Abstract
Human immunodeficiency virus (HIV) infection selectively targets the striatum, a region rich in opioid receptor-expressing neural cells, resulting in gliosis and neuronal losses. Opioids can be neuroprotective or can promote neurodegeneration. To determine whether opioids modify the response of neurons to human immunodeficiency virus type 1 (HIV-1) Tat protein-induced neurotoxicity, neural cell cultures from mouse striatum were initially characterized for μ and/or κ opioid receptor immunoreactivity. These cultures were continuously treated with morphine, the opioid antagonist naloxone, and/or HIV-1 Tat (1-72) protein, a non-neurotoxic HIV-1 Tat deletion mutant (TatΔ31-61) protein, or immunoneutralized HIV-1 Tat (1-72) protein. Neuronal and astrocyte viability was examined by ethidium monoazide exclusion, and by apoptotic changes in nuclear heterochromatin using Hoechst 33342. Morphine (10 nM, 100 nM or 1 μM) significantly increased Tat-induced (100 or 200 nM) neuronal losses by about two-fold at 24 h following exposure. The synergistic effects of morphine and Tat were prevented by naloxone (3 μM), indicating the involvement of opioid receptors. Furthermore, morphine was not toxic when combined with mutant Tat or immunoneutralized Tat. Neuronal losses were accompanied by chromatin condensation and pyknosis. Astrocyte viability was unaffected.
These findings demonstrate that acute opioid exposure can exacerbate the neurodegenerative effect of HIV-1 Tat protein in striatal neurons, and infer a means by which opioids may hasten the progression of HIV-associated dementia.
Keywords: morphine, heroin, drug abuse, acquired immunodeficiency syndrome (AIDS), basal ganglia, apoptosis
The human immunodeficiency virus (HIV) epidemic in North America and Western Europe is in part being driven by drug abuse.52 However, very little is known about the potential interaction of these drugs with HIV infection in the genesis of cerebral dysfunction. The observation of an increased frequency and severity of HIV encephalitis in an opiate abusing cohort suggests a relationship between HIV and drug abuse.7
HIV preferentially targets the basal ganglia.10,56 Interestingly, the basal ganglia also express high levels of opioid receptors.3,46,76 Based on this and other evidence, we recently proposed that drugs of abuse potentiate the effects of HIV by direct actions on neurons and glia.52 Despite the impairment in neuronal function and neuronal losses, the virus itself rarely targets neurons.5,36,53 Instead, HIV infects non-neuronal intermediates causing a productive infection in microglia and infiltrating monocytes. Recent evidence suggests that products released from HIV infected cells are neurotoxic (reviews20,36,40,53). These products can be broadly classified into two groups. “Virotoxins” are encoded by the viral genome, while “cellular toxins” are encoded by the host genome.53 Most evidence suggests that the virotoxins can induce the expression of cellular toxins initiating a spiraling cascade of events.53 Of the virotoxins produced, Tat is highly neurotoxic,23,39,44,58 secreted extracellularly from infected cells,13 and can be detected in the brains of patients with HIV encephalitis.29,31,38
Although there is a correlation between opioid drug abuse and accelerated HIV-induced damage to the brain,8 the nature of potential synergistic interactions is uncertain. Both opioids25,26,28 and Tat30,39,54,58 can disrupt cellular function or induce toxicity in neurons and astroglia. However, unlike Tat, opioids can have neuroprotective effects, preventing programmed cell death or allaying toxicity in some experimental systems.24,48,49,62 The purpose of this study was to determine whether opioid drugs (morphine) might interact with HIV Tat protein to modify the viability of striatal neurons. Morphine is the active metabolite (deacetylated) of heroin that functions in brain making it an appropriate prototypic opioid drug of abuse.
EXPERIMENTAL PROCEDURES
Mixed-striatal cultures
Striata were isolated from unsexed newborn or 1-day-old ICR mice (Harlen Sprague-Dawley, Indianapolis, IN) as previously described;73 however, using culture conditions that favored a balanced mixture of neurons and glia. The striata from two mice were pooled and considered as an independent sample (n=1). Growth medium contained Neurobasal™ medium (Gibco/Life Technologies, Grand Island, NY) supplemented with B-27 (2% v/v; Gibco/Life Technologies), L-glutamine (0.5 mM), and gentamicin (10 μg/ml). Briefly, striata were digested with 0.25% (w/v) trypsin in growth medium, centrifuged, and triturated in growth medium supplemented with 2.5% (v/v) donor horse serum. Cells were plated at a mean density of 100,000 cells/coverslip onto poly-L-lysine (0.1 mg/ml) coated-15 mm-diameter glass coverslips and incubated at 35°C in 5% CO2/95% air and high humidity. In addition, cells were maintained in serum-free growth medium transiently supplemented with 25 μM L-glutamate but then transferred into glutamate-free medium.24 Animal procedures were approved by the University of Kentucky Animal Care and Use Committee and were in accordance with the National Institute of Health guide for the care and use of laboratory animals.
Immunocytochemistry
Cultures were evaluated for the presence of opioid receptor containing neurons at 6 days in vitro. Immunodetection of μ and κ opioid receptors was performed as previously described.73 Rabbit anti-μ (MOR1; 1:2000), δ (DOR1; 1:2500), and κ (KOR1; 1:2000) affinity purified, polyclonal antisera (gift from Dr. Robert P. Elde) were used to detect μ, δ, and κ opioid receptors, respectively.2-4 Secondary biotinylated goat anti-rabbit antibodies (1:250) were conjugated to avidin-peroxidase (Vectastain ABC kit, Vector Laboratories, Burlingame, CA) or Cy2-avidin (1:250; Amersham Life Science, Pittsburgh, PA). Nickel-intensified diaminobenzidine (DAB) was used as a substrate for peroxidase.24
For triple label studies, neurons were detected using rabbit anti-human protein gene product (PGP) 9.5 antisera (1:1200 dilution; Ultraclone, Cambridge, UK) followed by goat anti-rabbit antibodies conjugated to Alexa 488 (Molecular Probes). PGP 9.5 is a neuronal ubiquitin carboxyl- terminal hydrolase.78 Astrocytes were detected using mouse anti-glial fibrillary acidic protein (GFAP) antibodies (1:300, Boehringer Mannheim, Indianapolis) followed by anti-mouse antibodies conjugated to Alexa 546 (Molecular Probes). Microglia were detected using biotinylated isolectin from Griffonia simplicifolia (IB4) (Sigma)12,17,61 (50 μg/ml for 16 h at 4°C in PBS) followed by incubation in streptavidin conjugated Alexa 350 (10 μg/ml in PBS, 1 h at room temperature; Molecular Probes). Once differential counts were performed on fluorescent neurons, astrocytes, and microglia, immature oligodendrocytes were identified in the same cultures using anti-O4 rat IgM monoclonal antibodies (1:1 dilution from ascites fluid; gift from Dr. M. Schachner, Zurich6,71). Primary O4 antibodies were detected with anti-rat IgM antibodies conjugated to peroxidase (Jackson ImmunoResearch, West Grove, PA) and visualized using nickel-intensified diaminobenzidine.33 For studies on astrocyte viability, rabbit anti-GFAP antisera (1:600 dilution; Chemicon, Temecula, CA), followed by goat anti-rabbit IgG conjugated to Cy3 (Jackson) were used to detect astroglia in combination with ethidium monoazide and Hoechst dyes.
Recombinant Tat protein
Recombinant Tat was prepared as described previously43 with minor modifications. The tat gene encoding the first 72 amino acids was amplified from HIV BRU obtained from Dr. Richard Gaynor through the AIDS repository at the NIH. Previous studies demonstrating neurotoxicity have used Tat derived from the HIV BRU strain and this facilitates comparisons among studies.47
Furthermore, we have previously shown that the neurotoxic domain of Tat is well conserved across various strains of HIV.47 The amplified tat gene was inserted into an Escherichia coli vector PinPoint Xa-2 (Promega). A deletion mutant from this plasmid was also prepared by deleting the sequence encoding amino acids 31-61 of Tat previously shown to contain the neurotoxic epitope.55 This construct allowed the expression of the Tat proteins as a fusion protein naturally biotinylated at the N-terminus. The biotinylated Tat proteins were purified on a column of soft release avidin resin and cleaved from the fusion protein using factor Xa and eluded from the column followed by desalting with a PD10 column. All purification steps contained dithiothreitol to prevent oxidation of the proteins. Tat proteins were >95% pure as determined by sodium dodecyl sulphate-polyacrylamide gel electrophoresis followed by silver staining, and analysis by HPLC using a C4 column showed a single symmetrical peak. Western blot analysis showed that these preparations contained both monomeric and dimeric forms of Tat1-72, but the monomeric form only for TatΔ31-61. The functional activity of Tat1-72 was confirmed using a transactivation assay in HL3T1 cells containing an HIV-1 LTR-CAT construct.43 The Tat preparation contained <1 pg/ml endotoxin as determined using a Pyrochrome Chromogenic test kit (Associates of Cape Cod, Inc., Falmouth, MA). The Tat protein was stored in a lyophilized form at −80°C in endotoxin-free siliconized microfuge tubes until taken for experimentation. Tat was immunoneutralized as described previously.55
Determination of toxicity
Viability assays were performed in striatal neuron cultures following exposure to opioids, HIV-1 Tat (1-72), mutant HIV-1 Tat (Δ31-61), and immunoneutralized HIV-1 Tat (1-72). Cells were continuously exposed to growth medium alone (untreated controls), morphine (10 nM, 100 nM, or 1 μM) (Sigma Chemical Co., St. Louis, MO), Tat (100 or 200 nM), mutant Tat (200 nM), immunoneutralized Tat (200 nM), morphine plus Tat, morphine plus mutant Tat, morphine plus immunoneutralized Tat, or morphine plus Tat and naloxone (3 μM; Research Biochemicals International, Natick, MA). To assess viability, the same neurons were photographed before and after treatment, and the proportion of dead cells was counted.24 A neuron was considered dead/dying if there was vacuolization in the cell body, nuclear fragmentation, and/or disrupted cell processes. In other experiments, viability was determined by double-labeling cells with ethidium monoazide bromide (Molecular Probes, Eugene, OR) and Hoechst 33342-trihydrochloride trihydrate (Molecular Probes). Neurons were incubated with ethidium monoazide bromide (0.5 μg/ml; w/v) in Dulbecco's phosphate buffered saline (PBS) (Gibco/Life Technologies). Dead cells that failed to exclude ethidium monoazide became permanently labeled after the ethidium was bound by photo-affinity to DNA using a 45 W fluorescent light (15 cm distance) for 30 minutes at room temperature.63 Cells were then fixed with Zamboni's fixative containing 3% paraformaldehyde and counterstained with Hoechst 33342 (15 μg/ml, Molecular Probes) in PBS for 15 minutes at room temperature. Hoechst labels DNA in all cells and permits assessment of nuclear morphology. The number of dead neurons (ethidium labeled nuclei) and total neuron numbers (Hoechst labeled nuclei) were counted and reported for individual cultures using a Nikon fluorescence microscope. Neurons were differentiated from other cell types based on morphologic criteria using Hoechst staining75 and by using cell-type-specific markers.
To arbitrarily sample cells, the x-axis microscope stage controller was moved along an equatorial line through the center of the coverslip and all cell clusters entering the field of vision were sampled. Between 500-1000 neurons were analyzed per culture and at least four cultures were assessed per treatment condition. Each culture consisted of cells derived from separate mice. Neuronal losses were reported as the percentage of total neurons relative to untreated control cultures. Astroglial identity was assessed by morphologic criteria confirmed by GFAP immunocytochemistry. Approximately 200-300 astrocytes were analyzed per culture.
Statistics
Statistical analyses were performed using analysis of variance (ANOVA) and Newman-Keuls post hoc test (if significant treatment effects were noted by ANOVA) (Statistica, StatSoft, Tulsa, OK). Treatment effects were considered significant if P <0.05. Photomicrographs were overlaid using Photoshop 5.0 (Adobe).
RESULTS
Characterization of phenotypes in mixed-striatal cultures
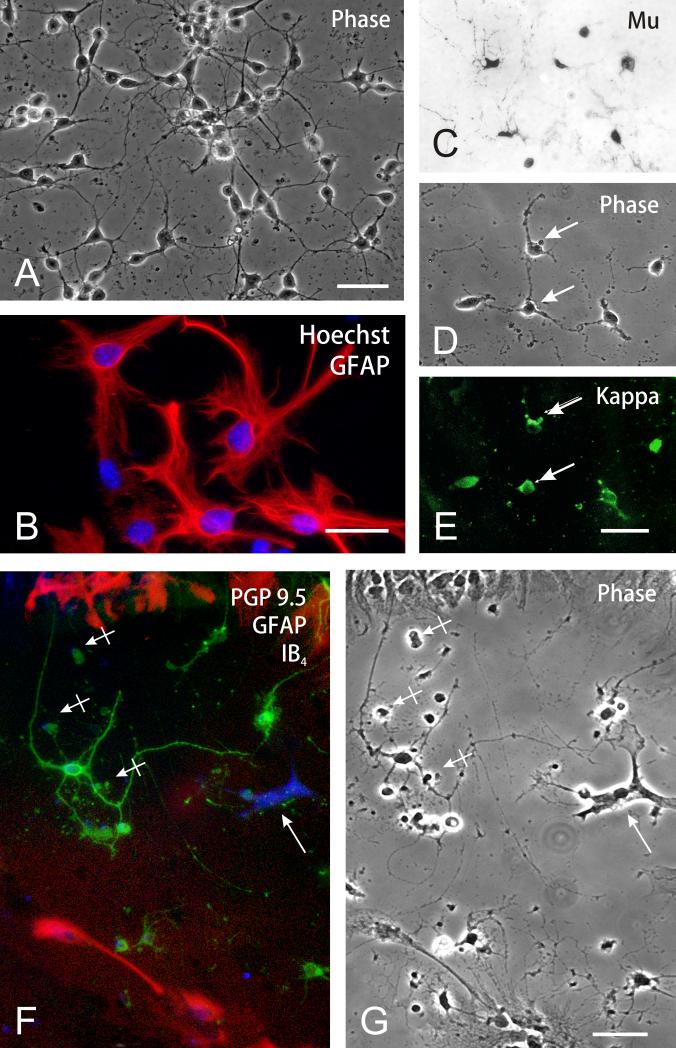
The main cell type in the striatal cultures was multipolar neurons, which were noted by 3 to 5 neurites and PGP 9.5 immunoreactivity (54.3 ± 1.6%; n = 4) (Fig. 1A,C-E). Astroglia were identified by GFAP immunoreactivity and comprised 41.8 ± 1.7% (n = 4) of total cells; of these most were the flat, polyhedral (type 1) astrocyte variant (Fig. 1B,F-G). Phenotypically, a majority of the neurons were immunopositive for both μ and κ opioid receptors (Fig. 1C-E). Fewer cells expressed δ receptor immunoreactivity (data not shown). Subsequent studies analyzed the effects of opioids and Tat on neurons. IB4 isolectin bound-microglia comprised 0.7 ± 0.3% (n = 4) of the total population (Fig. 1F); 2.6 ± 0.6% (n = 4) were O4 immunoreactive oligodendrocytes at various stages of development (not shown).
Fig. 1.
Characterization of neural cells in mouse striatal cultures (A-G). (A) Phase contrast photomicrograph of striatal cells at 5-7 days in vitro. A majority of the cells are neurons; scale bar = 20 μm. (B) Astrocytes were characterized by large nuclei with multiple nucleoli and dispersed heterochromatin (Hoechst 33342 blue immunofluorescence) and glial fibrillary acidic (GFAP) immunofluorescence (Cy3 red fluorochrome); scale bar = 25 μm. (C-E) Subpopulations of striatal neurons possessed μ (C), κ (E), and to a lesser extent δ (not shown), opioid receptor immunoreactivity. D & E show phase contrast and κ opioid receptor immunofluorescent images of the same cells; scale bar = 20 μm. (F-G) Triple-label-identification of neurons, astrocytes and microglia in striatal cultures. Neurons were identified using anti-PGP 9.5 indirect immunofluorescence (Alexa 488, green product), while astrocytes were labeled using anti-GFAP indirect immunofluorescence (Alexa 546, red product) and microglia were detected using IB4 biotinylated isolectin-conjugated (via avidin) to Alexa 350 (blue fluorescent product) (arrow) (F). A majority of the cells were neurons; microglia comprised 0.7% of the total cells. (G) A phase-contrast photomicrograph of the same cells as in Fig. 1F, some of which were non-viable/degenerating (hatched arrows); PGP 9.5 immunoreactivity is retained by many degenerating neurons; scale bar = 20 μm.
Morphine and HIV-1 Tat (1-72) neurotoxicity
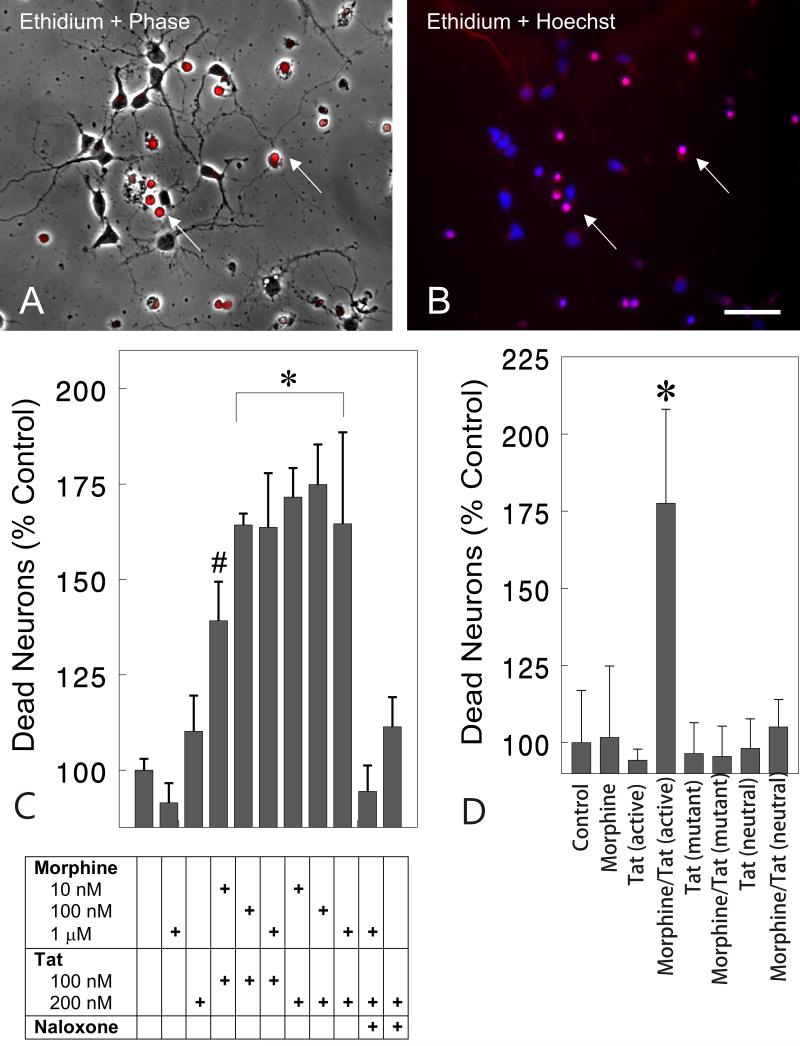
Toxicity between morphine and Tat was initially confirmed using repeated measures analysis (time lapse photomicrographs) of living neurons identified prior to treatment and at 16 h following treatment (data not shown). Marked synergistic toxicity between morphine (1 μM) and Tat (100 nM) was evident at 16 h that caused a 55.6% reduction in viable neurons compared to untreated groups or groups exposed to morphine alone (P<0.01). Neurotoxicity was denoted by shrunken, vacuolated cell bodies and retracted neurites. These initial observations prompted examination of the later 24-h time point using markers for cell viability. At 24 h, morphine and Tat together synergistically increased the proportion of dying cells, identified by their failure to exclude ethidium monoazide, by nearly two-fold (Fig. 2C). The ethidium monoazide-labeled dying cells often-displayed condensed, pyknotic nuclei using Hoechst 33342 blue-fluorescent dye (Fig. 2B). The combined toxicity was seen over a range of morphine (10 nM, 100 nM, or 1 μM) and Tat (100 nM or 200 nM) concentrations, and the effects of 1 μM morphine were prevented by naloxone (3 μM) (Fig. 2C). Importantly, neither the highest concentration of morphine (1 μM) nor Tat (200 nM) alone affected neuronal viability compared to untreated controls (Fig. 2C). Although Tat treatment alone resulted in a slight, albeit insignificant 10.2% increase in toxicity at 24 h, Tat alone can be neurotoxic with prolonged exposure (J.A. Gurwell and K.F. Hauser, unpublished).23,39,44,58 The proportion of ethidium monoazide-positive dying cells was 6 ± 2% in untreated control cultures.
Fig. 2.
Effect of opioids and/or Tat on the survival of striatal neurons at 24 h. Phase contrast microscopy (A) with ethidium monoazide (red fluorescent overlay in A & B) and Hoechst 33342-labeling (blue fluorescence) of nuclear chromatin patterns (B) were examined in the same cells at 24 h following morphine plus Tat exposure. Dying cells, noted by the inability to exclude ethidiummonoazide, lacked neuritic processes (A-B). Nonviable cells additionally displayed dense, pyknotic heterochromatin with Hoechst dye suggestive of apoptosis (B). (C). The viability assay shows morphine and Tat had synergistic effects on neuronal losses at 24 h. The effects of combined morphine (1 μM) and Tat (200 nM) toxicity was prevented by naloxone (3 μM) (C). The results are the mean ± SEM of 4-7 determinations on cells pooled from separate mice (*P < 0.02 versus untreated controls, treatment with Tat or morphine alone, or naloxone-exposed groups) (#P<0.05 versus untreated controls, Tat or morphine alone, or naloxone-exposed groups); morphine (Morph); naloxone (Nal); scale bar = 20 μm. (D). Tat Specificity. Synergistic toxicity of morphine (1 μM) and Tat (200 nM) (*P<0.03 versus untreated controls and all treatment groups) was attenuated in the presence of mutant Tat (31-61 deletion) (200 nM) and immunoneutralized Tat (200nM) (D). The results are the mean ± SEM of 5 determinations on cells pooled from separate mice.
The specificity of Tat toxicity was tested by treating cultures with morphine (1 μM) in combination with Tat (200 nM), TatΔ31-61 (200 nM), or immunoneutralized Tat (200 nM) (Fig. 2D). Previous studies have shown that Tat residues 31-61 have both neurotoxic and neuroexcitatory properties.55 Morphine (1 μM) and Tat (200 nM) synergistically increased neuronal death compared to all other treatment groups (P<0.03) (Fig. 2 D). Toxicity was not evident with either mutant Tat or immunoneutralized Tat by themselves, or with morphine (Fig. 2D).
Astrocyte viability
Dying cells were identified as neurons based on their morphology and the presence of PGP 9.5 immunoreactivity. Nevertheless, because some of the dying cells had degenerated so severely that they neither displayed evidence of a neuronal morphology nor possessed cell type specific immunoreactive markers, we examined the effect of opioids and/or Tat on the survival of astrocytes, the other major cell type present in our cultures, at 24 h (Table 1). Opioids and/or Tat had no effect on astrocyte viability, providing additional confirmation that the synergistic toxicity was restricted to neurons at 24 h. The proportion of ethidium monoazide-positive cells with astrocytic morphology was 1.5 ± 0.4% in untreated control cultures.
Table 1.
Effect of Opioids and/or Tat on Astrocyte Viability
| Treatment | Non-Viable Astrocytes (%)b |
|---|---|
| Untreated | 1.5 ± 0.4 |
| Morphine | 1.8 ± 0.3 |
| Tat | 0.9 ± 0.3 |
| Morphine + Tat | 1.6 ± 0.3 |
| Morphine + Tat + Naloxone | 1.3 ± 0.2 |
aCell cultures were continuously exposed to opioids and/or Tat for 24 h in vitro. The results are the mean ± SEM of four cultures, each containing cells derived from separate mice.
No significant effects on astrocyte survival were noted (ANOVA).
DISCUSSION
Morphine and HIV-1 Tat (1-72) display a synergistic striatal neurotoxicity
The findings present novel evidence that opioids and HIV-1 Tat protein are synergistically toxic to striatal neurons through a direct action on neural cell targets. Importantly, the enhanced toxicity was clearly mediated through opioid receptors, since the neurodegenerative effects of morphine were prevented by concurrent naloxone administration. Because morphine is a preferential μ opioid receptor agonist and a large proportion of the neurons in our cultures possess μ opioid receptor immunoreactivity as occurs in vivo,76 our results infer that μ receptors may be preferentially involved. Nevertheless, a definitive role for μ receptor involvement requires additional study using reagents that are more selective. Naloxone is a μ, δ, and κ opioid receptor antagonist. In addition, although subsets of the neurons in our cultures can possess μ, δ, and/or κ receptor immunoreactivity, it is uncertain whether opioids and Tat are directly affecting neurons or acting via glial intermediaries. Subpopulations of striatal astrocytes,73 as well as oligodendroglia33 and microglia14 can express opioid receptors and potentially mediate the effects of morphine and/or Tat in neurons.
Morphine and HIV-1 Tat (1-72) concentrations
Therapeutic levels of morphine in plasma following oral or intravenous administration for acute pain can approach 100 nmol/L (the concentrations in the central nervous system are likely to be lower79).41,42 In extreme instances, concentrations in human cerebrospinal fluid can be greater than 400 nM following intracerebroventricular administration for severe cancer pain.70 In contrast, opioid dependent individuals reportedly tolerate opioid blood levels that are 2.5 to 100-fold greater than typical therapeutic concentrations for acute pain.32 In rats, morphine concentrations in the nervous system can transiently approach 1.5-μg/g tissue weight following a 10-mg/kg intravenous dose.11 Thus, the morphine concentrations used that increase Tat toxicity in the present study are likely to mimic those seen with chronic drug abuse, but importantly are less likely to be realized at therapeutic dosages for pain management.
Tat protein is produced during HIV infection and can be released by infected cells.13,18,50,64,65 Furthermore, extracellular Tat is functionally active.19 The concentrations of Tat in our cultures are 1.5-3.0 μg/ml (100-200 nM, respectively). Concentrations of Tat are approximately 1 ng/ml in the serum of HIV infected patients and 4 ng/ml in the conditioned media of HIV infected cells.1,77 The extracellular space within the central nervous system comprises a small proportion of total brain volume,59 and may be dramatically reduced with pathophysiological changes.74 For this reason, it is conceivable that neurotoxic concentrations of Tat such as those used in this study may be achieved within the confined extracellular space of the HIV infected striatum.
Potential clinical significance of striatal neurotoxicity
Our basic experimental studies in vitro provide a possible explanation why opioid drug use causes increased dementia and motor dysfunction in a significant proportion of HIV-infected individuals. As recently noted by Dr. Alan Leshner, the Director of the National Institute on Drug Abuse, “Drug abuse and HIV are truly interlinked epidemics” (see52). “2.4 million Americans use heroin and as many as 30 % of injecting drug users are HIV positive52 (see also9)”. In a patient cohort in Scotland, 59% of HIV positive injecting drug users with an extensive (but not exclusive) history of opioid abuse have pathological changes associated with HIV encephalitis at autopsy.8,9 Within this cohort (homosexuals and drug users), cognitive impairment often coincided with HIV encephalitis. Similarly, in injecting drug users sampled throughout the United Kingdom, individuals with HIV infection are more likely to develop encephalitis.15
Morphine and HIV-1 Tat (1-72) can induce apoptotic pathways
The loss of neuronal viability (inability to exclude ethidium) was accompanied by nuclear shrinkage and chromatin compaction (pyknosis) and/or fragmentation (apoptotic bodies). Tat has been shown to activate apoptotic cascades such as caspase and Par-4 in primary neurons in culture.39 Interestingly, depending on the cell type and dosage, opioids can have paradoxical neuroprotective or neurodegenerative effects, which likely relates to the ability of opioid receptors to couple to cell death signaling pathways in different systems.27 In some experimental paradigms, opioids are neuroprotective.24,48,49,62 Interestingly, morphine may exaggerate HIV-envelope protein gp120-induced early proliferative increases in kidney fibroblasts68 and in gp120 interactions with substances of abuse can at times be protective.72 Clearly, however, HIV is a multisystem disease, and opioids themselves have diverse effects that are system specific.27 More typically, when opioids affect cell viability, they exacerbate apoptotic events or induce cytotoxicity. Morphine can induce toxicity in cerebellar Purkinje cells.25 Fentanyl, a selective μ agonist drug, is neurotoxic to the limbic system of rats at high dosages35 and can exacerbate the effects of ischemia-induced damage to the basal ganglia.34 Morphiceptin, a μ agonist, enhances staurosporine or wortmannin-induced apoptosis in embryonic chick neurons.21,51,66,67,69,80 Alternatively, in other studies, opioids have no intrinsic toxicity, but will synergistically enhance cell losses only if apoptosis is induced by other factors,16,21,22,45 which is similar to the present findings. It is important to note that morphine alone is not toxic to our striatal neurons, even following prolonged exposure (7 days) to high concentrations (1 or 10 μM), whereas prolonged exposure to Tat (100 or 200 nM > 72 h) is intrinsically neurotoxic (K.F. Hauser, unpublished).38,39 Thus, morphine is accelerating the toxic effects of Tat, and studies now in progress are addressing the nature of the interactions and the particular signaling pathways involved.
Conclusions
Our present results should be interpreted with some caution, because they represent an acute response of cells isolated in vitro to relatively high concentrations of morphine. Many of the detrimental effects of opioids result from high, fluctuating levels and a failure to accommodate to changes in opioid signal intensity.37,57 Steady state or moderate therapeutic exposures have few side effects. Moreover, with chronic opioid exposure, tolerance develops at the molecular, cellular, and systems levels. It is yet uncertain whether Tat would be more toxic in opioid tolerant cells. In addition, non-opioid factors present in vivo, such as specific EGF-family ligand-erbB signaling events, may normally modify or negate the neuronal response to opioids.60 The particular signaling pathways or temporal relatedness involved in opioid-Tat interactions remain speculative. Lastly, although we have assessed overt losses of neuronal populations, it is uncertain whether the effects are direct or indirect since the astroglia (~40%) or microglia (albeit <1%) may intervene and mediate aspects of the opioid-Tat synergism. Irrespective of these issues, our findings are an important first step and suggest that opioid drug abuse may contribute to the progression of HIV-associated dementia through a direct neurotoxic mechanism.
ACKNOWLEDGMENTS
This work was supported by NIH grants DA 06204, DA13559 and NS 39253. We thank Dr. Robert P. Elde for providing anti-μ and κ opioid receptor antisera, and Carol Anderson for technical assistance.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- ANOVA
analysis of variance
- DAB
diaminobenzidine
- GFAP
glial fibrillary acidic protein
- HIV
human immunodeficiency virus
- HIV-1
human immunodeficiency virus type 1
- HPLC
high performance liquid chromatography
- IB4
isolectin from Griffonia simplicifolia
- LTR
long terminal repeat
- PBS
phosphate buffered saline
- PGP 9.5
protein gene product 9.5
References
- 1.Albini A, Benelli R, Giunciuglio D, Cai T, Mariani G, Ferrini S, Noonan DM. Identification of a novel domain of HIV tat involved in monocyte chemotaxis. J. Biol. Chem. 1998;273:15895–900. doi: 10.1074/jbc.273.26.15895. [DOI] [PubMed] [Google Scholar]
- 2.Arvidsson U, Dado RJ, Riedl M, Lee J-H, Law PY, Loh H.H., Elde R, Wessendorf MW. δ-opioid receptor immunoreactivity: Distribution in brainstem and spinal cord, and relationship to biogenic amines and enkephalin. J. Neurosci. 1995;15:1215–1235. doi: 10.1523/JNEUROSCI.15-02-01215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvidsson U, Riedl M, Chakrabarti S, Lee J-H, Nakano AH, Dado RJ, Loh HH, Law P-Y, Wessendorf MW, Elde R. Distribution and targeting of a μ-opioid receptor (MOR1) in brain and spinal cord. J. Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee J-H, Nakano AH, Lin X, Loh HH, Law P-Y, Wessendorf MW, Elde R. The kappa-opioid receptor is primarily postsynaptic: Combined immunohistochemical localization of the receptor and endogenous opioids. Proc. Natl. Acad. Sci. USA. 1995;92:5062–5066. doi: 10.1073/pnas.92.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bansal R, Warrington A, Gard A, Ranscht B, Pfeiffer S. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mab used in the analysis of oligodendrocyte development. J. Neurosci. Res. 1989;24:548–557. doi: 10.1002/jnr.490240413. [DOI] [PubMed] [Google Scholar]
- 7.Bell JE. The neuropathology of adult HIV infection. Rev. Neurol. (Paris) 1998;154:816–829. [PubMed] [Google Scholar]
- 8.Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121:2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- 9.Bell JE, Donaldson YK, Lowrie S, McKenzie CA, Elton RA, Chiswick A, Brettle RP, Ironside JW, Simmonds P. Influence of risk group and zidovudine therapy on the development of HIV encephalitis and cognitive impairment in AIDS patients. AIDS. 1996;10:493–499. doi: 10.1097/00002030-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Berger JR, Nath A. HIV dementia and the basal ganglia. Intervirology. 1997;40:122–131. doi: 10.1159/000150539. [DOI] [PubMed] [Google Scholar]
- 11.Bhargava HN, Villar VM, Rahmani NH, Larsen AK. Time course of the distribution of morphine in brain regions, spinal cord and serum following intravenous injection to rats of differing ages. Pharmacology. 1993;47:13–23. doi: 10.1159/000139073. [DOI] [PubMed] [Google Scholar]
- 12.Brown H, Townsend M, Fearn S, Perry VH. The monoclonal antibody HB1 recognizes an adhesion molecule for macrophages in the brain. J Neurocytol. 1998;27:867–876. doi: 10.1023/a:1006932505819. [DOI] [PubMed] [Google Scholar]
- 13.Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 tat protein exits from cells via a leaderless secretory pathway and binds to extracellelar matrix-associated heparan sulfate proteoglycan through its basic region. AIDS. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Chao CC, Hu S, Peterson PK. Opiates, glia, and neurotoxicity. Adv. Exp. Med. Biol. 1996;402:29–33. doi: 10.1007/978-1-4613-0407-4_5. [DOI] [PubMed] [Google Scholar]
- 15.Davies J, Everall IP, Weich S, McLaughlin J, Scaravilli F, Lantos PL. HIV-associated brain pathology in the United Kingdom: an epidemiological study. AIDS. 1997;11:1145–50. doi: 10.1097/00002030-199709000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Dawson G, Dawson SA, Goswami R. Chronic exposure to kappa-opioids enhances the susceptibility of immortalized neurons (F-11kappa 7) to apoptosis-inducing drugs by a mechanism that may involve ceramide. J. Neurochem. 1997;68:2363–2370. doi: 10.1046/j.1471-4159.1997.68062363.x. [DOI] [PubMed] [Google Scholar]
- 17.Earle KL, Mitrofanis J. Identification of transient microglial cell colonies in the forebrain white matter of developing rats. J. Comp. Neurol. 1997;387:371–384. doi: 10.1002/(sici)1096-9861(19971027)387:3<371::aid-cne4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 18.Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990;345:84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- 19.Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan R, Wingfield P, Gallo R. Release, uptake, and effects of extracellular human immunodeficiency virus type-1 Tat protein on cell growth and viral replication. J. Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein LG, Gendelman HE. Human immunodeficiency virus type 1 infection of the nervous system: pathogenic mechanisms. Ann. Neurol. 1993;33:429–436. doi: 10.1002/ana.410330502. [DOI] [PubMed] [Google Scholar]
- 21.Goswami R, Dawson SA, Dawson G. Cyclic AMP protects against staurosporine and wortmannin-induced apoptosis and opioid-enhanced apoptosis in both embryonic and immortalized (F-11kappa7) neurons. J. Neurochem. 1998;70:1376–1382. doi: 10.1046/j.1471-4159.1998.70041376.x. [DOI] [PubMed] [Google Scholar]
- 22.Goswami R, Dawson SA, Dawson G. Multiple polyphosphoinositide pathways regulate apoptotic signalling in a dorsal root ganglion derived cell line [In Process Citation]. J. Neurosci. Res. 2000;59:136–144. [PubMed] [Google Scholar]
- 23.Haughey NJ, Holden CP, Nath A, Geiger JD. Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 Protein Tat. J. Neurochem. 1999;73:1363–1374. doi: 10.1046/j.1471-4159.1999.0731363.x. [DOI] [PubMed] [Google Scholar]
- 24.Hauser KF, Foldes JK, Turbek CS. Dynorphin A (1-13) neurotoxicity in vitro: Opioid and non-opioid mechanisms in spinal cord neurodegeneration. Exp. Neurol. 1999;160:361–375. doi: 10.1006/exnr.1999.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauser KF, Gurwell JA, Turbek CS. Morphine inhibits Purkinje cell survival and dendritic differentiation in organotypic cultures of the mouse cerebellum. Exp. Neurol. 1994;130:95–105. doi: 10.1006/exnr.1994.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauser KF, Harris-White ME, Jackson JA, Opanashuk LA, Carney JM. Opioids disrupt Ca2+ homeostasis and induce carbonyl oxyradical production in mouse astrocytes in vitro: transient increases and adaptation to sustained exposure. Exp. Neurol. 1998;151:70–76. doi: 10.1006/exnr.1998.6788. [DOI] [PubMed] [Google Scholar]
- 27.Hauser KF, Mangoura D. Diversity of the endogenous opioid system in development: novel signal transduction translates multiple extracellular signals into neural cell growth and differentiation. Perspect. Dev. Neurobiol. 1998;5:337–449. [PubMed] [Google Scholar]
- 28.Hauser KF, Stiene-Martin A, Mattson MP, Elde RP, Ryan SE, Godleske CC. μ-Opioid receptor-induced Ca2+ mobilization and astroglial development: Morphine inhibits DNA synthesis and stimulates cellular hypertrophy through a Ca2+-dependent mechanism. Brain Res. 1996;720:191–203. doi: 10.1016/0006-8993(96)00103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofman FM, Dohadwala MM, Wright AD, Hinton DR, Walker SM. Exogenous tat protein activates central nervous system-derived endothelial cells. J. Neuroimmunol. 1994;54:19–28. doi: 10.1016/0165-5728(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 30.Holden CP, Nath A, Haughey NJ, Geiger JD. Involvement of Na+/H+ exchangers, Ca2+ channels, and excitatory amino acid receptors in intracellular Ca2+ responses to HIV-1 gp120 in cultured human fetal brain cells. Neuroscience. 1999;91:1369–1378. doi: 10.1016/s0306-4522(98)00714-3. [DOI] [PubMed] [Google Scholar]
- 31.Hudson L, Liu J, Nath A, Narayan O, Male D, Jones M, Everall I. Detection of human immunodeficiency virus regulatory protein tat in CNS tissues. J. Neurovirol. 2000;6:145–155. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- 32.Jaffe JH. Drug addition and drug abuse. In: Gilman AG, Goodman LS, Rall TW, Murad F, editors. The Pharmacological Basis of Therapeutics. MacMillan Pub. Co.; New York: 1985. pp. 491–531. [Google Scholar]
- 33.Knapp PE, Maderspach K, Hauser KF. Endogenous opioid system in developing normal and jimpy oligodendrocytes: μ and κ opioid receptors mediate differential mitogenic and growth responses. Glia. 1998;22:189–201. doi: 10.1002/(sici)1098-1136(199802)22:2<189::aid-glia10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 34.Kofke WA, Garman RH, Garman R, Rose ME. Opioid neurotoxicity: fentanyl-induced exacerbation of cerebral ischemia in rats. Brain Res. 1999;818:326–334. doi: 10.1016/s0006-8993(98)01228-1. [DOI] [PubMed] [Google Scholar]
- 35.Kofke WA, Garman RH, Stiller RL, Rose ME, Garman R. Opioid neurotoxicity: Fentanyl dose-response effects in rats. Anesth. Analg. 1996;83:1298–1306. doi: 10.1097/00000539-199612000-00029. [DOI] [PubMed] [Google Scholar]
- 36.Kolson DL, Lavi E, Gonzalez-Scarano F. The effects of human immunodeficiency virus in the central nervous system. Adv Virus Res. 1998;50:1–47. doi: 10.1016/s0065-3527(08)60804-0. [DOI] [PubMed] [Google Scholar]
- 37.Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 38.Kruman II, Nath A, Maragos WF, Chan SL, Jones M, Rangnekar VM, Jakel RJ, Mattson MP. Evidence that Par-4 participates in the pathogenesis of HIV encephalitis. Am. J. Pathol. 1999;155:39–46. doi: 10.1016/S0002-9440(10)65096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp. Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- 40.Lipton SA. HIV-related neuronal injury. Potential therapeutic intervention with calcium channel antagonists and NMDA antagonists. Mol. Neurobiol. 1994;8:181–196. doi: 10.1007/BF02780669. [DOI] [PubMed] [Google Scholar]
- 41.Lotsch J, Weiss M, Ahne G, Kobal G, Geisslinger G. Pharmacokinetic modeling of M6G formation after oral administration of morphine in healthy volunteers. Anesthesiology. 1999;90:1026–1038. doi: 10.1097/00000542-199904000-00016. [DOI] [PubMed] [Google Scholar]
- 42.Lotsch J, Weiss M, Kobal G, Geisslinger G. Pharmacokinetics of morphine-6-glucuronide and its formation from morphine after intravenous administration. Clin. Pharmacol. Ther. 1998;63:629–639. doi: 10.1016/S0009-9236(98)90086-8. [DOI] [PubMed] [Google Scholar]
- 43.Ma M, Nath A. Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J. Virol. 1997;71:2495–2499. doi: 10.1128/jvi.71.3.2495-2499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magnuson DS, Knudsen BE, Geiger JD, Brownstone RM, Nath A. Human immunodeficiency virus type 1 tat activates non-N-methyl-D- aspartate excitatory amino acid receptors and causes neurotoxicity. Ann. Neurol. 1995;37:373–380. doi: 10.1002/ana.410370314. [DOI] [PubMed] [Google Scholar]
- 45.Maneckjee R, Minna JD. Opioids induce while nicotine suppresses apoptosis in human lung cancer cells. Cell Growth Differ. 1994;5:1033–1040. [PubMed] [Google Scholar]
- 46.Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: An in situ hybridization study. J. Comp. Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- 47.Mayne M, Bratanich AC, Chen P, Rana F, Nath A, Power C. HIV-1 tat molecular diversity and induction of TNF-alpha: implications for HIV-induced neurological disease. Neuroimmunomodulation. 1998;5:184–192. doi: 10.1159/000026336. [DOI] [PubMed] [Google Scholar]
- 48.Meriney SD, Ford MJ, Oliva D, Pilar G. Endogenous opioids modulate neuronal survival in the developing avian ciliary ganglion. J. Neurosci. 1991;11:3705–3717. doi: 10.1523/JNEUROSCI.11-12-03705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meriney SD, Gray DB, Pilar G. Morphine-induced delay of normal cell death in the avian ciliary ganglion. Science. 1985;228:1451–1453. doi: 10.1126/science.2990029. [DOI] [PubMed] [Google Scholar]
- 50.Munis JR, Kornbluth RS, Guatelli JC, Richman DD. Ordered appearance of human immunodeficiency virus type 1 nucleic acids following high multiplicity infection of macrophages. J. Gen. Virol. 1992;73:1899–1906. doi: 10.1099/0022-1317-73-8-1899. [DOI] [PubMed] [Google Scholar]
- 51.Nair MP, Schwartz SA, Polasani R, Hou J, Sweet A, Chadha KC. Immunoregulatory effects of morphine on human lymphocytes. Clin. Diagn. Lab Immunol. 1997;4:127–132. doi: 10.1128/cdli.4.2.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nath A, Booze RM, Hauser KF, Mactutus CF, Bell JE, Maragos WF, Berger JR. Critical questions for neuroscientists in interactions of drugs of abuse and HIV infection. NeuroAIDS. 1999 Nov;2 ( www.sciencemag.org/NAIDS) [Google Scholar]
- 53.Nath A, Geiger J. Neurobiological aspects of human immunodeficiency virus infection: neurotoxic mechanisms. Prog. Neurobiol. 1998;54:19–33. doi: 10.1016/s0301-0082(97)00053-1. [DOI] [PubMed] [Google Scholar]
- 54.Nath A, Geiger J, Mattson M, Magnuson D, Jones M, Berger J. Role of viral proteins in neuropathogenesis of HIV infection with emphasis on Tat. NeuroAIDS. 1998 Oct;1 ( www.sciencemag.org/NAIDS) [Google Scholar]
- 55.Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, Geiger JD. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J. Virol. 1996;70:1475–1480. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II Neuropathology. Ann. Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- 57.Nestler EJ. Under siege: The brain on opiates. Neuron. 1996;16:897–900. doi: 10.1016/s0896-6273(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 58.New DR, Ma M, Epstein LG, Nath A, Gelbard HA. Human immunodeficiency virus type 1 Tat protein induces death by apoptosis in primary human neuron cultures. J. Neurovirol. 1997;3:168–173. doi: 10.3109/13550289709015806. [DOI] [PubMed] [Google Scholar]
- 59.Nicholson C, Sykova E. Extracellular space structure revealed by diffusion. Trends Neurosci. 1998;21:207–215. doi: 10.1016/s0166-2236(98)01261-2. [DOI] [PubMed] [Google Scholar]
- 60.Opanashuk LA, Hauser KF. Opposing actions of the EGF family and opioids: Heparin binding-epidermal growth factor (HB-EGF) protects mouse cerebellar neuroblasts against the antiproliferative effect of morphine. Brain Res. 1998;804:87–94. doi: 10.1016/s0006-8993(98)00647-7. [DOI] [PubMed] [Google Scholar]
- 61.Papavasiliou AK, Mehler MF, Dobrenis K, Marmur R, Mabie PC, Kessler JA. Microglial lineage species are expressed in mammalian epidermal growth factor-generated embryonic neurospheres. J. Neurosci. Res. 1996;46:49–57. doi: 10.1002/(SICI)1097-4547(19961001)46:1<49::AID-JNR7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 62.Polakiewicz RD, Schieferl SM, Gingras AC, Sonenberg N, Comb MJ. mu-Opioid receptor activates signaling pathways implicated in cell survival and translational control. J. Biol. Chem. 1998;273:23534–23541. doi: 10.1074/jbc.273.36.23534. [DOI] [PubMed] [Google Scholar]
- 63.Riedy MC, Muirhead KA, Jensen CP, Stewart CC. Use of a photolabeling technique to identify nonviable cells in fixed homologous or heterologous cell populations. Cytometry. 1991;12:133–139. doi: 10.1002/cyto.990120206. [DOI] [PubMed] [Google Scholar]
- 64.Robert-Guroff M, Popovic M, Gartner S, Markham P, Gallo RC, Reitz MS. Structure and expression of tat-, rev-, and nef-specific transcripts of human immunodeficiency virus type 1 in infected lymphocytes and macrophages. J. Virol. 1990;64:3391–3398. doi: 10.1128/jvi.64.7.3391-3398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shutt DC, Soll DR. HIV-induced T-cell syncytia release a two component T-helper cell chemoattractant composed of Nef and Tat. J. Cell Sci. 1999;112:3931–3941. doi: 10.1242/jcs.112.22.3931. [DOI] [PubMed] [Google Scholar]
- 66.Singhal PC, Kapasi AA, Reddy K, Franki N, Gibbons N, Ding G. Morphine promotes apoptosis in Jurkat cells. J. Leukoc. Biol. 1999;66:650–658. doi: 10.1002/jlb.66.4.650. [DOI] [PubMed] [Google Scholar]
- 67.Singhal PC, Reddy K, Franki N, Sanwal V, Gibbons N. Morphine induces splenocyte apoptosis and enhanced mRNA expression of cathepsin-B. Inflammation. 1997;21:609–617. doi: 10.1023/a:1027334122387. [DOI] [PubMed] [Google Scholar]
- 68.Singhal PC, Sagar S, Reddy K, Sharma P, Ranjan R, Franki N. HIV-1 gp120 envelope protein and morphine-tubular cell interaction products modulate kidney fibroblast proliferation. J. Investig. Med. 1998;46:243–248. [PubMed] [Google Scholar]
- 69.Singhal PC, Sharma P, Kapasi AA, Reddy K, Franki N, Gibbons N. Morphine enhances macrophage apoptosis. J. Immunol. 1998;160:1886–1893. [PubMed] [Google Scholar]
- 70.Smith MT, Wright AW, Williams BE, Stuart G, Cramond T. Cerebrospinal fluid and plasma concentrations of morphine, morphine-3-glucuronide, and morphine-6-glucuronide in patients before and after initiation of intracerebroventricular morphine for cancer pain management. Anesth. Analg. 1999;88:109–116. [PubMed] [Google Scholar]
- 71.Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces. An immunocytochemical study in the central nervous system. Dev. Biol. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- 72.Stefano GB. Substance abuse and HIV-gp120: are opiates protective? Arch. Immunol. Ther. Exp. (Warsz.) 1999;47:99–106. [PubMed] [Google Scholar]
- 73.Stiene-Martin A, Zhou R, Hauser KF. Regional, developmental, and cell cycle-dependent differences in μ, δ, and kappa opioid receptor expression among cultured mouse astrocytes. Glia. 1998;22:249–259. [PMC free article] [PubMed] [Google Scholar]
- 74.Sykova E. The extracellular space in the CNS: Its regulation, volume and geometry in normal and pathological neuronal function. Neuroscientist. 1997;3:28–41. [Google Scholar]
- 75.Tucker LM, Morton AJ. A simple method for quantifying changes in neuronal populations in primary cultures of dissociated rat brain. J. Neurosci. Methods. 1995;59:217–223. doi: 10.1016/0165-0270(94)00207-w. [DOI] [PubMed] [Google Scholar]
- 76.Wang H, Gracy KN, Pickel VM. Mu-opioid and NMDA-type glutamate receptors are often colocalized in spiny neurons within patches of the caudate-putamen nucleus. J. Comp Neurol. 1999;412:132–146. doi: 10.1002/(sici)1096-9861(19990913)412:1<132::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 77.Westendorp MO, Shatrov VA, Schulze-Osthoff K, Frank R, Kraft M, Los M, Krammer PH, Droge W, Lehmann V. HIV-1 Tat potentiates TNF-induced NF-kappa B activation and cytotoxicity by altering the cellular redox state. Embo. J. 1995;14:546–54. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilkinson KD, Lee K, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- 79.Wolff T, Samuelsson H, Hedner T. Concentrations of morphine and morphine metabolites in CSF and plasma during continuous subcutaneous morphine administration in cancer pain patients. Pain. 1996;68:209–216. doi: 10.1016/s0304-3959(96)03102-8. [DOI] [PubMed] [Google Scholar]
- 80.Yin DL, Ren XH, Zheng ZL, Pu L, Jiang LZ, Ma L, Pei G. Etorphine inhibits cell growth and induces apoptosis in SK-N-SH cells: involvement of pertussis toxin-sensitive G proteins. Neurosci. Res. 1997;29:121–127. doi: 10.1016/s0168-0102(97)00080-1. [DOI] [PubMed] [Google Scholar]