Abstract
Background
The effects of intermittent, high dose vitamin D treatment in older adults have not been documented. We conducted a meta-analysis to provide a quantitative assessment of the efficiency of intermittent, high dose vitamin D treatment on falls, fractures, and mortality among older adults.
Methods
Electronic databases were searched for randomized controlled trials (RCTs) on high dose, intermittent vitamin D supplementation among older adults. Two researchers independently screened the literature according to specified inclusive and exclusive criteria to extract the data. Meta-analysis was performed by using Review Manager 5.1.0 software.
Results
Nine trials were included in this meta-analysis. High dose, intermittent vitamin D therapy did not decrease all-cause mortality among older adults. The risk ratio (95% CI) was 1.04 (0.91–1.17). No benefit was seen in fracture or fall prevention. The risk ratio for hip fractures (95% CI) was 1.17 (0.97–1.41) while for non-vertebral fractures (95% CI) it was 1.06 (0.91–1.22), and the risk ratio for falls (95% CI) was 1.02 (0.96–1.08). Results remained robust after sensitivity analysis.
Conclusion
Supplementation of intermittent, high dose vitamin D may not be effective in preventing overall mortality, fractures, or falls among older adults. The route of administration of vitamin D supplements may well change the physiological effects.
Introduction
Vitamin D plays a key role in human biology [1], and the beneficial effects of vitamin D supplementation have been documented. A large number of randomized controlled trials (RCTs) and meta-analyses have investigated the effects of vitamin D treatment on fractures and falls and suggest that vitamin D supplementation is effective in preventing fractures and falls among older adults [2–7]. Several meta-analyses of RCTs on the effect of vitamin D supplementation on total mortality have been published and found that when given together with calcium, vitamin D supplementation reduced total mortality but not when given alone [8–11].
In recent years, many researchers have focused on supplementing individuals with intermittent, high-dose vitamin D. A study of supplementation with 100,000 IU of oral vitamin D3 every three months demonstrated an increase in mean serum 25-hydroxy vitamin D, from 36.4 nmol/L at baseline to 124.0nmol/L at six months [12]. Another study compared the effects of vitamin D3 in a single dose of 500,000 IU with 50,000IU per month among older adults, concluding that large loading doses of vitamin D3 rapidly and safely normalized 25-hydroxy vitamin D levels in the frail older adult [13]. Finally, a RCT suggested that supplementation with cholecalciferol 100,000 IU every four months may prevent fractures without adverse effects in older adults living in the general community [14].
However, several RCTs using high dose, intermittent vitamin D reported an increase rather than a decrease, in the primary outcome of falls [15] and fractures [15,16]. This concern leaves the field with a challenge when considering the use of high dose of vitamin D supplementation among older adults. Thus, a meta-analysis that addresses this issue is clearly needed.
The objective of the current study was to perform a comprehensive systematic review and meta-analysis of RCTs to observe the effects of high dose, intermittent vitamin D on fall, fracture, and overall mortality prevention in older adults.
Materials and Methods
Search strategy
We performed a literature search for the purpose of identifying RCTs. We searched the electronic databases of Medline, Embase, and The Cochrane Central Register of Controlled Trials up to January 2013. The search strategy combined terms relating to study design (RCTs), intervention of vitamin D (vitamin D, vitamin D2, vitamin D3, ergocalciferol, cholecalciferol), and outcomes (falls, fractures, mortality). We also searched for any additional studies in the reference lists of recent reviewers of vitamin D treatment on older adults. Our searches were limited to human trials with no language restriction.
Eligibility criteria
The search results were then screened on the basis of the following criteria: (1) RCTs referring to an annual high dose (greater than 100,000 IU) or intermittent dose (interval time longer than one month); (2) The participants of this study were individuals aged 65 years or over. Unstable conditions, such as stroke and Parkinson’s disease, were excluded; (3) The treatment group was restricted to high dose, intermittent vitamin D alone, or in combination with calcium. The control group had no treatment or calcium therapy. Studies of patients receiving active vitamin D were excluded from the present study; (4) The number of participants with one or more falls, fractures, and deaths was reported separately for the vitamin D treatment group and the control group; (5) Review articles, commentaries, letters, observational studies were excluded.
Data extraction and quality assessment
Two researchers independently and in duplicate abstracted data using a standardized form. Data collected from studies included first author, publishing year, sample size, duration, dwelling, intervention, serum 25-hydroxy vitamin D levels at baseline, and primary results (the number of participants who suffered at least one fall, fracture, or death). Total number of falls and fractures were not used in the present study, because individuals with recurrent falls and fractures may have other significant risk factors, which may exaggerate the estimated risk. Quality assessment was performed by two independent researchers using the Cochrane Collaboration’s tool [17].
Data synthesis and analysis
The primary outcome was the number of participants who died during follow-up. Secondary outcomes were the number of participants who suffered hip fractures, non-vertebrae fractures, and falls. Mantel-Haenszel method was used to calculate risk ratios (RRs) and their 95% confidence intervals (CI). The I2 statistic was used to assess the presence of heterogeneity, I2 range from 0% to 100%. An I2 statistic greater than 50% suggested moderate heterogeneity [18], and a random effects model was used. A fixed-effects model was used for I2 statistic less than 50%, which showed that heterogeneity could be neglected [18]. We used Begg test and Egger test to evaluate the publication bias regarding the RR of mortality [19,20]. A p value less than 0.05 was considered to be statistically significant.
Sensitivity analysis and subgroup analysis
A sensitivity analysis was conducted by using the trim and fill method. For the overall results of mortality and fractures, we performed a sensitivity analysis by omitting one study at a time and then repeating the analysis. For the falls outcome, sensitivity analysis was conducted by excluding trials which did not have an explicit fall definition.
To explore the causes of inconsistency and sub-group treatment interactions, we conducted sub-group analyses according to type of residence (institutionalized vs. community dwelling), type of vitamin D (ergocalciferol vs. cholecalciferol), route of administration (intramuscular vs. oral), baseline level of 25-hydroxy vitamin D (≤ 50 nmol/liter vs. > 50 nmol/liter), and duration of vitamin D treatment (< 3 years vs. ≥ 3 years).
Results
Search Results
Eight hundred and eleven potentially relevant publications were found after an initial independent search of the electronic database. After scrutinizing the titles and abstracts, 767 articles were excluded according to the inclusion criteria. Among the remaining forty-four articles, twenty-six were excluded because vitamin D was used daily, nine were excluded because they did not provide acquired data. Subsequently, a total of nine RCTs were included in the final analysis [14–16, 21–26]. The details of study selection flow were explicitly described in Fig. 1.
Figure 1. Study flow diagram.
Study Characteristics
The main characteristics of the included studies are shown in Table 1. Nine trials containing 22,012 patients (10,950 in the vitamin D group and 11,062 in control group) were included in the present study. Mortality data was available in seven trials [14–16, 21, 22, 24, 25], four trials had hip fracture data [14, 16, 24, 25], and five trials had non-vertebral fracture data [14–16, 24, 25]. Eight trials reported fall data but only three were included in the primary analysis for their definition of falls and how they were assessed [15, 23, 26]. Duration of follow-up ranged from six months to five years. The trials were published from 2003 to 2012. Mean age of participants ranged from seventy-seven to eighty-five years. Vitamin D2 was used in six studies [16, 21–25] and vitamin D3 in the remaining three studies. Six trials [14, 15, 21, 24–26] used oral vitamin D while intramuscular injection was used in the remaining three trials. Calcium supplementation was used in two trials [22, 26]. All trials reported the baseline vitamin D status of participants based on serum 25-hydroxy vitamin D levels. Participants in six trials [14–16, 24–26] had baseline 25-hydroxy vitamin D levels at or above vitamin D adequacy (20 ng/ml or 50 nmol/L). Participants in the remaining three trials had baseline 25-hydroxy vitamin D levels in a range considered to be vitamin D deficient (< 20 ng/ml). Two had a high risk bias [22, 24], and the other seven studies had a low risk bias (Table 2).
Table 1. Characteristics of studies included in primary analysis.
| Study | Population characteristics | Treatment groups | Number of participants | Mean age (years) | Pre/post 25(OH)D (nmol/l;mean) | Follow up (moths) | Outcomes |
|---|---|---|---|---|---|---|---|
| Latham 2003 [21] | Recruited from geriatric rehabilitation center, institutionalized | Oral vit D2 300000 IU once | 108 | 80 | 37 to 60 at 3 months | 6 | Mortality |
| Placebo | 114 | 79 | 48 to 48 at 3 months | ||||
| Trivedi 2003 [14] | Elderly man and woman, community dwelling | Oral vit D3 100000 IU every 4 months | 1027 | 75 | 74 at 48 months | 60 | Mortality, fracture |
| Placebo | 1011 | 75 | 53 at 48 months | ||||
| Harwood 2004 [22] | Elderly women after hip fracture, community dwelling | Vit D2 300,000 IU/im/once | 30 | 80 | 28 to 41 at 12 months | 12 | Mortality |
| Vit D2 300,000 IU/im/once + 1,000 mg calcium | 25 | 81 | 30 to 48 at 12 months | ||||
| No treatment | 35 | 81 | 30 to 27 at 12 months | ||||
| Dhesi 2004 [23] | Ambulatory elderly with a history of fall institutionalized | Vit D2600000IU/im/once | 62 | 77 | 27 to 44 at 6 months | 6 | Fall |
| Placebo | 61 | 77 | 25 to 31 at 6 months | ||||
| Law 2006 [24] | Recruited from residential care home institutionalized | Oral vit D2 100000 IU every 3 months | 1762 | 85 | 59 to 77 at 3 months | 10 | Mortality Fracture |
| No treatment | 1955 | 85 | NA | ||||
| Lyons 2007 [25] | Nursing home residents institutionalized | Oral vit D2 100000 IU every 4 months | 1725 | 84 | 80/NA | 36 | Mortality fracture |
| Placebo | 1715 | 84 | 54/NA | ||||
| Smith 2007 [16] | Elderly man and woman, community dwelling | Vit D2 300,000 IU/im/year | 4727 | 79 | 56.5 to 68 at 4 months | 36 | Mortality fracture |
| Placebo | 4713 | 79 | NA | ||||
| Sanders 2010 [15] | Ambulatory elderly women at risk for fractures, community dwelling | Oral vit D3 500000 IU annually for 3–5 yerrs | 1131 | 77 | 53/NA | 36–60 | Mortality fracture |
| Placebo | 1125 | 77 | 45/NA | fall | |||
| Glendenning 2012 [26] | Older women, community dwelling | Oral vit D3150000 IU every 3 months + calcium 1300 daily | 353 | 77 | 65 to 75 at 9 months | 9 | Fall |
| Calcium 1300 daily | 333 | 77 | 66 to 60 at 9 months |
Table 2. Quality assessment of the included studies.
| Study[ref] | Random sequence generation | Allocation concealment | Blinding of participant and personnel | Blinding of outcome assessment | Incomplete outcome data addressed | Nonelective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Latham 2003 | L | L | L | L | L | L | L |
| Trivedi 2003 | L | L | L | L | L | L | L |
| Harwood 2004 | L | L | H | H | L | L | H |
| Dhesi 2004 | L | L | L | L | L | L | L |
| Law 2006 | L | H | H | H | L | L | U |
| Lyons 2007 | L | L | L | L | L | L | L |
| Smith 2007 | L | L | L | L | L | L | L |
| Sanders 2010 | L | L | L | L | L | L | L |
| Glendenning 2012 | L | L | L | L | L | L | L |
L, low risk; H, high risk; U, unclear
Meta-analysis of high dose, intermittent vitamin D treatment on overall mortality
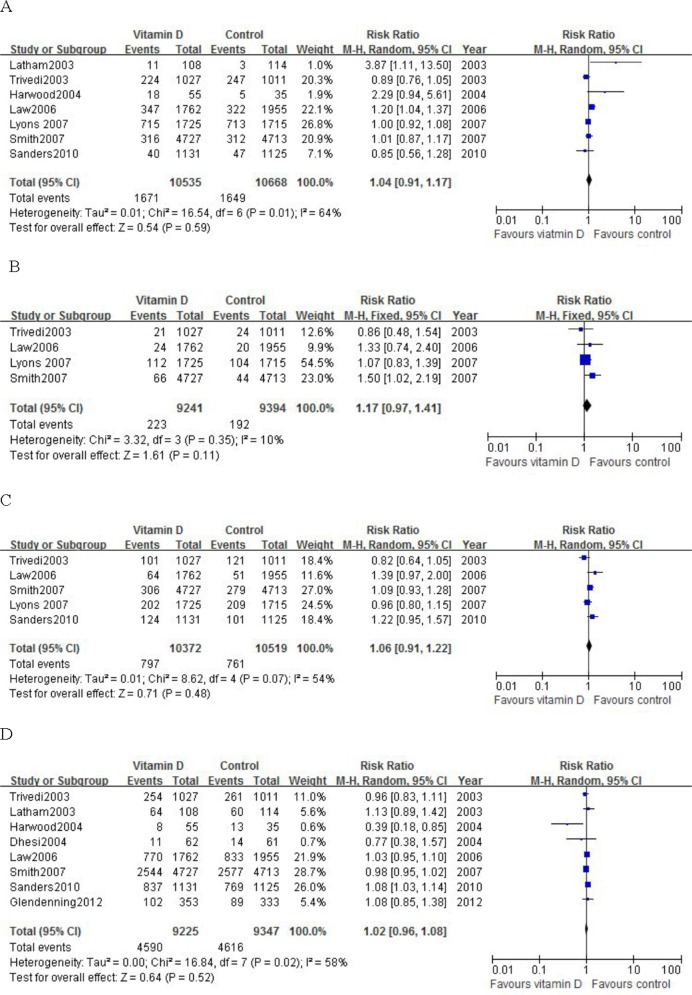
The risk ratio (95% CI) of mortality for patients treated with high dose, intermittent vitamin D compared with control was 1.04 (0.91–1.17), which was not statistically significant (p = 0.59). Heterogeneity was noted for this outcome (I2 = 64%) (Fig. 2A). A total of 1671 of 10,535 participants (15.9%) randomized to the vitamin D group and 1649 of 10,668 participants (15.5%) randomized to the placebo or no intervention group died. Results remained robust after sensitivity analysis.
Figure 2. Meta analysis of overall mortality (A), hip fracture (B), non-vertebral fracture (C), fall (D) in participants treatment with high dose, intermittent vitamin D compare with control.
Sub-group analysis (Table 3) showed no appreciable change in risk ratio according to gender, participant residency, type of vitamin D, route of administration, baseline 25-hydroxy vitamin D, or trial duration.
Table 3. Subgroup analyses of high-dose, intermittent vitamin D treatment on mortality.
| Subgroup | Studies,n | Participants, n | Death, n | Risk Ratio | P value | I2value | ||
|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | (95% CI) | ||||
| Participant dwelling | ||||||||
| Institutionalized | 3 | 3595 | 3784 | 1073 | 1038 | 1.13 (0.90, 1.41) | 0.29 | 79% |
| Community-dwelling | 4 | 6940 | 6884 | 598 | 611 | 0.96 (0.87, 1.07) | 0.48 | 43% |
| Gender | ||||||||
| Women only | 3 | 1512 | 1483 | 83 | 79 | 1.06 (0.66, 1.68) | 0.82 | 50% |
| Men only | 1 | 1019 | 1018 | 199 | 220 | 0.90 (0.76, 1.07) | 0.25 | 0% |
| Type of vitamin D | ||||||||
| Ergocalciferol | 5 | 8377 | 8532 | 1407 | 1355 | 1.10 (0.95, 1.29) | 0.21 | 68% |
| Cholecalciferol | 2 | 2158 | 2136 | 264 | 294 | 0.89 (0.76, 1.03) | 0.11 | 0% |
| Administration route | ||||||||
| Intramuscular | 2 | 4682 | 4748 | 334 | 317 | 1.03 (0.89, 1.20) | 0.66 | 68% |
| Oral | 5 | 5753 | 5920 | 1337 | 1332 | 1.02 (0.96, 1.09) | 0.46 | 70% |
| Baseline 25-hydroxy vitamin D | ||||||||
| ≤ 50 nmol/liter | 2 | 163 | 149 | 29 | 8 | 2.80 (1.36, 5.78) | 0.005 | 0% |
| > 50 nmol/liter | 5 | 10372 | 10519 | 1642 | 1641 | 1.01 (0.91, 1.12) | 0.83 | 55% |
| Duration of vitamin D treatment | ||||||||
| ≥ 3 years | 4 | 8610 | 8564 | 1295 | 1319 | 0.98 (0.91, 1.04) | 0.44 | 0% |
| < 3 years | 3 | 1925 | 2104 | 376 | 330 | 1.80 (0.90, 3.60) | 0.09 | 62% |
For I2 ≥50%, random effects model was used; for I2 <50%, fixed-effects model was used
Meta-analysis of high dose, intermittent vitamin D treatment on fractures
The risk ratio (95% CI) of hip fracture for patients treated with high dose, intermittent vitamin D compared with control was 1.17 (0.97–1.41), which was not statistically significant (p = 0.11). Heterogeneity was insignificant for this outcome (I2 = 10%) (Fig. 2B). The risk ratio (95% CI) of non-vertebral fracture for patients treated with high dose, intermittent vitamin D compared with control was 1.06 (0.91–1.22), which was not statistically significant (p = 0.48). Heterogeneity was noted for this outcome (I2 = 54%) (Fig. 2C). Results were robust in the sensitivity analysis by excluding individual trials one by one.
Meta-analysis of high dose, intermittent vitamin D treatment on falls
The risk ratio (95% CI) of falls for patients treated with high dose, intermittent vitamin D compared with control was 1.02 (0.96–1.08), which was not statistically significant (p = 0.52). Heterogeneity was significant for this outcome (I2 = 58%) (Fig. 2D). In sensitivity analysis of vitamin D treatment on falls, we excluded the trials where the definition of falls was not explicitly given. The risk ratio (95% CI) of falls for patients treated with high dose, intermittent vitamin D compared with control after sensitivity analysis was 1.08 (1.02–1.14), which was statistically significant (p = 0.006). Heterogeneity was insignificant for this outcome (I2 = 0%).
Publication bias
No evidence of publication bias was detected for the RR of mortality in the present study by either Begg or Egger’s test (Begg’s test, p = 0.764; Egger’s test, p = 0.295).
Discussion
We aimed to bring together the results of all eligible RCTs to gauge the effect of high dose, intermittent vitamin D supplementation among older adults concerning mortality, and fracture and fall prevention. Our results demonstrated that high dose, intermittent vitamin D supplementation was ineffective in preventing mortality. It was fairly consistent across sub-groups defined by participant residency, type of vitamin D, route of administration, baseline 25-hydroxy vitamin D, and trial duration. In addition, no benefit was seen in fracture and fall prevention.
Several previous meta-analyses suggested that oral vitamin D treatment reduced the risk of fractures and falls among older individuals [4–7], and further meta-analyses suggested that vitamin D was effective in reducing mortality [8–11]. Most studies included in these meta-analyses [4–11] were referring to vitamin D intake in the form of a daily tablet. Different from previous studies, the present study only involved trials using high dose (a single dose larger than 100,000 IU) or intermittent vitamin D (interval time longer than one month). The results contradicted previous findings.
A fall endpoint study of oral cholecalciferol 1000 IU plus calcium 1000 mg daily for one year in 302 community-dwelling older women living in Australia demonstrated a 19% reduction of first fall events [27]. Another RCT conducted by Flick et al [28] including 625 older people residing in sixty hostels and eighty-nine nursing homes across Australia demonstrated that ergocalciferol administered 10,000 IU once weekly and then 1,000 IU daily was associated with a significant reduced risk of falls and fractures. The results of these two trials indicated that high daily dose vitamin D was effective in preventing falls and fractures. In contrast, our results indicated that high dose, intermittent vitamin D was not effective in preventing falls and fractures. This may suggest that the dosing regimen, rather than the total dose, might determine the outcome [29]. Annual single-dose or intermittent, high dose vitamin D may not provide adequate concentrations in the blood over a whole year. Thus, negative outcomes may occur due to failure to maintain serum vitamin D over time, as with monthly, quarterly, or yearly bolus vitamin D dosing [30].
A systematic review concluded that cholecalciferol significantly decreased mortality while the effect of ergocalciferol may be neutral or even detrimental [10]. One included trial in the present study by Trivedi et al [14] suggested that four monthly supplementations with 100,000 IU cholecalciferol were beneficial for fractures and falls, and may prevent overall mortality in elderly living in the general community. However, four other studies demonstrated that ergocalciferol 300,000 IU once yearly or 100,000 every four months was not effective in preventing mortality and fractures. These five trials used the same total annual dose of vitamin D, but outcomes were entirely different. It may indicate that annual high doses of cholecalciferol may be more beneficial than ergocalciferol with certain time intervals.
The deleterious effect of high dose, intermittent vitamin D was observed in two studies [15,16]. Sanders et al [15] concluded that annual oral administration of 500,000 IU cholecalciferol resulted in an increased risk of falls and fractures; Smith et al [16] demonstrated that vitamin D was associated with a significantly increased risk of hip fractures. In the present study, the primary analysis of high dose, intermittent vitamin D treatment on falls found a significantly increased risk. The mechanism of the deleterious effect of supplemental intermittent, high-dose of vitamin D remains uncertain. The effect of vitamin D on muscle tissue is thought to occur through specific vitamin D receptors, and vitamin D receptor expression is decreased in older adults [31]. When vitamin D is given intermittently or daily, it has positive effects on muscle function, but when a very high dose of vitamin D is given, there is a negative effect on muscle function due to a sudden increase in vitamin D receptor occupancy [29]. Vitamin D receptors also exist in the central nervous system [32], so a deleterious effect on falls is also possible [29]. It has been recently reported that mega-doses of vitamin D were associated with transient increases in serum 25-hydroxy vitamin D level and in bone turnover markers [33]. This might explain the transient increases in fracture rate observed by Sanders et al [15].
Our review has several limitations. First, most of the participants in the present study were older women. The effects of intermittent, high-dose of vitamin D in younger, healthy persons and males are still inconclusive. Second, ascertainment of falls and fractures varied between studies, which likely produced inaccuracies in outcome reporting. Third, the falls analysis was heavily influenced by two studies [15, 26], both of which used participants with a relatively high baseline 25-hydroxy vitamin D concentration (50 nmol/L). This means that the fall risk calculated here may not be applicable to persons with a low 25-hydroxy vitamin D concentration (50 nmol/L). It is possible that high dose, intermittent supplementation of vitamin D may be detrimental to those with a sufficient vitamin D status, but not to those with a poor vitamin D status. Fourth, though our results indicated that intermittent, high-dose of vitamin D was ineffective in preventing fractures, falls, and mortality, the threshold of high dose, interval time, and dosing regimen still have not been documented. It is still unknown what the optimal dosing strategy is.
In conclusion, high-dose, intermittent vitamin D may not have a beneficial effect on general health among older adults as has been previously described with a normal daily dose of vitamin D. The effect was likely neutral. For those with high levels of vitamin D (25-hydroxy vitamin D levels > 50 nmol/L) to begin with, there may be a deleterious effect on falls. However, randomized controlled trials with physical outcomes as primary end points, using a number of different regimens are still needed to definitively resolve this challenging issue.
Supporting Information
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Wahl DA, Cooper C, Ebeling PR, Eggersdorfer M, Hilger J, et al. (2012) A global representation of vitamin D status in healthy populations. Arch Osteoporos 7:155–72. 10.1007/s11657-012-0093-0 [DOI] [PubMed] [Google Scholar]
- 2. Bischoff HA, Stähelin HB, Dick W, Akos R, Knecht M, et al. (2003) Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res 18:343–51. 10.1359/jbmr.2003.18.2.343 [DOI] [PubMed] [Google Scholar]
- 3. Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, et al. (2009) Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int 20:315–22. 10.1007/s00198-008-0662-7 [DOI] [PubMed] [Google Scholar]
- 4. DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group (2010) Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ 340:b5463 10.1136/bmj.b5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, et al. (2011) Clinical review: The effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab 96:2997–3006. 10.1210/jc.2011-1193 [DOI] [PubMed] [Google Scholar]
- 6. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, et al. (2009) Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 339:b3692 10.1136/bmj.b3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, et al. (2012) A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med 367:40–9. 10.1056/NEJMoa1109617 [DOI] [PubMed] [Google Scholar]
- 8. Autier P, Gandini S (2007) Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 167:1730–7. 10.1001/archinte.167.16.1730 [DOI] [PubMed] [Google Scholar]
- 9. Chung M, Balk EM, Brendel M, Ip S, Lau J, et al. (2009) Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep) 183:1–420. [PMC free article] [PubMed] [Google Scholar]
- 10. Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, et al. (2011) Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev 2011;7:CD007470 10.1002/14651858.CD007470.pub2 [DOI] [PubMed] [Google Scholar]
- 11. Rejnmark L, Avenell A, Masud T, Anderson F, Meyer HE, et al. (2012) Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. J Clin Endocrinol Metab 97:2670–81. 10.1210/jc.2011-3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wigg AE, Prest C, Slobodian P, Need AG, Cleland LG (2006) A system for improving vitamin D nutrition in residential care. Med J Aust 185:195–8. [DOI] [PubMed] [Google Scholar]
- 13. Bacon CJ, Gamble GD, Horne AM, Scott MA, Reid IR (2009) High-dose oral vitamin D3 supplementation in the older adult. Osteoporosis Int 20:1407–15. 10.1007/s00198-008-0814-9 [DOI] [PubMed] [Google Scholar]
- 14. Trivedi D, Doll R, Khaw K (2003) Effect of four monthly oral vitamin D supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 326:469–75. 10.1136/bmj.326.7387.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, et al. (2010) Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 303:1815–22. 10.1001/jama.2010.594 [DOI] [PubMed] [Google Scholar]
- 16. Smith H, Anderson F, Raphael H, Maslin P, Crozier S, et al. (2007) Effect of annual intramuscular vitamin D on fracture risk in older adult men and women—a population-based, randomized, double-blind, placebo controlled trial. Rheumatology (Oxford) 46:1852–7. 10.1093/rheumatology/kem240 [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, et al. (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JP, Green S, Cochrane Collaboration (2008) Cochrane Handbook for Systematic Reviews of Interventions. Chichester, England; Hoboken, NJ:Wiley-Blackwell, 2008. [Google Scholar]
- 19. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4): 1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 20. Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Latham NK, Anderson CS, Lee A, Bennett DA, Moseley A, et al. (2003) A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in older adult Subjects (FITNESS). J Am Geriatr Soc 51:291–9. 10.1046/j.1532-5415.2003.51101.x [DOI] [PubMed] [Google Scholar]
- 22. Harwood RH, Sahota O, Gaynor K, Masud T, Hosking DJ (2004) A randomised, controlled comparison of different calcium and vitamin D supplementation regimens in older adult women after hip fracture: The Nottingham Neck of Femur (NONOF) Study. Age Ageing 33:45–51. 10.1093/ageing/afh002 [DOI] [PubMed] [Google Scholar]
- 23. Dhesi JK, Jackson SH, Bearne LM, Moniz C, Hurley MV, et al. (2004) Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing 33:589–95. 10.1093/ageing/afh209 [DOI] [PubMed] [Google Scholar]
- 24. Law M, Withers H, Morris J, Anderson F (2006) Vitamin D supplementation and the prevention of fractures and falls: results of a randomised trial in older adult people in residential accommodation. Age Ageing 35:482–6. 10.1093/ageing/afj080 [DOI] [PubMed] [Google Scholar]
- 25. Lyons RA, Johansen A, Brophy S, Newcombe RG, Phillips CJ, et al. (2007) Preventing fractures among older people living in institutional care: a pragmatic randomised double blind placebo controlled trial of vitamin D supplementation. Osteoporos Int 18:811–8. 10.1007/s00198-006-0309-5 [DOI] [PubMed] [Google Scholar]
- 26. Glendenning P, Zhu K, Inderjeeth C, Howat P, Lewis JR, et al. (2012) Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: a randomized controlled trial. J Bone Miner Res 27:170–6. 10.1002/jbmr.524 [DOI] [PubMed] [Google Scholar]
- 27. Prince RL, Austin N, Devine A, Dick IM, Bruce D, et al. (2008) Effects of ergocalciferol added to calcium on the risk of falls in older adult high-risk women. Arch Intern Med 168:103–8. 10.1001/archinternmed.2007.31 [DOI] [PubMed] [Google Scholar]
- 28. Flicker L, MacInnis RJ, Stein MS, Scherer SC, Mead KE, et al. (2005) Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J Am Geriatr Soc 253:1881–8. 10.1111/j.1532-5415.2005.00468.x [DOI] [PubMed] [Google Scholar]
- 29. Sanders KM, Nicholson GC, Ebeling PR (2013) Is high dose vitamin D harmful? Calcif Tissue Int. 92:191–206. 10.1007/s00223-012-9679-1 [DOI] [PubMed] [Google Scholar]
- 30. Hollis BW, Wagner CL (2013) The role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab 98:4619–28. 10.1210/jc.2013-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van den Bergh JP, Bours SP, van Geel TA, Geusens PP (2011) Optimal use of vitamin D when treating osteoporosis. Curr Osteoporos Rep 9:36–42. 10.1007/s11914-010-0041-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buell JS, Dawson-Hughes B (2008) Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med 29:415–22. 10.1016/j.mam.2008.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rossini M, Gatti D, Viapiana O, Fracassi E, Idolazzi L, et al. (2012) Short-term effects on bone turnover markers of a single high dose of oral vitamin D3. J Clin Endocrinol Metab 2012;97:E622–6. 10.1210/jc.2011-2448 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)