Abstract
Background
The prevalence of low bone mineral density (BMD) in adult survivors of childhood acute lymphoblastic leukemia (ALL), and the degree of recovery or decline, are not well elucidated.
Procedure
Study subjects (age ≥ 18 years and ≥ 10 years post-diagnosis) participated in an institutional follow-up protocol and risk-based clinical evaluation based on Children’s Oncology Group guidelines. Trabecular volumetric BMD was ascertained using quantitative computed tomography, reported as age- and sex-specific Z-scores.
Results
At median age 31 years, 5.7% of 845 subjects had a BMD Z-score of ≤ - 2 and 23.8% had a Z-score of - 1 to -2. Cranial radiation dose of ≥ 24 Gray, but not cumulative methotrexate or prednisone equivalence doses, was associated with a 2-fold elevated risk of a BMD Z-score of ≤ -1. The cranial radiation effect was stronger in females than in males. In a subset of 400 subjects, 67% of those who previously had a BMD Z-score of ≤ -2 improved by one or more categories a median of 8.5 years later.
Conclusions
Very low BMD was relatively uncommon in this sample of adult survivors of childhood ALL, and BMD Z-scores tended to improve from adolescence to young adulthood. High dose cranial or craniospinal radiation exposure was the primary predictor of suboptimal BMD in our study. Given that cranial radiation treatment for childhood ALL is used far more sparingly now than in earlier treatment eras, concerns about persistently low BMD among most current childhood ALL patients may be unwarranted.
Keywords: epidemiology, cranial radiation, late effects, osteoporosis, osteopenia
INTRODUCTION
Children treated for acute lymphoblastic leukemia (ALL), the most common pediatric malignancy, [1] are at elevated risk for bone mineral density (BMD) deficits [2–4] that may persist into adulthood.[5–8] The harmful effect of cytotoxic treatment on BMD in ALL survivors may be due to the long treatment period of 2 to 3 years, which includes high-dose methotrexate and glucocorticoids, and in select cases, cranial or craniospinal radiation.[9] Glucocorticoids and methotrexate can interfere with skeletal growth and BMD recovery.[8, 10–14] Cranial radiation can impair hypothalamic-pituitary function, negatively impacting sex and growth hormone secretions with deleterious consequences on bone metabolism and BMD.[8, 10, 13, 15] In addition, impaired bone metabolism and diminished bone mass in newly diagnosed ALL patients suggest a direct disease effect on BMD from leukemic infiltration of bone marrow.[16–19] The prevalence of low BMD in long-term childhood ALL survivors, and the degree to which BMD recovers or declines over time, have not been well elucidated. The purpose of this study was to evaluate the prevalence of low BMD in adulthood among childhood ALL survivors and to explore clinical factors related to persistently low BMD.
METHODS
Subjects
Study subjects are participants in the St. Jude Lifetime Cohort Study (SJLIFE). SJLIFE is an institutional follow-up protocol with a staged and ongoing recruitment process that began in September 2007. Recruitment and data collection methods for SJLIFE have been described in detail previously.[20, 21] Eligibility for the IRB-approved study is restricted to individuals age 18 years or older who were treated for cancer at St. Jude Children’s Research Hospital (St. Jude), are ≥ 10 years post-diagnosis, and provided written informed consent. Participation involves a risk-based clinical surveillance evaluation consistent with Children’s Oncology Group guidelines for long term follow-up care, [22, 23] which includes a BMD assessment for ALL survivors. Medical record abstraction is conducted to document type and cumulative doses of individual chemotherapeutic agents, surgical interventions, and radiation therapy. Participants also complete a battery of health surveys. The study was approved by the Human Subjects Institutional Review Board at St. Jude.
Relevant to this analysis, St. Jude has an After Completion of Therapy (ACT) clinic for medical and psychosocial monitoring of late effects among patients who remain in remission for at least 2 years following completion of antineoplastic therapy and are at least 5 years from diagnosis. Patients are referred to the ACT clinic at the discretion of the primary treating service at St. Jude and may be followed until they are age 18 years or older and at least 10 years post diagnosis. At that point, ACT patients are discharged to the care of their community physician and they become eligible for recruitment into the SJLIFE research study. Subsequent to the Children’s Oncology Group follow-up guidelines that were first published in 2004, [23] ALL patients monitored in the ACT clinic may have received a BMD test for medical surveillance purposes. Some ACT ALL patients also participated in research protocols that included a BMD test. SJLIFE ALL participants who were followed in the ACT clinic and had an ACT BMD evaluation were considered in a subgroup analysis in the present study to quantify change in BMD over time.
BMD assessment
Trabecular volumetric BMD was determined by obtaining 28 to 32 three millimeter contiguous slice images of the mid-bodies of the first and second lumbar vertebra from quantitative computed tomography (QCT) using a GE VCT Lightspeed 64 detector (GE Healthcare, Milwaukee, WI USA) and Mindways QCT calibration phantoms and software (Mindways Software Inc., Austin TX USA). The average of the two vertebral BMD measures was calculated, standardized to age- and sex-specific norms, and reported as a Z-score (standard deviation score).
Treatment exposures
Based on a priori knowledge, cumulative doses of methotrexate, glucocorticoids, and cranial radiation were considered as risk factors in the analysis for BMD effects. Glucocorticoids (dexamethasone and prednisone) are reported as prednisone equivalent units [24] (1 mg prednisone = 0.15 mg dexamethasone). Cranial radiation exposure was classified into four mutually exclusive groups: 1) no radiation exposure; 2) <24 Gy; 3) ≥24Gy; or 4) any cranial radiation with spinal radiation (including 20 subjects with total body irradiation). By standard of care, spinal radiation dose was 15 Gy and almost every patient who received spinal radiation also received at least 24 Gy cranial radiation. In exploratory analyses, we also evaluated the impact of other treatment-related exposures including anthracyclines, cyclophosphamide, vincristine, etoposide and teniposide as possible risk factors for low BMD.
Endocrine Assessment
In the protocol applied to our study population, participants exposed to cranial radiotherapy were screened for GH deficiency (GHD) during the SJLIFE visit by measuring IGF-1 levels. Individuals with an IGF-1 Z-score of < -2 for age and sex and those who were diagnosed with GHD using stimulation testing during childhood or prior to SJLIFE were classified as having GHD.
Menstrual, pubertal, reproductive, and contraception history was obtained on all female participants; estradiol, LH and FSH levels were measured in all women and levels were interpreted based on history and ongoing hormonal therapies. Women were classified as having premature ovarian insufficiency (whether of central or primary origin) if they had a previously established diagnosis or if they experienced amenorrhea before the age of 40 years. Women receiving sex hormone replacement continuously since adolescence, and whose diagnosis of primary ovarian failure and/or hypogonadotropic hypogonadism was not established with certainty at initiation of treatment, were categorized as having an ‘unknown’ status (N=15).
All male participants were screened with AM testosterone, luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels. Individuals were classified as having testosterone deficiency if they had a previous diagnosis associated with this condition (whether of central or primary origin) or if their screening study at SJLIFE revealed an AM testosterone level < 200 ng/dL.
Statistical approach
The first analytic objective was to compare the cross-sectional distribution of potential risk factors across three levels of BMD Z-score: 1) > -1; 2) -1 to -2; 3) ≤ -2. Chi square statistics were used for categorical variables to test for homogeneity of treatment and demographic factors across the three BMD Z-score groups (Table 1). Kruskal-Wallis statistics were used for comparison of continuous variables across the BMD Z-score groups (Table 2). We also calculated age- and sex-specific cumulative prevalence of having a BMD Z-score of ≤ -1. The second analytic objective was to estimate the magnitude of association (as adjusted odds ratios) between risk factors and BMD in a multivariable logistic regression model stratified by sex. For these analyses, BMD was dichotomized into two groups (≤ -1 vs. > -1) and covariate selection was guided by an iterative identification of factors that may have confounding effects on the exposure-outcome risk estimates.[25] Because of our interest in treatment effects on BMD, the variables selected for inclusion in the multivariable model were cranial radiation dose (four indicator categories), cumulative prednisone equivalent dose (per 1000 mg/m2 units), cumulative methotrexate dose (per 1000 mg/m2 units), age in years at the SJLIFE BMD evaluation, and sex (for the overall model). Factors such as history of growth hormone deficiency, gonadal insufficiency, or body mass index were not included as potential confounders in the regression models because they were judged to be in the causal pathway (intermediates) between ALL treatment and BMD status, [26] especially from cranial irradiation. The third analytic objective was to compare change in BMD Z-score between two time points among the subgroup of SJLIFE participants who had a BMD test during a previous ACT clinic visit. If more than one ACT BMD test was available, the earliest test was used. A paired t-test was calculated to assess the mean difference in BMD Z-score between the ACT and SJLIFE BMD evaluations and a three by three contingency table was used to illustrate change (or lack thereof) in BMD Z-score category between the initial and subsequent tests.
Table 1.
Univariable characteristics of the 845 study participants stratified by BMD Z-score (categorical variables)
| Factor | Subgroup | Overall | BMD Z-score ≤ -2 | BMD Z-score -2 to -1 | BMD Z-score > -1 | P-value |
|---|---|---|---|---|---|---|
| Count (%) | Count (%) | Count (%) | Count (%) | |||
| Total | 845 (100) | 48 (5.7) | 201 (23.8) | 596 (70.5) | ||
| Age at Diagnosis | Age < 10 years | 652 (77.2) | 40 (6.1) | 155 (23.8) | 457 (70.1) | |
| Age ≥ 10 years | 193 (22.8) | 8 (4.1) | 46 (23.8) | 139 (72.0) | 0.57 | |
| Gender | Female | 429 (50.8) | 17 (4.0) | 71 (16.6) | 341 (79.5) | |
| Male | 416 (49.2) | 31 (7.5) | 130 (31.3) | 255 (61.3) | <0.0001 | |
| Race | White | 767 (90.8) | 48 (6.3) | 192 (25.0) | 527 (68.7) | |
| Non-White | 78 (9.2) | 0 (0.00) | 9 (11.5) | 69 (88.5) | 0.001 | |
| Cranial irradiation (CRT) dose | No CRT | 327 (38.7) | 17 (5.2) | 71 (21.7) | 239 (73.1) | |
| CRT < 24Gy | 212 (25.1) | 14 (6.6) | 56 (26.4) | 142 (67.0) | ||
| CRT ≥ 24Gy | 200 (23.7) | 4 (2.0) | 50 (25.0) | 146 (73.0) | ||
| CRT with spinal irradiation or total body irradiation | 106 (12.5) | 13 (12.3) | 24 (22.6) | 69 (65.1) | 0.01 | |
| Body mass index (BMI) at study visit | BMI ≥ 30 | 359 (42.5) | 18 (5.0) | 77 (21.4) | 264 (73.5) | |
| BMI 25 – 29 | 233 (27.6) | 11 (4.7) | 48 (20.6) | 174 (74.7) | ||
| BMI 18.5 – 24 | 236 (27.9) | 15 (6.4) | 72 (30.5) | 149 (63.1) | ||
| BMI < 18.5 | 17 (2.0) | 4 (23.5) | 4 (23.5) | 9 (52.9) | 0.002 | |
| History of growth hormone deficiency | Yes | 326 (38.6) | 23 (7.1) | 90 (27.6) | 213 (65.3) | 0.081 |
| No | 460 (54.4) | 21 (4.6) | 96 (20.9) | 343 (74.6) | ||
| Unknown | 59 (7.0) | 4 (6.8) | 15 (25.4) | 40 (67.8) | ||
| History of premature ovarian insufficiency (females only) | Yes | 47 (11.0) | 2 (4.3) | 11 (23.4) | 34 (72.3) | 0.24 |
| No | 367 (85.5) | 13 (3.5) | 58 (15.8) | 296 (80.7) | ||
| Unknown | 15 (3.5) | 2 (13.3) | 2 (13.3) | 11 (73.3)) | ||
| History of testosterone insufficiency (males only) | Yes | 102 (24.6) | 11 (10.8) | 25 (24.5) | 66 (64.7) | 0.18 |
| No | 313 (75.2) | 20 (6.4) | 104 (33.2) | 189 (60.4) | ||
| Unknown | 1 (0.2) | 0 (0) | 1 (100) | 0 (0) |
Table 2.
Univariable characteristics of the 845 study participants stratified by BMD Z-score (continuous variables)
| Variable | Median (interquartile range) | ||||
|---|---|---|---|---|---|
| Overall | BMD Z-score ≤ -2 | BMD Z-score -2 to -1 | BMD Z-score > -1 | P-value | |
| Age at diagnosis, years | 5.02 (3.07–9.33) | 5.57 (3.32–8.58) | 4.97 (3.03–9.59) | 4.94 (3.07–9.31) | 1.0 |
| Age at study visit, years | 31.3 (25.6–37.4) | 28.7 (24.3–35.3) | 30.5 (25.3–37.4) | 31.7 (26.1–37.7) | 0.08 |
| Body mass index at study visit | 28.5 (24.2–33.5) | 27.0 (23.0–33.7) | 27.0 (23.8–33.6) | 29.0 (24.7–33.5) | 0.06 |
| Cumulative methotrexate dose, mg/m2 | 5426 (2596–18332) | 6679 (2369–21971) | 5431 (2622–18263) | 5417 (2580–18217) | 0.4 |
| Cumulative prednisone equivalent dose, mg/m2 | 9520 (1120–10400) | 9520 (1620–13067) | 9520 (1120–10160) | 8960 (1120–10280) | 0.02 |
| Cumulative anthracyclines dose, mg/m2 | 46 (0.0–88) | 84 (41–127) | 81.6 (41–94) | 42 (0.0–87) | 0.004 |
| Cumulative cyclophosphamide dose, mg/m2 | 3490 (0.0–9556) | 4924 (0.0–8786) | 6757 (0.0–9869) | 1809 (0.0–9511) | 0.19 |
| Cumulative etoposide dose, mg/m2 | 0.0 (0.0–9208) | 1064 (0.0–9807) | 907 (0.0–9806) | 0.0 (0.0–8467) | 0.009 |
| Cumulative teniposide dose, mg/m2 | 0.0 (0.0–3241) | 0.0 (0.0–1691) | 0.0 (0.0–4327) | 0.0 (0.0–3171) | 0.31 |
| Cumulative vincristine dose, mg/m2 | 41 (6.3–57) | 48 (17–63) | 48 (7.3–58) | 37 (6.1–57) | 0.005 |
RESULTS
Sample population
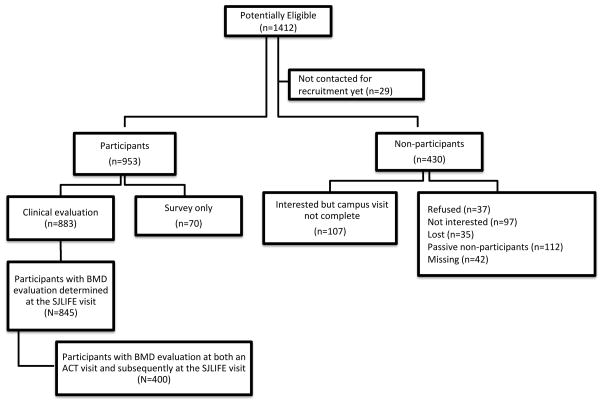
Figure 1 provides a flow (consort) diagram of the ALL survivors potentially eligible for this analysis. The source population for recruitment into SJLIFE as of the October 31, 2012 cutoff date included 1412 ALL survivors. Of those, 29 had not reached the interview pool for recruitment, 70 were participants in SJLIFE as health survey respondents only with no clinical or BMD examination, 107 had been contacted and expressed interest in participating but had not yet been scheduled, and 323 refused or could not be located. Of the 883 active participants, 845 had a clinical evaluation with a BMD test, which is 61% of the 1383 in the source population who had been offered enrollment. Of those, 400 had a prior BMD test conducted in the ACT clinic for analysis of BMD change over time. The evaluable cohort of 845 with a BMD test included 21 HSCT recipients (20 allogeneic HSCT for high risk or relapsed ALL or secondary AML and 1 autologous HSCT for a second primary brain tumor).
Figure 1.
Flow diagram of participation categories from the source population of potentially eligible ALL survivors in the St. Jude Lifetime Cohort Study as of October 2012.
Sample characteristics and distribution of BMD categories
Characteristics of the 845 study participants are shown in Tables 1 and 2. The median age at leukemia diagnosis was five years and the median age at the SJLIFE BMD evaluation was 31 years; neither factor differed statistically by BMD category. The presence of endocrine dysfunction was not significantly associated with BMD category (Table 1). Notably, the relatively low proportion of participants receiving hormonal therapy at the SJLIFE evaluation precluded assessment of the impact of replacement therapy on BMD (4 of 326 with GH deficiency, 10 of 47 with premature ovarian insufficiency, and 35 of 102 with testosterone insufficiency). A total of 205 (24%) survivors endorsed current use of vitamin D that was provided by prescription (n=3) or obtained as an over-the-counter medication or supplement (n=202). Current use of vitamin D was significantly associated with lower BMD Z-score group (p=0.047).
The overall prevalence with a BMD Z-score of ≤ - 2 was 5.7% and the prevalence with a Z-score - 1 to -2 was 23.8%. Thus, 70.5% of study participants had a BMD Z-score in the normal range (> -1). The cumulative prevalence of those with a BMD Z-score of ≤ -1 at age 40 years was 37.9% (95%CI 33.3%–42.5%) overall; 46.2% (95%CI 39.9%–52.4%) for males and 28.3% (95%CI 21.9%–34.9%) for females.
Multivariable models
As the number of subjects with very low BMD was too few to evaluate separately with statistical reliability, results of the multivariable models comparing BMD Z-score ≤ -1 with > -1 are presented in Table 3. For the overall model (males and females combined), neither methotrexate dose nor glucocorticoid dose were statistically associated with BMD category at the SJLIFE evaluation. In contrast, cranial radiation dose of ≥ 24 Gy was associated with an elevated risk of BMD Z-score ≤ -1 (adjusted odds ratio (OR) 2.05, 95%CI 1.21–3.46), as was craniospinal irradiation (adjusted OR 1.88, 95%CI 1.05–3.37), compared to those with no cranial or spinal radiation exposure. Because of our a priori hypothesis of a differential treatment effect by sex on BMD, sex-specific values are also presented in Table 3. The results show stronger radiation effects for female ALL survivors compared with male ALL survivors (P-value for effect heterogeneity between sex and CRT categories in the adjusted model = 0.01). Other chemotherapeutic exposures were not significantly associated with risk of BMD score of <-1, with the exception of etoposide exposure among males (p=0.015). The limited number of HSCT recipients precluded evaluation of this modality as a specific risk factor for low BMD.
Table 3.
Multivariable logistic regression analysis evaluating the relative odds of having a BMD Z-score of ≤ -1 versus > -1
| Overall | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factors in model | Adjusted odds ratio | 95% CI | p-value | Adjusted odds ratio | 95% CI | p-value | Adjusted odds ratio | 95% CI | p-value |
| No cranial radiation exposure | 1.00 | reference | |||||||
| Cranial radiation < 24Gy | 1.11 | 0.67–1.82 | 0.69 | 1.26 | 0.66–2.40 | 0.49 | 0.97 | 0.41–2.27 | 0.94 |
| Cranial radiation, ≥ 24Gy | 2.05 | 1.21–3.46 | 0.007 | 2.00 | 1.00–4.00 | 0.051 | 2.53 | 1.08–5.94 | 0.033 |
| Craniospinal radiationa | 1.88 | 1.05–3.37 | 0.033 | 1.23 | 0.57–2.75 | 0.57 | 3.27 | 1.35–7.93 | 0.009 |
| Cumulative methotrexate per 1000 mg/m2 unitsb | 1.00 | 0.97–1.02 | 0.73 | 0.98 | 0.95–1.01 | 0.21 | 1.03 | 0.98–1.07 | 0.26 |
| Cumulative prednisone equivalents per 1000 mg/m2 units | 1.00 | 0.96–1.04 | 0.95 | 0.97 | 0.92–1.03 | 0.32 | 1.03 | 0.96–1.11 | 0.36 |
| Cumulative anthracyclines dose per mg/m2 units | 1.00 | 0.99–1.00 | 0.24 | 1.00 | 1.00–1.01 | 0.11 | 1.00 | 0.99–1.00 | 0.89 |
| Cumulative etoposide dose per 100 mg/m2 units | 1.00 | 0.99–1.01 | 0.15 | 1.01 | 1.00–1.01 | 0.015 | 1.00 | 0.99–1.01 | 0.65 |
| Cumulative vincristine dose per mg/m2 units | 1.00 | 0.99–1.01 | 0.52 | 1.00 | 1.00–1.01 | 0.70 | 1.01 | 0.99–1.02 | 0.11 |
| Age at clinical visit, years | 0.97 | 0.94–0.99 | 0.010 | 0.95 | 0.92–0.98 | 0.004 | 0.99 | 0.95–1.04 | 0.68 |
| Female sex | 1.00 | reference | |||||||
| Male sex | 2.38 | 1.74–3.27 | <.0001 | ||||||
Total body irradiation or any cranial radiation dose with 15 gray spinal irradiation
Although not statistically significant in univariable analysis, methotrexate was included in the multivariable model because of its known adverse association with bone mineral density
Analysis of change in BMD category over time
An evaluation of the 400 study participants with both ACT and SJLIFE BMD evaluations compared to the 445 participants with only a SJLIFE BMD showed no statistically significant differences in age at diagnosis, gender or race. Consistent with the application of the Children’s Oncology Group follow-up guidelines in 2004, those with a prior BMD test tended to be younger (median age of 26 years) than did those without a prior BMD test (median age of 36 years). Also congruent with a younger ALL cohort, the subjects with an ACT BMD test were less likely to receive cranial radiation (42%) than were those without an ACT BMD test (79%) and those with an ACT BMD test received higher median cumulative doses of methotrexate (12867 vs. 2653 mg/m2) and prednisone equivalent units (9560 vs. 1120 mg/m2) than did the subjects without a prior BMD test.
Median time between the initial BMD test and the subsequent BMD test was 8.5 years (interquartile range = 6.6–10.7). The mean difference in BMD Z-scores between the two tests was −0.086 (95%CI −0.20 to 0.031, P=0.15). Table 4 compares BMD Z-score categories between the two evaluation times. At the initial test, 15.2% of participants had a BMD Z-score of ≤ -2; at the subsequent test that prevalence had decreased to 7.0%. In all, 367 (91.8%) either improved their BMD Z-score category or remained in the same category over the follow-up period. A suboptimal outcome group of 53 (13.3%) ALL survivors either did not improve beyond the ≤ -2 BMD category or had their BMD category worsen from baseline to follow-up (shaded area of Table 4).
Table 4.
Comparison of BMD Z-score categories at the initial visit (rows) and the subsequent visit (columns) in the subset of 400 participants with two BMD tests
| Subsequent BMD Z-score | ||||
|---|---|---|---|---|
| Initial BMD Z-score | > -1 | -1 to > -2 | ≤ -2 | Total at baseline |
| > -1 | 212 | 25 | 2 | 239 (59.8%) |
| -1 to > -2 | 40 | 54 | 6 | 100 (25.0%) |
| ≤ -2 | 11 | 30 | 20 | 61 (15.2%) |
| Total at follow-up | 263 (65.8%) | 109 (27.2%) | 28 (7.0%) | 400 (100%) |
The shaded area represents childhood ALL survivors with a suboptimal outcome, i.e. those whose BMD Z-score category either worsened over the time period or stayed within the low BMD category (N=53, 13.3%). The median follow-up time between BMD tests was 8.5 years (interquartile range 6.6 – 10.7).
DISCUSSION
Because of the known adverse effects of childhood ALL treatment on bone growth and metabolism, [13, 14] we hypothesized that a high percentage of survivors would have very low BMD in adulthood. We also surmised that BMD Z-scores would tend to decline over time, thus indicating an earlier onset of bone mineral loss than occurs in the ‘normal’ population. In contrast, although higher than would be expected from a standard normal distribution, we found that only 5.7% of our full study population (median age of 31 years) had a BMD Z-score consistent with osteoporosis (≤ -2). In addition, in the subset of subjects with two BMD measurements over time, we found that 67% of those who previously had a BMD Z-score of ≤ -2 improved by one or more categories a median of 8.5 years later. It should be noted that clinical management of inadequate BMD during the study period was limited to recommendations for calcium and vitamin D supplementation and/or lifestyle counseling to optimize bone health; bisphosphonates were not prescribed in the ACT clinic. Use of vitamin D at study evaluation was associated with a lower BMD Z-score, which could be related to suboptimal dosing from over the counter preparations in vitamin D deficient participants. Fortunately, it appears from our data that BMD values tend to improve over time in long-term childhood ALL survivors.
The strongest risk factor for a persistently low BMD (Z-score ≤ -1) during young adulthood was high dosage (≥24 Gy) of cranial radiation or craniospinal radiation. After adjusting for age and other treatment factors, this exposure dose, relative to no cranial radiation exposure, was associated with an approximate 2-fold increased risk of a BMD Z-score of ≤ -1. In addition, female survivors appeared to be more susceptible to craniospinal radiation effects on BMD than were males, which may reflect greater vulnerability to ovarian injury from scatter irradiation [10]. Males had a similar risk estimate to that of females for 24 Gy cranial irradiation without focal spinal radiation exposure. Increasing cumulative doses of glucocorticoid and methotrexate were not statistically associated with persistently low BMD for the overall group and among males in the cohort in the multivariable models controlling for treatment variables, age at SJLIFE evaluation, and sex, which may indicate long-term recovery from chemotherapy-related effects (Table 3). However, there was some indication that higher prednisone exposure was associated with lower BMD among female participants.
The low prevalence of BMD deficits, the cross-sectional analysis, and limited historical information and definitive tests to assess growth, testosterone, and ovarian hormone statuses over time precluded clinically meaningful analysis of the potential causal relation between endocrine organ dysfunction and BMD status as an adult. The observed effect of CRT on BMD is likely related to anterior pituitary deficiencies, including GH deficiency, which we found to be untreated in the majority of individuals evaluated in SJLIFE. Some patients who received CRT were not identified as being previously tested for GH deficiency and most who were tested as children for GHD were not tested again as an adult to meet the adult definition for GHD. We are unable to compare the impact of optimal replacement of GH in those deficient during childhood through adulthood on preventing low bone mineral density. As GH deficiency cannot be definitively assessed without GH stimulation testing, our data pertaining to GH status should be interpreted with caution.
Likewise, sex steroid production important in maintenance of BMD may be adversely affected by gonadotropin insufficiency associated with CRT or direct gonadal injury related to spinal irradiation [10]. Women with hypogonadism may not be receiving hormone replacement therapy at the time of the SJLIFE evaluation for a variety of reasons, including medical contraindications such as a history of thromboembolic disease or variable access to medical care. The frequency of untreated hypogonadism in males seemed to be even higher than that of females. However, the absence of confirmatory data regarding low testosterone and subsequent replacement in males diagnosed at the SJLIFE visit makes it difficult to assess the effect of hormonal replacement on BMD among men in this study.
Limitations of the study include the lack of BMD measurement before treatment initiation or at regular intervals following treatment completion. We could not, therefore, assess change from baseline or incremental recovery over time. Also important for consideration is the relatively young age of our study population and its lack of racial diversity. We could not address potential effects of ALL treatment on BMD trajectory at older ages when risk for osteopenia and osteoporosis increases significantly in the general population. In the general population, older age and body mass index are known to be correlated with BMD. In the current study population, an association with these factors and BMD was observed in univariate analyses, but not in multivariable models. As the SJLIFE cohort ages and continues to be followed, we can better evaluate the pattern of BMD changes over time. Another consideration is the potential for differential participation of subjects that could result in biased estimates of risk if the participants are not representative of the source population from which they were drawn. Our prior work evaluating representativeness of the SJLIFE cohort [21] provides some degree of confidence that we have a representative sample base, but we could not specifically evaluate differential BMD effects among non-participants.
Strengths of the study include the reasonably large sample size and relatively long follow-up period between BMD evaluations in our subgroup analysis. An additional study strength is the use of QCT rather than dual energy X-ray absorptiometry (DXA) as a measure of BMD. We elected to use QCT because it has several important advantages over DXA [27] in our patient population: 1) QCT measures trabecular bone, which is more sensitive to disease-related bone change; 2) QCT allows direct measurement of volumetric BMD, while DXA can only measure areal BMD and requires calculations for volumetric BMD that may be confounded by bone size and can be difficult to interpret in childhood cancer survivors;[13] and 3) QCT is less likely than DXA to overestimate BMD in obese individuals, whose DXA BMD values are elevated when soft tissue attenuates x-ray beams.[28]
In conclusion, very low BMD was relatively uncommon in this large sample of young adult survivors of childhood ALL, and BMD tended to improve between adolescence and young adulthood. Cranial or craniospinal radiation exposure (≥ 24 Gy) was the primary predictor of suboptimal BMD in our study; however, given that radiation treatment for childhood ALL is used far more sparingly now than in earlier treatment eras, [29] concerns about persistently low BMD among most current childhood ALL patients may be unwarranted, at least as suggested by these study results.
Acknowledgments
This work was supported by the Cancer Center Support (CORE) grant CA 21765 from the National Cancer Institute at the National Institutes of Health, and by the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1.Scheurer ME, Bondy ML, Gurney JG. Epidemiology of childhood cancer. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 6. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. pp. 2–16. [Google Scholar]
- 2.Mostoufi-Moab S, Brodsky J, Isaacoff EJ, et al. Longitudinal assessment of bone density and structure in childhood survivors of acute lymphoblastic leukemia without cranial radiation. J Clin Endocrinol Metab. 2012;97:3584–3592. doi: 10.1210/jc.2012-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arikoski P, Komulainen J, Voutilainen R, et al. Reduced bone mineral density in long-term survivors of childhood acute lymphoblastic leukemia. J Pediatr Hem Oncol. 1998;20:234–240. doi: 10.1097/00043426-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Gunes AM, Can E, Saglam H, et al. Assessment of bone mineral density and risk factors in children completing treatment for acute lymphoblastic leukemia. J Pediatr Hem Oncol. 2010;32:e102–107. doi: 10.1097/MPH.0b013e3181d32199. [DOI] [PubMed] [Google Scholar]
- 5.Kaste SC, Jones-Wallace D, Rose SR, et al. Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: frequency of occurrence and risk factors for their development. Leukemia. 2001;15:728–734. doi: 10.1038/sj.leu.2402078. [DOI] [PubMed] [Google Scholar]
- 6.Kaste SC, Rai SN, Fleming K, et al. Changes in bone mineral density in survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2006;46:77–87. doi: 10.1002/pbc.20553. [DOI] [PubMed] [Google Scholar]
- 7.Thomas I, Donohue JE, Ness KK, et al. Bone mineral density in young adult survivors of acute lymphoblastic leukemia. Cancer. 2008;113:3248–3256. doi: 10.1002/cncr.23912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasilewski-Masker K, Kaste SC, Hudson MM, et al. Bone Mineral Density Deficits in Survivors of Childhood Cancer: Long-term Follow-up Guidelines and Review of the Literature. Pediatrics. 2008;121:e705–e713. doi: 10.1542/peds.2007-1396. [DOI] [PubMed] [Google Scholar]
- 9.Margolin JF, Rabin KR, Steuber CP, Poplack DG. Acute Lymphoblastic Leukemia. In: Pizzo PA, Poplack DG, editors. Princples and Practice of Pediatric Oncology. 6. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. pp. 518–565. [Google Scholar]
- 10.Chemaitilly W, Sklar CA. Endocrine complications in long-term survivors of childhood cancers. Endocr-Relat Cancer. 2010;17:R141–159. doi: 10.1677/ERC-10-0002. [DOI] [PubMed] [Google Scholar]
- 11.Davies JH, Evans BA, Jones E, et al. Osteopenia, excess adiposity and hyperleptinaemia during 2 years of treatment for childhood acute lymphoblastic leukaemia without cranial irradiation. Clin Endocrinol (Oxf) 2004;60:358–365. doi: 10.1111/j.1365-2265.2003.01986.x. [DOI] [PubMed] [Google Scholar]
- 12.Mandel K, Atkinson S, Barr RD, Pencharz P. Skeletal morbidity in childhood acute lymphoblastic leukemia. J Clin Oncol. 2004;22:1215–1221. doi: 10.1200/JCO.2004.04.199. [DOI] [PubMed] [Google Scholar]
- 13.Crofton PM. Bone and bone turnover. Endocr Dev. 2009;15:77–100. doi: 10.1159/000207611. [DOI] [PubMed] [Google Scholar]
- 14.Fan C, Foster BK, Wallace WH, Xian CJ. Pathobiology and prevention of cancer chemotherapy-induced bone growth arrest, bone loss, and osteonecrosis. Curr Mol Med. 2011;11:140–151. doi: 10.2174/156652411794859223. [DOI] [PubMed] [Google Scholar]
- 15.Follin C, Link K, Wiebe T, et al. Bone loss after childhood acute lymphoblastic leukaemia: an observational study with and without GH therapy. Euro J Endocrinol. 2011;164:695–703. doi: 10.1530/EJE-10-1075. [DOI] [PubMed] [Google Scholar]
- 16.Halton JM, Atkinson SA, Fraher L, et al. Altered mineral metabolism and bone mass in children during treatment for acute lymphoblastic leukemia. J Bone Mineral Res. 1996;11:1774–1783. doi: 10.1002/jbmr.5650111122. [DOI] [PubMed] [Google Scholar]
- 17.Halton JM, Atkinson SA, Fraher L, et al. Mineral homeostasis and bone mass at diagnosis in children with acute lymphoblastic leukemia. J Pediatr. 1995;126:557–564. doi: 10.1016/s0022-3476(95)70349-7. [DOI] [PubMed] [Google Scholar]
- 18.Henderson RC, Madsen CD, Davis C, Gold SH. Longitudinal evaluation of bone mineral density in children receiving chemotherapy. J Pediatr Hem Oncol. 1998;20:322–326. doi: 10.1097/00043426-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Rayar MS, Nayiager T, Webber CE, et al. Predictors of bony morbidity in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;59:77–82. doi: 10.1002/pbc.24040. [DOI] [PubMed] [Google Scholar]
- 20.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ojha RP, Oancea SC, Ness KK, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: Results from the St. Jude Lifetime Cohort Study Pediatr Blood Cancer. 2013;60:856–864. doi: 10.1002/pbc.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Children’s Oncology Group. [Accessed February 4, 2014];Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. http://www.survivorshipguidelines.org/pdf/ltfuguidelines.pdf.
- 23.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 24.Meikle AW, Tyler FH. Potency and duration of action of glucocorticoids. Effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med. 1977;63:200–207. doi: 10.1016/0002-9343(77)90233-9. [DOI] [PubMed] [Google Scholar]
- 25.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiol. 1999;10:37–48. [PubMed] [Google Scholar]
- 26.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiol. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams JE. Quantitative computed tomography. Euro J Radiol. 2009;71:415–424. doi: 10.1016/j.ejrad.2009.04.074. [DOI] [PubMed] [Google Scholar]
- 28.Link TM. Osteoporosis imaging: state of the art and advanced imaging. Radiol. 2012;263:3–17. doi: 10.1148/radiol.12110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9:257–268. doi: 10.1016/S1470-2045(08)70070-6. [DOI] [PubMed] [Google Scholar]