Abstract
Secondary hyperparathyroidism (sHPT) is a major complication for patients with end-stage renal disease on long-term hemodialysis or peritoneal dialysis. When the disease is resistant to medical treatment, patients with severe sHPT are typically referred for parathyroidectomy (PTx), which usually improves biological parameters as well as clinical signs and symptoms. Unfortunately, early surgical failure with persistent disease may occur in 5%–10% of patients and recurrence reaches 20%–30% at 5 years. Presently, the use of parathyroid scintigraphy in sHPT is usually limited to the management of surgical failures after initial PTx. This review describes the strengths and limitations of typical 99mTc-sestamibi imaging protocols, and highlights the potential benefits of using parathyroid scintigraphy in the initial workup of surgical patients.
Keywords: parathyroid scintigraphy, 99mTc-MIBI, dual isotope, hemodialysis, secondary hyperparathyroidism, tertiary hyperparathyroidism, surgery
Secondary hyperparathyroidism (sHPT) is a frequent major complication for patients with end-stage renal disease on long-term hemodialysis or peritoneal dialysis. When the disease is resistant to medical treatment, patients with severe sHPT are typically referred for parathyroidectomy (PTx), which usually improves biological parameters as well as clinical signs and symptoms.1–6 PTx may also be necessary in a small subset of kidney transplant patients in whom tertiary hyperparathyroidism (tHPT) does not resolve spontaneously.7
Unfortunately, the rate of persistent or recurrent disease after PTx in patients with renal hyperparathyroidism is high.1,4,8–11 Parathyroid reoperation is challenging in this population because of frequent comorbidities and the risks of complications.9 Some early reports showed that parathyroid scintigraphy could be useful before initial PTx, providing both anatomic and functional information on individual parathyroid glands12–14; nevertheless, most expert surgeons have been skeptical and PTx without preoperative imaging remains the standard practice. Over the last few years, however, several reports, both from nephrologists and surgeons, suggest that parathyroid scintigraphy, alone or in combination with neck ultrasound, could be helpful when conducted before initial PTx in patients with sHPT.15–19 Parathyroid surgery is difficult and imaging studies are not a substitute to surgical skill. Preoperative imaging might, however, help surgeons to achieve optimal results. In a review on the surgical management of renal hyperparathyroidism, Madorin and colleagues support performing imaging studies “as they are non-invasive and may occasionally be helpful”.3 They do warn, however, that in many reports the sensitivity of imaging was poor.
This review begins with a brief description of the pathophysiology of sHPT and an overview of treatment goals. We then describe different 99mTc-sestamibi imaging protocols and try to answer the following questions: How best to conduct a parathyroid scan in the setting of renal hyperparathyroidism? What kind of information parathyroid scintigraphy can provide for lowering the risk of surgical failure at initial surgery and in case of reoperation?
PATHOPHYSIOLOGY OF RENAL HYPERPARATHYROIDISM, TREATMENT TARGETS, AND STRATEGIES
Secondary Hyperparathyroidism
With the decline of kidney function, sHPT develops due to a combination of factors, including hyperphosphatemia, elevated fibroblast growth factor 23 (FGF-23) levels, reduced vitamin D, and hypocalcemia.20 In patients on long-standing dialysis, parathyroid growth progressively shifts from diffuse hyperplasia of parathyroid glands to asymmetrical nodular and tumor-like monoclonal growth, with a high risk of functional autonomy due to the loss of expression of vitamin D receptor and calcium-sensing receptor from parathyroid cells. A shift from hypocalcemia to hypercalcemia is not unusual in advanced stages, especially when high doses of active vitamin D are used to control sHPT. All of these imbalances contribute to the mineral and bone metabolism disorders, vascular and soft-tissue calcification, cardiovascular events, and mortality.20–24 Elevated levels of phosphorous, calcium, calcium-phosphorus product, parathyroid hormone, alkaline phosphatase, and FGF23 have been associated with death and cardiovascular events.20–24
The arsenal of medical treatment includes dietary phosphorus restriction, phosphate binders, vitamin D sterols and analogs, and calcimimetics. The use of calcimimetics has increased the likelihood of achieving the targets set by either the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (NKF-K/DOQI) in the setting of dialysis25 [intact parathyroid hormone (iPTH) 150–300, calcium 8.4–9.5 mg/dL, phosphorus 3.5–5.5 mg/dL, and calcium-phosphorus product <55 mg2/dL2] or the Kidney Disease Improving Global Outcomes (KDIGO) in dialysis patients (iPTH: 2–9-fold the upper normal limits)26 and has reduced the number of patients referred to PTx. However, in the recently published report from the randomized trial “EVOLVE” that included 3883 hemodialysis patients, cinacalcet (Sensipar/Mimpara, Amgen) did not significantly reduce the risk of death or major cardiovascular events.27 Also, there was a higher risk of adverse events (gastrointestinal, hypocalcemic, and neoplastic events) in the cinacalcet group.
Presently, PTx is usually recommended when sHPT is refractory to medical therapy. Based on KDOQI guidelines, patients with PTH levels greater than 800 pg/mL with hypercalcemia and/or hyper-phosphatemia despite medical therapy should be offered PTx.25 Other strong indications for surgery are the presence of complications, such as intractable pruritus, bone pain, spontaneous tendon and bone fractures, metastatic calcifications, and calciphylaxis.1,3,5,6
Parathyroid gland volume greater than 500 mm3 at ultrasound, or maximum diameter greater than 1 cm, have also been suggested as criteria for surgery.3 It is still debated whether PTx should be offered earlier (eg, PTH >500 pg/mL instead of >800 pg/mL), as suggested by Japanese guidelines,25,28 or be favored over chronic use of calcimimetics in patients with severe sHPT. Recent findings from the EVOLVE trial27 might call into question the cost-effectiveness of chronic use of calcimimetics in patients with severe sHPT.
Parathyroidectomy has proved to be effective for reducing elevated levels of PTH, calcium, phosphorus, calcium × phosphorus product, and FGF-23 for improving symptoms, bone mass, anemia, hypertension, cardiac hypertrophy, quality of life, and for reducing mortality.1,6 However, PTx does not appear to be the optimal therapy for achieving the K-DOQI recommended values for calcium, phosphate, and PTH. In particular, undesirably low PTH may require increased clinical surveillance.11
The two most widely accepted surgical techniques are subtotal PTx and total PTx with autotransplantation. Based on a meta-analysis of 53 publications, neither technique has established superiority over the other in terms of surgical complications, or disease persistence or recurrence.4
Five to 10% of patients have persistent disease after surgery with PTH levels clearly higher than 300 pg/mL at 6 months.8,11 The rate of surgical failure due to recurrence reaches 20% to 30% at 5 years in patients continuing hemodialysis.10,11 On the other hand, some patients have permanent hypoparathyroidism with undetectable PTH levels due to necrosis of remnant tissue or because the surgeon has opted for total PTx without transplantation, an operation which is not favored by nephrologists due to the risk of adynamic bone disease.11 Many patients have detectable PTH levels that are, however, below the target. Whether the target of 150 pg/mL should also be applied to operated patients is not clear.3,8
Tertiary Hyperparathyroidism
The hypercalcemia that develops after renal transplantation is usually transient, and gradually resolves with the involution of parathyroid hyperplasia and associated decrease of serum PTH concentration. However, in about 10% of patients, tHPT persists beyond 6–12 months and requires surgery. Subtotal PTx seems to be the preferred procedure for tHPT.7
PARATHYROID SCINTIGRAPHY PROTOCOLS
Parathyroid scintigraphy uses sestamibi (methoxyisobutylisonitrile) radiolabeled with 99mTc (99mTc-MIBI), a tracer also used for myocardial scintigraphy. After intravenous administration, this cationic and lipophilic radiopharmaceutical is rapidly cleared from circulation and passively accumulates within the mitochondria of metabolically active cells. The imaged field should span from the angle of the mandible to the heart because ectopic glands may be widely distributed along the parathyroid cell migration routes.29
One difficulty with parathyroid imaging is that 99mTc-MIBI shows high uptake in the thyroid gland. Different protocols have been proposed to distinguish parathyroid from thyroid uptake (reviewed below). They use 99mTc-MIBI alone or in comparison to a thyroid tracer. There are also some technical considerations that can influence image resolution and sensitivity.
99mTc-MIBI “Dual-Phase” Protocol
This single-tracer approach is based on differential 99mTc-MIBI retention between parathyroid and thyroid tissue.12 99mTc-MIBI retention is prolonged in parathyroid lesions whereas the tracer washes out more rapidly from normal thyroid tissue. The original dual-phase protocol requires only 2 planar images, one recorded early (15 min) and the other late (2 h) after 99mTc-MIBI injection. Focal areas showing an increase in uptake over time (relative to the thyroid) are suggestive of parathyroid lesions. Parathyroid lesions with rapid tracer clearance can however be missed.
99mTc-MIBI and 99mTcO4− Comparison
In this protocol, images obtained with 99mTc-MIBI are compared to those obtained with 99mTc pertechnetate (99mTcO4−), a thyroid tracer. Because both tracers use the same isotope (99mTc), the thyroid image is acquired either before or after 99mTc-MIBI image acquisition. Patient motion between these 2 acquisitions may lead to artifacts on subtraction images.
99mTc-MIBI and 123I Simultaneous Acquisition With Subtraction
In this protocol, 99mTc-MIBI is used in conjunction with 123I. The main advantage of using 123I as a thyroid tracer is that thyroid and parathyroid images can be acquired simultaneously in a dual-energy window setup (for example a window of 130–150 keV for 99mTc-MIBI and 152–175 keV for 123I).13,30 123I is administered 2 hours before 99mTc-MIBI injection and the start of imaging.
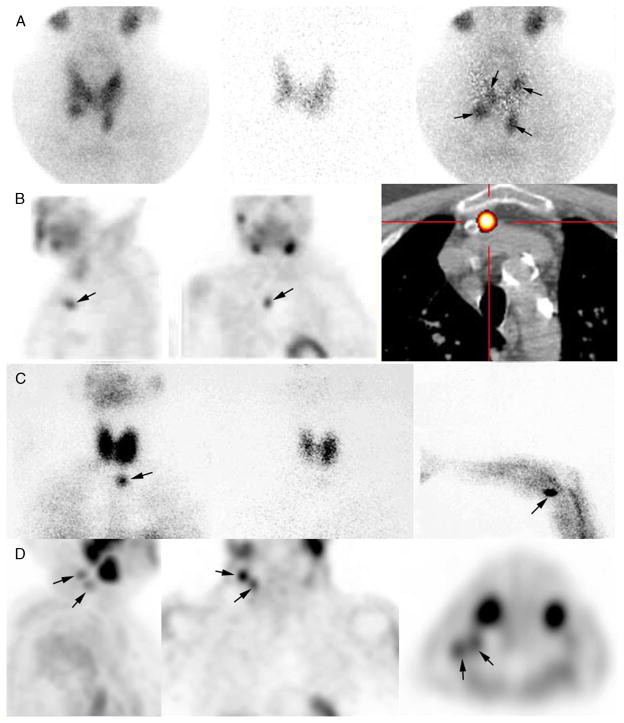
After acquisition, the 123I thyroid image is digitally subtracted from the 99mTc-MIBI image. The residual image corresponds to the parathyroid image (Fig. 1A).
FIGURE 1.
Case examples of parathyroid scintigraphy in sHPT. A, Parathyroid scintigraphy prior to initial surgery for secondary HPT. Subtraction protocol: 123I at 2 hours post-administration of 12 MBq from 740 MBq of 99mTc-sestamibi at time 0, simultaneous dual-tracer image recording (planar + pinhole), digital subtraction of images (99mTc-sestamibi minus 123I). Four enlarged parathyroid glands are seen on pinhole subtraction images in this patient, with asymmetrical gland locations (arrows). B–D, Parathyroid scintigraphy for persistent or recurrent sHPT. B, Recurrent tertiary HPT related to a supernumerary ectopic gland (left and middle: SPECT images; right: fusion SPECT/CT image). C, Recurrence caused by supernumerary ectopic “upper mediastinal” parathyroid gland and forearm graft hyperplasia (arrows). Left: planar static cervicomediastinal 99mTc-sestaMIBI; middle: planar static cervicomediastinal 123I image; right: 99mTc-sestaMIBI planar image centered over the graft. D, Recurrent sHPT related to parathyromatosis. The patient had total thyroid ablation during one of the previous parathyroid surgeries. Multiple foci of 99mTc-sestaMIBI uptake, corresponding to hyperfunctioning parathyroid tissue are seen in upper lateral right neck.
Pinhole Collimator
The large field-of-view image of the neck and the mediastinum is obtained with a parallel-holes collimator and is necessary to depict ectopia. The pinhole collimator offers an additional magnified view of the thyroid/parathyroid area.13
Tomoscintigraphy “SPECT”
Single-photon emission computed tomography (SPECT) provides 3D scintigraphy data and facilitates the location of parathyroid lesions in the anterior-posterior plane. The use of SPECT/CT hybrid instrument allows direct correlation of SPECT functional data with cross-sectional CT anatomic data, which is particularly helpful to pinpoint the position of mediastinal foci and other ectopic parathyroid glands29 (Fig. 1B).
Sensitivity of Different Protocols
Comparative studies in the setting of primary hyperparathyroidism have established the superiority of simultaneous dual-isotope imaging, with 99mTc-MIBI + 123I, over single-tracer techniques.31,32 Unfortunately, head-to-head comparisons in patients with sHPT are not available.
In a recent meta-analysis of parathyroid scintigraphy in sHPT, which included 471 patients from 24 studies, the pooled sensitivity in detecting hyperplastic glands was 58%.33 The authors pointed out technical heterogeneity and wide variations in sensitivity across different studies (35%–90%), but did not report on the individual sensitivities of different protocols.33
Each parathyroid protocol has its own strengths and limitations. However, there are significant differences in sensitivity that are outlined in Table 1 and discussed hereafter.
TABLE 1.
Performance of Different 99mTc-MIBI Parathyroid Planar Scintigraphy Protocols in Secondary Hyperparathyroidism
| Tracers Used | Pinhole Collimator | Studies/Patients/Number of Lesions* | Sensitivity (%)* | |
|---|---|---|---|---|
| A | 99mTc-MIBI only “dual-phase” | No | 15/308/899 | 56.2 (505/899) |
| B | 99mTc-MIBI only “dual-phase” | Yes | 4/60/196 | 63.2 (124/196) |
| C | 99mTc-MIBI + 123I (simultaneous acquisition plus subtraction) | Yes | 2/31/126 | 75.4 (95/126) |
| D | 99mTc-MIBI + 99mTcO4 (non-simultaneous) | No | 2/51/178 | 51.7 (92/178) |
| E | 99mTc-MIBI + 99mTcO4 (non-simultaneous) | Yes | 1/21/78 | 62.8 (49/78) |
Comparison between imaging protocols were performed using chi-squared test: A vs. B (P = 0.082); A vs. C (P < 0.001); B vs. C (P = 0.031).
Data were extracted from Tables 1 to 3 of the paper published by Caldarella,33 and sensitivity was calculated based on these data.
The original dual-phase protocol, which records only 2 planar images with a parallel-holes collimator—at 15 minutes and 2 hours after 99mTc-sestamibi injection, respectively—is a very simple and popular technique,12,14,16–18,33 but is associated with some limitations. While all protocols depend on tracer uptake by parathyroid lesions—which may vary according to mitochondria content, cell cycle, and hyperfunction—the dual-phase protocol further depends on tracer retention. The presence of P-glycoprotein (P-gp) or other efflux proteins might cause rapid tracer washout and reduced sensitivity. Moreover, relying solely on the parallel-holes collimator results in low image resolution with difficulty to detect small lesions or to individualize 2 contiguous hyperplastic glands. Sensitivity in the setting of first operation for sHPT is suboptimal, ranging from 35% to 83% in the studies included in the meta-analysis,33 with a pooled sensitivity of 56.2% (Table 1).
One way to improve sensitivity of the “single-tracer” dual-phase technique is to add pinhole imaging, offering a magnified view of the neck.18,19,34 Pinhole imaging has been a standard for optimal thyroid scintigraphy. In our opinion, its role is even more crucial for parathyroid imaging. When considering the 4 studies in the meta-analysis that added a pinhole image, sensitivity of single-tracer imaging ranged between 50% and 90%,33 with a pooled sensitivity of 63.2% (Table 1). 99mTc-MIBI single-tracer SPECT has higher sensitivity compared to planar imaging with a parallel-holes collimator, but still has lower sensitivity compared to pinhole imaging.34
It has been suggested that hyperplastic parathyroid glands are better detected by dual-tracer “subtraction” techniques.29 However, optimal results require the use of 123I as the thyroid tracer in order to benefit from simultaneous acquisition with 99mTc-MIBI, thus yielding subtraction images that are free from motion artifacts.13 The sensitivity offered by 99mTc-MIBI + 123I pinhole imaging ranged between 66% and 91%,15,30,33 with a pooled sensitivity of 75.4% (Table 1). This sensitivity is significantly higher than that offered by single-tracer parallel-hole imaging (56.2%; P < 0.001) and single-tracer pinhole imaging (63.2%; P = 0.031) (Table 1). Similar sensitivity (77%) was also reported using 99mTc-MIBI + 123I SPECT.35
99mTc-pertechnetate does not allow for simultaneous imaging with 99mTc-MIBI, and subtraction images are thus prone to motion artifacts. Based on data from studies included in the meta-analysis,33 the use of 99mTc-pertechnetate would not seem to offer improved sensitivity over single-tracer imaging, with an average sensitivity of 51.7% with parallel-holes collimator and 62.8% when using pinhole imaging (Table 1).
How to Best Conduct a Parathyroid Scan?
Based on data from several studies (Table 1), a pinhole view over the thyroid area improves resolution and should be part of any parathyroid imaging protocol.
Dual tracer using 99mTc-MIBI + 123I simultaneous acquisition with image subtraction offered the highest sensitivity so far. Also, differentiating thyroid nodules from parathyroid lesions is sometimes difficult with 99mTc-MIBI alone due to tracer retention in thyroid nodules. Specificity is improved with the use of 123I, a tracer specific to the thyroid.
Adding a SPECT/CT sequence does not further improve sensitivity in comparison to that already offered by pinhole subtraction.29 It, however, adds 3D topographic information which is often helpful to plan the surgical approach in the presence of a mediastinal focus or other suspicion of parathyroid ectopia on planar views. SPECT/CT acquisition can be performed as single-isotope 99mTc-MIBI imaging or as simultaneous dual-isotope imaging with 123I. The latter improves accuracy in the neck region. Given the high frequency of ectopic lesions in the setting of reoperative parathyroid surgery, a SPECT/CT sequence should be obtained. Future work should investigate whether SPECT/CT acquisition should be added routinely also before the first surgery.
Patient Preparation for Parathyroid Scintigraphy
Active vitamin D analogs and calcimimetics can lower 99mTc-MIBI uptake.36 Whenever possible, these treatments should be withheld for 2 weeks before scintigraphy to enhance sensitivity.
When using 123I (or 99mTc-pertechnetate), any treatment with thyroid hormones must be withheld for 2 weeks. Iodine-containing contrast media injections must also be avoided in the month preceding scintigraphy.
For convenience, scintigraphy is usually performed on a day when hemodialysis is not scheduled.
PARATHYROID SCINTIGRAPHY IN THE MANAGEMENT OF SECONDARY RENAL HYPERPARATHYROIDISM
Initial Surgery for sHPT
The successful outcome of PTx (ie, HPT suppression without hypoparathyroidism) is very dependent on the skills and experience of the surgeon. The surgeon will explore the 4 glands and either perform subtotal PTx (leaving a small part of one gland with its blood supply) or total PTx with autotransplantation (AT) of small pieces of parathyroid tissue (grafts) in a muscle of the forearm or neck (PTx + AT). Most surgeons will also routinely perform a trans-cervical bilateral thymectomy. With this standard surgery, and in the absence of preoperative imaging, the rates of persistent HPT and that of permanent hypoparathyroidism (due to loss of blood supply to the remnant) are about 5%–10% and 5%–20%, respectively.8,10,11 During long-term follow-up, disease recurrence may be observed in about 20%–30% of patients.10,11 Total PTx without transplantation can somewhat lower the overall failure rate; it is, however, associated with higher rates of permanent hypoparathyroidism and of adynamic bone disease.11
Below, we discuss the controversial role of imaging studies conducted prior to initial surgery.
Identification of Four Orthotopic Parathyroid Glands
Failure to identify 4 parathyroid glands usually results in persistent HPT.8 A missed parathyroid gland in the neck may be the cause of a difficult and extensive reoperation.
It is not unusual for parathyroid glands to show some asymmetrical positioning on both sides of the neck (Fig. 1A). Extensive surgical dissection would increase operative time and also increase the risk of morbidity (hematoma, recurrent laryngeal nerve palsy, necrosis of the glandular remnant, and hypoparathyroidism). This is particularly true in these patients who are at bleeding risk. When only 3 glands are identified during the operation, the information provided by preoperative scintigraphy may potentially modify the area of surgical exploration and reduce operating time and complications. Identifying all 4 parathyroid glands in patients with sHPT can also be difficult due to the high prevalence of nodular goiter in this patient population and the gross appearance of hyperplastic glands that may mimic thyroid tissue. Parathyroid scintigraphy, when performed as a dual-tracer protocol, facilitates the distinction between thyroid and parathyroid nodular tissue.
Localization of Ectopic and Supernumerary Parathyroid Glands
In experienced hands, the inability to identify an ectopic or supernumerary parathyroid is the main cause of failure, persistence, or early recurrence of sHPT.9 From a surgical point of view, a distinction exists between a minor ectopy (retrotracheal, in thyrothymic horn and upper anterior mediastinum, beneath thyroid capsule) and a major ectopy (low anterior mediastinal, aortopulmonary window, retroesophageal, undescended cervical glands and those located in the sheath of the carotid artery, or intrathyroidal parathyroid that is totally embedded in thyroid parenchyma). While the risk of major ectopy is about 2%–3% in primary HPT,37 the overall risk that at least one gland be ectopic in patients with sHPT is 4 times higher. Detection of ectopic parathyroid glands is probably the most important contribution of preoperative imaging. Also, the high sensitivity to visualize ectopic parathyroid glands is considered the main advantage of scintigraphy over ultrasound.19
A supernumerary “fifth” parathyroid gland is present in about 10% to 15% of individuals. About half of these glands are located in the thymus and the others in the neck. Some studies using dual-isotope 123I/99mTc-sestaMIBI subtraction scintigraphy, or dual-phase imaging with additional pinhole or SPECT acquisition, identified macroscopic supernumerary parathyroid glands before initial surgery,13,17–19 while other studies using the dual-phase protocol with only parallel-hole imaging were less successful.14,36 The dual-phase protocol with only parallel-hole imaging rarely identifies all 4 parathyroid glands.14,36 Therefore, it can be argued that some cervical or ectopic glands visualized on scintigraphy have not been declared as “supernumerary” on scintigraphy simply because fewer than 5 glands were visualized overall.
Choice of the Parathyroid Gland to be Partially Preserved
Preservation of some parathyroid tissue is needed to maintain a correct mineral balance and avoid adynamic bone disease. The optimal volume to be preserved as a glandular remnant or grafted tissue should be slightly above that of a normal parathyroid gland (about 60 mg). The choice of the gland depends on its gross appearance (the gland should be less likely to have severe nodular hyperplasia) as well as its anatomical situation.3 The functional information provided by parathyroid scintigraphy may guide surgeons towards the most suitable gland for preservation (least active/autonomous).12,13,16,19 Functional variation is especially seen in patients with severe sHPT who developed hypercalcemia and in patients with tHPT. 99mTc-MIBI uptake in these patients can vary widely from one parathyroid gland to another. Torregrosa and colleagues found that MIBI uptake is positively correlated to both iPTH concentration in the ipsilateral jugular vein and to proliferation indexes (the percentage of parathyroid cells in the S/G2 phase).14 In a retrospective analysis, a higher rate of recurrence was observed when remnant tissue was selected from a parathyroid gland with high 99mTc-sestamibi uptake.16
Several recent studies have shown that parathyroid scintigraphy is a helpful tool prior to initial PTx.15–19 By retrospective analysis, one team found that surgical failures have decreased since the introduction of sestamibi and intraoperative PTH assay.18 A randomized controlled study designed to assess the impact of parathyroid scintigraphy on the rate of persistence, morbidity, and recurrence of sHPT will be of particular interest.
Persistent or Recurrent sHPT
Persistent sHPT diagnosed in the early postoperative period, or during the first 6 months, results from missed orthotopic or ectopic glands, too large remnant/grafts, or supernumerary macroscopic parathyroid glands. In patients who undergo PTx + AT, the diagnosis of surgical failure is easy because parathyroid grafts are not immediately functional. After subtotal PTx for sHPT, there is no clear cutoff PTH level for early diagnosis of persistent disease. A maximal cutoff value of 150 pg/mL used by some authors18 may be more appropriate than the cutoff recommended by the K/DOQI (300 pg/mL), which is probably better suited to identifying recurrences. The magnitude in serum calcium levels decrease in the early postoperative period can also be a useful parameter.
Delayed recurrences due to growth of the remaining parathyroid tissue (from remnant/grafts) or supernumerary glands are sometimes observed after an appropriate initial PTx; this rate can reach 20%–30% after 5 years of continuing dialysis.1,10,11
Interestingly, patients who are referred for “recurrent HPT” not infrequently harbor 2 sites of autonomous disease38: one corresponding to a recurrence that developed within the preserved tissue and one corresponding to a “supernumerary parathyroid” (Fig. 1C). From an imaging perspective, the distinction is not always clear between persistent and recurrent disease, and it is possible that the identification of ectopic glands may also affect time to recurrence.
Recurrence may also rarely develop from parathyromatosis, which is caused by inadvertent rupture of the parathyroid capsule, with spilling of parathyroid cells in the surgical field. Parathyroid scintigraphy shows multiple hyperfunctioning foci in the neck (Fig. 1D), and recurrence is frequent despite reoperation.38
Preoperative imaging is considered mandatory before reoperation for persistent or recurrent disease.9,18 With the advance in imaging techniques, invasive procedures such as venous sampling are now rarely necessary.38 When parathyroid scintigraphy identifies an ectopic mediastinal gland, co-registered CT (SPECT/CT), diagnostic CT, or MRI is needed to confirm the location and provide anatomic information that will determine the most appropriate surgical approach29 (Fig. 1B). Due to the high sensitivity of sestamibi imaging in this situation, the role of invasive imaging techniques such as venous sampling is decreasing. Good sensitivity has also been reported with 11C-methionine PET/CT.39 Thus, in the rare cases of negative sestamibi imaging, there might be a role for this tracer. However, this short-life tracer requires an on-site cyclotron and is thus not widely available in clinical practice.
Research Avenues
Low parathyroid sestamibi uptake was found to correlate with positive response to vitamin D or calcimimetics in dialysis patients with sHPT.36 Scintigraphy can also point to differences in response from one parathyroid gland to another. Scintigraphy may then play a role in clinical research involving novel drugs.
Asymmetrical gland disease is often the case in renal transplant patients with tHPT. Subtotal PTx is recommended because more limited PTx is associated with a higher risk of persistence/recurrence.7 When the disease is limited to 1 or 2 glands, patients may benefit from more limited resection.40 However, this should be the case only in carefully selected patients, such as those showing absent or low sestamibi uptake from some parathyroid glands in addition to small size. Also, the role in this situation of some additional adjuncts, such as intraoperative PTH assessment and intraoperative radionuclide probing (gamma probe), or endoscopic surgery, should be investigated.29
CONCLUSION
The role of parathyroid scintigraphy in the setting of reoperation is well established. Useful information might be obtained by performing a parathyroid scintigraphy prior to initial PTx in patients with sHPT: detection of an eventual ectopic gland, thus avoiding surgical failure or reducing the extent of dissection; identification of an eventual supernumerary parathyroid gland; and identification of the parathyroid gland with the lowest 99mTc-MIBI uptake intensity, intended to be partially autografted or maintained. An adequate protocol is necessary to optimize detection. Comparative studies in the setting of primary hyperparathyroidism have established the superiority of simultaneous dual-isotope imaging, with 99mTc-MIBI + 123I, over single-tracer techniques.31,32 Optimal technique is even more crucial in patients with sHPT. Pinhole imaging over the thyroid area is indicated for high resolution. Dual-tracer 99mTc-MIBI + 123I imaging, with simultaneous acquisition and image subtraction, offers the highest sensitivity. A SPECT/CT sequence adds 3D topographic information, which is very helpful to plan the surgical approach in the case of mediastinal lesion or other ectopic parathyroid lesions, and should be part of the parathyroid imaging protocol in the setting of reoperative parathyroid surgery.
Acknowledgments
The authors gratefully acknowledge the support of the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRP–NIMH–NIH).
Footnotes
Conflicts of interest and sources of funding: none declared.
References
- 1.Jofre R, Lopez Gomez JM, Menarguez J, et al. Parathyroidectomy: whom and when? Kidney Int Suppl. 2003;85:S97–S100. doi: 10.1046/j.1523-1755.63.s85.23.x. [DOI] [PubMed] [Google Scholar]
- 2.Kestenbaum B, Andress DL, Schwartz SM, et al. Survival following parathyroidectomy among United States dialysis patients. Kidney Int. 2004;66:2010–2016. doi: 10.1111/j.1523-1755.2004.00972.x. [DOI] [PubMed] [Google Scholar]
- 3.Madorin C, Owen RP, Fraser WD, et al. The surgical management of renal hyperparathyroidism. Eur Arch Otorhinolaryngol. 2012;269:1565–1576. doi: 10.1007/s00405-011-1833-2. [DOI] [PubMed] [Google Scholar]
- 4.Richards ML, Wormuth J, Bingener J, et al. Parathyroidectomy in secondary hyperparathyroidism: is there an optimal operative management? Surgery. 2016;139:174–180. doi: 10.1016/j.surg.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 5.Sharma J, Raggi P, Kutner N, et al. Improved long-term survival of dialysis patients after near-total parathyroidectomy. J Am Coll Surg. 2012;214:400–407. doi: 10.1016/j.jamcollsurg.2011.12.046. discussion 407–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tominaga Y, Matsuoka S, Uno N. Surgical and medical treatment of secondary hyperparathyroidism in patients on continuous dialysis. World J Surg. 2009;33:2335–2342. doi: 10.1007/s00268-009-9943-3. [DOI] [PubMed] [Google Scholar]
- 7.Triponez F, Clark OH, Vanrenthergem Y, et al. Surgical treatment of persistent hyperparathyroidism after renal transplantation. Ann Surg. 2008;248:18–30. doi: 10.1097/SLA.0b013e3181728a2d. [DOI] [PubMed] [Google Scholar]
- 8.Kovacevic B, Ignjatovic M, Zivaljevic V, et al. Parathyroidectomy for the attainment of NKF-K/DOQI and KDIGO recommended values for bone and mineral metabolism in dialysis patients with uncontrollable secondary hyper-parathyroidism. Langenbecks Arch Surg. 2010;397:413–420. doi: 10.1007/s00423-011-0901-9. [DOI] [PubMed] [Google Scholar]
- 9.Dotzenrath C, Cupisti K, Goretzki E, et al. Operative treatment of renal autonomous hyperparathyroidism: cause of persistent or recurrent disease in 304 patients. Langenbecks Arch Surg. 2003;387:348–354. doi: 10.1007/s00423-002-0322-x. [DOI] [PubMed] [Google Scholar]
- 10.Gagne E, Urena P, Leite-Silva S, et al. Short- and long-term efficacy of total parathyroidectomy with immediate autografting compared with subtotal parathyroidectomy in hemodialysis patients. J Am Soc Nephrol. 2002;3:1008–1017. doi: 10.1681/ASN.V341008. [DOI] [PubMed] [Google Scholar]
- 11.Mazzaferro S, Pasquali M, Farcomeni A, et al. Parathyroidectomy as a therapeutic tool for targeting the recommended NKF-K/DOQI ranges for serum calcium, phosphate and parathyroid hormone in dialysis patients. Nephrol Dial Transplant. 2008;23:2319–2323. doi: 10.1093/ndt/gfm931. [DOI] [PubMed] [Google Scholar]
- 12.Piga M, Bolasco P, Satta L, et al. Double phase parathyroid technetium-99m-MIBI scintigraphy to identify functional autonomy in secondary hyperparathyroidism. J Nucl Med. 1996;37:565–569. [PubMed] [Google Scholar]
- 13.Hindié E, Urena P, Jeanguillaume C, et al. Preoperative imaging of parathyroid glands with technetium-99m-labelled sestamibi and iodine-123 subtraction scanning in secondary hyperparathyroidism. Lancet. 1999;353:2200–2204. doi: 10.1016/S0140-6736(98)09089-8. [DOI] [PubMed] [Google Scholar]
- 14.Torregrosa JV, Fernandez-Cruz L, Canalejo A, et al. (99m)Tc-sestamibi scintigraphy and cell cycle in parathyroid glands of secondary hyperparathyroidism. World J Surg. 2000;24:1386–1390. doi: 10.1007/s002680010229. [DOI] [PubMed] [Google Scholar]
- 15.Perie S, Fessi H, Tassart M, et al. Usefulness of combination of high-resolution ultrasonography and dual-phase dual-isotope iodine 123/technetium Tc 99m sestamibi scintigraphy for the preoperative localization of hyperplastic parathyroid glands in renal hyperparathyroidism. Am J Kidney Dis. 2005;45:344–352. doi: 10.1053/j.ajkd.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Fuster D, Ybarra J, Ortin J, et al. Role of pre-operative imaging using 99mTc-MIBI and neck ultrasound in patients with secondary hyperparathyroidism who are candidates for subtotal parathyroidectomy. Eur J Nucl Med Mol Imaging. 2006;33:467–473. doi: 10.1007/s00259-005-0021-2. [DOI] [PubMed] [Google Scholar]
- 17.de la Rosa A, Jimeno J, Membrilla E, et al. Usefulness of preoperative Tc-mibi parathyroid scintigraphy in secondary hyperparathyroidism. Langenbecks Arch Surg. 2008;393:21–24. doi: 10.1007/s00423-007-0151-z. [DOI] [PubMed] [Google Scholar]
- 18.Gasparri G, Camandona M, Bertoldo U, et al. The usefulness of preoperative dual-phase 99mTc MIBI-scintigraphy and IO-PTH assay in the treatment of secondary and tertiary hyperparathyroidism. Ann Surg. 2009;250:868–871. doi: 10.1097/SLA.0b013e3181b0c7f4. [DOI] [PubMed] [Google Scholar]
- 19.Vulpio C, Bossola M, De Gaetano A, et al. Usefulness of the combination of ultrasonography and 99mTc-sestamibi scintigraphy in the preoperative evaluation of uremic secondary hyperparathyroidism. Head Neck. 2010;32:1226–1235. doi: 10.1002/hed.21320. [DOI] [PubMed] [Google Scholar]
- 20.Quarles LD. Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease. Exp Cell Res. 2012;318:1040–1048. doi: 10.1016/j.yexcr.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2012;26:1948–1955. doi: 10.1093/ndt/gfq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganesh SK, Stack AG, Levin NW, et al. Association of elevated serum PO(4), Ca × PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 23.Tentori F, Blayne MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eknoyan G, Levin A, Levin NW. Bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 suppl 3):S1–S201. [PubMed] [Google Scholar]
- 26.Moe SM, Drüeke TB, Block GA, et al. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009;113:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 27.Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. New Engl J Med. 2012;367:2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 28.Guideline Working Group, Japanese Society for Dialysis Therapy. Clinical practice guideline for the management of secondary hyperparathyroidism in chronic dialysis patients. Ther Apher Dial. 2008;12:514–525. doi: 10.1111/j.1744-9987.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 29.Hindié E, Ugur O, Fuster D, et al. 2009 EANM parathyroid guidelines. Eur J Nucl Med Mol Imaging. 2009;36:1201–1216. doi: 10.1007/s00259-009-1131-z. [DOI] [PubMed] [Google Scholar]
- 30.Hindié E, Melliere D, Jeanguillaume G, et al. Parathyroid imaging using simultaneous double-window recording of technetium-99m-sestamibi and iodine-123. J Nucl Med. 1988;39:1100–1105. [PubMed] [Google Scholar]
- 31.Caveny SA, Klingensmith WC, 3rd, Martin WE, et al. Parathyroid imaging: the importance of dual-radiopharmaceutical simultaneous acquisition with 99mTc-sestamibi and 123I. J Nucl Med Technol. 2012;40:104–110. doi: 10.2967/jnmt.111.098400. [DOI] [PubMed] [Google Scholar]
- 32.Tunninen V, Varjo P, Schildt J, et al. Comparison of five parathyroid scintigraphic protocols. Int J Mol Imaging. 2013;2013:921260. doi: 10.1155/2013/921260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caldarella C, Treglia G, Pontecorvi A, et al. Diagnostic performance of planar scintigraphy using (99m)Tc-MIBI in patients with secondary hyperparathyroidism: a meta-analysis. Ann Nucl Med. 2012;26:794–803. doi: 10.1007/s12149-012-0643-y. [DOI] [PubMed] [Google Scholar]
- 34.Ali L, Loutfi I, Biswas G, et al. Improved delineation of parathyroid lesions in patients with chronic renal failure using magnified pinhole imaging. J Nucl Med Technol. 2011;39:35–39. doi: 10.2967/jnmt.110.076984. [DOI] [PubMed] [Google Scholar]
- 35.Neumann DR, Esselstyn CB, Jr, Go RT, et al. Comparison of double-phase 99mTc-sestamibi with 123I-99mTc-sestamibi subtraction SPECT in hyperparathyroidism. AJR Am J Roentgenol. 1997;169:1671–1674. doi: 10.2214/ajr.169.6.9393188. [DOI] [PubMed] [Google Scholar]
- 36.Fuster D, Torregrosa JV, Domenech B, et al. Dual-phase 99mTc-MIBI scintigraphy to assess calcimimetic effect in patients on haemodialysis with secondary hyperparathyroidism. Nucl Med Commun. 2009;30:890–894. doi: 10.1097/MNM.0b013e3283305df6. [DOI] [PubMed] [Google Scholar]
- 37.Hindié E, Melliere D, Perlemuter L, et al. Primary hyperparathyroidism: higher success rate of first surgery after preoperative Tc-99m sestamibi-I-123 subtraction scanning. Radiol. 1999;204:221–228. doi: 10.1148/radiology.204.1.9205251. [DOI] [PubMed] [Google Scholar]
- 38.Hindié E, Zanotti-Fregonara P, Just PA, et al. Parathyroid scintigraphy findings in chronic kidney disease patients with recurrent hyperparathyroidism. Eur J Nucl Med Mol Imaging. 2010;37:623–634. doi: 10.1007/s00259-009-1313-8. [DOI] [PubMed] [Google Scholar]
- 39.Rubello D, Fanti S, Nanni C, et al. 11C-methionine PET/CT in 99mTc-sestamibi-negative hyperparathyroidism in patients with renal failure on chronic haemodialysis. Eur J Nucl Med Mol Imaging. 2006;33:453–459. doi: 10.1007/s00259-005-0008-z. [DOI] [PubMed] [Google Scholar]
- 40.Pitt SC, Panneerselvan R, Chen H, et al. Tertiary hyperparathyroidism: is less than a subtotal resection ever appropriate? A study of long-term outcomes. Surgery. 2009;146:1130–1137. doi: 10.1016/j.surg.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]