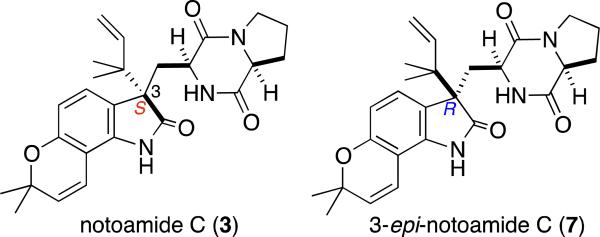
In Figure 1, the structures of notoamide C (3) and 3-epi-notoamide C (7) should be corrected as shown: This error was caused by the configuration of notoamide C, which was initially assigned as 3R based on biogenetic considerations.1 Recently, the absolute configuration of notoamide C was corrected to be 3S based on the biochemical conversion of notoamide E into notoamide C by recombinant NotB.2 Recently, the relative and absolute configuration of notoamide C was independently reported by Chen, et al. by single crystal X-ray analysis of notoamide C isolated from Aspergillus sp. XS-20090066.3 Accordingly the configuration of 7 was corrected to be 3R.
Contributor Information
Sachiko Tsukamoto, Kumamoto University, Graduate School of Pharmaceutical Sciences Kato, Hikaru.
Hikaru Kato, Kumamoto University, Graduate School of Pharmaceutical Sciences, Greshock, Thomas.
Thomas J. Greshock, Colorado State University, Chemistry Hirota, Hiroshi
Hiroshi Hirota, Riken Advanced Science Institute, Ohta, Tomihisa.
Tomihisa Ohta, Kanazawa University, Faculty of Pharmaceutical Sciences Williams, Robert.
Robert M. Williams, Colorado State University, Department of Chemistry
REFERENCES
- 1.Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, Williams RM, Tsukamoto S. Angew. Chem., Int. Ed. 2007;46:2254. doi: 10.1002/anie.200604381. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Finefield JM, Sunderhaus JD, McAfoos TJ, Williams RM, Sherman DH. J. Am. Chem. Soc. 2012;134:788. doi: 10.1021/ja2093212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen M, Shao C-L, Fu X-M, Xu R-F, Zheng J-J, Zhao D-L, She Z-G, Wang C-Y. J. Nat. Prod. 2013;76:547–553. doi: 10.1021/np300707x. [DOI] [PubMed] [Google Scholar]