Abstract
A number of factors affect the consumption risk from mercury in fish, including mercury levels, seasonal patterns of mercury concentrations, human consumption patterns, and sensitive populations (e.g. pregnant women, fetuses, young children, and yet unknown genetic factors). Recently the protective effects of selenium on methylmercury toxicity have been publicized, particularly for saltwater fish. We examine levels of mercury and selenium in several species of fish and seabirds from the Aleutians (Alaska), determine selenium:mercury molar ratios, and examine species-specific and individual variation in the ratios as a means of exploring the use of the ratio in risk assessment and risk management. Variation among species was similar for mercury and selenium. There was significant inter-specific and intraspecific variation in selenium:mercury molar ratios for fish, and for birds. The mean selenium:mercury molar ratios for all fish and bird species were above 1, meaning there was an excess of selenium relative to mercury. It has been suggested that an excess of selenium confers some protective advantage for salt water fish, although the degree of excess necessary is unclear. The selenium:mercury molar ratio was significantly correlated negatively with total length for most fish species, but not for dolly varden. Some individuals of Pacific cod, yellow irish lord, rock greenling, Pacific halibut, dolly varden, and to a lesser extent, flathead sole, had selenium:mercury ratios below 1. No bird muscle had an excess of mercury (ratio below 1), and only glaucous-winged gull and pigeon guillemot had ratios between 1 and 5. There was a great deal of variation in selenium:mercury molar ratios within fish species, and within bird species, making it difficult and impractical to use these ratios in risk assessment or management, for fish advisories, or for consumers, particularly given the difficulty of interpreting the ratios.
Keywords: Mercury, Selenium, Molar ratios, Fish consumption, Marine
1. Introduction
For many parts of the world, fishing, and fish and shellfish consumption are important aspects of their culture, as well as a method of obtaining protein (Toth and Brown, 1997). High fishing rates occur in many different cultures, including both rural and urban areas (Burger et al., 2001a, 2001b; Bienenfeld et al., 2003), among Native Americans (Harris and Harper, 2000; Burger et al., 2007a, 2007b), and in other regions of the world, particularly in Asia (Burger et al., 2003; Takezaki et al., 2003; Lu et al., 2008; Hsiao et al., 2011). Since well over half of the world’s population resides within 100 km of oceans, it is important to understand the factors that affect the health and safety of saltwater fish as a food source.
Fish provide many nutrients and high quality protein, yet many people in the world are faced with deciding whether the benefits of eating fish outweigh the risks from contaminants. Fisheries not only provide protein and fish oil for humans, but also provide fishmeal for aquaculture use (Brunner et al., 2009), as well as recreational opportunities, cultural benefits, and esthetic pleasures (Toth and Brown, 1997; Harris and Harper, 1998; Burger, 2000, 2002). In many places, fish and shellfish are the only readily available source of protein that people can self-harvest, often throughout the year.
Fish consumption is associated with low blood cholesterol (Anderson and Wiener, 1995), positive pregnancy outcomes, and better child cognitive test performances (Oken et al., 2008). Fish (and fish oil) contain omega-3 (n–3) fatty acids that reduce cholesterol levels and the incidence of heart disease, blood pressure, stroke, and pre-term delivery (Kris-Etherton et al., 2002; Daviglus et al., 2002; Patterson, 2002; Virtanen et al., 2008; Ramel et al., 2010).
Fishing and consumption of fish create three difficulties: 1) for many parts of the world fish and shellfish are a critical and important source of protein for people (Dorea et al., 1998; Pinheiro et al., 2009), 2) overfishing results in fish declines and a shifting of fish populations to smaller individuals (in some cases destroying subsistence and commercial fisheries, Pauly et al., 1998; Safina, 1998), and 3) some fish contain contaminants (methylmercury [MeHg], PCBs) at high enough levels to cause effects on the fish themselves (Eisler, 1987), and on top-level predators, including humans (WHO, 1989; NRC, 1996, 2000; Hightower and Moore, 2003). Fish consumption is the most significant source of methylmercury exposure for the public (Grandjean et al., 1997; Rice et al., 2000), and is one of the largest contributors for other vertebrates (Eisler, 1987).
High levels of methylmercury in some fish can cause adverse health effects in people consuming large quantities (IOM, 1991, 2006; Grandjean et al., 1997; Gochfeld, 2003; Hites et al., 2004). In fetuses and young children, effects include neurodevelopmental deficits (Crump et al., 1998; Steuerwald et al., 2000; NRC, 2000), behavioral deficits (JECFA, 2003), and poorer cognitive test performance from both fetal and childhood exposure (Oken et al., 2008; Freire et al., 2010). In adults, methylmercury exposure counteracts the cardioprotective effects of fish consumption (Rissanen et al., 2000; Guallar et al., 2002), promotes cardiovascular disease (Choi et al., 2009), and result in neurological and locomotory deficits (Hightower and Moore, 2003).
Methods for reducing the risk from contaminants in fish include reduction of mercury in the environment (source reduction), reducing the rates of methylation and behavioral modification of consumption or cooking patterns. One of the main sources of mercury in aquatic environments, and in the food chain, is from atmospheric deposition, which is difficult to control globally. Countries respond to high mercury levels in fish by issuing consumption advisories or fishing bans. In the United States, it is largely the responsibility of states to determine health risks and to issue fish and shellfish consumption advisories. The U.S. Food and Drug Administration (USFDA, 2001, 2005) has additional responsibilities, and issued a series of consumption advisories based on methylmercury for saltwater fish. The FDA suggested that pregnant women and women of childbearing age who may become pregnant should limit their fish consumption, should avoid eating four types of marine fish (shark, swordfish, king mackerel, tilefish), should limit their consumption of all other fish to just 12 ounces per week, and should also limit consumption of canned tuna (USFDA, 2001, 2003, 2005).
Another factor that may contribute to lowered mercury toxicity from fish consumption may be the co-occurrence of other elements or other foods. From the late 1960s to the 1980s experiments with rats and other laboratory animals demonstrated the protective effects of selenium on mercury toxicity (Satoh et al., 1985; Lindh and Johansson, 1987). However, thereafter field and laboratory studies identified the toxic effects of selenium in wildlife (Eisler, 2000), particularly at Kesterson in California (Ohlendorf and Hothem, 1995; Ohlendorf, 2000). Subsequently, however, Mozaffarian (2009) reported that lower levels of nonfatal heart attacks were associated with higher levels of selenium, and the positive benefits of selenium on mercury toxicity from salt water fish gained importance (Ralston, 2008). Selenium is an essential trace element (i.e. a deficiency state has been identified), and it is toxic at high levels. It is regulated in the body (Eisler, 2000). Mercury, on the other hand, has no known essential role.
Much of mercury toxicity is mediated through binding to sulfur of proteins. Recent attention has focused on whether any or most of the toxicity of methylmercury is due to impaired selenium-dependent enzyme synthesis or activity (Watanabe et al., 1999; Ralston, 2008, 2009; Ralston et al., 2008). Mercury and methylmercury are irreversible selenoenzyme inhibitors (Watanabe et al., 1999; Carvalho et al., 2008), and they thus impair selenoprotein form and function. Mercury binds to selenium with a high affinity, and high maternal exposure inhibits selenium-dependent enzyme activity in the brain (Berry and Ralston, 2008). Cell culture studies and animal experiments show adverse impacts of high methylmercury exposure on selenoenzymes (particularly glutathione peroxidase and thioredoxin reductase) occur as a result of selenium–mercury interaction, which may explain oxidative damage attributable to methylmercury (Beyrouty and Chan, 2006; Cabanero et al., 2007; Pinheiro et al., 2009; Ralston, 2009), although biokinetics differ depending on the forms of selenium and of mercury (Dang and Wang, 2011; Sormo et al., 2011). Thus it is clear that selenium, which is regulated in the body, can be limiting at low levels, is essential at intermediate (or required) levels, can be toxic at high levels, and has some potential to protect against mercury toxicity at some undetermined level (Eisler, 2000).
Ralston and others (Ralston, 2008; Peterson et al., 2009a, 2009b; Sormo et al., 2011) suggested that excess selenium protects against mercury toxicity, and that selenium:mercury molar ratios above 1 are largely protective for adverse mercury affects. The actual selenium: mercury ratio that would protect against mercury toxicity is unclear. This is still a controversial issue, although Ralston (2008, 2009) and others (Kaneko and Ralston, 2007; Raymond and Ralston, 2004, 2009; Peterson et al., 2009a, 2009b) have argued strongly for the molar ratio being an important value for risk assessment.
In this paper we examine inter- and intraspecific differences in selenium:mercury molar ratios in a range of fish from the Aleutian Islands (Alaska). These are species eaten by Aleuts and some species are also fished commercially (Burger et al., 2007a). Our objectives were to determine: 1) whether mean selenium:mercury molar ratios varied by species, 2) whether mean selenium:mercury molar ratios were related to fish species size [total length or weight], 3) whether there were individual differences in the molar ratio within species, 4) whether within species individual molar ratios were related to fish size, and 5) whether mean selenium:mercury molar ratios are sufficiently constant (e.g. low variation) to allow for use in risk assessment, risk management, or risk communication. The Bering Sea around the Aleutian Islands provides a significant portion of commercial fish consumed in the continental United States, and Dutch Harbor in the Aleutians often has the largest tonnage of fish landings in the world (AMAP, 1998). Further, subsistence fish are an important part of the diet of the Aleuts living in small and remote villages in the Aleutians (Hamrick and Smith, 2003; Fall et al., 2006; Burger et al., 2007a, 2007b).
2. Methods
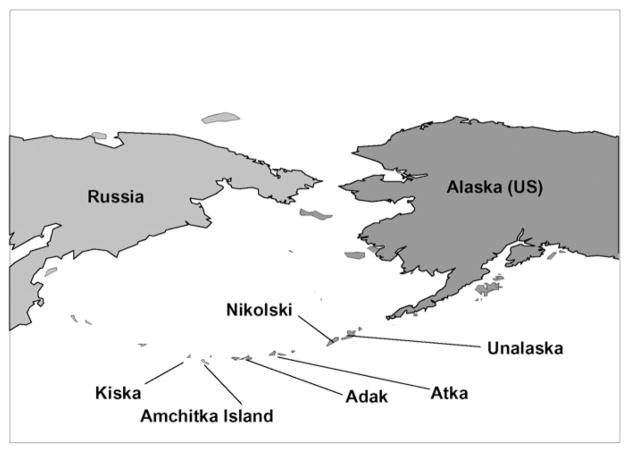
The chain of Aleutian Islands juts out from Alaska toward Russia (Fig. 1). Some of the islands are closer to Russia than to Alaska, and are quite small and isolated in one of the most dangerous and rugged seas (Bering Sea). The islands are inhabited by Aleuts, Alaskan Natives who rely on subsistence foods for much of the year. Aleut fishermen from Atka sometimes go as far as Amchitka to catch some fish, particularly halibut. Nikolski is the oldest continually-occupied community in North America (Black, 1974; Schlung, 2003). There are twice-weekly fiights to Adak, Atka, and Nikolski, which bring food, although most commercial food arrives by ship.
Fig. 1.
Map showing sampling locations for fish collected in the Aleutian Islands, Alaska, from 2003 to 2005.
Fish and birds were collected in July and August 2004 from the Aleutian Islands of Adak (52° N lat; 176° W long), Amchitka (51° N lat; 179° E long) and Kiska (51° N lat;177° E long, Fig. 1), and fish were collected in Spring 2005 from Nikolski (52 N lat; 168° W long) under appropriate permits from the State of Alaska’s Department of Fish and Game (# CF-04-043). Salmon samples were also collected from Atka (52 N lat; 174 W long) in August 2003. Amchitka and Kiska Islands are part of the Alaska Maritime National Wildlife Refuge that was established in 1913 by executive order of President Taft (ATSDR, 2000). There are small Aleut communities on Adak (ca 200), Nikolski (ca 35–38) and Atka (ca 85), but Amchitka and Kiska are currently uninhabited although they are traditional Aleut homelands.
Fish were collected from all five islands either from land or boat, with rod and reel by Aleuts and by scientists, and with trawling and by spearing while underwater by scientists while on the trawlers, Ocean Explorer or Gladiator (Fig. 1). Aleuts from Nikolski and Atka were on the expedition to Amchitka and Kiska, and collected samples in all locations, in the traditional manner used in their villages. Birds were collected by shotgun by Aleuts. Fish, birds, and bird eggs were immediately measured, weighed and dissected, and samples of muscle were frozen for later analysis. All samples were given a unique number and had Chain of Custody forms with the following information recorded: specimen number, species, age class where appropriate, date, island, location from that island, collector, and preparator. All samples were shipped frozen to the Environmental and Occupational Health Sciences Institute (EOHSI) of Rutgers University for metal analysis. Most samples were collected as part of research by the Consortium for Risk Evaluation with Stakeholder Participation (CRESP) to examine radionuclide levels in marine biota for the Department of Energy (Burger et al., 2007a). Levels of all anthropogenic radionuclides examined were well below safe human health risk guidance levels (Powers et al., 2005). Scientific names for all species examined in the present study are given in Table 1.
Table 1.
Mercury and selenium levels (ppm, wet weight) (μg/g), selenium:mercury molar ratios, and relationship to size for fish species collected from the Aleutian Islands, Alaska (2003–2005). Given are arithmetic means±SE, standard deviation and Kendall Tau correlation coefficients.
| Common name | Scientific name | n | Mercury mean±SE |
Selenium mean±SE |
Se:Hg ratio (Means)a | Se:Hg ratio correlation with Hg tau (p) | Se:Hg ratio correlation with length tau (p) | Se:Hg ratio correlation with Weight tau (p) |
|---|---|---|---|---|---|---|---|---|
| Fish | ||||||||
| Great sculpin | Myoxocephalus polyacanthocephalus | 27 | 0.37±0.06 | 0.55±0.03 | 3.80 | −0.78 (<0.0001) | −0.31 (0.03) | −0.55 (<0.0001) |
| Yellow irish lord | Hemilepidotus jordani | 68 | 0.28±0.02 | 0.34±0.02 | 3.09 | −0.70 (<0.0001) | −0.16 (NS) | −0.15 (0.07) |
| Flathead sole | Hippoglossoides elassodon | 39 | 0.28±0.01 | 0.40±0.04 | 3.65 | −0.3 (0.007) | −0.08 (NS) | −0.07 (NS) |
| Pacific cod | Gadus macrocephalus | 140 | 0.17±0.01 | 0.18±0.01 | 2.69 | −0.65 (<0.0001) | −0.39 (<0.0001) | −0.38 (<0.0001) |
| Black rockfish | Sebastes melanops | 65 | 0.17±0.02 | 0.57±0.02 | 8.73 | −0.73 (<0.0001) | −0.31 (0.0004) | −0.32 (0.0002) |
| Pacific halibut | Hippoglossus stenolepis | 24 | 0.16±0.04 | 0.37±0.03 | 6.00 | −0.75 (<0.0001) | −0.53 (0.0003) | 0.51 (0.0005) |
| Red irish lord | Hemilepidotus hemilepidotus | 56 | 0.13±0.01 | 0.24±0.04 | 4.73 | −0.57 (<0.0001) | −0.25 (0.009) | −0.23 (0.01) |
| Dolly varden | Salvelinus malma | 75 | 0.11±0.01 | 0.35±0.04 | 7.78 | −0.59 (<0.0001) | 0.23 (0.004) | 0.19 (0.02) |
| Rock greenling | Hexagrammos lagocephalus | 82 | 0.10±0.01 | 0.20±0.01 | 5.11 | −0.68 (<0.0001) | −0.20 (0.008) | −0.22 (0.004) |
| Rock sole | Lepidopsetta bilineate | 27 | 0.09±0.01 | 0.54±0.06 | 14.90 | −0.49 (0.0004) | −0.14 (NS) | −0.17 (NS) |
| Walleye pollock | Theragra chalcogramma | 12 | 0.07±0.02 | 0.46±0.02 | 15.85 | −0.85 (0.0001) | −0.43 (0.05) | −0.41 (0.06) |
| Northern rock sole | Lepidopsetta polyxystra | 15 | 0.07±0.01 | 0.47±0.03 | 17.58 | −0.50 (0.01) | 0.11 (NS) | −0.09 (NS) |
| Pacific Ocean perch | Sebastes alutus | 17 | 0.05±0.01 | 0.88±0.05 | 46.42 | −0.91 (<0.0001) | −0.62 (0.0007) | −0.60 (0.0007) |
| Atka mackerel | Pleurogrammus monopterygius | 19 | 0.05±0.00 | 0.41±0.04 | 22.56 | −0.54 (0.001) | −0.04 (NS) | −0.10 (NS) |
| Sockeye salmon | Oncorhynchus nerka | 15 | 0.04±0.01 | 0.25±0.03 | 14.92 | −0.46 (0.02) | b | b |
| Kruskal Wallis χ2 (p) | 222 (<0.0001) | 336 (<0.0001) | 242 (<0.0001) | |||||
| Invertebrate | ||||||||
| Octopus | Octopus dofleini | 5 | 0.04±0.01 | 0.23±0.04 | 15.44 | −1.00 (0.01) | b | −0.82 (NS) |
| Bird muscle | ||||||||
| Bald eagle | Haliaeetus leucocephalus | 1 | 1.74c | 3.9c | 5.71 | c | ||
| Pigeon guillemot | Cepphus columba | 21 | 0.49±0.04 | 1.04±0.08 | 5.35 | −0.30 (0.06) | ||
| Glaucous-winged gull | Larus glaucescens | 32 | 0.33±0.03 | 1.05±0.11 | 8.13 | −0.39 (0.002) | ||
| Common eider | Somateria mollissima | 20 | 0.12±0.01 | 0.78±0.07 | 16.19 | −0.19 (NS) | ||
| Tufted puffin | Fratercula cirrhata | 8 | 0.12±0.02 | 3.10±0.43 | 65.06 | −0.6 (0.03) | ||
| Kruskal Wallis χ2 (p) | 55.9 (<0.0001) | 20.6 (0.0004) | 45.6 (<0.0001) | |||||
| Bird eggs | ||||||||
| Glaucous-winged gull | Larus glaucescens | 21 | 0.70±0.07 | 1.57±0.09 | 5.67 | −0.61 (0.0001) | ||
| common eider | Somateria mollissima | 54 | 0.43±0.02 | 1.73±0.14 | 10.20 | −0.24 (0.01) | ||
| Kruskal Wallis χ2 (p) | 12.2 (0.0005) | 0.04 (NS) | 8.8 (0.003) | |||||
The Se/Hg molar ratios are calculated on unrounded mean Hg and Se values.
Length measurements are not available.
No standard error or correlation because N=1.
At EOHSI, a 2 g (wet weight) sample of tissue was digested in trace metal grade nitric acid in a microwave (MD 2000 CEM), using a digestion protocol of three stages of 10 min each under 50, 100 and 150 pounds per square inch (3.5, 7, and 10.6 kg/cm2) at 80% power. Digested samples were subsequently diluted to 25 ml with deionized water. Instruments and containers were washed in 10% HNO3 solution and rinsed with deionized water, prior to each use (Burger et al., 2001a, 2001b, 2007a).
Selenium was analyzed by graphite furnace atomic absorption, with a detection limit of 0.0007 μg/g. Mercury was analyzed by the cold vapor technique using the Perkin Elmer FIMS-100 mercury analyzer, with an instrument detection level of 0.0002 μg/g, and a matrix level of quantification of 0.002 μg/g. DORM-2 Certified dogfish tissue was used as the calibration verification standard. Recoveries between 90–110% were accepted to validate the calibration. All specimens were run in batches that included blanks, a standard calibration curve, one spiked specimen, and one duplicate. The accepted recoveries for spikes ranged from 85% to 115%; no batches were outside of these limits. 10% of samples were digested twice and analyzed as blind replicates (with agreement within 15%).
All concentrations (total mercury) are expressed in μg/g (=ppm=parts per million) on a wet weight basis. Ppm is the unit used by state and federal agencies when communicating with the public generally, and when discussing risk from fish consumption. People may dry some foods before consumption, for example salmon. We found that dry weight for cold-water fish examined in this study ranged from 18 to 24%. Thus a dry weight equivalent would be 4–5 × higher than the values published here.
This paper reports on total mercury and total selenium levels, without speciation. However, many studies have shown that almost all of the mercury in fish and avian tissue is methylmercury, and 90% is a reasonable approximation of this proportion, which varies somewhat among types, laboratories, and seasons (Lansens et al., 1991; Jewett et al., 2003; Cabanero et al., 2007; Scudder et al., 2009). However, future work should involve speciation, at least of a subsample, since it is important to speciate mercury when interpreting the protective effects of selenium (Khan and Wang, 2009; Lemes and Wang, 2009), especially for mammals (Lemes et al., 2011).
While dissecting Pacific cod, we removed a sample of 46 otoliths for age identification. Delsa Anderi of NOAA identified the ages of these samples.
For each species a mean selenium:mercury molar ratio was calculated from the average selenium and average mercury levels (see Table 1) by dividing concentration (in μg/g) by the molecular weight (200.59 for mercury and 78.96 for selenium). This is the usual method used to determine molar ratios in the literature, partly because some of the calculations have been done from the published literature, rather than by the authors conducting the studies (who had the original data). Therefore, we calculated the ratios from the means to be consistent with the literature and allow comparisons. Note that some papers report the mercury:selenium ratio rather than the selenium:mercury reported in this paper (e.g. Cappon and Smith, 1981). Se:Hg is the reciprocal of Hg:Se.
We used Kruskal Wallis X2 values to test for differences in mercury levels, selenium levels, and the selenium:mercury molar ratios among fish species and locations, and Kendall Tau correlations to examine the relationship between the molar ratios and mercury levels, and between the molar ratios and fish size (e.g. length) (SAS, 2005). The level for significance was designated as <0.05. Hereafter the term selenium:mercury refers to the selenium:mercury molar ratio.
3. Results
3.1. Interspecific differences
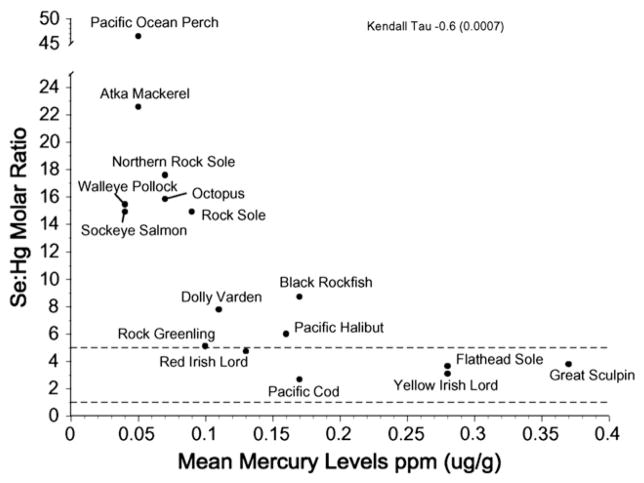
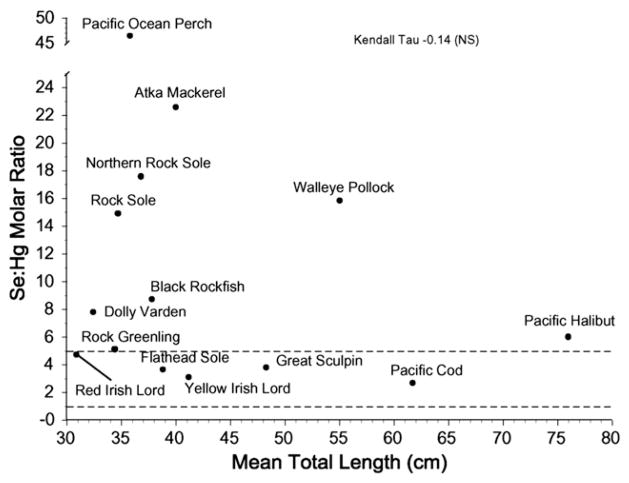
There are interspecific differences in mercury and selenium levels, and in the selenium:mercury molar ratio for fish and for bird muscle. For bird eggs (smaller sample of species), only mercury and the selenium:mercury molar ratio were significantly different (Table 1). When all fish species are considered together, the mean selenium:mercury ratio was negatively correlated with mean mercury levels (Fig. 2), but was not significantly correlated with mean total fish length (Fig. 3). This lack of correlation was not only due to walleye pollock. Similar relationships were not examined for the birds which have determinate growth.
Fig. 2.
Relationship between mean selenium:mercury molar ratios and mean mercury levels for fish from the Aleutians. Kendall tau= −0.6 for the relationship between selenium:mercury molar ratio and mean mercury levels.
Fig. 3.
Relationship between mean selenium:mercury molar ratios and mean total fish length for fish from the Aleutians. There was not a significant relationship between the ratio and mean fish length among fish species.
3.2. Intraspecific differences
For the 14 species for which length and weight data were available, the selenium:mercury molar ratio was significantly negatively correlated with length of fish for 8 of 14 species and with weight for 7 of 14 species (Table 1). The ratio was positively correlated with length and weight for dolly varden. For halibut the ratio was negatively correlated with length and positively with weight. Being negatively correlated means that as fish size increased, the selenium:mercury molar ratio decreased (i.e. selenium provides less protection from mercury toxicity).
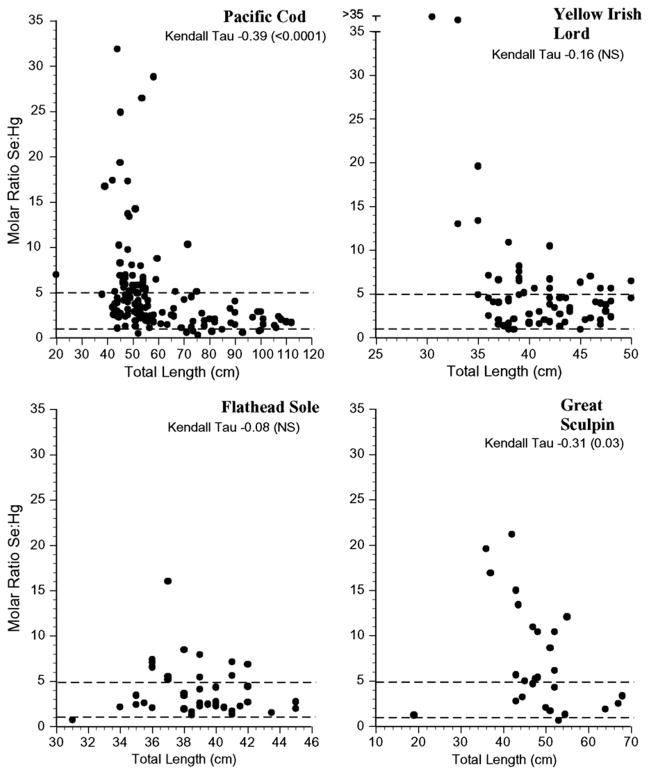
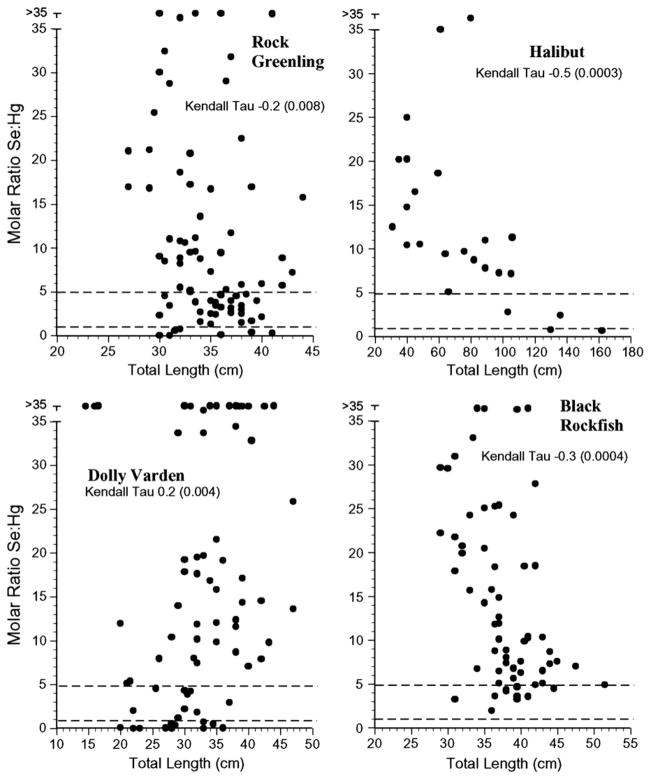
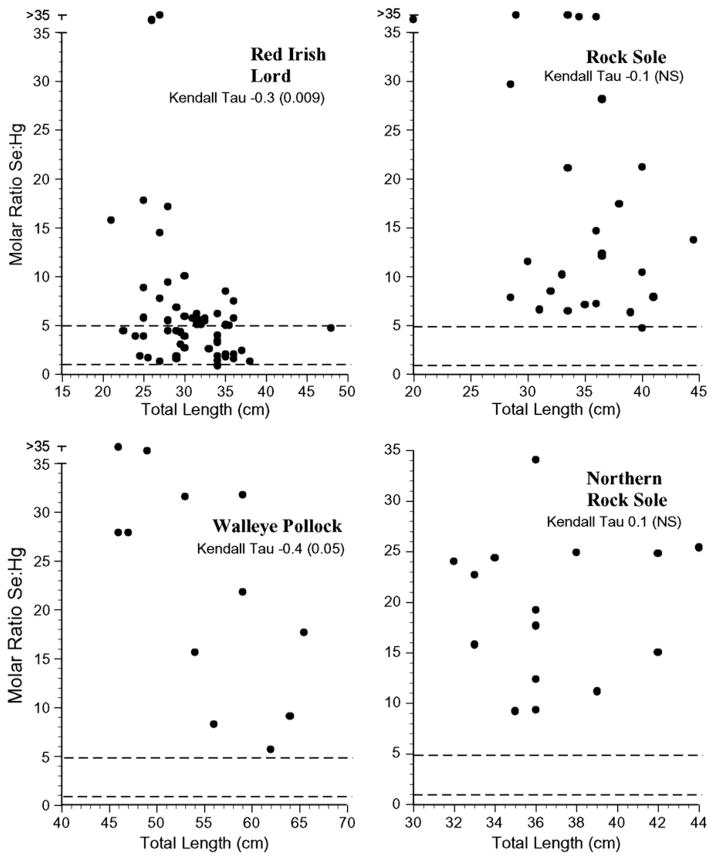
For risk assessors or managers to successfully use selenium: mercury relationships in risk assessment there should be low intra-specific variation, but if there is variation, it should relate to size. The selenium:mercury molar ratios for all individuals’ samples for each species examined are shown in Figs. 4–7. The variation within species was great, with some individuals falling below a selenium: mercury ratio of 1 for Pacific cod, yellow irish lord, flathead sole, great sculpin, rock greenling, halibut, and dolly varden (Figs. 4 and 5). For all fish, except walleye pollock, northern rock sole, Pacific ocean perch, and Atka mackerel, there were some Se:Hg values between 1 and 5.
Fig. 4.
Individual selenium:mercury molar ratios for Pacific cod, yellow irish lord, flathead sole and great sculpin collected from the Aleutians. Kendall tau correlations indicate whether there was a significant relationship between the ratio and length for each species.
Fig. 7.
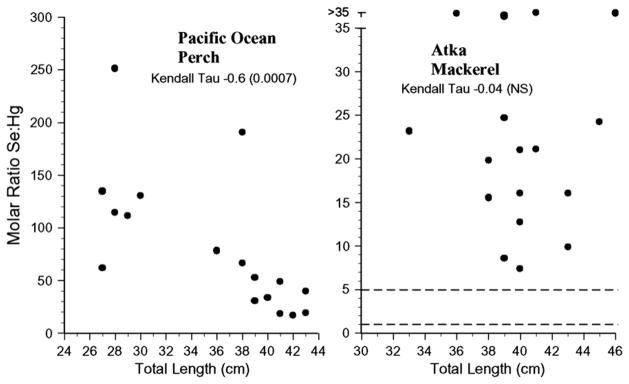
Individual selenium:mercury molar ratios for Pacific ocean perch and Atka mackerel collected from the Aleutians. Kendall tau correlations indicate whether there was a significant relationship between the ratio and length for each species.
Fig. 5.
Individual selenium:mercury molar ratios for rock greenling, halibut, dolly verden and black rockfish collected from the Aleutians. Kendall tau correlations indicate whether there was a significant relationship between the ratio and length for each species.
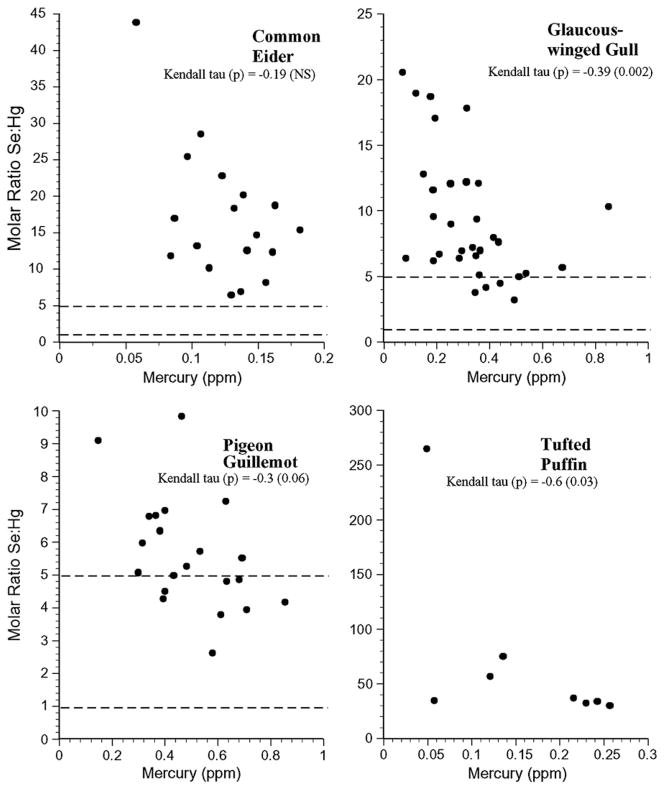
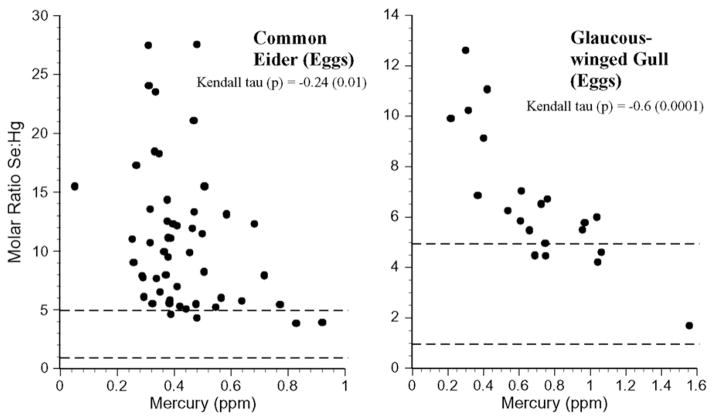
Similarly, there was individual variation within a species for all the bird species (Fig. 8). None of the birds had selenium:mercury molar ratios below 1, and only glaucous-winged gull and pigeon guillemot had values between 1 and 5. Both gull and eider eggs had selenium: mercury molar ratios between 1 and 5 (Fig. 9).
Fig. 8.
Individual selenium:mercury molar ratios for muscle of common eider, glaucous-winged gull, pigeon guillemot and tufted puffin collected from the Aleutians. Kendall tau correlations indicate whether there was a significant relationship between the ratio and mercury levels for each species.
Fig. 9.
Individual selenium:mercury molar ratios for eggs of common eider and glaucous-winged gull collected from the Aleutians. Kendall tau correlations indicate whether there was a significant relationship between the ratio and mercury levels for each species.
4. Discussion
4.1. Interspecific and intraspecific variation: trophic level considerations
In general, fish that are large and high on the trophic scale have higher mercury levels than smaller, herbivorous fish. Mercury levels are usually correlated with fish size (weight or length), both within and among species (Green and Knutzen, 2003; Storelli et al., 2002; Simonin et al., 2008; Burger and Gochfeld, 2011), and elimination rate is also negatively correlated with size (Trudel and Rasmussen, 1997). Thus, species of fish that are large may have low selenium:mercury ratios because selenium is regulated in the body (Eisler, 2000), and mercury levels increase with size and trophic level (Power et al., 2002). Small species of fish, on the contrary, may have a range of ratios from low to high because at low mercury levels, the size relationship may not hold (Park and Curtis, 1997). Further, some smaller fish species are bottom feeders, and acquire moderate or high mercury levels from prey that reside in the sediment (Campbell, 1994), and some herbivores can have high levels of metals (Tayel and Shriadah, 1996).
In this study of 15 species of fish from the Aleutians, we found that: 1) there were interspecific differences in mercury and selenium levels, and in the selenium:mercury molar ratios in both fish and bird muscle, 2) there was a significant difference in mercury levels but not selenium levels for eggs of gulls and eiders, 3) the ratios were negatively correlated with fish length for 8 of 14 species, 4) the ratios were negatively correlated with fish weight for 7 of 14 species with data, (only dolly varden showed a positive correlation of ratio with length and width, 5) mean selenium:mercury molar ratios were negatively correlated with mean mercury levels for the fish species, but not with mean length, 6) individuals of many fish species had some ratios below 1, and more ratios between 1 and 5, and 7) no individual birds had ratios below 1.
These data indicate that for fish species, there is sometimes a significant relationship between the selenium:mercury molar ratio and length, but the relationship is generally negative. This means that as the fish get bigger and mercury levels increase, the ratio decreases, and the potential protective effect of selenium decreases. There was a great deal of variation among individuals of the same species. That is, knowing the mean selenium:mercury molar ratio for a given species did not necessarily predict whether any individuals had ratios below 5, or below 1, or even the frequency of low ratios. This is the result of individual variation in both selenium and mercury levels, which is partly a result of differences in prey, the proportion of different prey items in the diet, and foraging location (Watras et al., 1998; Snodgrass et al., 2000; Burger and Gochfeld, 2011). Dietary uptake accounts for more than 90% of the total uptake (Wiener et al., 2003).
In this study, the species that had the highest trophic level (cod, sculpin, Irish lords), and were predators, had the lowest selenium:mercury molar ratios, and those that were lower, had higher selenium: mercury molar ratios (perch, sole). Further, fish that had longer lifespans (halibut, greenling, rockfish) tended to have lower selenium: mercury molar ratios. Thus, fish species that have longer lifespans and are at a higher trophic levels had lower selenium:mercury ratios, and thus selenium levels would likely be less protective than fish with higher ratios.
4.2. Age vs size variation in Pacific cod
As mentioned above, mercury levels usually increase with size of the fish (both within and among species), and are generally higher in higher trophic level fish (Watras et al., 1998; Wiener et al., 2003). Size is usually related to age within a given species, as with time, fish grow larger. Usually, however, age is not known, and size is used as a surrogate for age (Boening, 2000). Where age was known, age was more strongly correlated with mercury levels than was size (although size was highly correlated; Braune, 1987; Burger and Gochfeld, 2007). Age and mercury are more highly correlated in food limited environments because growth (either weight gain or size) is stunted, resulting in older fish that are not significantly larger than younger fish.
Otoliths can be used to age fish, and we did so in the study of mercury in Pacific cod from the Aleutians (Burger and Gochfeld, 2007, the same cod examined in the present paper). In this study, the selenium: mercury molar ratio was correlated with age, and the correlation was about the same (0.31) compared to length=0.39.
4.3. Selenium:mercury molar ratios in saltwater fish
It is important to understand mercury levels in saltwater fish because they are an important food source for people, and other predators, although ratios in freshwater fish are also important, particularly for recreational fishers (Burger, 2012). Large predatory fish, such as shark, swordfish, and tuna bioaccumulate high levels of mercury, and may have mechanisms for demethylating mercury. For decades, studies mainly reported levels of mercury in different fish species, but attention is now focusing on levels of selenium as well (Kaneko and Ralston, 2007; Burger and Gochfeld, 2011; Burger, 2012). Although most studies still do not report selenium levels, it is essential to understand variations in selenium:mercury molar ratios before interspecific and geographical patterns can be identified.
Because of the wide range in fish sizes, trophic levels, and foraging methods, there can be a great deal of variation in selenium and mercury levels, and in the selenium:mercury molar ratios. Thus, the range in the mean selenium:mercury molar ratios for marine fish varies markedly among regions: 1) 0.46 to 17.6 for 15 species from Hawaii (Kaneko and Ralston, 2007), 2) 0.58 to 12.5 for 11 commercial species (Cappon and Smith, 1981; but they selected mainly predatory species and evaluated only 1–4 individuals/species each), 3) 3–22 for 4 species from Spain and Portugal (Cabanero et al., 2005), 4) 2.0 to 17.3 for three species from Spain (Cabanero et al., 2007), and 5) 0.36 to over 60 for 19 recreationally caught species from New Jersey (Burger and Gochfeld, 2012).
In the present study, we found that the mean selenium:mercury molar ratios for 15 marine fish ranged from 2.7 to 45.4. Considerably more data are required before generalizations about selenium:mercury ratios can be made. It is also useful to compare the molar ratios for the same species from different regions or circumstances. For example, the selenium:mercury molar ratio for Pacific cod collected from supermarkets in New Jersey was 16.5, whereas in the present study it was 2.69. It is possible that the supermarket sample was either heterogeneous or mislabeled (Lowenstein et al., 2010), or that very different sized fish were sampled (it is difficult to determine size in fish purchased from markets). Further, those from the Aleutians were from a rather narrow zone, while those that reach the commercial market could be from the entire Bering Sea.
Selenium:mercury molar ratios have not been considered in birds, largely because there is no commercial sale of birds, and scientists and others examining the potential positive benefit of these ratios have not considered birds as a important subsistence food nor as an important source of mercury exposure. In this study, the mean selenium: mercury molar ratios for birds were all above 5, and in general, few individuals within each species had ratios below 5, and none had ratios below 1.
4.4. Protective effect of selenium and risk assessment
Understanding the factors that affect methylmercury toxicity is critical to reducing potential effects, particularly for fetuses and young children. One conservative estimate is that 250,000 women may be exposing their fetuses to levels of methylmercury above federal health guidelines (i.e. the EPA Reference dose; Hughner et al., 2008), while Trasande et al. (2005) estimate the proportion of fetuses in jeopardy is much higher (7.8 to 15.7%). There is some indication that the FDA warnings about fish consumption have resulted in a reduction in the consumption of fish generally, and of canned tuna specifically (Shimshack et al., 2007). This may not be a positive outcome, given that fish are a healthy source of protein that has a number of health benefits. Further, Groth (2010) recently showed that, with the exception of swordfish, relatively high mercury fish make up a small share of fish and shellfish consumption in the U.S. To make sure that warnings about mercury do not reduce consumption of low mercury species, the fish consuming public, particularly sensitive populations, need information to make informed decisions about seafood safety (Gochfeld and Burger, 2005; IOM, 2006).
The potential ability of selenium to reduce mercury toxicity suggests that risk assessors and managers can consider this factor (Ralston, 2009). Ralston and others suggested that selenium:mercury molar ratios above 1 (or some other ratio) are protective for adverse mercury affects (Ralston, 2008; Peterson et al., 2009a, 2009b), but the actual ratio that is protective is unclear (Ralston et al., 2008; Burger and Gochfeld, 2012). There is no biological basis for identifying an absolute selenium:mercury ratio that is protective; indeed selenium binds many other cations, and any protection conferred would be relative (Lemire et al., 2010).
It is clear that selenium confers some protective benefits, on genetic damage and on cancer (El-Bayoumy, 2001), as well as on mercury toxicity (Tran et al., 2007; Ralston, 2008; Peterson et al., 2009a; Chatziargyriou and Dailianis, 2010, references in introduction). A range of epidemiologic, laboratory and human clinical intervention trials support a protective role for selenium against cancer development, and that selenium plays a protective role with respect to mercury (El-Bayoumy, 2001; Chatziargyriou and Dailianis, 2010). Selenium plays a role in antioxidant enzymes (Tran et al., 2007), especially for mammals (Speier et al., 1985) and bivalves (Chatziargyriou and Dailianis, 2010).
4.5. Risk management
If selenium in marine fish, especially commercial marine fish, can reduce the toxicity of mercury, then it is another factor that should be considered in risk assessment and risk management. The practical implications of the modification of mercury toxicity by selenium are unclear (Watanabe, 2002). We suggest caution before selenium:mercury ratios become part of the considerations for mercury toxicity regulations or advisories, and that selenium:mercury ratios should be used only in conjunction with mercury levels.
In this study, with fish species that are consumed by Aleuts (often subsistence fishing) and are important commercial species, we found several key aspects of relevance for risk assessors, risk managers, and consumers: 1) all mean selenium:mercury molar ratios for fish, birds, and bird eggs were above 1, 2) there was a great deal of variation both within and among species in these ratios, 3) the ratio (and any protectiveness) generally decreased with increasing fish length and increasing mercury level, and 4) the individual variation in selenium: mercury molar ratios for all the fish and bird species, which often involved some individuals with values below 1, was sufficiently great to make it difficult to generalize either about species, or individuals within a species, or size relationships within a species. Thus risk assessors and managers, public health officials, and consumers cannot easily use these ratios in making decisions.
Finally, it should be mentioned that mercury is only one contaminant of concern for saltwater fish. Polychlorinated biphenyls (PCBs), organochlorine pesticides, and other contaminants can pose a risk, and should be considered when evaluating and managing risk. For example, the levels of PCBs in the fish reported in this sample pose a risk to people who consume large quantities, particularly of rock greenling, dolly varden, and flathead sole, all fish that are relatively low on the food chain (Hardell et al., 2010) and all fish that had some of the lowest selenium:mercury molar ratios in the present study. This study is one of the few where mercury (Burger et al., 2007a), PCBs and organochlorine pesticides (Hardell et al., 2010) were examined in the same fish. Some of the fish that had low mercury levels, had high PCB levels, suggesting the importance of doing multi-contaminant risk assessments, with appropriate risk management.
Fig. 6.
Individual selenium:mercury molar ratios for red irish lord, rock sole, walleye Pollock and northern rock sole collected from the Aleutians. Kendall tau correlations indicate whether there was a significant relationship between the ratio and length for each species.
Acknowledgments
We thank the many people who contributed to the development and execution of CRESP’s Amchitka Geophysical and Biological Project, especially C. W. Powers, D. Kosson, L. Bliss, B. Friedlander, D. Volz, M. Greenberg, and H. Mayer, and the following for their help throughout the project, S. Jewett, D. Barnes, L. Duffy, J. Weston, M. Stabin, A. Morkill, R. Patrick, D. Rogers, D. Dasher, J. Halverson, P. Sanders, J. Alchowiak and the people of the villages of Unalaska, Nikolski, Atka, and Adak in the Aleutians. We thank the entire crew of the Ocean Explorer, Captain Ray Haddon, mate Glenn Jahnke, cook Don Dela Cruz, and Bill Dixon, Joao Do Mar, and Walter Pestka, as well as the Captain of the Gladiator trawler and his crew for aiding our collection, Delsa Anderi for analyzing otoliths and M. E. Wilkins for allowing us to participate on their cruise. We thank several agencies who contributed to this research, including the U.S. Fish & Wildlife Service, and the Alaska Department of Environmental Conservation. This research was funded by the Consortium for Risk Evaluation with Stakeholder Participation (CRESP) through the Department of Energy (DE-FG 26-00NT 40938, DE-FC01-06EW07053) and by NIEHS P30ES005022. The results, conclusions and interpretations reported herein are the sole responsibility of the authors, and should not in any way be interpreted as representing the views of the funding agencies.
References
- AMAP. Oslo: Arctic Monitoring and Assessment Porgramme AMAP. 1998. AMAP assessment report: arctic pollution issues; p. 859. [Google Scholar]
- Anderson PD, Wiener JB. Eating fish. In: Graham JD, Wiener JB, editors. Risk versus risk: tradeoffs in protecting health and the environment. Cambridge, Mass: Harvard Univ Press; 1995. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological profile for arsenic. Atlanta, GA, USA: Agency for Toxic Substances and Disease Registry; 2000. [Google Scholar]
- Berry MJ, Ralston NVC. Mercury toxicity and the mitigating role of selenium. Ecohealth. 2008;5:456–9. doi: 10.1007/s10393-008-0204-y. [DOI] [PubMed] [Google Scholar]
- Beyrouty P, Chan HM. Co-consumption of selenium and vitamin E altered the reproductive and developmental toxicity of methylmercury in rats. Neurotoxicol Teratol. 2006;28:49–58. doi: 10.1016/j.ntt.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Bienenfeld LS, Golden AL, Garland EJ. Consumption of fish from polluted waters by WIC participants in East Harlem. J Urban Health. 2003;80:349–58. doi: 10.1093/jurban/jtg036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RF. Geology and ancient Aleuts, Amchitka and Umnak Islands, Aleutians. Arct Archeol. 1974;11:126–40. [Google Scholar]
- Boening DW. Ecological effects, transport, and fate of mercury: a general review. Che-mosphere. 2000;40:1335–51. doi: 10.1016/s0045-6535(99)00283-0. [DOI] [PubMed] [Google Scholar]
- Braune BM. Mercury accumulation in relation to size and age of Atlantic Herring (Clupea harengus harengus) from the Southwestern Bay of Fundy, Canada. Arch Environ Contam Toxicol. 1987;16:311–20. doi: 10.1007/BF01054948. [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Jones PJS, Friel S, Bartley M. Fish, human health and marine ecosystem health: policies in collision. Int J Epidemiol. 2009;38:91–100. doi: 10.1093/ije/dyn157. [DOI] [PubMed] [Google Scholar]
- Burger J. Consumption advisories and compliance: the fishing public and the deamplification of risk. J Environ Plan Manage. 2000;43:471–88. [Google Scholar]
- Burger J. Consumption patterns and why people fish. Environ Res. 2002;90:125–35. doi: 10.1006/enrs.2002.4391. [DOI] [PubMed] [Google Scholar]
- Burger J. Selenium:mercury molar ratios in fish from the Savannah River: implications for risk management. J Risk Res. 2012:1–18. [Google Scholar]
- Burger J, Gochfeld M. Risk to consumers from mercury in Pacific cod (Gadus macrocephalus) from the Aleutians: fish age and size effects. Environ Res. 2007;105:276–84. doi: 10.1016/j.envres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Mercury and selenium levels in 19 species of saltwater fish from New Jersey as a function of species, size, and season. Sci Total Environ. 2011;409:1418–29. doi: 10.1016/j.scitotenv.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Selenium and mercury ratios in saltwater fish from New Jersey: individual and species variations complicate possible use in human health consumption advisories. Environ Res. 2012;114:12–23. doi: 10.1016/j.envres.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gaines KF, Gochfeld M. Ethnic differences in risk from mercury among Savannah River fishermen. Risk Anal. 2001a;21:533–44. doi: 10.1111/0272-4332.213130. [DOI] [PubMed] [Google Scholar]
- Burger J, Gaines KF, Boring CS, Stephens WL, Jr, Snodgrass J, Gochfeld M. Mercury and selenium in fish from the Savannah River: species, trophic level, and locational differences. Environ Res. 2001b;87:108–18. doi: 10.1006/enrs.2001.4294. [DOI] [PubMed] [Google Scholar]
- Burger J, Fleischer J, Gochfeld M. Fish, shellfish, and meat meals of the public in Singapore. Environ Res. 2003;93:254–61. doi: 10.1016/s0013-9351(03)00015-x. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Jeitner C, Burke S, Stamm T, Snigaroff R, et al. Mercury levels and potential risk from subsistence foods from the Aleutians. Sci Total Environ. 2007a;384:93–105. doi: 10.1016/j.scitotenv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Jeitner C, Burke S, Stamm T. Metal levels in flathead sole (Hippoglossoides elassodon) and great sculpin (Myoxocephalus polyacanthocephalus) from Adak Island, Alaska: potential risk to predators and fishermen. Environ Res. 2007b;103:62–9. doi: 10.1016/j.envres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Cabanero AI, Carvalho C, Madrid Y, Batoreu C, Camara C. Quantification and speciation of mercury and selenium in fish samples of high consumption in Spain and Portugal. Biol Trace Elem Res. 2005;103:17–35. doi: 10.1385/BTER:103:1:017. [DOI] [PubMed] [Google Scholar]
- Cabanero AI, Madrid Y, Camara C. Mercury–selenium species ratio in representative fish samples and their bioaccessibility by an in vitro digestion method. Biol Trace Elem Res. 2007;119:195–211. doi: 10.1007/s12011-007-8007-5. [DOI] [PubMed] [Google Scholar]
- Campbell KR. Concentrations of heavy metals associated with urban runoff in fish living in stormwater treatment ponds. Arch Environ Contam Toxicol. 1994;27:352–6. [Google Scholar]
- Cappon CJ, Smith JC. Mercury and selenium content and chemical form in fish muscle. Arch Environ Contam Toxicol. 1981;10:305–19. doi: 10.1007/BF01055632. [DOI] [PubMed] [Google Scholar]
- Carvalho CML, Chew EH, Hashemy LI, Lu J, Holmgren A. Inhibition of the human thioredoxin system: a molecular mechanism of mercury toxicity. J Biol Chem. 2008;283:11913–23. doi: 10.1074/jbc.M710133200. [DOI] [PubMed] [Google Scholar]
- Chatziargyriou V, Dailianis S. The role of selenium-dependent glutathione perocidase (Se-GPx) against oxidative and genotoxic effects of mercury in haemocytes of mussel Mytilus galloprovincialis (LmK.) Toxicol In Vitro. 2010;24:1363–72. doi: 10.1016/j.tiv.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Choi AL, Budtz-Jorgensen E, Jorgensen PJ, Salonen JT, Tuomainen T, Murata K, et al. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ Health Perspect. 2009;117:367–72. doi: 10.1289/ehp.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump KS, Kjellstrom T, Shipp AM, Silvers A, Stewart A. Influence of prenatal mercury exposure upon scholastic and psychological test performance: benchmark analysis of a New Zealand cohort. Risk Anal. 1998;18:701–13. doi: 10.1023/b:rian.0000005917.52151.e6. [DOI] [PubMed] [Google Scholar]
- Dang F, Wang W. Antagonistic interaction of mercury and selenium in a marine fish is dependent on their chemical species. Environ Sci Technol. 2011;45:3116–22. doi: 10.1021/es103705a. [DOI] [PubMed] [Google Scholar]
- Daviglus M, Sheeshka J, Murkin E. Health benefits from eating fish. Comment Toxicol. 2002;8:345–74. [Google Scholar]
- Dorea JG, Moreira MB, East G, Barbosa AC. Selenium and mercury concentrations in some fish species of the Madeira River, Amazon Basin, Brazil. Biol Trace Elem Res. 1998;65:211–20. doi: 10.1007/BF02789097. [DOI] [PubMed] [Google Scholar]
- Eisler R. Mercury hazards to fish, wildlife, and invertebrates: a synoptic review, 85. 1.10 Washington, D.C: US Fish and Wildlife Service Re; 1987. [Google Scholar]
- Selenium Eisler R. Handbook of chemical risk assessment: health hazards to humans, plants, and animals. Vol. 3. Boca Raton, FL: CRC Press; 2000. [Google Scholar]
- El-Bayoumy K. The protective role of selenium on genetic damage and on cancer. Mutat Res. 2001;475:123–39. doi: 10.1016/s0027-5107(01)00075-6. [DOI] [PubMed] [Google Scholar]
- Fall JA, Stanek RT, Brown L, Utermohle C. Alaska Dept. of Fish & Game; Juneau, AK: 2006. The harvest and use of fish, wildlife and plant resources in False Pass, Unimak Island, Alaska. Paper No. 183. http://nativeknowlege.org/db/files/tp183.htm. [Google Scholar]
- Freire C, Ramos R, Lopez-Expinosa M, Diez S, Vioque J, Ballester F, et al. Hair mercury levels, fish consumption, and cognitive development in preschool children from Granada, Spain. Environ Res. 2010;110:96–104. doi: 10.1016/j.envres.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Gochfeld M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol Environ Saf. 2003;56:174–9. doi: 10.1016/s0147-6513(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Gochfeld M, Burger J. Good fish/bad fish: a composite benefit–risk by dose curve. Neurotoxicology. 2005;26:511–20. doi: 10.1016/j.neuro.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:418–28. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Green NW, Knutzen J. Organohalogens and metals in marine fish and mussels and some relationships to biological variables at reference localities in Norway. Mar Pollut Bull. 2003;46:362–77. doi: 10.1016/S0025-326X(02)00515-5. [DOI] [PubMed] [Google Scholar]
- Groth G., III Ranking the contributions of commercial fish and shellfish varieties to mercury exposure in the United States: implications for risk communication. Environ Res. 2010;110:226–36. doi: 10.1016/j.envres.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Guallar E, Sanz-Gallardo MI, van’t Veer P, Bode P, Aro A, Gomez-Aracena J. Heavy metals and myocardial infarction study group: mercury, fish oils, and the risk of myocardial infarction. N Engl J Med. 2002;347:1747–54. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- Hamrick K, Smith J. Subsistence food use in Unalaska and Nikolski. Anchorage, AK: Aleutian/Pribilof Island Association; 2003. [Google Scholar]
- Hardell S, Tilander H, Welfinger-Smith G, Burger J, Carpenter DO. Levels of poly-chlorinated biphenyls (PCBs) and three organochlorine pesticides in fish from the Aleutian Islands of Alaska. PLoS One. 2010;5:11. doi: 10.1371/journal.pone.0012396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SG, Harper BL. Native American exposure scenarios and a tribal risk model. Risk Anal. 1998;17:789–5. doi: 10.1111/j.1539-6924.1997.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Harris SG, Harper BL. Using eco-cultural dependency webs in risk assessment and characterization of risks to tribal health and cultures. Environ Sci Pollut Res Int. 2000;2:91–100. [Google Scholar]
- Hightower JM, Moore D. Mercury levels in high-end consumers of fish. Environ Health Perspect. 2003;111:604–8. doi: 10.1289/ehp.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites RA, Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. Global assessment of organic contaminants in farmed salmon. Science. 2004;303:226–9. doi: 10.1126/science.1091447. [DOI] [PubMed] [Google Scholar]
- Hsiao H, Ullrich SM, Tanton TW. Burdens of mercury in residents of Temirtau, Kazakhstan. 1: hair mercury concentrations and factors of elevated hair mercury levels. Sci Total Environ. 2011;409:2272–80. doi: 10.1016/j.scitotenv.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Hughner RS, Maher JK, Childs NM. Review of food policy and consumer issues of mercury in fish. J Am Coll Nutr. 2008;27:185–94. doi: 10.1080/07315724.2008.10719690. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (IOM) Seafood safety. Washington, DC: National Academy Press; 1991. [Google Scholar]
- Institute of Medicine (IOM) Seafood choices. Balancing benefits and risks. Washington, DC: National Academy Press; 2006. [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) [Accessed March 2005.];2003 www.who.int/pcs/jecfa/jecra-htm.
- Jewett SC, Zhang X, Naidu AS, Kelley JJ, Dasher D, Duffy LK. Comparison of mercury and methylmercury in northern pike and Arctic grayling from western Alaska rivers. Chemosphere. 2003;50:383–92. doi: 10.1016/s0045-6535(02)00421-6. [DOI] [PubMed] [Google Scholar]
- Kaneko JJ, Ralston NV. Selenium and mercury in pelagic fish in the central north Pacific near Hawaii. Biol Trace Elem Res. 2007;119:242–54. doi: 10.1007/s12011-007-8004-8. [DOI] [PubMed] [Google Scholar]
- Khan MAK, Wang F. Mercury-selenium compounds and their toxicological significance: toward a molecular understanding of the mercury–selenium antagonism. Environ Toxicol Chem. 2009;28:1567–77. doi: 10.1897/08-375.1. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- Lansens P, Leermakers M, Waeyens W. Determination of methylmercury in fish by headspace-gas chromatography with microwave-induced plasma detection. Water Air Soil Pollut. 1991;56:103–15. [Google Scholar]
- Lemes M, Wang F. Methylmercury speciation in fish muscle by HPLC-ICP-MS following enzymatic hydrolysis. J Anal At Spectrom. 2009;24:663–8. [Google Scholar]
- Lemes M, Wang F, Stern GA, Ostertag SK, Chan HM. Methylmercury and selenium speciation in different tissues of Beluga Whales (Delphinapterus leucas) from the Western Canadian Arctic. Environ Toxicol Chem. 2011;30:2732–8. doi: 10.1002/etc.684. [DOI] [PubMed] [Google Scholar]
- Lemire M, Fillion M, Frenette B, Mayer A, Philibert A, Passon CJS, et al. Selenium and mercury in the Brazalian Amazon: opposing influences on age-related cataracts. Environ Health Perspect. 2010;118:1584–9. doi: 10.1289/ehp.0901284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh U, Johansson E. Protective effects of selenium against mercury toxicity as syudied in the rat liver and kidney by nuclear analytical techniques. Biol Trace Elem Res. 1987;12:109–20. doi: 10.1007/BF02796669. [DOI] [PubMed] [Google Scholar]
- Lowenstein JH, Burger J, Jeitner CW, Amato G, Kolokotronis SO, Gochfeld M. DNA barcodes revewal species-specific mercury levels in tuna sushi that pose a health risk to consumers. Biol Lett. 2010;6:692–5. doi: 10.1098/rsbl.2010.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YZ, Yan BX, Wang MJ, Guo LY. The evolution rule and ecology risk assessment of mercury in fish of Gonghua River. J Agro-Environ Sci. 2008;27:2430–3. [Google Scholar]
- Mozaffarian D. Fish, mercury, selenium and cardiovascular risk: current evidence and unanswered questions. Int J Environ Res Public Health. 2009;6:1894–916. doi: 10.3390/ijerph6061894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (NRC) The Bering Sea ecosystem. Washington DC: National Academy Press; 1996. [Google Scholar]
- National Research Council (NRC) Toxicological effects of methylmercury. Washington DC: National Academy Press; 2000. [Google Scholar]
- Ohlendorf HM. Ecotoxicology of selenium. In: Hoffman DJ, Rattner BA, Burton GS Jr, Cairns J Jr, editors. Handbook of ecotoxicology. Boca Raton, FL: Lewis Publ; 2000. pp. 465–500. [Google Scholar]
- Ohlendorf HM, Hothem RL. Agricultural drainage effects on wildlife in Central California. In: Hoffman DJ, Rattner BA, Burgon GS Jr, Cairns J Jr, editors. Handbook of ecotoxicology. Boca Raton, FL: Lewis Publ; 1995. pp. 577–85. [Google Scholar]
- Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, et al. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am J Epidemiol. 2008;167:1171–81. doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JG, Curtis LR. Mercury distribution in sediments and bioaccumulation by fish two Oregon reservoirs: point-source and nonpoint source impacted systems. Arch Environ Contam Toxicol. 1997;33:423–9. doi: 10.1007/s002449900272. [DOI] [PubMed] [Google Scholar]
- Patterson J. Introduction—comparative dietary risk: balance the risks and benefits of fish consumption. Comment Toxicol. 2002;8:337–44. [Google Scholar]
- Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F., Jr Fishing down marine food webs. Science. 1998;279:860–3. doi: 10.1126/science.279.5352.860. [DOI] [PubMed] [Google Scholar]
- Peterson SA, Ralston NVC, Whanger PD, Oldfield JE, Mosher WD. Selenium and mercury interactions with emphasis on fish tissue. Environ Bioindic. 2009a;4:318–34. [Google Scholar]
- Peterson SA, Ralston NVC, Peck DV, Van Sickle J, Robertson JD, Spate VL, et al. How might selenium oderate the toxic effects of mercury in stream fish in western US? Environ Sci Technol. 2009b;43:3919–25. doi: 10.1021/es803203g. [DOI] [PubMed] [Google Scholar]
- Pinheiro MCN, de Nascimento JLM, Silveira LCL, daRocha JBT, Aschner M. Mercury and selenium — a review on aspects related to the health of human populations in the Amazon. Environ Bioindic. 2009;4:222–45. doi: 10.1080/15555270903143440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power M, Klein GM, Guiguer RRA, Kwan MKH. Mercury accumulation in the fish community of a sub-Arctic lake in relation to trophic position and carbon sources. J Appl Ecol. 2002;39:819–90. [Google Scholar]
- Powers CW, Burger J, Kosson D, Gochfeld M, Barnes D, editors. Piscataway, New Jersey: CRESP; 2005. Biological and geophysical aspects of potential radionuclide exposure in the Amchitka marine environment. (available at www.cresp.org) [Google Scholar]
- Ralston NVC. Selenium health benefit values as seafood safety criteria. Eco-Health. 2008;5:442–55. doi: 10.1007/s10393-008-0202-0. [DOI] [PubMed] [Google Scholar]
- Ralston NVC. Introduction to 2nd issue on special topic: selenium and mercury as interactive environmental indicators. Environ Bioindic. 2009;4:286–90. [Google Scholar]
- Ralston NVC, Ralston CR, Blackwell JL, III, Raymond LJ. Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology. 2008;29:802–11. doi: 10.1016/j.neuro.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Ramel A, Martinez JA, Kiely M, Bandarra NM, Thorsdottir I. Moderate consumption of fatty fish reduces diastolic blood pressure in overweight and obese European young adults during energy restriction. Nutrition. 2010;26:168–74. doi: 10.1016/j.nut.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Raymond LJ, Ralston NVC. Mercury:selenium interactions and health implications. SMDJ Seychelles Med Dent J. 2004;17:72–7. doi: 10.1016/j.neuro.2020.09.020. [DOI] [PubMed] [Google Scholar]
- Raymond LJ, Ralston NVC. Selenium’s importance in regulatory issues regarding mercury. Fuel Proc Technol. 2009;90:1333–8. [Google Scholar]
- Rice G, Swartout J, Mahaffey K, Schoeny R. Derivation of U.S. EPS’s oral Reference Dose (RfD) for methylmercury. Drug Chem Toxicol. 2000;23:41–54. doi: 10.1081/dct-100100101. [DOI] [PubMed] [Google Scholar]
- Rissanen T, Voutilainen S, Nyyssonen K, Lakka TA, Salonen JT. Fish oil-derived fatty acids, docosahexaenoic acid and docosaphentaenoic acid, and the risk of a coronary events: the Kuopio ischaemic heart disease risk factor study. Circulation. 2000;102:2677–9. doi: 10.1161/01.cir.102.22.2677. [DOI] [PubMed] [Google Scholar]
- Safina C. Song for a blue ocean. New York NY: Henry Holt & Co; 1998. [Google Scholar]
- SAS. Statistical analysis. Cary, NC: SAS Inc; 2005. [Google Scholar]
- Satoh H, Yasuda N, Shimai S. Development of reflexes in neonatal mice prenatally exposed to methylmercury and selenite. Toxicol Lett. 1985;25:199–203. doi: 10.1016/0378-4274(85)90082-7. [DOI] [PubMed] [Google Scholar]
- Schlung TM. Umnak: the people remembered. Walnut Creek, California: Hardscratch Press; 2003. [Google Scholar]
- Scudder BC, Chaser LC, Wentz DA, Bauch NJ, Brigham ME, Moran PW, et al. US Dept of Interior Report 2009–5109. Reston, Va: 2009. Mercury in fish, bed sediments, and water from streams across the United States, 1998–2005; p. 74. [Google Scholar]
- Shimshack JP, Ward MB, Beatty KM. Mercury advisories: information, education, and fish consumption. J Environ Econ Manage. 2007;53:158–79. [Google Scholar]
- Simonin HA, Loukmas JJ, Skinner LC, Roy KM. Lake variability: key factors controlling mercury concentrations in New York state fish. Environ Pollut. 2008;154:107–15. doi: 10.1016/j.envpol.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Snodgrass JW, Jagoe CH, Bryan AL, Jr, Burger J. Effects of trophic status, and wetland morphology, hydroperiod and water chemistry on mercury concentrations in fish. Can J Fish Aquat Sci. 2000;57:171–80. [Google Scholar]
- Sormo EG, Ciesielski TM, Overjordet IB, Lierhagen S, Eggen GS, Berg T, et al. Selenium moderates mercury toxicity in free-ranging freshwater fish. Environ Sci Technol. 2011;45:6561–6. doi: 10.1021/es200478b. [DOI] [PubMed] [Google Scholar]
- Speier C, Baker SS, Newburger PE. Relationship between in vitro selenium supply, glutathione peroxidase activity, and phagocytic function in the HL-60 human myeloid cell line. J Biol Chem. 1985;260:8951–5. [PubMed] [Google Scholar]
- Steuerwald U, Weihe P, Jorgansen PJ, Bjerve K, Brock J, Heinzow B, et al. Maternal seafood diet, methylmercury exposure, and neonatal neurological function. J Pediatr. 2000;136:599–605. doi: 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- Storelli MM, Stuffler RG, Marcotrigiano GO. Total and methylmercury residues in tuna-fish from the Mediterranean Sea. Food Addit Contam. 2002;19:715–20. doi: 10.1080/02652030210153569. [DOI] [PubMed] [Google Scholar]
- Takezaki T, Inoue M, Kataoka H, Ikeda S, Yoshida M, Ohashi Y, et al. Diet and lung cancer risk from a 14-year population-based prospective study in Japan: with special reference to fish consumption. Nutr Cancer. 2003;45:160–7. doi: 10.1207/S15327914NC4502_04. [DOI] [PubMed] [Google Scholar]
- Tayel FTR, Shriadah MMA. Fe, Cu, Mn, Pb and Cd in some fish species from Western Harbor of Alexandria, Egypt. Bull Natl Inst Oceanogr Fish. 1996;22:85–96. [Google Scholar]
- Toth JF, Jr, Brown RB. Racial and gender meanings of why people participate in recreational fishing. Leis Sci. 1997;19:129–46. [Google Scholar]
- Tran D, Moody AJ, Fisher AS, Foulkes ME, Jha AN. Protective effects of selenium on mercury-induced DNA damage in mussel haemocytes. Aquat Toxicol. 2007;84:11–8. doi: 10.1016/j.aquatox.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Trasande L, Landrigan PJ, Schechter C. Public health and economic consequences of methylmercury toxicity to the developing brain. Environ Health Perspect. 2005;113:590–6. doi: 10.1289/ehp.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel M, Rasmussen JB. Modeling the elimination of mercury by fish. Environ Sci Technol. 1997;31:1716–22. [Google Scholar]
- US Food and Drug Administration (US FDA) FDA consumer advisory. Washington, DC: U.S. Food and Drug Administration; 2001. [accessed 1 December 2001]. Available: http//www.fda.gov/bbs/topics/ANSWERS/2000/advisory.html. [Google Scholar]
- US Food and Drug Administration (USFDA) FDA consumer advisory. Washington, DC: U.S. Food and Drug Administration; 2003. [accessed 1 January 2004]. Available: http//www.fda.gov/bbs/topics/ANSWERS/2000/advisory.html. [Google Scholar]
- US Food and Drug Administration (USFDA) Mercury levels in commercial fish and shellfish. Washington, DC: U.S. Food and Drug Administration; Washington, D.C: U.S. Food and Drug Administration; 2005. [accessed 1 January 2005]. Available http//www.fda.gov/bbs/topics/ANSWERS/2000/advisory.html. [Google Scholar]
- Virtanen JK, Mozaffarian D, Chiuve SE, Rimm EB. Fish consumption and risk of major chronic disease in men. Am J Clin Nutr. 2008;88:1618–25. doi: 10.3945/ajcn.2007.25816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe C. Modification of mercury toxicity by selenium: practical importance. Tohoku J Exp Med. 2002;196:71–7. doi: 10.1620/tjem.196.71. [DOI] [PubMed] [Google Scholar]
- Watanabe C, Yoshida K, Kasanuma Y, Satoh H. In utero methylmercury exposure differentially affects the activities of selenoenzymes in the fetal mouse brain. Environ Res. 1999;80:208–14. doi: 10.1006/enrs.1998.3889. [DOI] [PubMed] [Google Scholar]
- Watras CJ, Back RC, Halvorsen S, Hudson RJM, Morrison KA, Wente SP. Bioaccumulation of mercury in pelagic freshwater food webs. Sci Total Environ. 1998;219:183–208. doi: 10.1016/s0048-9697(98)00228-9. [DOI] [PubMed] [Google Scholar]
- Wiener JG, Krabbenhoft DP, Heinz GH, Scheuhammer M. Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr, editors. Handbook of ecotoxicology. Boca Raton, FL: Lewis Publications; 2003. pp. 409–63. [Google Scholar]
- World Health Organization (WHO) Mercury-environmental aspects. Geneva, Switzerland: WHO; 1989. [Google Scholar]