Abstract
Individuals who fish, and their families that ingest self-caught fish, make decisions about where to fish, what type of fish to eat, and the quantity of fish to eat. While federal and state agencies often issue consumption advisories for some fish with high mercury (Hg) concentrations, advisories seldom provide the actual metal levels to the general public. There are few data for most saltwater fish, and even less information on variations in Hg levels in fish within a state or geographical region. The objective of this study was to provide Hg concentrations from 19 species of fish caught in different locations in New Jersey to (1) test the hypothesis that mean metal levels vary geographically, (2) provide this information to individuals who fish these coastal waters, and (3) provide a range of values for risk assessors who deal with saltwater fish exposure in the Northeastern United States. Selenium (Se) was also examined because of its purported moderating effect on the toxicity of Hg. Hg levels showed significant geographical variation for 10 of 14 species that were caught in more than one region of New Jersey, but there were significant locational differences for Se in only 5 of the fish. Mercury levels were significantly lower in fish collected from northern New Jersey (except for ling, Molva molva), compared to other regions. As might be expected, locational differences in Hg levels were greatest for fish species with the highest Hg concentrations (shark, Isurus oxyrinchus; tuna, Thunnus thynnus and T. albacares; striped bass, Morone saxatilis; bluefish, Pomatomus saltatrix). Fishers and their families might reduce their risk from Hg exposure not only by selecting fish generally lower in Hg, but by fishing predominantly in some regions over others, further lowering the potential risk. Health professionals might use these data to advise patients on which fish are safest to consume (in terms of Hg exposure) from particular geographical regions.
Increasingly governmental agencies, public policymakers, managers, and the public are concerned about making decisions to clean up the environment, and to determine methods to reduce exposure to the environment and humans from contaminants. Optimally, risk reduction might be accomplished by source removal—that is, eliminating pollutants from the environment and the food chain. However, source reduction does not always occur, and then governmental or individual choices may be necessary to diminish risks to consumers. The risk from mercury (Hg) to fish consumers is such an issue. Even though there has been some source reduction, Hg levels in fish remain a health concern to species that consume them, including humans.
Methylmercury (MeHg) and other contaminants in some fish are high enough to potentially produce effects on the fish themselves, and top-level predators (World Health Organization [WHO] 1989; Ratcliffe et al. 1996; National Research Council [NRC] 2000; Sweet and Zelikoff 2001; Consumer Reports 2003). Levels of MeHg are sufficiently high in some fish to cause adverse health effects in people consuming large quantities (Institute of Medicine [IOM] 1991; 2006; Grandjean et al. 1997; Sweet and Zelikoff 2001; Gochfeld 2003; Hightower and Moore 2003; Hites et al. 2004), with neurodevelopmental effects from fetal exposure the most prominent effect (Steuerwald et al. 2000; NRC 2000; Counter et al. 2002). Prenatal MeHg has led to behavioral deficits in infants (Joint FAO/WHO Expert Committee on Food Additives [JECFA] 2003) and to poorer cognitive test performance (Oken et al. 2008). Methylmercury counteracted the cardioprotective effects attributed to fish consumption (Rissanen et al. 2000; Guallar et al. 2002).
Fish consumption is the primary significant source of MeHg exposure for the public today (Rice et al. 2000). Hg occurs naturally in seawater, and coastal waters receive Hg runoff from land, input from rivers, and airborne deposition. Biomethylation, which occurs in sediment, converts Hg to the form more toxic to animals. The U.S. Food and Drug Administration (FDA 2001; 2005) issued a series of consumption advisories based on MeHg that suggested that pregnant women and women of childbearing age who may become pregnant should avoid eating 4 types of marine fish (shark, swordfish, king mackerel, tilefish), and limit their consumption of all other fish to just 12 ounces (=342 g) per week (FDA 2001; 2003). In addition, there are also recent warnings about canned tuna (FDA 2005).
Saltwater fishing remains an important commercial, recreational, and subsistence activity in many coastal states and countries around the world. Fish are an important dietary item for individuals living along coastal New Jersey, and recreational fishers often freeze fish for consumption at all times of the year (Burger 2005; Gobeille et al. 2005). Fish are an excellent, low-fat source of protein that contributes to (1) lower blood cholesterol, (2) positive pregnancy outcomes, and (3) better child cognitive test performances (Oken et al. 2008). Fish contain omega-3 (n-3) fatty acids that reduce cholesterol levels and the incidence of heart disease, stroke, and preterm delivery (Patterson 2002; Virtanen et al. 2008). Further, fish, particularly oceanic fish, are relatively rich in Se, necessary for seleno-enzyme functions, and Se has long been known to offer some protection against Hg-induced toxicity.
It is therefore important to understand how to reduce the risk from Hg exposure, and to provide the public with useful information on fish that are low or high in Hg. The public thus needs to choose whether to eat fish, which species to eat, and where to fish. Some New Jersey fisherfolk, and some of those fishing in other regions along the Atlantic Coast of North American, fish from boats that go out in the ocean, or move up and down the coast to select among fishing locations. After extensive media coverage of Hg in fish, the fishers requested information in locally caught fish and contributed to this study by providing fish samples.
In this study, the Hg concentrations were examined in a wide range of fish species from coastal New Jersey to provide information on differences among species and fishing locations. Unlike many studies, this investigation did not focus only on those species expected to have high levels (and thus pose the greatest risk), but examined levels in the wide range of fish caught by local recreational fishermen. Risk balancing by the public is possible when information is freely available, and if Hg concentrations are known for a range of fish, in a range of locations.
Selenium concentrations were also analyzed because this metal offers some protection against Hg exposure (Satoh et al. 1985; Ralston 2009; Lémire et al. 2010). Lower prevalence of nonfatal heart attacks is associated with higher Se concentrations (Mozaffarian 2009). Recent studies with animal models suggested that some (if not most) of the adverse impacts of high MeHg exposure occur as a result of Hg’s impairment of Se-dependent enzyme activities (Ralston 2009; Ralston et al. 2008). Mercury and MeHg are irreversible selenoenzyme inhibitors (Watanabe et al. 1999; Carvalho et al. 2008) and impair selenoproteins and function. Ralston (2008) suggested that where the Se:Hg molar ratio exceeds 1:1, there is adequate Se to prevent Hg-induced toxicity, although there is contradictory evidence (Lemire et al. 2010). It is therefore important to report both Hg and Se levels such that an adequate database exists to examine Se levels in fish, and the relationship between the two metals.
Thus, the objectives of this paper were to (1) provide Hg concentrations from 19 species of fish caught in different locations in New Jersey to test the hypothesis that levels vary geographically, (2) give this information to individuals who fish these coastal waters, (3) provide a range of Hg values for risk assessors who deal with saltwater fish exposure in the Northeastern United States, and (4) make available information on Se levels for interpretation of their potential adverse effects and evaluate Se purported moderating effect on toxicity of Hg. It was postulated that there would be locational differences, with levels of Hg being higher in the New York/New Jersey harbor area, associated with high levels of industrialization.
METHODS
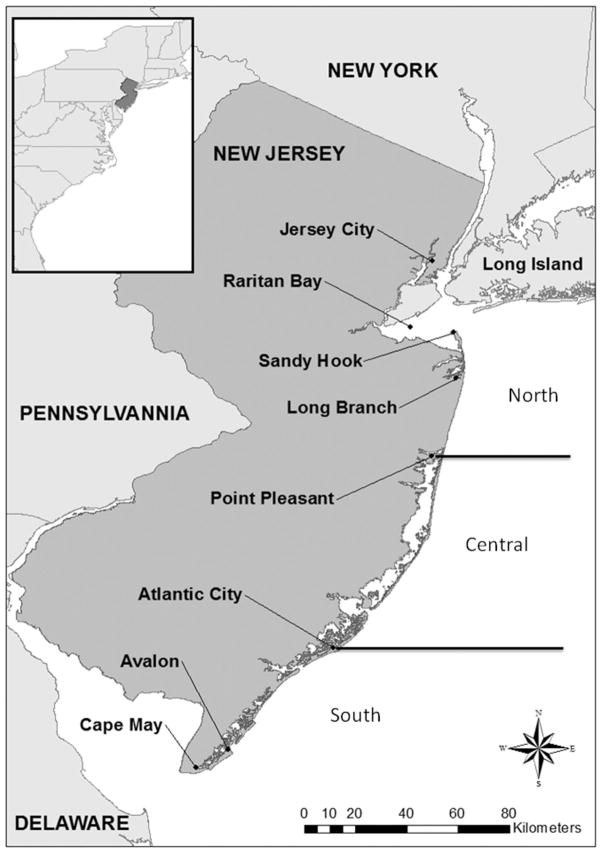
Samples from 19 marine fish species were collected (2003–2008) from several sites along the New Jersey shore (Figure 1; scientific names and trophic levels information found in Table 1), in collaboration with recreational fishers (Jersey Coast Anglers Association, Jersey Coast Shark Angler) and others. The actual sample collection (removal of sample from the fish caught by fisherfolk) was done by Rutgers personnel who went to local docks and fishing sites to meet recreational fishers. The 19 species are the fish most often caught by NJ fishers, and were selected because they are most relevant to recreational fishers in the region. Fish from individual fishers were taken home to eat, and those from tournaments were either taken home for consumption by the families of the fishers, or were donated to orphanages or other facilities. In addition, a small number of individual fish below the recreational size limits of some species (bluefish, striped bass) were collected by the NJ Department of Environmental Protection (DEP) trawls.
FIGURE 1.
Map showing the locations of sampling for fish from New Jersey, 2005–2008.
TABLE 1.
Prey Types, Adult Trophic Level, and Maximum Life Sspan for 19 Species of New Jersey Fish
| Species | Maximum years | Prey items | Trophic Level | |
|---|---|---|---|---|
| Shortfin Mako | Isurus oxyrinchus | 25 | bony fishes, other sharks, cephalopods; larger individuals may feed on larger prey such as billfish and small cetaceans | 4.3–5.4 |
| Atlantic bluefin tuna | Thunnus thynnus | 15 | small schooling fishes (anchovies, sauries, hakes) or on squids and red crabs | 4.4 |
| Striped bass | Morone saxatilis | 30 | wide variety of fishes and invertebrates | 3.3–4.9 |
| Bluefish | Pomatomus saltatrix | 9 | fish, crustaceans and cephalopods | 4.5 |
| Tautog | Tautoga onitis | 34 | mussels, gastropods, other mollusks and crustaceans | |
| Yellowfin tuna | Thunnus albacares | 9 | fishes, crustaceans and squids | 4.2–4.5 |
| Windowpane flounder | Scophthalmus aquosus | 7 | benthic crustaceans, mollusks, echinoderms, finfish | 3.5–4.2 |
| Dolphin (mahi-mahi) | Coryphaena hippurus | 4 | fish, zooplankton, crustaceans, squid | 4.4–4.5 |
| Southern kingfish | Menticirrhus americanus | ? | bethnic worms and crustaceans | 3.5–3.9 |
| Black seabass | Centropristis striata | 10 | amphipods, crabs, shrimp | 3.7–4.1 |
| Weakfish | Cynoscion regalis | 18 | crustaceans and fishes | 3.8–4.5 |
| Cunner | Tautogolabrus adspersus | 6 | benthic crustaceans, crabs, finfish | 3.3–4.0 |
| Northern kingfish | Menticirrhus saxatilis | benthic worms and crustaceans | 3.6–4.5 | |
| Summer flounder (fluke) | Paralichthys dentatus | 9 | benthic crustaceans, finfish, cephalopods | 4.5 |
| Atlantic croaker | Micropogonias undulatus | 5 | worms, crustaceans and fishes | 3.1–3.7a |
| Scup (porgie) | Stenotomus chrysops | amphipods, worms, sand dollars and young squid | 3.2–4.5 | |
| Winter flounder | Pseudopleuronectes americanus | 14 | organisms living in, on, or near the bottom; shrimps, amphipods, crabs, sea urchins, and snails | 2.8–3.6 |
| Ling | Molva molva | 25 | fishes (cod, herring, flatfish), lobsters, cephalopods, and starfishes | |
| Atlantic menhaden | Brevoortia tyrannus | feed by filtering phytoplankton (diatoms) and zooplankton (small crustaceans, annelid worms, and detritus) | 2.3–4.2 |
Note. Species listed by mean levels of mercury (see figure 2). Prey items, trophic level, and maximum life span are from www.fishbase.org; trophic level given is for recruits and juveniles (not adults).
An approximately 50-g sample plug biopsy was removed from the side of the fish, over the lateral line just anterior to the tail. Samples were immediately placed in plastic bags, labeled with information about species, length and weight, location collected, and how obtained. Fish or samples were kept in coolers and brought for element analysis to the Environmental and Occupational Health Sciences Institute (EOHSI) of Rutgers University, where they were frozen immediately, or dissected and frozen immediately.
Fish tissue samples were analyzed in the Elemental Analysis Laboratory of the Environmental and Occupational Health Sciences Institute in Piscataway, NJ. Total Hg was analyzed using a Perkin Elmer FIMS-100 mercury analyzer by cold vapor atomic absorption spectrometry. Selenium was analyzed using a Perkin Elmer 5100 graphite furnace atomic absorption spectrometer with Zeeman correction. Metal concentrations are expressed as micrograms per gram (ppm) on a wet weight basis. Molar concentrations were obtained by dividing by the molecular weight (200.59 for Hg and 78.9 for Se). Total Hg was analyzed and reported, although many studies show that almost all of Hg in fish tissue is MeHg. Ninety percent is a reasonable approximation of this proportion (Duffy et al. 1999), which varies among fish types and laboratories (Lansens et al. 1991). Similarly, Freije and Awadh (2009) reported that more than 90% of total Hg was MeHg in marine fish. Recently, Scudder et al. (2009) suggested that about 95% of Hg present in fish is MeHg, and that lower levels may have been biased by analytical and homogeneity variability (Bloom 1991)
All laboratory equipment and containers were washed in 10% HNO solution and rinsed with deionized water prior to each use (Burger et al. 2001). A 2-g (wet weight) sample of tissue was digested in 4 ml trace metal grade nitric acid and 3 ml deionized water in a microwave (MDS 2000 CEM). Digested samples were subsequently diluted to 15 ml with deionized water.
A calibration curve was constructed which included a blank and 4 levels of standards. Correlation coefficients on the calibration curve above 0.995 were accepted. All samples were analyzed twice for each metal. Selenium was analyzed straight and then spiked with 4 μl of 50-μg/L standard. In addition to the rigorous screening and clean collection protocol followed, standard reference material, spiked specimens, and blanks were determined along with samples to evaluate analytical control and accuracy. Certified reference material (CRM) DORM-2, “dogfish muscle certified reference material for trace metals,” from the National Institute of Standards and Technology (NIST), was used for cold vapor atomic absorption spectroscopy (Hg). DORM-2 recoveries ranged from 93% to 108%. Standard reference material (SRM) 1640, “trace metals in natural water,” from the National Institute of Standards and Technology (NIST), was used for Zeeman graphite furnace atomic absorption spectroscopy (Se) quality control evaluation, with recoveries ranging from 85 to 112%. In addition, spikes were prepared with PE Pure atomic spectroscopy standard and standard reference material (SRM) 3133, “mercury standard solution,” to further establish quality control. The accepted recoveries for reference material and spikes ranged from 85 to 115%; no batches were outside of these limits.
For further quality control on Hg, our laboratory periodically has run a random subset of samples in the Quebec Laboratory of Public Health; the correlation between the two laboratories was over 0.90 ( p < .0001; Burger and Gochfeld 2004). All results are reported as parts per million (ppm = μg/g) on wet weight basis. See Burger et al. (2011) for further description of methods and other aspects of Hg concentrations.
Kruskall–Wallis chi-squared tests were used to identify the significant differences (SAS Institute, Inc. 1995). This test was used because it is more tolerant, given the variation within so many different species of fish. The level for significance was designated as p < .05.
RESULTS
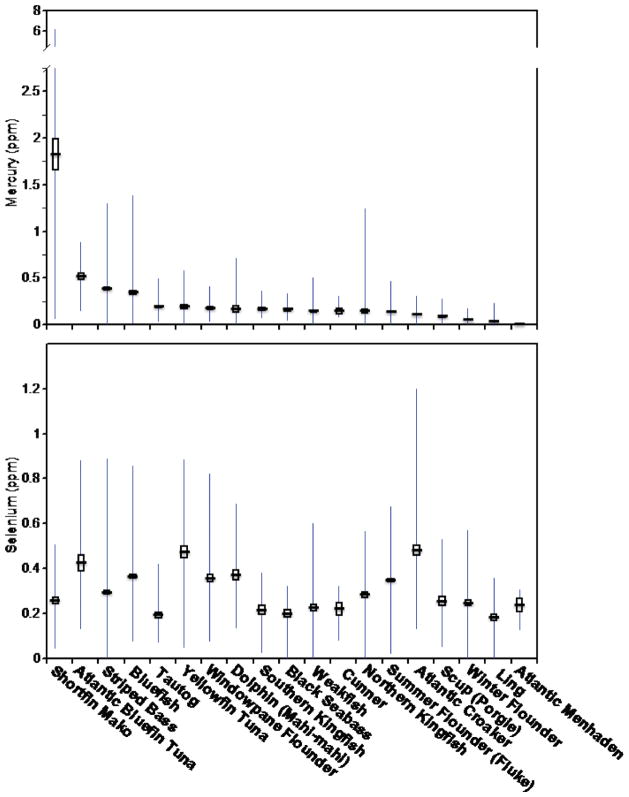
There were significant interspecific differences in Hg and Se levels (Figure 2), as well as locational differences. The interspecific differences differed by two orders of magnification for menhaden (0.01 ppm Hg) and Mako shark (1.83 ppm Hg). Four fish species were below 0.1 ppm Hg, which is below risk level.
FIGURE 2.
Mean, standard error, and range for Hg levels (ppm, wet weight) and Se levels for fish collected from coastal New Jersey, 2003–2008.
Mercury levels differed significantly for 10 of 14 species that were collected for at least two regions (Table 2). Mercury levels were highest in fish from the south for bluefin tuna, striped bass, fluke, and Atlantic croaker. The highest Hg levels were detected in fish from central New Jersey for bluefish, yellowfin tuna, weakfish, and northern kingfish, and highest from northern New Jersey only for windowpane (which did not include any samples from the south).
TABLE 2.
Mean Total Mercury Levels (ppm) by Region
| Species | Mercury
|
χ 2 (p) | |||||
|---|---|---|---|---|---|---|---|
| South
|
Central
|
North
|
|||||
| n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | ||
| Shortfin mako | 12 | 1.76 ± 0.18 | 40 | 1.86 ± 0.22 | NS | ||
| Atlantic bluefin tuna | 21 | 0.55 ± 0.03 | 2 | 0.30 ± 0.06 | 4.3 (.04) | ||
| Striped bass | 77 | 0.48 ± 0.04 | 101 | 0.33 ± 0.02 | 9.3 (.002) | ||
| Bluefish | 59 | 0.19 ± 0.02 | 79 | 0.49 ± 0.04 | 68 | 0.32 ± 0.03 | 40.6 (<.0001) |
| Tautog | 24 | 0.17 ± 0.02 | 1 | 0.26 a | 22 | 0.23 ± 0.02 | NS |
| Yellowfin tuna | 43 | 0.17 ± 0.02 | 4 | 0.49 ± 0.04 | 8.0 (.005) | ||
| Windowpane flounder | 32 | 0.17 ± 0.02 | 16 | 0.22 ± 0.02 | 3.9 (.05) | ||
| Dolphin (mahi-mahi) | 23 | 0.19 ± 0.04 | 3 | 0.10 ± 0.05 | 1 | 0.04 | NS |
| Southern kingfish | 23 | 0.17 ± 0.02 | |||||
| Black seabass | 5 | 0.18 ± 0.02 | 14 | 0.16 ± 0.02 | NS | ||
| Weakfish (Squeteague) | 21 | 0.16 ± 0.03 | 15 | 0.21 ± 0.02 | 24 | 0.11 ± 0.02 | 11.1 (.004) |
| Cunner | 7 | 0.15 ± 0.03 | |||||
| Northern kingfish | 4 | 0.13 ± 0.07 | 44 | 0.21 ± 0.03 | 24 | 0.06 ± 0.01 | 26.7 (<.0001) |
| Summer flounder (fluke) | 109 | 0.15 ± 0.01 | 42 | 0.11 ± 0.02 | 107 | 0.14 ± 0.01 | 14.9 (.0006) |
| Atlantic croaker | 24 | 0.15 ± 0.01 | 37 | 0.10 ± 0.01 | 2 | 0.14 ± 0.04 | 9.8 (.008) |
| Scup (porgy) | 27 | 0.09 ± 0.02 | |||||
| Winter flounder | 58 | 0.06 ± 0.00 | |||||
| Ling | 28 | 0.03 ± 0.00 | 11 | 0.07 ± 0.02 | 16.4 (<.0001) | ||
| Atlantic menhaden | 5 | 0.01 ± 0.01 | |||||
Note. North includes fish from Jersey City, Raritan Bay, and Sandy Hook. Central includes fish caught between Point Pleasant and Atlantic City. South includes all locations south of Atlantic City. Given are arithmetic means ± SE with Kruska–Wallis chi-squared values and p values for each species comparing among regions.
In contrast, there were significant locational differences in Se for only five species (Table 3). Two were higher in the south, one in central, and one in the north. Croaker was low in the central area.
TABLE 3.
Mean Selenium Levels by Region
| Species | Selenium (ppm)
|
χ 2 (p) | |||||
|---|---|---|---|---|---|---|---|
| South
|
Central
|
North
|
|||||
| n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | ||
| Shortfin mako | 12 | 0.34 ± 0.02 | 40 | 0.23 ± 0.01 | 12.2 (.0005) | ||
| Atlantic bluefin tuna | 21 | 0.43 ± 0.04 | 2 | 0.45 ± 0.06 | NS | ||
| Striped bass | 77 | 0.30 ± 0.02 | 101 | 0.29 ± 0.01 | NS | ||
| Bluefish | 59 | 0.36 ± 0.02 | 79 | 0.38 ± 0.01 | 68 | 0.35 ± 0.02 | NS |
| Tautog | 24 | 0.19 ± 0.02 | 1 | 0.15 ± a | 22 | 0.20 ± 0.02 | NS |
| Yellowfin tuna | 43 | 0.49 ± 0.03 | 4 | 0.29 ± 0.06 | 5.0 (.03) | ||
| Windowpane flounder | 32 | 0.40 ± 0.02 | 16 | 0.27 ± 0.02 | 12.9 (.0003) | ||
| Dolphin (mahi-mahi) | 23 | 0.37 ± 0.02 | 3 | 0.30 ± 0.01 | 1 | 0.69 | NS |
| Southern kingfish | 23 | 0.22 ± 0.02 | |||||
| Black seabass | 5 | 0.19 ± 0.05 | 14 | 0.21 ± 0.02 | NS | ||
| Weakfish (Squeteague) | 21 | 0.22 ± 0.02 | 15 | 0.29 ± 0.04 | 24 | 0.20 ± 0.02 | NS |
| Cunner | 7 | 0.22 ± 0.03 | |||||
| Northern kingfish | 4 | 0.22 ± 0.13 | 44 | 0.29 ± 0.02 | 24 | 0.28 ± 0.02 | NS |
| Summer flounder (fluke) | 109 | 0.36 ± 0.01 | 42 | 0.34 ± 0.02 | 107 | 0.34 ± 0.01 | NS |
| Atlantic croaker | 24 | 0.58 ± 0.05 | 37 | 0.42 ± 0.02 | 2 | 0.58 ± 0.12 | 13.5 (.001) |
| Scup (porgy) | 27 | 0.26 ± 0.02 | |||||
| Winter flounder | 58 | 0.25 ± 0.01 | |||||
| Ling | 28 | 0.16 ± 0.02 | 11 | 0.23 ± 0.03 | 3.8 (.05) | ||
| Atlantic menhaden | 5 | 0.24 ± 0.03 | |||||
North includes fish from Jersey City, Raritan Bay, and Sandy Hook. Central includes fish caught between Point Pleasant and Atlantic City. South includes all locations south of Atlantic City. Given are arithmetic means ± SE with Kruskal–Wallis chi-squared values and p values for each species comparing among regions.
DISCUSSION
Trophic Level Differences
As might be expected, there were differences in Hg levels among species that related to trophic level. Top-level predators, such as shark and tuna, had higher levels than other species, followed by bass and bluefish (Table 1). The relationships, however, are complex, and may relate to prey type (and differences in percent prey types) as well as age. Information on prey types is not specific to the locations where fish were collected for this study, making it difficult to know whether specific fish specialized on only some of the listed prey items.
Data indicated, however, several species with relatively low Hg concentrations, rendering them considerably less risky to consume on a regular basis, particularly by sensitive populations, see Burger and Gochfeld (2011) for further discussion of species and size relationships. Overall, there were few interspecific and locational differences in Se concentrations, partly because Se, as a trace element, is regulated in the body.
Locational Differences
Locational differences in Hg levels might be expected (for species with sufficient samples for the analysis), even for a small state such as New Jersey, when there are areas of high urbanization and industrialization (such as the New York/New Jersey harbor, and Delaware Bay). Both the NY/NJ harbor and Delaware Bay are two of the most active seaports in the United States. At the start of the study, the prediction was made that Hg concentrations would be higher in the north because of the high industrialization, urbanization, and heavy ocean traffic, particularly in the New York/New Jersey harbor area. However, this was not the case. Overall these data indicate that levels are not generally highest in fish caught in the more industrialized northern part of the state as the fishers suspected. Indeed, industrialized Delaware Bay may contribute significantly to contamination in the southern part. Hg levels were highest in fish from the southern part for bluefin tuna, striped bass, fluke, and Atlantic croaker, were highest in fish from central New Jersey for bluefish, yellowfin tuna, weakfish, and northern kingfish, and were highest from northern New Jersey only for windowpane.
The general lack of large locational difference may be due to the migratory nature of some species, to the general oceanic lifestyle of some of the fish, to homogenization of waters along the Jersey shore, or to a real lack of differences in Hg in waters of this region. The two simplest explanations are not mutually exclusive: (1) Fish in different places feed on different local foods with different sources and amounts of local pollution, or (2) migratory fish from different populations move along the Jersey Shore at different times. Predators must rely on local food sources, and many of their prey species are sedentary (many predators eat young fish, which remain in coastal and bay nurseries for a year or two) (Able and Fahay 1998). Thus, Hg levels depend upon the local prey base.
As an example, species such as striped bass and bluefish migrate and move within the region. Bluefish are migratory, spending their summers in the north and their winters around Florida and the Gulf Stream (Pottern et al. 1989). Large fish move into the bays and estuaries to spawn in late spring–early summer, then move offshore, while schools of intermediate-sized fish and smaller fish remain inshore in bays, estuaries, and creeks (Able and Fahay 1998; Neuman et al. 2004). “Snapper blues” (bluefish in the 1-kg size range) are regularly caught from the beach or in bays. Similarly, striped bass migrate seasonally to the south in the fall and winter and to the north in spring and summer (Waldman et al. 1990; Secor et al. 2001). However, some striped bass are sedentary for many months, remaining within an area of only meters (Ng et al. 2007). Further, tuna are migratory, and their migrations are perhaps less well known for specific regions, such as near the New Jersey shore.
These migratory patterns may be partly responsible for the differences in Hg levels, but trophic level clearly plays a role, both in explaining interspecific differences and in explaining locational differences. Predators must rely on local food sources, and many of their prey species are sedentary (many predators eat young fish, which remain in coastal and bay nurseries for a year or two). Thus, Hg levels would somewhat depend upon the local prey base.
Finally, locational differences might be due entirely to differences in sizes of fish collected at different locations. Mercury levels were significantly related to fish size for 10 fish species, and Se showed no consistent correlation to fish length (Burger and Gochfeld 2011). Further, the effect of fish size X location on Hg levels did not enter any of the models as a significant contributor to variation in Hg or Se levels (Burger and Gochfeld 2011).
Locational Differences and Risk
From a human perspective, the question is whether the levels in New Jersey saltwater fish exceed health guidance or standards. It is difficult to clearly establish “safe” limits for fish consumption. States generally set advisories to be protective of the most sensitive populations. However, there are also guidance levels set by different nations and states. For example, the FDA (2001) has set 1 ppm as the allowable limit for interstate commerce (FDA 2001), and the U.S. Environmental Protection Agency (EPA) (2008) provided guidelines for the consumption of fish (4 meals/month of 8 ounces [=227 g] each), suggesting that people eat only fish with values between 0.12 ppm and 0.23 ppm. Most fish in this study averaged within this range.
The United Kingdom and the European Union established criteria for certain metals in fish (e.g., the level for Hg is 0.5 ppm in edible fish, with up to 1 ppm allowed for certain exempt predatory fish species). China has set standards for MeHg in canned fish (ppm wet weight) of 0.5 ppm (except 1 ppm is allowed in shark, sailfish, tuna, pike, and other high-Hg fish). In 1982 the European Commission set an Environmental Quality Standard for Hg: The mean concentration of Hg in a representative sample of fish shall not exceed 0.3 ppm (wet weight), although recently maximum limits were set for foodstuffs at 1 ppm (European Commission 2008). The World Health Organization has set the maximum consumption level of 0.5 ppm total Hg (Marrugo-Negrete et al. 2008).
In terms of risk, the question is whether locational differences are sufficiently high to influence risk to humans that consume these fish. If one assumes that from a risk perspective individuals should avoid consuming fish with levels above 0.3 ppm, then only a few species of fish in this study averaged above these levels. However, high level fish consumers (over 2 meals/week) should avoid fish >0.1 ppm. The locational differences, although significant, were relatively small except for the fish species with the highest Hg levels (tuna, striped bass, bluefish). For the species with relatively low levels (e.g., below 0.2 ppm), the differences were rather minor. This suggests that if individuals avoid fish with relatively high Hg levels, then locational differences in fishing sites exert little effect. If, on the other hand, people are fishing for tuna, bass, or bluefish, then sensitive populations would do well to consider where they are fishing, to avoid regions with the highest levels, and to avoid top trophic fish species where possible.
Acknowledgments
We thank T. Fote for advice and support throughout the study, M. Donio for field and laboratory assistance, and the many anglers in New Jersey who allowed us to collect samples from their fish or who collected the samples for us. This research was partly supported by the Jersey Coast Angler’s Association (JCAA), the Jersey Coast Shark Anglers Association (JCSA), NIEHS Center Grant (P30ES005022), and the Consortium for Risk Evaluation with Stakeholder Participation (Department of Energy, DE-FC01-06EW07053), Wildlife Trust, and EOHSI. This research was conducted under a Rutgers University protocol, and fish samples were obtained from recreational anglers and NJ DEP trawls. The views and conclusions expressed in this paper are solely those of the authors, and do not reflect the funding agencies.
References
- Able KW, Fahay MP. The first year in the life of estuarine fishes in the middle Atlantic Bight. New Brunswick, NJ: Rutgers University Press; 1998. [Google Scholar]
- Bloom NS. On the chemical form of Hg in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci. 1991;49:1010–17. [Google Scholar]
- Burger J. Fishing, fish consumption, and knowledge about advisories in college students and others in central New Jersey. Environ Res. 2005;98:268–75. doi: 10.1016/j.envres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Hg in canned tuna: White versus light and temporal variation. Environ Res. 2004;96:239–49. doi: 10.1016/j.envres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Burger J, Gaines KF, Gochfeld M. Ethnic differences in risk from Hg among Savannah River fishermen. Risk Anal. 2001;21:533–44. doi: 10.1111/0272-4332.213130. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Mercury and selenium levels in 19 species of salt-water fish from New Jersey as a function of species, size, and season. Sci Total Environ. 2011;409:1418–1429. doi: 10.1016/j.scitotenv.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho CML, Chew EH, Hashemy LI, Lu J, Holmgren A. Inhibition of the human thioredoxin system: A molecular mechanism of mercury toxicity. J Biol Chem. 2008;283:11913–23. doi: 10.1074/jbc.M710133200. [DOI] [PubMed] [Google Scholar]
- Consumer Reports. American’s fish: Fair or foul? New York: Consumers Union; 2003. [accessed 1 April 2004]. http://www.consumerreports.org/special/consumerInteret/Reports/0102fis0.html. [Google Scholar]
- Counter SA, Buchanan LH, Ortega F, Laurell G. Elevated blood mercury and neuro-otological observations in children of the Ecuadorian gold mines. J Toxicol Environ Health A. 2002;65:149–63. doi: 10.1080/152873902753396785. [DOI] [PubMed] [Google Scholar]
- Duffy LK, Scofield E, Rodgers T, Patton M, Bowyer RT. Comparative baseline mercury concentrations, HSP 70 and HSP 60 in subsistence fish from the Y-K Delta region of Alaska. Comp Biochem Physiol. 1999;124C:181–86. doi: 10.1016/s0742-8413(99)00055-9. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. FDA consumer advisory. Washington, DC: U.S. Food and Drug Administration; 2001. [accessed 1 December 2001]. http//www.fda.gov/bbs/topics/ANSWERS/2000/advisory.html. [Google Scholar]
- Food and Drug Administration. FDA consumer advisory. Washington, DC: U.S. Food and Drug Administration; 2003. [accessed 1 January 2004]. http//www.fda.gov/bbs/topics/ANSWERS/2000/advisory.html. [Google Scholar]
- Food and Drug Administration. Hg levels in commercial fish and shellfish. Washington, DC: U.S. Food and Drug Administration; 2005. [accessed 1 January 2005]. http//www.fda.gov/bbs/topics/ANSWERS/2000/advisory.html. [Google Scholar]
- Freije A, Awadh M. Total and methylmercury intake associated with fish consumption in Bahrain. Water Environ J. 2009;23:155–64. [Google Scholar]
- Gobeille AK, Morland KV, Bopp RF, Godbold JH, Landrigan PJ. Body burdens of mercury in lower Hudson River area anglers. Environ Res. 2005;101:205–12. doi: 10.1016/j.envres.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Gochfeld M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol Environ Safety. 2003;56:174–79. doi: 10.1016/s0147-6513(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorenson N, Jorgensen PJ. Cognitive deficit in 7-year old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:418–28. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Guallar E, Sanz-Gallardo MI, van’t Veer P, Bode P, Aro A, Gomez-Aracena J. Heavy metals and Myocardial Infarction Study Group: Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med. 2002;347:1747–54. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- Hightower JM, Moore D. Mercury levels in high-end consumers of fish. Environ Health Perspect. 2003;111:604–608. doi: 10.1289/ehp.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites RA, Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. Global assessment of organic contaminants in farmed salmon. Science. 2004;303:226–29. doi: 10.1126/science.1091447. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Seafood safety. Washington, DC: National Academy Press; 1991. [Google Scholar]
- Institute of Medicine. Seafood choices: Balancing benefits and risks. Washington, DC: National Academy Press; 2006. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. [accessed March 2005];Evaluation of Certain Food Additives and Contaminants. 2004 http://whqlibdoc.who.int/trs/WHO_TRS_922.pdf.
- Lansens P, Leermakers M, Vaeyens W. Determination of methylmercury in fish by headspace-gas chromatography with microwave-induced-plasma detection. Water Air Soil Pollut. 1991;56:103–15. [Google Scholar]
- Lémire M, Fillion M, Frenette B, Mayer A, Philibert A, Passos CJS, Guimarães JRD, Barbosa F, Jr, Mergler D. Selenium and mercury in the Brazilian Amazon: Opposing influences on age-related cataracts. Environ Health Perspect. 2010;118(11):1584–1589. doi: 10.1289/ehp.0901284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrugo-Negrete J, Verbel JO, Ceballos EL, Benitez LN. Total mercury and methylmercury concentrations in fish from the Mojana region of Columbia. Environ Geochem Health. 2008;30:21–30. doi: 10.1007/s10653-007-9104-2. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D. Fish, mercury, selenium and cardiovascular risk: Current evidence and unanswered questions. Int J Environ Res Public Health. 2009;6:1894–1916. doi: 10.3390/ijerph6061894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Toxicological effects of methylHg. Washington, DC: National Academy Press; 2000. [Google Scholar]
- Neuman MJ, Ruess G, Able KW. Species composition and food habits of dominant fish predators in salt marshes of an urbanized estuary, the Hackensack Meadowlands, New Jersey. Urban Hab. 2004;2:3–22. [Google Scholar]
- Ng CL, Able KW, Grothues TM. Habitat use, site fidelity, and movement of adult striped bass in a southern New Jersey estuary based on mobile acoustic telemetry. Trans Am Fish Soc. 2007;136:1344–55. [Google Scholar]
- Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, Hu H, Gillman MW. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am J Epidemiol. 2008;167:1171–81. doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. Introduction—Comparative dietary risk: Balance the risks and benefits of fish consumption. Comments Toxicol. 2002;8:337–44. [Google Scholar]
- Pottern GB, Huish MT, Kerby JH. Species profiles: Life histories and environmental requirements of coastal fishes and invertebrates (mid-Atlantic): Bluefish. US Fish & Wildlife Service, Biol Rep. 1989;82(11.94):1–21. [Google Scholar]
- Ralston NVC. Selenium health benefit values as seafood safety criteria. Eco-Health. 2008;5:442–55. doi: 10.1007/s10393-008-0202-0. [DOI] [PubMed] [Google Scholar]
- Ralston NVC. Introduction to 2nd issue on special topic: Selenium and mercury as interactive environmental indicators. Environ Bioindicat. 2009;4:286–90. [Google Scholar]
- Ralston NVC, Ralston CR, Blackwell JL, III, Raymond LJ. Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology. 2008;29:802–11. doi: 10.1016/j.neuro.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Ratcliffe HE, Swanson GM, Fischer LJ. Human exposure to mercury: A critical assessment of the evidence of adverse health effects. J Toxicol Environ Health. 1996;49:221–70. doi: 10.1080/713851079. [DOI] [PubMed] [Google Scholar]
- Rice G, Swartout J, Mahaffey K, Schoeny R. Derivation of U.S. EPA’s oral reference dose (RfD) for methylmercury. Drug Chem Toxicol. 2000;23:41–54. doi: 10.1081/dct-100100101. [DOI] [PubMed] [Google Scholar]
- Rissanen T, Voutilainen S, Nyyssonen K, Lakka TA, Salonen JT. Fish oil-derived fatty acids, docosahexaenoic acid and docosaphentaenoic acid, and the risk of acute coronary events: The Kuopio ischaemic heart disease risk factor study. Circulation. 2000;102:2677–79. doi: 10.1161/01.cir.102.22.2677. [DOI] [PubMed] [Google Scholar]
- SAS Institute, Inc. SAS users’ guide. Cary, NC: Statistical Institute, Inc; 1995. [Google Scholar]
- Satoh H, Yasuda N, Shimai S. Development of reflexes in neonatal mice prenatally exposed to methylmercury and selenite. Toxicol Lett. 1985;25:199–203. doi: 10.1016/0378-4274(85)90082-7. [DOI] [PubMed] [Google Scholar]
- Scudder BC, Chaser LC, Wentz DA, Bauch NJ, Brigham ME, Moran PW, Krabbenhoft DP. Mercury in fish, bed sediment, and water from streams across the United States, 1998–2005. Reston, VA: U.S. Department of the Interior; 2009. report 2009–5109. [Google Scholar]
- Secor DH, Rooker JR, Zlokovitz E, Zdanowcz VS. Identification of the riverine, estuarine, and coastal contingents of Hudson River striped bass based upon elemental fingerprints. Mar Ecol Prog Ser. 2001;211:245–53. [Google Scholar]
- Steuerwald U, Weihe P, Jorgansen PJ, Bjerve K, Brock J, Heinzow B, Budtz-Jorgensen E, Grandjean P. Maternal seafood diet, methylmercury exposure, and neonatal neurological function. J Pediatr. 2000;136:599–605. doi: 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- Sweet LI, Zelikoff JT. Toxicology and immunotoxicology of mercury: A comparative review in fish and humans. J Toxicol Environ Health B. 2001;4:161–205. doi: 10.1080/109374001300339809. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. EPA’s 2008 report on the environment (final report) Washington, DC: U.S. Environmental Protection Agency; 2008. (EPA/600/R-07/045F) [Google Scholar]
- Virtanen JK, Mozaffarian D, Chiuve SE, Rimm EB. Fish consumption and risk of major chronic disease in men. Am J Clin Nutr. 2008;88:1618–25. doi: 10.3945/ajcn.2007.25816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman JR, Dunning DJ, Ross QE, Mattson MT. Range dynamics of Hudson River striped bass along the Atlantic coast. Trans Am Fish Soc. 1990;119:910–19. [Google Scholar]
- Watanabe C, Yin K, Kasanuma Y, Satoh H. In utero exposure to methylmercury and selenium deficiency converge on the neurobehavioral outcome in mice. Neurotoxicol Teratol. 1999;21:83–88. doi: 10.1016/s0892-0362(98)00036-1. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Hg—Environmental aspects. Geneva, Switzerland: WHO; 1989. [Google Scholar]