska. This suite of metals was chosen because they are the primary metals of concern for marine organisms (mercury, cadmium, lead) and because there are other comparative data for the other species (see Burger 1993). Technically, arsenic and selenium are metalloids, but they will be included under the term “metals” in this article. We test the null hypotheses that (1) there were no interspecific differences in metal levels in feathers, (2) there were no interisland differences in metal levels in feathers, (3) there were no locality differences in feathers of adults that nested near the three underground nuclear tests on Amchitka (Long Shot 1965; Milrow 1969; Cannikin 1971), and (4) there were no gender differences in feather levels in common eiders or tufted puffins. Partly we chose these species because eiders are relatively low on the food chain, and puffins are higher on the food chain.
Materials and Analytical Methods
Study Sites and Species
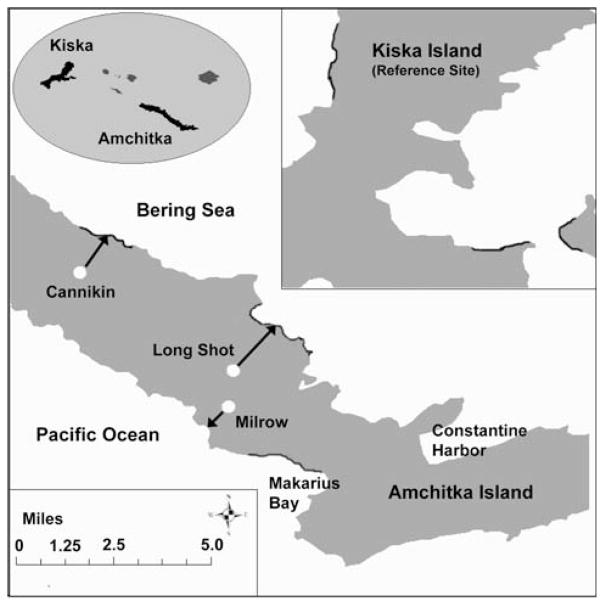
Amchitka Island (Fig. 1; 51° N lat; 179° E long) is part of the Alaska Maritime National Wildlife Refuge system. It contains important ecological resources (Burger et al 2005, 2006a, 2006b; Merritt and Fuller 1977). Amchitka is the only island where the United States detonated underground tests called Long Shot (1965), Milrow (1969), and Cannikin (1971) (Fig. 2). Amchitka is unusual because of its remoteness (remote from coastal runoff with associated metals and other contaminants), depth below ground surface of some of the contamination (radionuclides), and the importance of its ecological resources and seafood productivity that could be at risk if there were significant seepage of radionuclides from Amchitka tests to the marine environment (Burger et al 2006b).
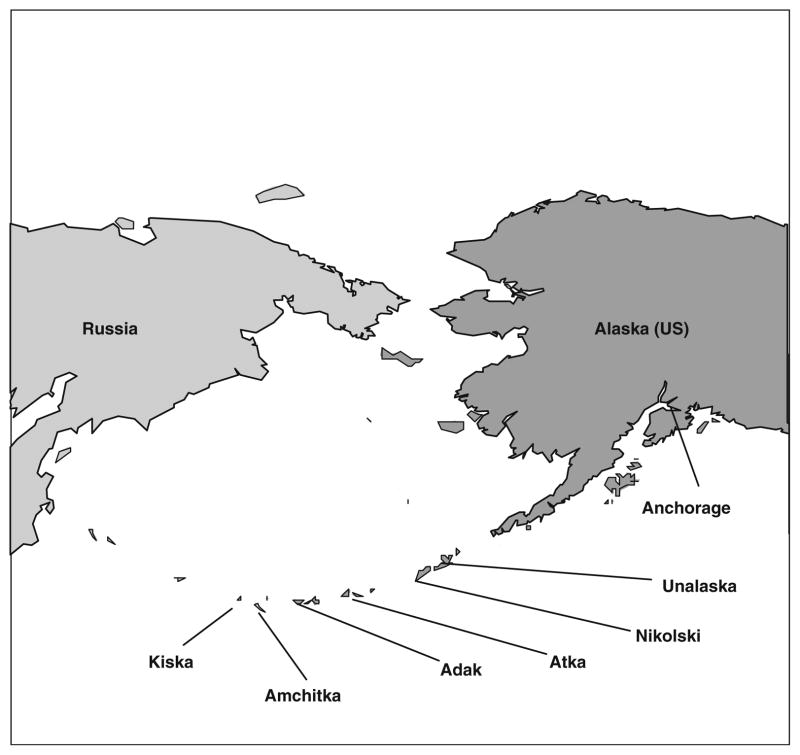
Fig. 1.
Map showing the collecting locations for eiders and puffins at Amchitka and Kiska islands in the Aleutians
Fig. 2.
Map showing the locations of the three underground nuclear test sites on Amchitka. Shading indicates areas where birds were collected
Kiska (51° N lat; 177° E long) had Japanese military occupation during the Second World War but has not been occupied since then. It contains many of the same terrestrial and benthic environments as Amchitka (Burger et al 2006b) and was selected as a reference site. The collection sites on Kiska were at least 140 km west of Amchitka. Thus, these sites were at a sufficient distance from one another that interchange of birds between the islands was very unlikely. Both islands are part of the Alaska Maritime National Wildlife Refuge, originally established in 1913 by executive order of President Taft (ATSDR 2004).
Common eiders reach adult breeding plumage at 2–4 years (Goudie et al 2000) and tufted puffin reach adult plumage at 2–3 years (Piatt and Kitaysky 2002). Tufted puffins breed from warm coastal islands off California to frozen Alaskan habitats (Piatt and Kitaysky 2002), whereas common eiders have a circumpolar breeding distribution (Goudie et al 2000). Both species are highly marine and feed on invertebrates, although puffins also eat small forage fish and squid. On Kiska and Amchitka, common eiders breed in small colonies in grass along flat coastal zones, whereas puffins breed in cracks and crevices on rocky slopes and cliffs.
Collection Protocol
Under appropriate federal and state permits, breast feathers were collected from adult common eiders and tufted puffins nesting at Amchitka and Kiska islands in the Aleutian Chain of Alaska. All birds were collected in July, during the breeding season; when both species were incubating eggs or had recently-hatched chicks. Birds were shot by Aleut hunters (both species are subsistence foods for Aleuts) for the radionuclide work, and samples were taken at this time for metals analysis. Noting the exact timing of collecting is critical because Wayland et al (2005) found that levels of cadmium, mercury, and selenium can vary by time of the year. Breast feathers were placed in individual envelopes and labeled for later identification and were then shipped to Rutgers University for analysis.
Breast feathers were selected because they are considered to be more representative of exposure to metals than other feathers (Burger 1993; Furness et al 1986). Metals enter feathers during the 2–3 weeks it takes for them to grow, then the blood supply atrophies, and there is no further uptake of metals (Burger 1993; Thompson et al 1998). Thus, feathers are an archive of metal exposure during feather formation (Braune and Gaskin 1987). Although there is some variation in metals levels among breast feathers, by using several feathers the differences are averaged.
Adults are exposed to heavy metals and metalloids through their food and water. Once ingested, contaminants can be excreted directly or absorbed into the bloodstream. Subsequently, absorbed metals are delivered to target or storage organs or sequestered in feathers (Braune 1987; Lewis and Furness 1991) or other tissues. In some environments, feathers are susceptible to increased metal accumulation by external contamination (Goede and deBruin 1986; Rose and Parker 1982), although such external contamination might be much less in remote areas, and vigorous washing removes significant external contamination.
Kim et al. (1996) recently suggested that some pelagic seabirds (albatrosses and petrels) are capable of demethylating methylmercury in the liver and storing it as an immobilizable inorganic form; however, the mercury in feathers has been reported to be as high as 100% methylmercury (Thompson et al 1991). Heavy metals in the breast feathers represent circulating concentrations in the blood during the few weeks of feather formation, which, in turn, represents both local exposure and mobilization from internal tissues (Lewis and Furness 1991; Monteiro 1996). Both the eiders and puffins in the western Aleutians remain in the vicinity of the islands, moving offshore between October and April (Kenyon 1961).
The initial study of the marine ecosystem around Amchitka Island, by the multiuniversity Consortium for Risk Evaluation with Stakeholder Participation, was conducted to provide the Department of Energy and the state and federal resource trustees evidence regarding current radionuclide contamination at Amchitka, as well as baseline data for future biomonitoring (Powers et al 2005). Material collected as part of that study was then used for the present metal analysis.
Analytic Methods
All feathers were analyzed in the Elemental Analysis Laboratory of the Environmental and Occupational Health Sciences Institute in Piscataway, NJ. Feathers were washed three times with acetone, washed once with deionized water, air-dried, and then digested individually. A 0.05-g (dry weight) sample was digested in 3 mL ultrex ultrapure nitric acid and 2 mL deionized water in a microwave (MD 2000 CEM), using a digestion protocol of three stages of 10 min each under 50, 100, and 150 psi (3.5, 7, and 10.6 kg/cm2) at 70X power. Digested samples were subsequently diluted to 10 mL with deionized water. All laboratory equipment and containers were washed in 10% HNO3 solution and a deionized water rinse prior to each use (Burger et al. 2001).
Mercury was analyzed by the cold vapor technique, and the other elements were analyzed by graphite furnace–atomic absorption (Burger and Gochfeld 1991). All concentrations are expressed in nanograms per gram (ppb) on a dry weight basis using weights obtained from air-dried specimens.
Detection limit were: 0.02 ppb for cadmium, 0.08 ppb for chromium, 0.15 ppb for lead, 0.09 ppb for manganese, 0.2 ppb for mercury, and 0.7 ppb for selenium. All specimens were analyzed in batches with calibration standards, spiked specimens, and a reference material (DORM-2). Recoveries ranged from 88% to 102%. Batches with recoveries of less than 85% were reanalyzed. The coefficient of variation on replicate, spiked samples ranged up to 10%.
Kruskal–Wallis nonparametric analysis of variance was performed using the SAS PROC NPAR1WAY with the Wilcoxon option yielding a chi square statistic (SAS 1995). Both arithmetic and geometric means are given to facilitate comparisons with other studies. We accept a probability level of 0.05 as significance, but in view of sample size limitations present, all probability values below 0.10 are given to allow the reader to assess the significance for themselves.
Results
There were interspecific differences in the concentrations of metals in the feathers, except for arsenic and lead (Table 1). Puffins had significantly higher levels of chromium (10 times higher), selenium (7.5 times higher), and mercury (3 times higher) in their feathers than did common eiders. Eiders had significantly higher levels of manganese (three times higher) in their feathers than did puffins.
Table 1.
Metal levels (ng/g = ppb, dry weight) in Common eider feathers and tufted puffin feathers collected at Amchitka and Kiska islands, Aleutians, Alaska
| Feathers | Common eider (n = 26) | Tufted puffin (n = 39) | Kruskal–Wallis χ2 (p) |
|---|---|---|---|
| Arsenic | 138 ± 18.0 107 |
136 ± 25.6 15.0 |
1.1 (NS) |
| Cadmium | 79.8 ± 4.0 77.0 |
80.3 ± 12.9 49.1 |
4.2 (0.04) |
| Chromium | 172 ± 49.9 34.6 |
1820 ± 230 1450 |
42.0 ( <0.0001) |
| Lead | 992 ± 132 828 |
1260 ± 339 104 |
2.2 (NS) |
| Manganese | 1870 ± 267 1460 |
622 ± 58.1 531 |
23.6 ( <0.0001) |
| Mercury | 840 ± 81.5 715 |
2540 ± 195 2120 |
26.9 ( <0.0001) |
| Selenium | 878 ± 88.3 731 |
6600 ± 344 6110 |
42.7 ( <0.0001) |
Note: For each metal, the first row provides the arithmetic mean ± standard error and the second row gives geometric means. Statistical comparison using Kruskal–Wallis nonparametric ANOVA yielding chi square statistics (p-values). All numbers are rounded to three significant figures
There were few interisland differences in metal levels in feathers from Amchitka and Kiska, and where there were differences, they were not great (Table 2). For the three test sites on Amchitka, we combined Long Shot and Cannikin because they are quite close and located on the same side of Amchitka (Bering Sea), whereas Milrow is on the Pacific Ocean side. There were no significant differences for feathers, except that manganese levels were higher in puffin feathers from near Long Shot than near Milrow (Table 3).
Table 2.
Metal levels (ng/g = ppb dry weight) in feathers of adult common eider and adult tufted puffin collected from Amchitka and Kiska
| Feathers Common eider | Amchitka n = 13 | Kiska n = 13 | Kruskal–Wallis χ2 (p) |
|---|---|---|---|
| Arsenic | 126 ± 25.3 101 |
151 ± 26.1 114 |
0.8 (NS) |
| Cadmium | 80.9 ± 6.0 77.5 |
78.6 ± 5.4 76.4 |
0.6 (NS) |
| Chromium | 103 ± 30.3 14.8 |
242 ± 92.9 81.0 |
1.5 (NS) |
| Lead | 832 ± 212 653 |
1154 ± 151 1050 |
4.8 (0.03) |
| Manganese | 2560 ± 440 1990 |
1177 ± 154 1070 |
4.8 (0.03) |
| Mercury | 846 ± 143 659 |
834 ± 84.9 776 |
0.3 (NS) |
| Selenium | 868 ± 132 722 |
888 ± 123 741 |
0.1 (NS) |
|
| |||
| Tufted puffin | n = 22 | n = 17 | |
|
| |||
| Arsenic | 161 ± 39.8 20.9 |
103 ± 27.8 9.8 |
0.8 (NS) |
| Cadmium | 103 ± 20.1 66.6 |
51.4 ± 11.3 33.1 |
3.7 (0.05) |
| Chromium | 2040 ± 342 1590 |
1520 ± 284 1280 |
0.2 (NS) |
| Lead | 926 ± 423 29.6 |
1680 ± 550 525 |
5.7 (0.02) |
| Manganese | 696 ± 83.4 601 |
527 ± 74.9 452 |
2.1 (NS) |
| Mercury | 2590 ± 290 2020 |
2480 ± 254 2260 |
0.1 (NS) |
| Selenium | 6580 ± 523 5910 |
6620 ± 424 6390 |
0.0 (NS) |
Note: For each metal, the first row provides the arithmetic mean ± standard error and the second row provides geometric means. Comparison with Kruskal–Wallis nonparametric ANOVA yielding chi square statistics and p values. All entities are rounded to three significant figures
Table 3.
Comparison of metal levels in adult tufted puffin feathers from Milrow area and Long Shot area
| Feathers | Milrow (n = 4) | Long Shot–Cannikin (n = 18) | Kruskal–Wallis χ2 (p) |
|---|---|---|---|
| Arsenic | 131 ± 77.7 19.2 |
166 ± 49.1 18.8 |
0.05 (NS) |
| Cadmium | 82.8 ± 54.2 37.9 |
107 ± 23.4 73.9 |
0.8 (NS) |
| Chromium | 1780 ± 452 1610 |
2130 ± 432 1590 |
0.07 (NS) |
| Lead | 510 ± 220 37 |
1080 ± 543 45.6 |
0.07 (NS) |
| Manganese | 365 ± 81.2 342 |
786 ± 96 695 |
5.4 (0.02) |
| Mercury | 3250 ± 401 3170 |
2450 ± 358 1780 |
0.8 (NS) |
| Selenium | 5670 ± 711 5510 |
6780 ± 654 5970 |
1.1 (NS) |
| Weight | 754 ± 41.5 | 806 ± 18.3 | 1.4 (NS) |
Note: The rirst row provides the arithmatic mean ± standard error. The second row provides the geometric mean. Comparison by Kruskal–Wallis nonparametric one-way ANOVA yielding the chi square statistic
There were no gender-related differences in metal levels in eiders, and there was only one such difference for tufted puffins (Table 4). Female puffins had significantly higher levels of mercury in feathers than did males (geometric mean 2720 vs. 1850 ppb), which is surprising given that females can rid their bodies of heavy metals through sequestration in eggs.
Table 4.
Metal concentrations (ng/g = ppb on dry weight) in adult common eider and adult tufted puffin feathers by gender
| Feathers Common eider | Male n = 6 | Female n = 20 | χ2 (p) |
|---|---|---|---|
| Body weight (g) | 2260 ± 85.1 | 1670 ± 52.6 | 11.4 (0.0007) |
| Arsenic | 169 ± 46.7 139 |
128 ± 18.5 97.9 |
1.1 (NS) |
| Cadmium | 78.8 ± 5.2 77.9 |
80.0 ± 4.97 76.7 |
0.1 (NS) |
| Chromium | 89.9 ± 44.7 7.7 |
197 ± 52.9 54.3 |
1.1 (NS) |
| Lead | 743 ± 138 682 |
1070 ± 164 877 |
0.9 (NS) |
| Manganese | 1270 ± 397 1010 |
2040 ± 320 1630 |
1.5 (NS) |
| Mercury | 782 ± 166 566 |
857 ± 95.5 767 |
0.0 (NS) |
| Selenium | 665 ± 144 533 |
942 ± 104 804 |
1.3 (NS) |
|
| |||
| Tufted puffin | n = 25 | n = 13 | |
|
| |||
| Body weight (g) | 819 ± 11.4 | 738 ± 15.1 | 14.0 (0.0002) |
| Arsenic | 119 ± 34.3 5.9 |
164 ± 39.8 75.6 |
2.3 (NS) |
| Cadmium | 68.8 ± 14.9 39.7 |
101 ± 25.8 69.5 |
1.1 (NS) |
| Chromium | 1960 ± 336 1470 |
1550 ± 242 1390 |
0.0 (NS) |
| Lead | 1570 ± 505 339 |
750 ± 252 22.1 |
1.1 (NS) |
| Manganese | 694 ± 80.7 594 |
495 ± 68.8 430 |
2.0 (NS) |
| Mercury | 2270 ± 231 1850 |
3060 ± 350 2230 |
4.0 (0.05) |
| Selenium | 6600 ± 430 6280 |
6590 ± 643 5770 |
0.3 (NS) |
Note: The first row provides the arithmetic mean ± SE and the second row provides the geometric mean. The right-hand column compares differences using Kruskal–Wallis nonparametric one-way ANOVA, yielding chi square values and p-values. All numbers are rounded to three significant figures
The correlations among metals levels within feathers were generally weak, although there were some significant relationships (Table 5). The strongest correlation was between chromium and mercury in the feathers of both puffins and eiders. Many studies have reported a correlation between mercury and selenium for internal tissues, and in this study, they were significantly correlated only for eider feathers (Table 5). Overall, there were few correlations for the feathers of eiders (4 of 21) and puffins (7 of 21; Table 5). Arsenic and manganese were correlated with body weight for eiders, and manganese and mercury were correlated with weight for puffins.
Table 5.
Intermetal correlations for common eider (above the diagonal, n = 26) and tufted puffins (below the diagonal, n = 39) from Amchitka and Kiska combined
| Arsenic | Cadmium | Chromium | Lead | Manganese | Mercury | Selenium | Weight | |
|---|---|---|---|---|---|---|---|---|
| Arsenic | ********* | NS | NS | NS | NS | NS | NS | 0.3 (0.03) |
| Cadmium | NS | ********* | NS | NS | NS | NS | NS | NS |
| Chromium | NS | 0.3 (0.008) | ********* | 0.3 (0.02) | NS | 0.4 (0.01) | 0.3 (0.05) | NS |
| Lead | NS | NS | NS | ********* | NS | NS | NS | NS |
| Manganese | NS | 0.3 (0.01) | NS | NS | ********* | NS | NS | −0.3 (0.02) |
| Mercury | NS | 0.2 (0.03) | 0.4 (0.0005) | NS | NS | ********* | 0.3 (0.02) | NS |
| Selenium | 0.3 (0.06) | NS | NS | NS | NS | NS | ********* | NS |
| Weight | NS | NS | NS | NS | 0.2 (0.07) | −0.1 (0.03) | NS | ********* |
Note: Kendall tau correlations (p-values); NS indicates p-values>0.10
Discussion
To varying extents, metals can bioaccumulate to reach toxic levels that can lower survival and reproductive success in birds (Bargagli et al 1998; Burger 1993; Eisler 1987; Scheifler et al 2005). Because some pollutants undergo biomagnification up the food chain, their concentrations have often been studied in top-level predators such as raptors and fish-eating birds (Burger 2002; Burger and Gochfeld 1997, 2002, 2004; van Straalen and Ernst 1991). In this study, however, we examined mercury, lead, cadmium, and other metals in the feathers of eiders (relatively low on the food chain) and puffins (higher on the food chain). There are three key questions concerning the metals data from this study: (1) What are the causes of differences among the species? (2) What accounts for any interisland or within-Amchitka differences? (3) How does one think about the lack of gender-related differences? (4) How do these levels compare to those of birds from other areas? (5) Are the levels sufficiently high to cause adverse effects in the birds themselves or in consumers of the birds? Each of these questions is discussed next.
Interspecific Differences
Interspecific differences in metals levels in the feathers of birds could result from differences in diet (in either foods eaten, sizes of prey, or proportion of prey or other food items), absorption rates, time in the vicinity of the colony, and location of the birds at the time of molt when breast feathers were formed, as well as a possible genetic component (Kelsall and Calaprice 1972). Although both species are resident in the Aleutians, the degree that they wander from the immediate vicinity of the breeding colonies is unknown.
In this study, tufted puffins had significantly higher levels of chromium, lead, mercury, and selenium in their feathers than did common eiders, and eiders had significantly higher levels of manganese. In general, we attribute these differences to differences in diet. Common eiders eat mainly lower-trophic-level benthic invertebrates in the intertidal and subtidal regions, but sometimes they eat crustaceans and echinoderms (Goudie et al 2000). In the Pacific/Bering Sea regions, the diet of common eiders was 46% mollusks, 31% crustaceans, and 14% echinoderms (Cottam 1939). In contrast, tufted puffins feed mainly on small schooling fishes during the breeding season, although they also eat invertebrates such as squid (Piatt and Kitaysky 2002). In the Aleutians, however, diets vary by location, with squid accounting for 80% of the items in puffin diets in the western Aleutians but only 17% of diets in the eastern Aleutians (Piatt and Kitaysky 2002); fish made up 22% of diets in the eastern Aleutians. Walleye pollock, sandlance, and greenling were all important fish in the diet of puffins. Thus, to truly understand the diet of puffins, site-specific information is necessary, derived from either stomach content analysis or stable isotopic studies (Braune et al 2002). Nonetheless, it is clear that puffins normally eat a higher percent of higher-trophic-level fish and squid than do eiders, perhaps accounting for the differences.
The greatest differences between species were for chromium (10 times higher in puffins than eiders) and selenium (7.5 times higher in puffins than eiders). Mercury levels were only three times higher in puffins than eiders. These differences should reflect differences in diet for the birds foraging around Amchitka and Kiska islands, a region in which dietary data were not reported (see Piatt and Kitaysky 2002). The data that are reported, however, indicated that puffins eat more fish and higher-trophic-level invertebrates than eiders (references above), accounting for these differences.
The one unexpected result was that eiders had significantly higher levels of manganese than puffins (by three times). Further, manganese levels were significantly higher in eiders collected from the Long Shot/Cannikin area compared to those from Milrow. Manganese is a metal that is regulated internally, suggesting that eiders can sequester manganese in their feathers to rid internal tissues of manganese. The differences might also suggest that eiders can tolerate higher levels and that they were exposed to higher levels, perhaps through their invertebrate prey. Relatively few studies with feathers (or with internal tissues) examine manganese, making it difficult to compare eiders with other species. These findings suggest a need to examine manganese levels in feathers of other seabirds as a bioindicator of possible exposure and bioaccumulation. Because manganese has been used as a fuel additive and increased exposure has some human health implications (Cooper 1984), it is important to learn more about its movement through the food chain.
Interisland and Within-Amchitka Differences
Locational differences in metal levels could derive from differences in local exposure, atmospheric deposition, or foraging regimes; that is, adult eiders and puffins living on the different islands could eat different foods, of different sizes, and in different proportions. Further, exposure on Amchitka and Kiska could differ in the same foods due to differences in anthropogenic sources. Both islands were occupied by military operations in World War II. Amchitka was also occupied during the underground nuclear test shots and again during Department of Energy cleanup. Kiska Island was occupied by the Japanese during World War II. In addition to the point sources (such as military activities on both islands, nuclear testing on Amchitka), metals derive from global transport and fallout and oceanic transport. Global transport generally confers a uniform distribution within a local area, although there are regional variations attributable to precipitation regime (Simon et al 2004).
For most studies of metals in feathers, it is also important to understand both the timing and pattern of molt and where birds were at the time of the molt. Both species remain in the Aleutians all year, although they might wander offshore. We found few significant interisland differences, and, more importantly, these differences were not large, suggesting that they might have been due to small differences in diet of birds on the different islands. The only difference that was consistent for the two species was lead, which was higher on Kiska than Amchitka. The only military difference between Amchitka and Kiska is that Kiska was also occupied by the Japanese during World War II, and extensive bombing by Americans, sinking of ships, and other activities may have resulted in higher lead levels in the environment.
In a related study examining radionuclides and mercury in gulls (Larus glaucescens) and eiders (Somateria mollissima) from these same islands, we found that gull feather (L. glaucescens) levels were significantly higher for lead and manganese on Kiska than on Amchitka (Burger and Gochfeld 2007; Burger et al 2008). Thus, the finding of higher levels of lead on Kiska than on Amchitka was consistent for feathers of eiders, gulls, and puffins, birds of different trophic levels. This suggests that lead levels are higher in a wide range of prey of these species around Kiska than Amchitka. This was surprising to us, given that Amchitka had a much longer period of military and nuclear-related occupation.
Rocque and Winker (2004) examined levels of several metals in the livers of rock sandpipers (Calidris ptilocnemis) in Attu, Adak, Amlia, and the Alaskan Peninsula and found that arsenic was highest in Attu, mercury was highest in the Alaska Peninsula, and there were no differences in the other metals. For cormorant (Phalacrocorax spp.) livers, Attu had the highest levels of cadmium, and there were no significant differences for the other metals. Using a conversion factor from Burger (1993), mercury levels were higher in sandpipers from the Alaska Peninsula (converted, 5888 ppb) than in puffins from the Amchitka/Kiska Islands (this study, 2541 ppb). For cadmium, sandpiper levels at Attu were higher (converted, 960 ppb) than for the puffins (80 ppb) from Amchitka/Kiska. For chromium, the mean levels in sandpipers in Adak were higher (2524 ppb) than those we found for puffins (1816 ppb) at Amchitka/Kiska. For selenium, the mean levels for cormorant (4708 ppb) were also higher than for the feathers puffins (6597 ppb) at Amchitka/Kiska. There are not as much data for arsenic in feathers. However, in Franklin’s gulls (Larus pipixcan), the ratio of feather: liver for arsenic was 1.5 for adults (Burger and Gochfeld 1996). Using this conversion, the concentration of arsenic in sandpipers (1090 ppb) was lower than for the puffins (1360 ppb) from Amchitka/Kiska. These conclusions, however, are based on two conversions: extrapolating the levels in feathers from those in liver, and converting dry weight to wet weight. Nonetheless, Rocque and Winker (2004) found that levels were generally higher in the sandpipers than the cormorants, the fish-eating species.
It is remarkable that most studies that examine metal levels in internal tissues of birds do not also measure levels in feathers (which would contribute to finer feather:tissue ratios). We suggest that scientists dealing with metals need to provide wet–dry conversions (because in drying specimens, such conversions are determined) and that there is a need for more studies that examine levels in feathers and other tissues to allow for conversions. This is particularly important because feathers are often used as convenient, noninvasive bioindicators. Further, feathers from museum collections can be used to examine temporal trends for metals not used in the preservation process (e.g., arsenic, mercury), and it is often possible to collect feathers from endangered or threatened species (whereas the collection of other tissues are prohibited to preserve dwindling populations). Alternatively, eggs can prove useful as bioindicators, particularly when birds lay large clutches or will lay replacement clutches.
Gender Differences
Most studies of metals levels in birds that use feathers as a bioindicator do not know the sex of the birds because many species, particularly seabirds, do not exhibit sexual dimorphisms. However, in this study, we collected the birds and identified them to sex. There were no sexual difference in metals levels in the feathers of eider (even though males were significantly larger) and only one difference for puffins. Male puffins had lower mercury levels in their feathers than did females, even though there was no significant weight difference. We had expected that if there were sexual differences, males would have higher levels because females have an additional method of ridding their body of mercury; females can sequester metals, such as mercury, in their eggs (Burger 1993; Fimreite et al 1974, 1982).
Burger (1993) summarized studies of gender-related differences in metal levels in feathers and noted that for eight studies, differences were noted only for three of the eight species. In only one species were levels higher in males than females (Bonaparte’s gull, Larus philadelphia). Since then, few studies have examined these differences. Gender did not enter as a significant variable for metal levels in feathers of albatrosses (Diomedea immutabilis; Burger and Gochfeld 2000d), but Gochfeld et al (1996) found that male laughing gulls (Larus atricilla) had higher levels of lead in feathers than did females (they examined five other metals without effect).
One difficulty with using feathers as bioindicators of internal dose is that it is often impossible to identify the sex, especially in seabirds. Even studies of metal levels in internal tissues often fail to examine gender differences because outside of the breeding season, identification by morphological examination is also often difficult. This illustrates the importance of noting gender where possible, even if the differences in metals levels by gender are not significant.
Regional Differences
It is also useful to examine metal levels in feathers of other seabirds from the Pacific region and for the same species of birds from other regions. Burger and Gochfeld (2000a) examined metals levels in the feathers of 12 species of seabirds from Midway Atoll (28° 15′ N, 177° 20′ W) in the northern Pacific Ocean, some 2875 km south from Amchitka and Kiska. Midway was used for communications since the early 1900 s and served as a military base during and after World War II until 1997, when it was taken over by the US Fish & Wildlife Service. Midway was thus used for military operations for longer, and more intensely, than the Aleutian Islands, suggesting that contaminant levels from local sources would be higher than those found in Amchitka birds. Species examined from Midway included terns, noddies, shearwaters, tropicbirds, and albatrosses. For all metals, the mean levels in feathers of puffins and eiders in the Aleutians were well below the species with the highest means from Midway: arsenic: mean of 136 ppb for puffin, 459 ppb for white tern Gygis alba; cadmium: 80 ppb for puffin, 950 ppb for gray-backed tern Sterna lunata; chromium: 1816 ppb for puffin, 6570 ppb for Laysan albatross (Diomedea immutabillis); lead: 1255 ppb for puffin, 2380 ppb for Christmas shearwater (Puffinus nativitatis); manganese: 1866 ppb for eider, 2050 ppb for Christmas shearwater; mercury: 2541 ppb for puffin, 19,700 ppb for Bonin petrel (Pterodroma hypoleuca); selenium: 6597 ppb for puffin, 10,100 ppb for Christmas shearwater (Burger and Gochfeld 2000a, d). In most of these studies, the variation was similar. Given the higher human use of Midway, compared to the Aleutian Islands, it is not surprising the levels were higher in the feathers of birds from Midway compared to Amchitka and Kiska.
Significance of Levels
Feathers can serve as an indicator of internal contamination (Burger 1993; Goede and deBruin 1986; Furness et al 1986) and can then be used to assess whether there are potential behavioral or reproductive deficits in populations (Burger and Gochfeld 2001). Laboratory studies have been used to identify the levels of metals that result in adverse impacts on the behavior, physiology, or reproductive success of birds. In general, mercury, cadmium, and lead are of primary concern in marine environments (Thompson and Furness 1998), and each will be discussed next. Levels of metals in birds are also of interest because of potential effects on predators that might eat them (either as prey or as carrion). Both puffins and eiders are also a preferred Aleut food, and bird eggs are a delicacy during the early egg-laying and incubation period (Fish & Wildlife Service 2004; Hamrick and Smith 2003; Patrick 2002).
Mercury is the contaminant of most concern in marine ecosystems (Mailman 1980; Thompson and Furness 1998). In general, mercury levels in feathers that are associated with adverse reproductive effects in birds are 5000 ppb (Burger and Gochfeld 2000b; Eisler 1987). Concentrations of 15,000 ppb mercury are required for adverse effects in some predatory fish (Spry and Wiener 1991; Wiener and Spry 1996). Thus, the levels in the feathers of both puffins and common eiders from Amchitka and Kiska were well below the levels known to cause adverse effects in the birds themselves, or in their predators.
Lead is a second contaminant of concern in marine environments. Adverse effects in birds occur at lead levels of 4000 ppb in feathers (Burger and Gochfeld 2000c; Custer and Hoffman 1994). The levels in feathers of eiders and puffins from Amchitka and Kiska were well below this threshold level, suggesting that they are not adversely impacted.
Cadmium causes sublethal and behavioral effects at lower concentrations than lead and mercury (Eisler 1985), but feather levels known to cause adverse effects in the birds themselves have not been determined from laboratory studies. However, conversion factors developed from Burger (1993) suggest that feather levels that are associated with adverse effects would range from 100 ppb (shearwaters) to 2000 ppb (terns). Thus, the cadmium levels found in puffin and eider feathers from Amchitka and Kiska were well below the calculated effects level.
There are few controlled laboratory studies for the other metals, making it difficult to interpret the significance of these levels. However, for selenium, feather levels of 3800–26,000 ppb (depending on species) result in mortality (converted after Burger 1993), and 1800 ppb results in sublethal adverse effects (after Heinz 1996), but these conclusions are very tentative, and laboratory studies are required to establish the effects levels. The levels of selenium in feathers of puffins (6597 ppb) exceeded the 1800-ppb level and those of eiders were similar (1728 ppb), suggesting that selenium should receive more study for birds from the region.
Thus, overall, the levels of metals in feathers do not suggest any adverse effects in the birds from the Aleutians.
Conclusion
In many remote oceanic islands and arctic regions, birds are an important part of the food chain and of subsistence diets. The levels of mercury and other metals in feathers of eiders and tufted puffins were used as bioindicators of potential hazard to the birds themselves, to predators that eat them, and to humans who consume them as part of a subsistence diet. Levels of mercury and other metals were sufficiently low that they did not pose a risk to the birds themselves and to their consumers. Puffins, higher on the food chain, had much higher levels of chromium, selenium, and mercury than did eiders. There differences in levels of metals among islands were small, and probably insignificant biologically.
Acknowledgments
Feathers were collected under appropriate state and federal permits (04-079, MBO-86658-0, and 22833), and our studies were approved by the Rutgers University Animal Review Board. We thank the many people who contributed to the development and execution of CRESP’s Amchitka Geophysical and Biological Project, especially C. W. Powers, D. Kosson, B. Friedlander, C. Jeitner, S. Burke, D. Volz, and S. Jewett. We also thank the following for help throughout the project: D. Barnes, L. Duffy, A. Morkill, R. Patrick, D. Rogers, D. Dasher, and the people of the villages of Unalaska, Nikolski, Atka, and Adak in the Aleutians. Technical help was provided by T. Shukla, S. Shukla, M. Donio, C. Chin, A. Qu, and C. Lamptey. We thank the entire crew of the Ocean Explorer, Captain Ray Haddon, mate Glenn Jahnke, cook Don Dela Cruz, and Bill Dixon, Joao Do Mar, and Walter Pestka, for making our field work possible and pleasant and for bringing us safely back to port. This research was funded by the Consortium for Risk Evaluation with Stakeholder Participation (CRESP) through the Department of Energy (DE-FG 26-00NT 40938, DE-FC01-86EW07053), the Division of Life Sciences of Rutgers University, by Wildlife Trust, and by NIEHS ESO 5022.
Footnotes
The results, conclusions, and interpretations reported herein are the sole responsibility of the authors and should not in any way be interpreted as representing the views of the funding agencies.
Contributor Information
Joanna Burger, Email: burger@biology.rutgers.edu, Division of Life Sciences, Rutgers University, 604 Allison Road, Piscataway, NJ 08854-8082, USA. Consortium for Risk Evaluation with Stakeholder Participation (CRESP), Piscataway, NJ, USA. Environmental and Occupational Health Sciences Institute (EOHSI), Piscataway, NJ, USA.
Michael Gochfeld, Division of Life Sciences, Rutgers University, 604 Allison Road, Piscataway, NJ 08854-8082, USA. Environmental and Occupational Health Sciences Institute (EOHSI), Piscataway, NJ, USA. Environmental and Occupational Medicine, UMDNJ-Robert Wood Johnson Medical School, Piscataway, NJ, USA.
References
- AFSC (Alaska Fisheries Science Center) [accessed 8 August 2008];Alaska fisheries: Pollock 2007. 2006 Available from http://www.afsc.noaa.gov/species/pollock.php.
- ATSDR. [accessed 8 August 2008];Public health assessment: Naval Air Facility, Adak. 2004 Available from http://www.atsdr.cdc.gov/hac/PHA/adak/ada_toc.html.
- Bargagli R, Monaci F, Sanchez-Hernandez JC, Cateni D. Biomagnification of mercury in an Antarctic marine coastal food web. Marine Ecol Progr Series. 1998;169:65–76. [Google Scholar]
- Braune BW. Comparison of total mercury levels in relation to diet and molt for nine species of marine birds. Arch Environ Contam Toxicol. 1987;16:217–224. [Google Scholar]
- Braune BW, Donaldson GM, Hobson KA. Contaminant residues in seabird eggs from the Canadian Arctic. II. Spatial trends and evidence from stable isotopes for intercolony differences. Environ Poll. 2002;117:133–145. doi: 10.1016/s0269-7491(01)00186-5. [DOI] [PubMed] [Google Scholar]
- Braune BW, Gaskin DE. Mercury levels in Bonaparte’s gull (Larus Philadelphia) during autumn molt in the Quoddy region, New Brunswick, Canada. Arch Environ Contam Toxicol. 1987;16:539–549. [Google Scholar]
- Burger J. Metals in avian feathers: bioindicators of environmental pollution. Rev Environ Toxicol. 1993;5:203–311. [Google Scholar]
- Burger J. Food chain differences affect heavy metals in bird eggs in Barnegat Bay, New Jersey. Environ Res. 2002;90:33–39. doi: 10.1006/enrs.2002.4381. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Cadmium and lead in common terns (Aves: Sterna hirundo): relationship between levels in parents and eggs. Environ Monit Assess. 1991;16:253–258. doi: 10.1007/BF00397612. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Heavy metal and selenium levels in Franklin’s Gull (Larus pipixcan) parents and their eggs. Arch Environ Contam Toxicol. 1996;30:487–491. doi: 10.1007/BF00213400. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Risk, mercury levels, and birds: relating adverse laboratory effects to field biomonitoring. Environ Res. 1997;75:160–172. doi: 10.1006/enrs.1997.3778. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Heavy metals in Franklin’s gull tissues: age and tissue differences. Environ Toxicol Chem. 1999;18:673–678. [Google Scholar]
- Burger J, Gochfeld M. Metal levels in feathers of 12 species of seabirds from Midway Atoll in the northern Pacific Ocean. Sci Total Environ. 2000a;257:37–52. doi: 10.1016/s0048-9697(00)00496-4. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Metals in albatross feathers from Midway Atoll: influence of species, age, and nest location. Environ Res. 2000b;82:207–221. doi: 10.1006/enrs.1999.4015. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Effects of lead on birds (Laridae): a review of laboratory and field studies. J Toxicol Environ Health. 2000c;3:59–78. doi: 10.1080/109374000281096. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Metals in Laysan albatrosses from Midway Atoll. Arch Environ Contamin Toxicol. 2000d;38:254–259. doi: 10.1007/s002449910033. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Effects of chemicals and pollution on seabirds. In: Schreiber EA, Burger J, editors. Biology of marine birds. CRC Press; Boca Raton, FL: 2001. pp. 485–525. [Google Scholar]
- Burger J, Gochfeld M. Metal levels in eggs of common terns (Sterna hirundo) in New Jersey: temporal trends from 1971 to 2002. Environ Res. 2004;94:336–343. doi: 10.1016/S0013-9351(03)00081-1. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Metals and radionuclides in birds and eggs from Amchitka and Kiska Islands in the Bering Sea/Pacific Ocean ecosystem. Environ Monit Assess. 2007;127:105–117. doi: 10.1007/s10661-006-9264-z. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Jeitner C, et al. Mercury and other metals in feathers of glaucous-winged gulls (Larus glaucescens) in the Aleutians. Environ Monit Assess. 2008 Jul 15; doi: 10.1007/s10661-008-0306-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Kosson D, et al. Science, policy, and stakeholders: developing a consensus science plan for Amchitka Island, Aleutians, Alaska. Environ Manage. 2005;35:557–568. doi: 10.1007/s00267-004-0126-6. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Kosson DS, Powers CW. Biomonitoring for ecosystem and human health protection at Amchitka Island. CRESP; Piscataway, NJ: 2006a. [Google Scholar]
- Burger J, Jewett S, Gochfeld M, et al. Can biota sampling for environmental monitoring be used to characterize benthic communities in the Aleutians? Sci Total Environ. 2006b;369:393–402. doi: 10.1016/j.scitotenv.2006.02.045. [DOI] [PubMed] [Google Scholar]
- Burger J, Shukla T, Dixon C, et al. Metals in feathers of sooty tern, white tern, gray-backed tern, and brown noddy from islands in the North Pacific. Environ Monit Assess. 2001;71:71–89. doi: 10.1023/a:1011695829296. [DOI] [PubMed] [Google Scholar]
- Cooper WC. The health implications of increased manganese in the environment resulting from the combustion of fuel additives: a review of the literature. J Toxicol Environ Health. 1984;14:23–46. doi: 10.1080/15287398409530561. [DOI] [PubMed] [Google Scholar]
- Cottam C. Techical Bulletin No. 643. US Department of Agriculture; Washington, DC: 1939. Food habits of North American diving ducks. [Google Scholar]
- Custer TW, Hoffman WL. Trace elements in canvasback (Aytha valisineria) wintering in Louisiana, USA, 1987–1988. Environ Poll. 1994;84:253–259. doi: 10.1016/0269-7491(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Eisler R. Biological Report 85 (1.2) US Fish & Wildlife Service; Washington, DC: 1985. Cadmium hazards to fish, wildlife and invertebrates: a synoptic review. [Google Scholar]
- Eisler R. Biological Report 85 (1.1) US Fish & Wildlife Service; Washington, DC: 1987. Mercury hazards to fish, wildlife and invertebrates: a synoptic review. [Google Scholar]
- Fimreite N, Brevik F, Trop R. Mercury and organochlorine in eggs from a Norwegian gannet colony. Arch Environ Contam Toxicol. 1982;28:58–60. doi: 10.1007/BF01608413. [DOI] [PubMed] [Google Scholar]
- Fimreite N, Brun E, Froslie A, Fredrickson P, Gundersen N. Mercury in eggs of Norwegian seabirds. Astarte. 1974;1:71–75. [Google Scholar]
- Wildlife Fish Service. Alaska subsistence spring/summer migratory bird harvest. U.S. Fish & Wildlife Service; Anchorage, AK: 2004. [Google Scholar]
- Fitzgerald WF. Atmospheric and oceanic cycling of mercury. In: Riley JP, Chester R, editors. Chemical oceanography. Vol. 10. Academic Press; New York: 1989. pp. 151–186. [Google Scholar]
- Furness RW. Birds as monitors of pollutants. In: Furness RW, Greenwood JJD, editors. Birds as monitors of environmental change. Chapman & Hall; London: 1993. pp. 86–143. [Google Scholar]
- Furness RW, Camphuysen KCJ. Seabirds as monitors of the marine environment. J Marine Sci. 1997;54:726–723. [Google Scholar]
- Furness RW, Muirhead SJ, Woodburn M. Using bird feathers to measure mercury in the environment: relationship between mercury content and moult. Marine Poll Bull. 1986;17:27–37. [Google Scholar]
- Furness RW, Rainbow PS, editors. Heavy metals in the marine environment. CRC Press; Boca Raton, FL: 1990. [Google Scholar]
- Gochfeld M. Mercury levels in some seabirds of the Humboldt Current, Peru. Environ Pollut A. 1980;22:197–205. [Google Scholar]
- Gochfeld M, Beland J, Shukla T, Benson T, Burger J. Heavy metals in laughing gulls: gender, age and tissue differences. Environ Toxicol Chem. 1996;15:2275–2283. [Google Scholar]
- Goede AA, deBruin M. The use of feathers for indicating heavy metal pollution. Environ Monit Assess. 1986;7:249–256. doi: 10.1007/BF00418017. [DOI] [PubMed] [Google Scholar]
- Goudie RI, Robertson GJ, Reed A. Common eider. Birds North Am. 2000;546:1–32. [Google Scholar]
- Houghton JT, Callander BA, Varney SK. Climate change 1992. Cambridge University Press; Cambridge: 1992. [Google Scholar]
- Hammerschmidt CR, Fitzgerald WF, Lamborg CH, Balcom PH, Tseng CM. Biogeochemical cycling of methylmercury in lakes and tundra watersheds of Arctic Alaska. Environ Sci Technol. 2006;40:1204–1211. doi: 10.1021/es051322b. [DOI] [PubMed] [Google Scholar]
- Hamrick K, Smith J. Subsistence food use in Unalaska and Nikolski. Aleutian Pribilof Island Association; Anchorage, AK: 2003. [Google Scholar]
- Heinz GH. Selenium in birds. In: Beyer WM, Heinz WM, editors. Environmental contaminants in wildlife: Interpreting tissue concentrations. Lewis; Boca Raton, FL: 1996. pp. 447–458. [Google Scholar]
- Kelsall JP, Calaprice JR. Chemical content of waterfowl plumage as a potential diagnostic tool. J Wildlife Manage. 1972;36:1088–1097. [Google Scholar]
- Kenyon KW. Birds of Amchitka Island, Alaska. Auk. 1961;78:305– 326. [Google Scholar]
- Kim EY, Murakami T, Saeki D, Tatsukawa R. Mercury levels and its chemical form in tissues and organs of seabirds. Arch Environ Contam Toxicol. 1996;30:259–266. [Google Scholar]
- Lewis SA, Furness RW. Mercury accumulation and excretion by laboratory reared black-headed gulls (Larus ridibundus) chicks. Arch Environ Contam Toxicol. 1991;21:316–320. [Google Scholar]
- Mailman RB. Heavy metals. In: Perry JJ, editor. Introduction to environmental toxicology. Elsevier; New York: 1980. pp. 34–43. [Google Scholar]
- Merritt ML, Fuller RG, editors. The environment of Amchitka Island, Alaska. Report NVO-79. Technical Information Center. Energy Research and Development Administration; Washington, DC: 1977. [Google Scholar]
- Metcheva R, Yurukova L, Teodorova S, Nikolova E. The penguin feathers as bioindicator of Antarctic environmental state. Sci Total Environ. 2006;362:259–265. doi: 10.1016/j.scitotenv.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Monteiro LR. Seabirds as monitors of mercury in the marine environment. Water Air Soil Pollut. 1996;80:851–870. [Google Scholar]
- Monteiro LR, Furness RW. Seabirds as monitors of mercury in the marine environment. Water Air Soil Pollut. 1995;80:831–870. [Google Scholar]
- NOAA (National Oceanic and Atmospheric Administration) [accessed 26 May 2006];Dutch Harbor—Unalaska, in Alaska, Top US port for landings in 2003. NOAA report 04-096. 2004 Available from www.nmfs.noaa.gov/docs/04-096_top_ports.pdf.
- Nygard T, Lie E, Roy N, Steinnes E. Metal dynamics in an Antarctic food chain. Marine Pollut Bull. 2001;42:598–602. doi: 10.1016/s0025-326x(00)00206-x. [DOI] [PubMed] [Google Scholar]
- Patrick R. Proceedings of the Amchitka long-term stewardship workshop. CRESP/University of Alaska; Fairbanks, AK: 2002. How local Alaska native communities view the Amchitka issue. [Google Scholar]
- Peakall DB. Animal biomarkers as pollution indicators. Chapman & Hall; London: 1992. [Google Scholar]
- Piatt JF, Kitaysky AS. Tufted puffin. Birds North Am. 2002;708:1–32. [Google Scholar]
- Powers CW, Burger J, Kosson D, Gochfeld M, Barnes D, editors. Biological and geophysical aspects of potential radionuclide exposure in the Amchitka marine environment. CRESP; Piscataway, NJ: 2005. [Google Scholar]
- Rocque DA, Winker K. Biomonitoring of contaminants in birds from two trophic levels in the North Pacific. Environ Toxicol Chem. 2004;23:759–766. doi: 10.1897/03-182. [DOI] [PubMed] [Google Scholar]
- Rose GA, Parker GH. Effects of smelter emissions on metal levels in the plumage of ruffed grouse near Sudbury, Ontario. Can J Zool. 1982;60:2659–2667. [Google Scholar]
- SAS (Statistical Analysis System) SAS users’ guide. SAS Institute; Cary, NC: 1995. [Google Scholar]
- Savinov VM, Gabrielsen GW, Savinova TN. Cadmium, zinc, copper, arsenic, selenium and mercury in seabirds from the Barents Sea: levels, inter-specific and geographical differences. Sci Total Environ. 2003;306:133–158. doi: 10.1016/S0048-9697(02)00489-8. [DOI] [PubMed] [Google Scholar]
- Schiefler R, Gauthier-Clerc M, LeBohec C, et al. Mercury concentrations in king penguins (Aptenodytes patagonicus) feathers at Crozet Islands (Sub-Antarctica): temporal trend between 1966–1974 and 2000–2001. Environ Toxicol Chem. 2005;24:125–128. doi: 10.1897/03-446.1. [DOI] [PubMed] [Google Scholar]
- Simon SL, Bouville A, Beck HL. The geographic distribution of radionuclide deposition across the continental U.S. from atmospheric nuclear testing. J Environ Radioact. 2004;74:91–105. doi: 10.1016/j.jenvrad.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Spry DJ, Wiener JG. Metal bioavailability and toxicity to fish in low-alkalinity lakes: a critical review. Environ Poll. 1991;71:243–304. doi: 10.1016/0269-7491(91)90034-t. [DOI] [PubMed] [Google Scholar]
- Thompson DR, Furness RW. Seabirds as biomonitors of mercury inputs to epipelagic and mesopelagic marine food chains. Sci Total Environ. 1998;213:299–305. [Google Scholar]
- Thompson DR, Hamer KC, Furness RW. Comparison of the levels of total and organic mercury in seabird feathers. Marine Pollut Bull. 1991;20:577–579. [Google Scholar]
- Thompson DR, Bearhop S, Speakman JR, Furness RW. Feathers as a means of monitoring mercury in seabirds: insights from stable isotope analysis. Environ Pollut. 1998;101:193–200. doi: 10.1016/s0269-7491(98)00078-5. [DOI] [PubMed] [Google Scholar]
- van Straalen NM, Ernst WHO. Metal biomagnification may endanger species in critical pathways. Oikos. 1991;62:255–265. [Google Scholar]
- Walsh PM. The use of seabirds as monitors of heavy metals in the marine environment. In: Furness RW, Rainbow PS, editors. Heavy metals in the marine environment. CRC Press; Boca Raton, FL: 1990. pp. 183–204. [Google Scholar]
- Wayland M, Gilchrist HG, Neugebauer E. Concentrations of cadmium, mercury and selenium in common eider ducks in the eastern Canadian arctic: influence of reproductive stage. Sci Total Environ. 2005;351:323–332. doi: 10.1016/j.scitotenv.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Wiener JG, Spry DJ. Toxicological significance of mercury in freshwater fish. In: Beyer WN, Heinz GH, Redmon-Norwood AW, editors. Environmental contaminants in wildlife: Interpreting tissue concentrations. SETAC Special Publications, Lewis Publishers; Boca Raton, FL: 1996. pp. 297–339. [Google Scholar]