Abstract
Background
Infections caused by non-O1 Vibrio cholera are uncommon. The aim of our study was to investigate the clinical and microbiological characteristics of patients with non-O1 V. cholera infections.
Methods
The clinical charts of all patients with non-O1 V. cholera infections and who were treated in two hospitals in Taiwan were retrospectively reviewed.
Results
From July 2009 to June 2014, a total of 83 patients with non-O1 V. cholera infections were identified based on the databank of the bacteriology laboratories of two hospitals. The overall mean age was 53.3 years, and men comprised 53 (63.9%) of the patients. Liver cirrhosis and diabetes mellitus were the two most common underlying diseases, followed by malignancy. The most common type of infection was acute gastroenteritis (n = 45, 54.2%), followed by biliary tract infection (n = 12, 14.5%) and primary bacteremia (n = 11, 13.3%). Other types of infection, such as peritonitis (n = 5, 6.0%), skin and soft tissue infection (SSTI) (n = 5, 6.0%), urinary tract infection (n = 3, 3.6%) and pneumonia (2, 2.4%), were rare. July and June were the most common months of occurrence of V. cholera infections. The overall in-hospital mortality of 83 patients with V. cholera infections was 7.2%, but it was significantly higher for patients with primary bacteremia, hemorrhage bullae, acute kidney injury, acute respiratory failure, or admission to an ICU. Furthermore, multivariate analysis showed that in-hospital mortality was significantly associated with acute respiratory failure (odds ratio, 60.47; 95% CI, 4.79-763.90, P = 0.002).
Conclusions
Non-O1 V. cholera infections can cause protean disease, especially in patients with risk factors and during warm-weather months. The overall mortality of 83 patients with non-O1 V. cholera infections was only 7.2%; however, this value varied among different types of infection.
Introduction
Vibrio cholerae, gram-negative bacteria, are ubiquitous in the aquatic environment. Till now, there are more than 200 serogroups of V. cholerae within the species. Although V. cholerae O1 is the notorious strain that can cause epidemic or pandemic diarrheal disease—cholera, emerging infections due to non-O1 V. cholerae has become another unneglectable problem. Acute gastroenteritis is the most common clinical manifestation of non-O1 V. cholerae infection for both sporadic and outbreaks cases [1]. In contrast, non-O1 V. cholerae has been rarely involved in extra-intestinal infections, which include primary bacteremia, skin and soft tissue infections (SSTI), pneumonia, acute pyosalpinx, acute cholecystitis, endophthalmitis, peritonitis, urinary tract infection, splenic abscess, liver abscess, intracerebral abscess, meningitis, cholangitis, and empyema [2–17]. However, most of the previous studies are cases reports because of its rare occurrences. Therefore, a large-scale study about this issue is warranted to help us better understand the clinical manifestations and prognostic factors of non-O1 V. cholerae infections.
As we know, V. cholerae infections is strongly associated with water exposure or ingestion of raw food [2, 3]. The coastal area in southern Taiwan, located in subtropical area, is the kind of environment suitable for the growth of V. cholerae. Taking advantage of the geographic characteristics, we conduct this retrospective study to investigate the clinical manifestations, microbiological characteristics, response to treatment, and outcome in a medical center and a regional hospital in southern Taiwan.
Materials and Methods
Hospital setting and patient selection
This study design is a retrospective study and was conducted at two hospitals, Chi Mei Medical Center, a 1290-bed referral medical center, and Chi Mei Medical Center, Liouying branch, a 900-bed regional hospital, located in Tainan, Taiwan. From the computerized database of the bacteriology laboratory from July 2009 to June 2014, patients whose cultures yielded non-O1 V. cholerae species were identified. Because this study only focused on V. cholerae infection, polymicrobial infections were excluded. The clinical charts of all patients included in this study were retrospectively reviewed. Information regarding age, gender, underlying immunocompromising conditions including history of immunosuppressant drug use, diabetes mellitus, liver cirrhosis, chronic kidney disease, and malignancy were collected. The data was collected on a routine basis and the analysis was carried out retrospectively. The records and information of patients were anonymized and de-identified prior to analysis. Therefore, no informed consent was required and it was specifically waived by Institution Review Board. Ethics approval was obtained from Institution Review Board of Chi Mei Medical Center.
Bacterial isolates
V. cholerae by conventional identification methods and two commercial systems, including the API 20E system (bioMerieux, Vitek, Inc., Hazelwood, MO, USA) and the Phoenix system (NMIC/ID-72, Becton Dickinson, Sparks, MD). All of the clinical isolates were identified as V. cholerae non-serogroup O1 based on negative reaction by slide agglutination with polyvalent O1 (Difco, Becton Dickinson, Sparks, MD). However, V. cholerae serogroup O139 was not tested due to lack of reagents.
Definitions
The diagnosis of the bacteremia infection focus was made based on clinical, and bacteriological investigations. Acute gastroenteritis was classified as clinical diarrhea plus a positive stool culture. SSTI was defined as clinical soft tissue inflammation plus positive soft tissue or pus culture. Peritonitis was diagnosed in patients with clinical peritoneum inflammation plus positive culture of ascites. Biliary tract infections were diagnosed in patients with clinical hepatobiliary tract inflammation plus positive bile cultures. The bile specimens were collected by percutaneous transhepatic cholangiodrainage, percutaneous gallbladder drainage, or operation. Pneumonia was defined as a positive culture for V. cholerae in purulent sputum samples and the presence of newly developed lung infiltrates. Genitourinary tract infection (UTI) was defined as positive urine culture with growth of 105 CFU/ml and pyuria. If no primary focus could be identified, the bacteremia was classified as primary. Shock was diagnosed in patients with a systolic blood pressure < 90 mm Hg or in patients who required inotropic agents to maintain blood pressure. In-hospital mortality was defined as death from all causes during the study episode of hospitalization.
Statistical analysis
Data were analyzed using SPSS version 11.0 software. Continuous variables were expressed as mean ± standard deviation. Comparisons between continuous variables were analyzed using the Wilcoxon rank sum test or Student’s independent t test, as appropriate. Comparisons between or among categorical variables were made using the chi-square or Fisher’s exact test. The analyses of risk factors and outcomes were performed using the chi-square test. A multivariable forward logistic regression model was used to identify risk factors for mortality. Statistical significance was set at P < 0.05.
Results
Clinical characteristics
During the study period, a total of 83 patients with non-O1 V. cholerae infections were identified based on the databank of the bacteriology laboratories of two hospitals. The clinical characteristics of all patients with non-O1 V. cholerae infections are summarized in Table 1. The overall mean age was 53.3 years, and men comprised 53 (63.9%). Liver cirrhosis and diabetes mellitus were the two most common underlying diseases, followed by malignancy. Among 20 patients with liver cirrhosis, 10 cases had history of alcoholism, and 8 patients had ascites. Hepatocellular carcinoma was the most common type of cancer (n = 7), followed by pancreatic cancer (n = 3). Abdominal pain (n = 60, 72.3%) was the most common presentation, followed by diarrhea (n = 52, 62.7%), and fever (n = 34, 41.0%). Additionally, initial shock and the presence of hemorrhagic bullae were noted in 9 (10.8%) and 5 (6.0%) patients, respectively. The most common type of infection was acute gastroenteritis (n = 45, 54.2%), followed by biliary tract infection (n = 12, 14.5%) and primary bacteremia (n = 11, 13.3%). Other types of infection were rare, such as peritonitis (n = 5, 6.0%), SSTI (n = 5, 6.0%), urinary tract infection (n = 3, 3.6%) and pneumonia (2, 2.4%). Secondary V. cholerae bacteremia was noted in 22 episodes of various type of infections, including acute gastroenteritis (n = 7), biliary tract infection (n = 7), peritonitis (n = 5), SSTI (n = 2), and urinary tract infection (n = 1). Among 45 patients with acute gastroenteritis, three had initial shock. However, none of them had blood stool or resemble dysentery. 3 of five patients with SSTI had presentation of necrotizing fasciitis, and all of them had underlying conditions, such as liver cirrhosis, diabetes mellitus, and chronic kidney disease. Only one had concomitant V. cholerae bacteremia and in-hospital mortality.
Table 1. Demographic characteristic of 83 patients with non-O1 Vibrio cholerae infections.
| Characteristic | No. (%) of all patients (n = 83) | No. (%) of patients with acute gastroenteritis (n = 45) | No. (%) of patients with biliary tract infections (n = 12) | No. (%) of patients with primary bacteremia (n = 11) |
|---|---|---|---|---|
| Age, mean ± SD (years) | 53.3 ± 19.3 | 45.3 ± 17.0* , † | 66.5 ± 16.7* | 61.0 ± 14.1 † |
| Male (%) | 53 (63.9) | 23 (51.1) † | 10 (83.7) | 10 (90.9) † |
| Underlying conditions | ||||
| Liver cirrhosis | 20 (24.1) | 6 (13.3) † | 1 (8.3) # | 8 (72.7) † , # |
| Diabetes mellitus | 19 (22.9) | 8 (17.8) † | 2 (16.7) | 6 (54.5) † |
| Cancer | 15 (18.1) | 2 (4.4) | 2 (16.7) | 1 (9.1) |
| Hepatitis B infection | 10 (12.0) | 2 (4.4) | 0 (0.0) | 3 (27.3) |
| Hepatitis C infection | 10 (12.0) | 4 (8.9) | 2 (16.7) | 4 (36.4) |
| Chronic kidney disease | 7 (8.4) | 2 (4.4) | 0 (0.0) | 1 (9.1) |
| Immunosuppressant use | 3 (3.6) | 1 (2.2) | 1 (8.3) | 0 (0.0) |
| Steroid use | 4 (4.8) | 1 (2.2) | 1 (8.3) | 0 (0.0) |
| Alcoholism | 14 (16.9) | 5 (11.1) | 2 (16.7) | 4 (36.4) |
| Initial presentation | ||||
| Abdominal pain | 60 (72.3) | 37 (82.2) | 10 (83.3) | 6 (54.5) |
| Diarrhea | 52 (62.7) | 45 (100.0)* , † | 2 (66.7)* | 2 (18.2) † |
| Fever | 34 (41.0) | 11 (24.4) † | 34 (41.0) | 8 (72.7) † |
| Shock | 9 (10.8) | 3 (6.7) | 0 (0.0) | 2 (18.2) |
| Hemorrhagic bullae | 5 (6.0) | 0 (0.0) † | 0 (0.0) | 2 (18.2) † |
| Laboratory examinations | ||||
| White blood cell | 12116.4 ± 9661.1 | 13154.3 ± 11159.8 | 15833.3 ± 10879.9 | 7727.3 ± 3596.4 |
| Hemoglobin | 12.7 ± 3.6 | 13.9 ± 4.3 † | 13.7 ± 1.9 # | 10.6 ± 2.0 † , # |
| Creatinine | 1.7 ± 2.0 | 1.9 ± 2.8 | 1.2 ± 0.4 | 1.6 ± 0.5 |
| Glutamic oxaloacetate transaminase | 127.9 ± 256.3 | 47.4 ± 38.6* , † | 286.8± 498.0 | 155.5 ± 244.2 † |
| Total bilirubin | 3.7 ± 3.2 | 2.4 ± 2.1 | 3.3 ± 2.4 | 4.4 ± 3.5 |
| C-reactive protein | 38.8 ± 62.3 | 11.8 ± 16.1* , † | 75.2 ± 92.9* | 61.2 ± 66.4 † |
| Concomitant bacteremia | 33 (39.8) | 7 (15.6)* | 7 (58.3)* , # | 11 (100.0) † , # |
| Intensive care unit admission | 14 (16.9) | 3 (6.7) † | 0 (0.0) # | 6 (54.5) † , # |
| Acute respiratory failure | 9 (10.8) | 3 (6.7) | 0 (0.0) | 3 (27.3) |
| Acute kidney injury | 15 (18.1) | 5 (11.1) | 2 (16.7) | 4 (36.4) |
| In-hospital mortality | 6 (7.2) | 1 (2.2) † | 0 (0.0) | 3 (27.3) † |
*Significant difference between acute gastroenteritis and biliary tract infection
† Significant difference between acute gastroenteritis and primary bacteremia
#Significant difference between biliary tract infection and primary bacteremia
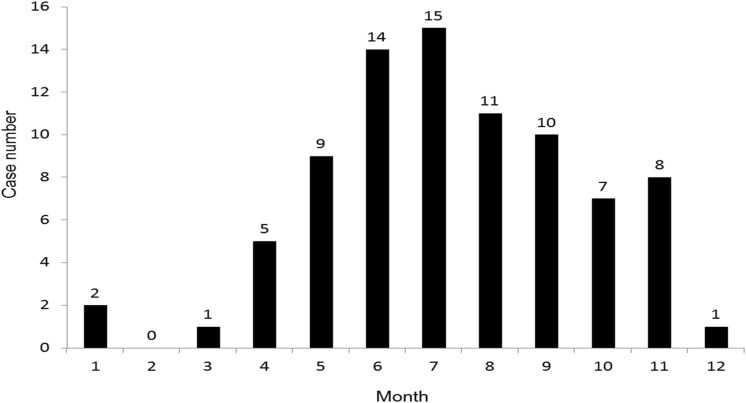
July and June were the most common months of occurrence of V. cholerae infections, followed by August, September, and May (Fig. 1). The patients with acute gastroenteritis was significant younger than the patients with biliary tract infection and primary bacteremia. The patients with primary bacteremia had more liver cirrhosis than the patients with acute gastroenteritis and biliary tract infection. Fever was more common among the patients with primary bacteremia than with acute gastroenteritis. C-reactive protein was significant higher among the patients with biliary tract infection and primary bacteremia than the patients with acute gastroenteritis. Three mortalities developed in patients with primary bacteremia, and each one developed in patient with SSTI, peritonitis, and acute gastroenteritis. The patients with primary bacteremia had higher in-hospital mortality than the patients with biliary tract infections.
Figure 1. Distribution of non-O1 Vibrio cholerae infections by month.
Comparison between patients with mortality and survival
Fifteen (18.1%) and 9 (10.8%) patients had the complication as acute kidney injury requiring emergent hemodialysis and acute respiratory failure requiring mechanical ventilator, respectively. Overall, 14 patients required intensive care unit admission, and in-hospital mortality was 7.2%. Analysis of risk factor for mortality among 83 patients with V. cholerae infections was shown in table 2. The in-hospital mortality was significantly associated with the presence of hemorrhage bullae, acute kidney injury, acute respiratory failure, and admission to ICU. However, a multivariable analysis showed that the in-hospital mortality was only significantly associated with acute respiratory failure (odds ratio, 60.47; 95% CI, 4.79–763.90, P = 0.002).
Table 2. Comparison between patients with survival and mortality.
| No (%) of patients with survival (n = 77) | No (%) of patients with mortality (n = 6) | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | P value | 95% CI | Odds ratio | P value | 95% CI | |||
| Fever | 31 (40.3) | 3 (50.0) | 1.484 | 0.685 | 0.281–7.834 | |||
| Shock on presentation | 7 (9.1) | 2 (33.3) | 5.000 | 0.13 | 0.773–32.336 | |||
| Hemorrhagic bullae | 2 (2.6) | 3 (50.0) | 37.500 | 0.002 | 4.461–315.263 | 15.971 | 0.082 | 0.703–363.022 |
| Diabetes mellitus | 17 (22.1) | 2 (33.3) | 1.765 | 0.61 | 0.297–10.472 | |||
| Liver cirrhosis | 17 (22.1) | 3 (50.0) | 3.529 | 0.15 | 0.652–19.099 | |||
| Chronic kidney disease | 7 (9.1) | 0 (0.0) | 0.909 | 1.00 | 0.847–0.976 | |||
| Cancer | 14 (18.2) | 1 (16.7) | 1.125 | 1.00 | 0.117–10.852 | |||
| Concomitant bacteremia | 28 (36.4) | 5 (83.3) | 8.750 | 0.034 | 0.973–78.706 | |||
| Acute respiratory failure | 4 (5.2) | 5 (83.3) | 91.250 | <0.001 | 8.520–977.340 | 60.469 | 0.002 | 4.790–763.90 |
| Acute kidney injury | 10 (13.0) | 5 (83.3) | 33.5 | 0.001 | 3.540–317.044 | |||
| ICU admission | 9 (11.7) | 5 (83.3) | 37.778 | <0.001 | 3.955–360.865 | |||
Discussion
As toxigenic V. cholerae draws the most attention of V. cholerae infections, the clinical data for non-O1 V. cholerae infections are limited. During this 5-year survey, a total of 83 patients with non-O1 V. cholerae infections were identified from two hospitals in southern Taiwan. To the best of our knowledge, this study is the largest series of patients with non-O1 V. cholerae infections, and we have several significant findings. In this study, we found the clinical manifestations of non-O1 V. cholerae infections are various. In addition to the most common presentation—acute gastroenteritis, non-O1 V. cholerae infections can presented as biliary tract infection, primary bacteremia, peritonitis, SSTI, urinary tract infection and pneumonia. Moreover, we noted that among the different types of clinical infections, the characteristics of the infected patients differed accordingly. For example, patients with biliary tract infections and primary bacteremia had greater rates of diabetes mellitus, and liver cirrhosis, malignancy, and higher level of C-reactive protein than patients with acute gastroenteritis. This is consistent with previous studies showing that persons with known liver disease, and diabetes mellitus, are at high risk of invasive Vibrio infections [18–21]. Therefore, clinicians should consider non-O1 V. cholerae as etiologic agents in cases of invasive infections in persons with liver cirrhosis and diabetes mellitus.
In the present work, the overall mortality rate of patients with non-O1 V. cholerae infections was only 7.2% (n = 6). For each type of infection, the mortality was 27.3% (3/11), 20% (1/5), 20% (1/5) and 7.2% (6/83) among of patients with primary bacteremia, SSTI, peritonitis, and acute gastroenteritis, respectively. Furthermore, the mortality rate of patients with primary bacteremia was significant higher than patients with acute gastroenteritis (p < 0.05). By univariable analysis, we found that the presence of hemorrhagic bullae, concomitant bacteremia, acute kidney injury, acute respiratory failure and ICU admission were poor prognostic factors. Moreover, by multivariable analysis, acute respiratory failure was found to be the only independent and significant risk factors associated with mortality. Although the impact of hemorrhagic bullae on mortality did not reach statistical significance (p = 0.082) by multivariable analysis, it may be caused by limited cases number. However, the trend should be evident; therefore, clinicians should be alert about the presence of hemorrhage bullae as the risk factor of poor outcome.
In this study, we also assess the association between seasonality and non-O1 V. cholerae infections. We found that June and July are the most common months of non-O1 V. cholerae infections, and about 80% of cases occur between May and October. It indicates the strong relationship between warm weather and the occurrence of non-O1 V. cholerae infections. As previous report [2], physicians should consider this pathogen as possible infectious agent for patients with risk factor, especially in the warm weather.
This study had several limitations. First, we did not determine the in vitro antibiotic susceptibility profiles of the Vibrio species. Therefore, we cannot evaluate the association between antimicrobial agent and outcome. Second, this study is a retrospective investigation that may suffer from sources of bias, such missing data. We cannot obtain the exposure history of aquatic environment and seafood. Third, we used all-cause mortality for outcome analysis and did not evaluate the mortality attributable to non-O1 V. cholerae infections. Forth, both the biochemical based test used in this study may wrongly identify some of the other vibrios/aeromonads as V. cholerae., and molecular based identification should be adopted for confirmation of V cholerae isolates. In addition, analysis of virulence gene properties of V. cholerae isolated is important, especially the strains associated with diarrhea. In our country, cholera is a notifiable disease. If clinicians suspect that the V. cholera infection may be cholera, they need to report the case to Taiwan CDC and send the strain for further confirmatory microbiology study. Both of two hospitals in the present work should follow this policy, therefore, all of the cases in this study are supposed not to be toxigenic strain despite we did not keep clinical isolate for further molecular-method based confirmation test and analysis of virulence gene. Finally, the cases number remains limited and further large-scale study is warranted to better understand the clinical manifestations of non-O1 V. cholerae infections.
In conclusion, non-O1 V. cholerae infections are not uncommon pathogens that can cause protean disease, especially for patients with risk factors and in warm weather months. The overall mortality is only 7.2%; however, it varies among different type of infection. Moreover, the mortality is significant associated with the presence of hemorrhagic bullae, concomitant bacteremia, acute kidney injury, acute respiratory failure and ICU admission by univariable analysis and, acute respiratory failure by multivariable analysis.
Data Availability
All relevant data are included within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Janda JM, Powers C, Bryant RG, Abbott S (1988) Current perspectives on the epidemiology and pathogenesis of clinically significant Vibrio spp. Clin Microbiol Rev 1:245–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin CJ, Chiu CT, Lin DY, Sheen IS, Lien JM (1996) Non-O1 Vibrio cholerae bacteremia in patients with cirrhosis: 5-yr experience from a single medical center. Am J Gastroenterol 91: 336–40. [PubMed] [Google Scholar]
- 3. Ku WC, Chuang YC, Huang GC, Hsu SY (1998) Infections due to non-O1 Vibrio cholerae in southern Taiwan: predominance in cirrhotic patients. Clin Infect Dis 27: 774–80. 10.1086/514947 [DOI] [PubMed] [Google Scholar]
- 4. Vogt AP, Doshi RK, Higgins JE, Burd EM, Ribner BS, et al. (2010) Acute cholecystitis caused by nontoxigenic Vibrio cholerae O1 Inaba. J Clin Micriobiol 48: 1002–4. 10.1128/JCM.02198-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang CC, Lee BJ, Yang SS, Lin YH, Lee YL (2008) A case of non-O1 and non-O139 Vibrio cholerae septicemia with endophthalmitis in a cirrhotic patient. Jpn J Infect Dis 61: 475–6. [PubMed] [Google Scholar]
- 6. Petsaris O, Nousbaum JB, Quilici ML, Le Coadou G, Payan C, et al. (2010) Vibrio cholerae non-O1, non-O139 bacteremia in a cirrhotic patient. J Med Microbiol. [Epub ahead of print]. [DOI] [PubMed]
- 7. Aguinaga A, Portillo ME, Yuste JR, del Pozo JL, Garcia-Tutor E, et al. (2009) Non-O1 Vibrio cholerae inguinal skin and soft tissue infection with bullous skin lesions in a patient with a penis squamous cell carcinoma. Ann Clin Microbiol Antimicrob 8:17 10.1186/1476-0711-8-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wiwatworapan W, Insiripong S (2008) Non-O1/non-O139 Vibrio cholerae septicemia with peritonitis. Southeast Asian J Trop Med Public Health 39: 1098–101. [PubMed] [Google Scholar]
- 9. Sangaré L, Sanou I, Somé D, et al. (2006)Urinary infection due to non-01, non-0139 Vibrio cholerae . Med Trop (Mars) 66: 513–4. [PubMed] [Google Scholar]
- 10. Shannon JD, Kimbrough RC 3rd (2006) Pulmonary cholera due to infection with a non-O1 Vibrio cholerae strain. J Clin Microbiol 44: 3459–60. 10.1128/JCM.02343-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cavuoti D, Fogli M, Quinton R, Gander RM, Southern PM (2002) Splenic abscess with Vibrio cholerae masking pancreatic cancer. Diagn Microbiol Infect Dis 43: 311–3. 10.1016/S0732-8893(02)00412-1 [DOI] [PubMed] [Google Scholar]
- 12. Liou CW, Lui CC, Cheng MH (2001) A case of intracerebral abscess caused by non-O1 Vibrio cholerae . Eur J Clin Microbiol Infect Dis 20: 678–80. 10.1007/s100960100569 [DOI] [PubMed] [Google Scholar]
- 13. Kerketta JA, Paul AC, Kirubakaran VB, Jesudason MV, Moses PD (2002) Non-01 Vibrio cholerae septicemia and meningitis in a neonate. Indian J Pediatr. 69: 909–10. 10.1007/BF02723720 [DOI] [PubMed] [Google Scholar]
- 14. West BC, Silberman R, Otterson WN (1998) Acalculous cholecystitis and septicemia caused by non-O1 Vibrio cholerae: first reported case and review of biliary infections with Vibrio cholerae . Diagn Microbiol Infect Dis. 30: 187–91. 10.1016/S0732-8893(97)00235-6 [DOI] [PubMed] [Google Scholar]
- 15. Nishikawa M, Hamanaka Y, Suzuki T (1989) A case report of acute obstructive suppurative cholangitis in a non-0–1 Vibrio cholerae biliary carrier. Nippon Geka Hokan. 58: 155–61. [PubMed] [Google Scholar]
- 16. Lai CC, Liu WL, Chiu YH, Gau SJ, Hsueh PR (2012) Spontaneous bacterial empyema due to non-O1, non-O139 Vibrio cholerae in a cirrhotic patient with hepatocellular carcinoma. Diagn Microbiol Infect Dis 73:84–5. 10.1016/j.diagmicrobio.2012.01.011 [DOI] [PubMed] [Google Scholar]
- 17. Lai CC, Liu WL, Chiu YH, Chao CM, Gau SJ, et al. (2011) Liver abscess due to non-O1 Vibrio cholerae in a cirrhotic patient with hepatocellular carcinoma. J Infect 62:235–7. 10.1016/j.jinf.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 18. Daniel NA, MacKinnon L, Bishop R, Altekruse S, Ray B, et al. (2000) Vibrio parahaemolyticus infection in the United States, 1973–1998. J Infect Dis 181: 1661–6. 10.1086/315459 [DOI] [PubMed] [Google Scholar]
- 19. Dechet Am, Yu PA, Koram N, Painter J (2008) Non foodborne Vibrio infections: an important cause of morbidity and mortality in the United States, 1997–2006. Clin Infect Dis 46: 970–6. 10.1086/529148 [DOI] [PubMed] [Google Scholar]
- 20. Blake PA, Merson MH, Weaver RE, Hollis DG, Heublein PC (1979) Disease caused by a marine Vibrio. Clinical characteristics and epidemiology. N Engl J Med 300: 1–5. [DOI] [PubMed] [Google Scholar]
- 21. Tacket CO, Brenner F, Blake PA (1984) Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis 149: 558–61. 10.1093/infdis/149.4.558 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are included within the paper.