Abstract
HIV epidemiology informs prevention trial design and program planning. Nine clinical research centers (CRC) in sub-Saharan Africa conducted HIV observational epidemiology studies in populations at risk for HIV infection as part of an HIV prevention and vaccine trial network. Annual HIV incidence ranged from below 2% to above 10% and varied by CRC and risk group, with rates above 5% observed in Zambian men in an HIV-discordant relationship, Ugandan men from Lake Victoria fishing communities, men who have sex with men, and several cohorts of women. HIV incidence tended to fall after the first three months in the study and over calendar time. Among suspected transmission pairs, 28% of HIV infections were not from the reported partner. Volunteers with high incidence were successfully identified and enrolled into large scale cohort studies. Over a quarter of new cases in couples acquired infection from persons other than the suspected transmitting partner.
Introduction
The African continent is disproportionately affected by the HIV pandemic. Despite representing only 12% of the world’s population, sub-Saharan Africa bears nearly 70% of the world’s cases of HIV [1] and the epidemic there is complex [2]. The greatest diversity of HIV subtypes is found across Africa, primarily subtypes A, C, D and their recombinants, and this varies by region [3]. Modes of transmission and affected populations are equally diverse, and can change over time. While the epidemic is primarily heterosexual [4], other key populations including men who have sex with men [5] may also contribute to HIV transmission regionally. Policy makers and clinical trialists depend on epidemiology data to guide effective prevention planning, service delivery [6, 7] and design and conduct of prevention trials [2, 8, 9].
HIV surveillance systems have often relied on cross sectional surveys of HIV infection [10]. While prevalence data may provide some indication of risk groups and can provide guidance for service delivery, they fail to identify recently infected persons. Though there is some progress being made on assays to estimate incidence from prevalent samples [11], these assays have yet to be proven valid, particularly in the African context [12, 13]. Furthermore, the increasing availability of antiretroviral therapy throughout Africa is allowing persons with HIV to live longer, making prevalence data even less relevant [14]. Research towards understanding and preventing HIV transmission should ideally focus on incident cases of HIV. Large-scale HIV incidence cohorts remain the gold standard for collecting these data.
Beginning in 1999 the International AIDS Vaccine Initiative (IAVI) undertook the funding and development of in-country laboratory and clinical capacity at nine research centers in Kenya, Uganda, Rwanda, Zambia and South Africa to support HIV vaccine trials. This work included observational epidemiology studies designed to recruit key populations suitable for trials, provide critical experience to the research teams, and inform the design of future HIV prevention trials. In 2004 this network began multiple, large-scale studies of HIV prevalence, incidence and early infection. Here we present the findings of these observational epidemiology studies.
Methods
Clinical Research Network
This work was conducted at nine sites in South Africa, Zambia, Kenya, Uganda and Rwanda (Fig. 1). All clinical research centers (CRC) are affiliated with existing institutions, locally staffed and most are led by local clinician-scientists who are recognized experts on HIV. To ensure that studies are conducted according to the International Conference on Harmonization Good Clinical Practices (ICH-GCP), GCP training is provided. HIV testing and counseling training and support are provided to counseling staff through a continuous improvement process. Specialized training addresses the needs of key populations such as men who have sex with men [15] or married/cohabiting couples [16, 17]. Study clinics are equipped to provide some care to volunteers (e.g., diagnosis and treatment of sexually transmitted diseases) and local health care resources available for referral have been assessed and strengthened where possible [18].
Figure 1. Map of the collaborating research centers of the IAVI Africa HIV Prevention Partnership.
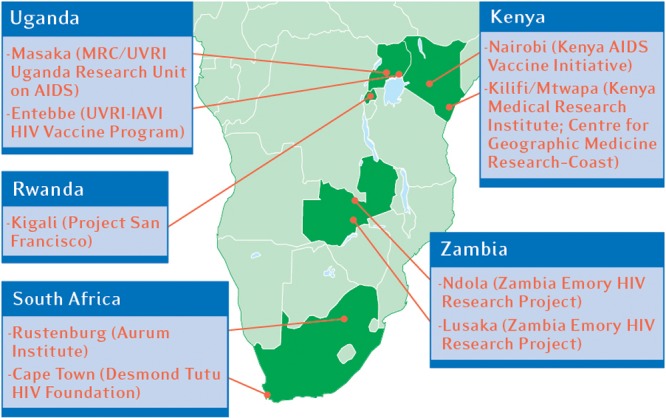
MRC/UVRI: Medical Research Council/Uganda Virus Research Institute, IAVI: International AIDS Vaccine Initiative
Laboratory facilities
All laboratories are equipped to perform tests for HIV antibody (rapid test for HIV, HIV ELISA, and p24 ELISA), syphilis and pregnancy. To assure assay comparability across CRC, quality assurance and control (QA/QC) is performed by Clinical Laboratory Services in Johannesburg, South Africa. Laboratory personnel are trained in GCLP, and 8 of the nine research centers in the network have now received GCLP accreditation through Qualogy (www.qualogy.co.uk). The Rustenburg CRC did not adopt PBMC processing facilities, instead sending sample to Johannesburg for processing, and therefore never qualified for GCLP accreditation. Three labs were accredited in 2007 (Nairobi, Kilifi, Entebbe), one in 2008 (Kigali), one in 2009 (Lusaka), one in 2010 (Ndola) and one in 2011 (Masaka).
HIV prevalence studies
Three CRCs (Nairobi, Masaka and Kilifi) enrolled 1,000–2,000 study volunteers, 18 years or older, for a single visit to characterize local HIV prevalence and risk factors. HIV VCT employed two rapid tests (typically Determine and Unigold; with tiebreakers Murex in Masaka, Vironostika in Nairobi, and Instascreen in Kilifi). A brief, site-specific, demographic and risk assessment questionnaire was administered. In Nairobi, volunteers included sex workers and their clients. In Masaka, the goal was to obtain a population-based estimate of prevalence and therefore recruitment included all residents of three rural communities, recruited and provided with HIV counseling and results at their homes. Home based counseling included pre-test counseling and sample draw in the home, with delivery of results within 1–4 weeks. Subsequent testing and counseling (in the incidence studies, below) was done in a local research clinic. In Kilifi, study volunteers were recruited from three populations: 1) randomly selected residents of the Kilifi Demographic Surveillance System (DSS), 2) walk-in VCT clients at the study site, and 3) the referred sexual partners of DSS and walk-in volunteers.
HIV incidence studies
Methods for each cohort have been published separately (Table 1). Briefly, each CRC performed community mapping, community-sensitization exercises, and/or prevalence studies, and based on the results and their own area(s) of expertise, each team selected risk screening criteria to enroll key populations. Teams reviewed enrollment criteria and HIV incidence in real time to confirm enrollment of true key populations. The cohorts included: 1) HIV negative partners of co-habiting, sexually active couples with an HIV infected, antiretroviral therapy (ART)-naïve partner (Zambia; Rwanda; Entebbe, Uganda) or irrespective of ART status (Masaka, Uganda); 2) sexually active sex workers and their clients (Kenya); 3) members of fishing communities aged 13–49 years and reporting at least one of the following in the past 3 months: more than one partner, a new partner, a sexually transmitted infection (STI), absence from home for more than 2 nights, regular sex partner who is HIV infected (Uganda; in January 2012, criteria were modified to ages 18–49 years, ART-naïve, report of alcohol/recreational drug use, report of unprotected sex; more than one partner was dropped); 4) Men who have sex with men (MSM; Kenya and Rustenburg); 4) younger participants recruited in Cape Town (≥16 years) and in the first fishing community study (Uganda, ≥13 years); and 5) geography as a screening criterion, as in the recruitment of adult members of several study villages (Masaka) and women living in peri-urban, economically disadvantaged regions with high HIV prevalence (Rustenburg).
Table 1. HIV prevalence and multivariate predictors of prevalent HIV infection in Masaka, Kilifi and Nairobi.
| Masaka | Kilifi | Nairobi Total | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | HIV | Total | HIV | Total | HIV | ||||||||||||||||
| Characteristic | N | % | N | % | PR | p value | 95% CI | N | % | N | % | PR | p value | 95% CI | N | % | N | % | PR | p value | 95% CI |
| Total | 1663 | 100 | 187 | 11.2 | -- | 1769 | 100 | 146 | 8.3 | -- | 996 | 100 | 163 | 16.4 | -- | ||||||
| Volunteer sex | |||||||||||||||||||||
| Male | 650 | 39.1 | 56 | 8.6 | ref. | 726 | 41.0 | 39 | 5.4 | ref. | 296 | 29.7 | 20 | 6.8 | ref. | ||||||
| Female | 1013 | 60.9 | 131 | 12.9 | 1.80 | 0.001 | (1.28, 2.53) | 1043 | 59.0 | 107 | 10.3 | 1.65 | 0.007 | (1.15. 2.38) | 700 | 70.3 | 143 | 20.4 | 3.05 | <0.001 | (1.95, 4.78) |
| Volunteer age (years) | |||||||||||||||||||||
| 18–24 | 425 | 25.6 | 30 | 7.1 | ref. | 596 | 33.7 | 29 | 4.9 | ref. | 287 | 28.8 | 14 | 4.9 | ref. | ||||||
| 25–29 | 311 | 18.7 | 29 | 9.3 | 1.34 | 0.230 | (0.83, 2.18) | 356 | 20.1 | 38 | 10.7 | 1.75 | 0.016 | (1.11, 2.76) | 221 | 22.2 | 37 | 16.7 | 3.38 | <0.001 | (1.89, 6.06) |
| 30–34 | 261 | 15.7 | 43 | 16.5 | 2.39 | <0.001 | (1.55, 3.69) | 326 | 18.4 | 32 | 9.8 | 1.58 | 0.065 | (0.97, 2.56) | 150 | 15.1 | 36 | 24.0 | 4.83 | <0.001 | (2.71, 8.62) |
| 35+ | 666 | 40.0 | 85 | 12.8 | 1.99 | 0.001 | (1.33, 2.97) | 491 | 27.8 | 47 | 9.6 | 1.37 | 0.183 | (0.86, 2.19 | 338 | 33.9 | 76 | 22.5 | 4.44 | <0.001 | (2.58, 7.65) |
| Marital status | |||||||||||||||||||||
| Single, never married | 274 | 16.5 | 30 | 10.9 | NS | 461 | 26.1 | 18 | 3.9 | ref. | 521 | 52.3 | 68 | 13.1 | NS | ||||||
| Divorced or widowed | 239 | 14.4 | 53 | 22.2 | 167 | 9.4 | 43 | 25.7 | 3.56 | <0.001 | (2.02, 6.29) | 385 | 38.7 | 84 | 21.8 | ||||||
| Married | 1150 | 69.2 | 104 | 9.0 | 1141 | 64.5 | 85 | 7.4 | NA | -- | -- | 90 | 9.0 | 11 | 12.2 | ||||||
| Married monogamous | NA | -- | -- | -- | 985 | 55.7 | 64 | 6.5 | 1.13 | 0.646 | (0.66, 1.94) | NA | -- | -- | -- | ||||||
| Married polygamous | NA | -- | -- | -- | 156 | 8.8 | 21 | 13.5 | 2.27 | 0.011 | (1.21, 4.28) | NA | -- | -- | -- | ||||||
| Location of home village | |||||||||||||||||||||
| Distal from paved road | 1286 | 77.3 | 128 | 10.0 | ref. | NA | -- | -- | -- | NA | -- | -- | -- | ||||||||
| Adjacent to paved road | 377 | 22.7 | 59 | 15.6 | 1.54 | 0.004 | (1.15, 2.06) | NA | -- | -- | -- | NA | -- | -- | -- | ||||||
| Location of current residence | |||||||||||||||||||||
| Rural | NA | -- | -- | -- | 171 | 9.7 | 4 | 2.3 | ref. | NA | -- | -- | -- | ||||||||
| Urban | NA | -- | -- | -- | 1598 | 90.3 | 142 | 8.9 | 4.06 | 0.005 | (1.52, 10.85) | NA | -- | -- | -- | ||||||
| Source of study volunteers | |||||||||||||||||||||
| Randomly selected from community | NA | -- | -- | -- | 821 | 46.4 | 60 | 7.3 | NS | NA | -- | -- | -- | ||||||||
| Walk-in VCT clients | NA | -- | -- | -- | 315 | 17.8 | 35 | 11.1 | NA | -- | -- | -- | |||||||||
| Partners of above volunteers | NA | -- | -- | -- | 633 | 35.8 | 51 | 8.1 | NA | -- | -- | -- | |||||||||
| Reported condom use * | |||||||||||||||||||||
| None of the time | 1127 | 67.8 | 114 | 10.1 | ref. | NA | -- | -- | -- | NA | -- | -- | -- | ||||||||
| Half or more of the time | 167 | 10.0 | 17 | 10.2 | 1.06 | 0.809 | (0.66, 1.71) | NA | -- | -- | -- | NA | -- | -- | -- | ||||||
| Less than half of the time | 369 | 22.2 | 56 | 15.2 | 1.54 | 0.006 | (1.13, 2.08) | NA | -- | -- | -- | NA | -- | -- | -- | ||||||
| Never | NA | -- | -- | -- | 1095 | 61.9 | 96 | 8.8 | NS | NA | -- | -- | -- | ||||||||
| Sometimes | NA | -- | -- | -- | 342 | 19.3 | 21 | 6.1 | NA | -- | -- | -- | |||||||||
| Always | NA | -- | -- | -- | 108 | 6.1 | 6 | 5.6 | NA | -- | -- | -- | |||||||||
| Not asked | NA | -- | -- | -- | 224 | 12.7 | 23 | 10.3 | NA | -- | -- | -- | |||||||||
| No | NA | -- | -- | -- | NA | -- | -- | -- | 163 | 16.4 | 17 | 10.4 | ref. | ||||||||
| Yes | NA | -- | -- | -- | NA | -- | -- | -- | 833 | 83.6 | 146 | 17.5 | 1.64 | 0.036 | (1.03, 2.59) | ||||||
| Sexually transmitted diseases reported in past 12 months (Masaka) or ever (Kilifi, Nairobi) | |||||||||||||||||||||
| None | 1000 | 60.1 | 85 | 8.5 | ref. | 1100 | 62.2 | 56 | 5.1 | ref. | NA | -- | -- | -- | |||||||
| Urethral discharge | 180 | 10.8 | 16 | 8.9 | 0.91 | 0.725 | (0.55, 1.51) | 556 | 31.4 | 73 | 13.1 | 1.24 | 0.202 | (0.89, 1.72) | NA | -- | -- | -- | |||
| Genital ulcers | 264 | 15.9 | 40 | 15.2 | 1.57 | 0.010 | (1.11, 2.22) | 280 | 15.8 | 55 | 19.6 | 2.48 | <0.001 | (1.79, 3.43) | NA | -- | -- | -- | |||
| Both ulcers and discharge | 219 | 13.2 | 46 | 21.0 | 2.16 | <0.001 | (1.56, 3.01) | NA ** | -- | -- | -- | NA | -- | -- | -- | ||||||
| No | NA | -- | -- | -- | NA | -- | -- | -- | 642 | 64.5 | 102 | 15.9 | NS | ||||||||
| Yes (no details collected) | NA | -- | -- | -- | NA | -- | -- | -- | 354 | 35.5 | 61 | 17.2 | |||||||||
| Reported current substance use | |||||||||||||||||||||
| None | NA | -- | -- | -- | NA | -- | -- | -- | 444 | 44.6 | 78 | 17.6 | ref. | ||||||||
| Alcohol | NA | -- | -- | -- | NA | -- | -- | -- | 547 | 54.9 | 85 | 15.5 | NS | ||||||||
| Marijuana | NA | -- | -- | -- | NA | -- | -- | -- | 85 | 8.5 | 14 | 16.5 | 1.99 | 0.002 | (1.28, 3.08) | ||||||
| Number and type of reported sexual partners in past 12 months (Masaka, Kilifi) or 7 days (Nairobi) | |||||||||||||||||||||
| None | 229 | 13.8 | 41 | 17.9 | ref. | 326 | 18.4 | 34 | 10.4 | NS | 109 | 10.9 | 21 | 19.3 | NS | ||||||
| one steady partner | 1118 | 67.2 | 102 | 9.1 | 0.55 | <0.001 | (0.40, 0.77) | 764 | 43.2 | 61 | 8.0 | 140 | 14.1 | 16 | 11.4 | ||||||
| casual or >1 partner | 316 | 19.0 | 44 | 13.9 | 0.98 | 0.910 | (0.63, 1.51) | 241 | 13.6 | 24 | 10.0 | 747 | 75.0 | 126 | 16.9 | ||||||
| Not asked | 0 | -- | 0 | -- | 438 | 24.8 | 27 | 6.2 | 0 | -- | 0 | -- | |||||||||
| Report of more than one current sexual partner *** | |||||||||||||||||||||
| No, only one | NA | -- | -- | -- | NA | -- | -- | -- | 37 | 3.7 | 4 | 10.8 | NS | ||||||||
| Yes, more than one | NA | -- | -- | -- | NA | -- | -- | -- | 963 | 96.7 | 159 | 16.5 | |||||||||
PR: Prevalence Ratio, CI: Confidence Interval, ref: reference group, NA: data were not collected at this CRC, NS: data were not significant predictors of prevalent HIV in multivariate modeling
* In Masaka condom use was measured with the question: Have you and your partner ever used a condom (yes/no), and if so, how often do you use a condom (Always, more than half of the time, about half of the time, rarely or less than half of the time)? In Kilifi: In the past 12 months, have you used a condom never, sometimes or always during sex? In Kangemi: Have you used a condom before (yes/no)?
** Due to collinearity in the covariates, model failed to converge with discharge and ulcers considered together
*** In Kangemi, all volunteers were sexually active, but not all volunteers were sexually active in the past 7 days
Volunteers were followed quarterly or monthly. Cohort sizes were selected to provide a robust estimate of HIV incidence in light of the challenges of recruiting marginalized populations, and ranged from 250–1,000 volunteers. At every visit, volunteers were given HIV counseling and testing by both rapid test and p24 ELISA to detect HIV infection prior to antibody seroconversion (see references in Table 2). Rapid tests were typically performed by venipuncture and done in parallel. Condoms were provided, as was lubricant for the cohorts where anal sex was prevalent. Sexual behavior questionnaires were administered quarterly in Kenya, South Africa, Rwanda and Zambia; and every six months in Masaka. Cohorts with HIV incidence persistently <2% are not presented here (e.g., [19, 20]). Volunteers with incident HIV infection were invited to enroll in the early HIV infection study, 2006–2011, and had their CD4 T cell counts and viral load done at regular intervals. These data were used to inform referrals for antiretroviral therapy provided per the national guidelines. CRCs maintained strong relationships with local health care facilities to assure volunteers would be kept aware of and referred to as needed additional care and treatment as it became available (e.g., medical male circumcision) [21].
Table 2. Enrollment and HIV incidence among at-risk volunteers enrolled for HIV prevention preparatory studies stratified by CRC and, where appropriate, sex.
| CRC and study population | Enrollment years | Year of last study visit | Total enrolled | Total PY | HIV cases detected | HIV incidence | 95% CI | p value ** | ref. *** |
|---|---|---|---|---|---|---|---|---|---|
| HIV discordant couples | |||||||||
| Kigali, Rwanda | Stephenson, 2008; Wall, 2012 | ||||||||
| DC male negative | 2002–11 * | 2011 | 972 | 1844 | 64 | 3.5 | (2.7, 4.4) | 0.16 | |
| DC female negative | 2002–11 * | 2011 | 884 | 1705 | 45 | 2.6 | (1.9, 3.5) | ||
| Lusaka, Zambia | Kempf, 2008; Stephenson, 2008 | ||||||||
| DC male negative | 1995–11 * | 2011 | 1656 | 3367 | 226 | 6.7 | (5.9, 7.7) | 0.003 | |
| DC female negative | 1995–11 * | 2011 | 1393 | 2848 | 252 | 8.9 | (7.8, 10.0) | ||
| Ndola, Zambia | Lambdin, 2011 | ||||||||
| DC male negative | 2004–11 | 2011 | 416 | 575 | 35 | 6.1 | (4.2, 8.5) | 0.01 | |
| DC female negative | 2004–11 | 2011 | 335 | 399 | 43 | 10.8 | (7.8, 14.5) | ||
| Masaka, Uganda | Ruzagira, 2011 (a) | ||||||||
| DC male negative | 2006–11 | 2011 | 581 | 1143 | 44 | 3.8 | (2.9, 5.2) | 0.44 | |
| DC female negative | 2006–11 | 2011 | 313 | 514 | 24 | 4.7 | (3.1, 7.0) | ||
| Masaka, Uganda | Ruzagira, 2011 (a) | ||||||||
| DC on ART ∞ | 2006–09 | 2009 | 152 | 271 | 3 | 1.1 | (0, 2.4) | — | |
| Entebbe, Uganda | NA | ||||||||
| DC male negative | 2006–09 | 2010 | 276 | 345 | 5 | 1.4 | (0.6, 3.5) | 0.27 | |
| DC female negative | 2006–09 | 2010 | 322 | 416 | 11 | 2.6 | (1.5, 4.8) | ||
| Men who have sex with men | |||||||||
| Kilifi, Kenya | 2005–2012 ▪ | 2013 ▪ | 726 | 996 | 73 | 7.3 | (5.8–9.2) | — | Price, 2012; Sanders, 2013 |
| Nairobi, Kenya | 2006–09 | 2010 | 303 | 338 | 19 | 5.6 | (3.6, 8.8) | — | Price, 2012 |
| Rustenburg, South Africa | 2009–10 | 2011 | 29 | 21 | 2 | 9.5 | (2.4, 38.0) | — | NA |
| At-risk women | |||||||||
| Cape Town, South Africa | Price, 2012 | ||||||||
| Younger women ▪▪ | 2006–7 | 2008 | 337 | 284 | 8 | 2.8 | (1.4, 5.6) | — | |
| Rustenburg, South Africa | |||||||||
| Women, clinic and community based | 2008–9 | 2009 | 223 | 67 | 2 | 3.0 | (0.4, 10.8) | — | Feldblum, 2012 |
| Peripheral community residents | 2010–11 | 2012 | 323 | 213 | 19 | 8.9 | (5.7, 14.0) | — | NA |
| Kilifi, Kenya | Price, 2012 | ||||||||
| Female sex workers | 2005–2010 | 2012 | 315 | 552 | 13 | 2.4 | (1.4, 4.1) | — | |
| Fishing Community members | |||||||||
| Masaka, Uganda | NA | ||||||||
| Male | 2012–13 | 2013 ▪ | 327 | 244 | 13 | 5.3 | (3.1, 9.2) | 0.10 | |
| Female | 2012–13 | 2013 ▪ | 217 | 139 | 14 | 10.1 | (6.0, 17.0) | ||
| Masaka and Entebbe, Uganda | Seely, 2012 | ||||||||
| Male | 2009–10 | 2011 | 450 | 652 | 34 | 4.5 | (3.1, 6.7) | 0.59 | |
| Female | 2009–10 | 2011 | 550 | 554 | 25 | 5.2 | (3.7, 7.3) |
* IAVI support initiated in 2004
** p value for the incident rate ratio calculator comparing HIV incidence by sex, where study populations are stratified by sex
*** For additional details on recruitment, demographics, follow up and earlier reports of HIV incidence. With the exception of Feldblum 2012, all the data above and in Table 3 represents new analyses and additional follow up time.
▪ Cohort enrollment currently open
▪▪ Recruited women from 16 years old; the only cohort to recruit <18 year olds
∞ HIV positive partner in the DC cohort is on ART, too few to analyze by volunteer sex (87 negative men, 65 women)
PY: Person years, IQR: Interquartile range, CI: Confidence Interval, CRC: Clinical Research Center, DC: Discordant Couple (reported sex applies to enrolled HIV uninfected volunteers, HIV infected volunteers were all ART-naive except where noted), NA: reference not available, data not previously published
Early HIV infection study
The Early HIV Infection Study has been described previously [22, 23]. Briefly, volunteers identified with incident HIV infection were invited to enroll as early as possible (typically within 2 months of their estimated date of infection (EDI)) and follow-up is ongoing. Viral subtype was determined by sequencing the pol region of HIV from an early sample [22]. Suspected transmitting partners were invited to enroll, and molecular epidemiologic linkages were determined by sequence comparison of pol, gag, gp120, gp41, and/or long terminal repeat regions [24].
Data collection and management
Data from the HIV prevalence studies were managed by CRC staff. HIV incidence and early infection study data were managed by the Perinatal HIV Research Unit (PHRU) in Johannesburg, South Africa, except the incidence studies in Rwanda, Zambia, and some in Uganda, which were managed by the CRC staff. Data were transcribed onto a DataFax (Clinical DataFax Systems, Inc., Ontario, Canada) form and digitally faxed to a central server; manual and automatic queries were received then resolved by the CRC data management team. An epidemiologist reviewed the data prior to analysis.
Data analysis
Inferential statistics include chi square or Wilcoxon rank-sum as appropriate. Predictors of prevalent HIV were evaluated separately for different key populations. Covariates were selected to remain in the multivariable model based on a p-value<0.05. Modeling results are shown as p-values, prevalence ratios, and 95% confidence intervals. Comparisons of HIV incidence across CRC or by gender were done by Cox Proportional Hazards modeling; analyses comparing HIV incidence across time was done using the Stata incidence rate ratio calculator. Only those cohorts with recruitment spread over > 3 years and with adequate follow-up (>400 PY) were analyzed by year. Estimates that vary from published results are due to additional follow-up time included in this report. Data analysis was done with Stata (v12, College Park, TX, USA).
Ethical approval of clinical research
Prior to enrollment, all volunteers underwent an informed consent procedure and written informed consent was documented. Ethical approvals in each country were obtained from the Kenya Medical Research Institute Ethical Review Committee, the Kenyatta National Hospital Ethical Review Committee of the University of Nairobi, the Rwanda National Ethics Committee, the Uganda Virus Research Institute Science and Ethics Committee, the Uganda National Council of Science and Technology, the University of Cape Town Health Science Research and Ethics Committee, the University of Zambia Research Ethics Committee, the Bio-Medical Research Ethics Committee at the University of KwaZulu Natal, and the Emory University Institutional Review Board.
Results
1. HIV prevalence and associated risk factors in Masaka, Nairobi and Kilifi
From January to December 2004, 4,428 volunteers were enrolled at three CRCs in a cross-sectional study of HIV prevalence. Prevalence varied from 8% to 16%; women and older volunteers had a significantly higher HIV prevalence at each CRC (Table 1). Marital status was associated with HIV infection only at Kilifi, with divorced/widowed and polygamously married volunteers two to three times more likely to be HIV infected than volunteers who had never been married. In Masaka, residing in the community situated along the Kampala-Kigali trans-African highway was associated with a 50% higher HIV prevalence compared to two villages off the highway. In Kilifi, residents of urban areas were four times as likely to be HIV infected as were rural volunteers. In Masaka, inconsistent condom use relative to never using a condom was associated with HIV, and in Nairobi report of ever having used a condom was associated with HIV infection. Report of genital ulcers was associated with a 1.5 to 2.5-fold increase in HIV in Masaka and Kilifi. In Nairobi, marijuana use was associated with a two-fold increased likelihood of HIV infection. In Masaka, report of a single, steady partner was inversely associated with HIV infection relative to report of no sexual partners in the past 3 months.
2a. HIV incidence in discordant couples
From 2002 to 2011, 1,856 couples discordant for HIV status were enrolled in Kigali (Table 2). HIV incidence was similar among women and men (p = 0.16). HIV incidence was nearly 2.5 times higher in the first three months of follow-up compared to subsequent visits for men (p = 0.003, Table 3), but for women this only achieved borderline significance. HIV incidence did not drop significantly with calendar year in Kigali (Table 3).
Table 3. HIV incidence stratified by duration of time in study follow up, and (where data were available) by study year.
| CRC and study population | Total PY | HIV cases detected | HIV incidence | 95% CI | p value * |
|---|---|---|---|---|---|
| HIV discordant couples | |||||
| Kigali, Rwanda: male negative | |||||
| First 3 months of follow up | 238 | 17 | 7.2 | (4.2, 11.5) | 0.003 |
| >3 months of follow up | 1615 | 47 | 2.9 | (2.1, 3.9) | |
| 2002–2006 | 947 | 38 | 4.0 | (2.8, 5.5) | 0.20 |
| 2007–2011 | 896 | 26 | 2.9 | (1.9, 4.3) | |
| Kigali, Rwanda: female negative | |||||
| First 3 months of follow up | 219 | 10 | 4.6 | (2.2, 8.4) | 0.08 |
| >3 months of follow up | 1494 | 35 | 2.3 | (1.6, 3.3) | |
| 2002–2006 | 863 | 19 | 2.2 | (1.3, 3.4) | 0.27 |
| 2007–2011 | 842 | 26 | 3.1 | (2.0, 4.5) | |
| Lusaka, Zambia: male negative | |||||
| First 3 months of follow up | 404 | 39 | 9.7 | (6.9, 13.2) | 0.02 |
| >3 months of follow up | 2979 | 187 | 6.3 | (5.4, 7.2) | |
| 2003–2006 | 1152 | 75 | 6.5 | (5.1, 8.2) | 0.67 |
| 2007–2011 | 1058 | 64 | 6.1 | (4.7, 7.7) | |
| Lusaka, Zambia: female negative | |||||
| First 3 months of follow up | 341 | 51 | 15.0 | (11.1, 19.7) | <0.001 |
| >3 months of follow up | 2520 | 201 | 8.0 | (6.9, 9.2) | |
| –2006 | 848 | 91 | 10.7 | (8.6, 13.2) | 0.001 |
| 2007–2011 | 741 | 44 | 5.9 | (4.3, 8.0) | |
| Ndola, Zambia: male negative | |||||
| First 3 months of follow up | 99 | 6 | 6.1 | (2.2, 13.2) | 0.96 |
| >3 months of follow up | 479 | 29 | 6.1 | (4.1, 8.7) | |
| 2004–2007 | 290 | 23 | 7.9 | (5.0, 11.9) | 0.07 |
| 2008–2011 | 284 | 12 | 4.2 | (2.2, 7.4) | |
| Ndola, Zambia: female negative | |||||
| First 3 months of follow up | 79 | 15 | 19.1 | (10.7, 31.5) | 0.02 |
| >3 months of follow up | 322 | 28 | 8.7 | (5.8, 12.6) | |
| 2004–2007 | 168 | 23 | 13.7 | (8.7, 20.5) | 0.14 |
| 2008–2011 | 231 | 20 | 8.7 | (5.3, 13.4) | |
| Masaka, Uganda: male negative (excluding females on ART) | |||||
| First 3 months of follow up | 127 | 3 | 2.4 | (0.8, 7.4) | 0.39 |
| >3 months of follow up | 1017 | 41 | 4.0 | (3.0, 5.5) | |
| 2006–2008 | 442 | 20 | 4.5 | (2.9, 7.0) | 0.36 |
| 2009–2011 | 701 | 24 | 3.4 | (2.3, 5.1) | |
| Masaka, Uganda: female negative (excluding males on ART) | |||||
| First 3 months of follow up | 70 | 4 | 5.8 | (2.2, 15.3) | 0.64 |
| >3 months of follow up | 445 | 20 | 4.5 | (2.9, 7.0) | |
| 2006–2008 | 150 | 10 | 6.7 | (3.6, 12.4) | 0.19 |
| 2009–2011 | 365 | 14 | 3.8 | (2.3, 6.5) | |
| Entebbe, Uganda: male negative | |||||
| First 3 months of follow up | 61 | 2 | 3.3 | (0.8, 13.2) | 0.36 |
| >3 months of follow up | 284 | 4 | 1.4 | (0.5, 3.7) | |
| Entebbe, Uganda: female negative | |||||
| First 3 months of follow up | 72 | 2 | 2.8 | (0.7, 11.1) | 0.89 |
| >3 months of follow up | 344 | 9 | 2.6 | (1.4, 5.0) | |
| Men who have sex with men | |||||
| Nairobi, Kenya | |||||
| First 3 months of follow up | 64 | 5 | 7.8 | (3.2, 18.7) | 0.42 |
| >3 months of follow up | 274 | 14 | 5.1 | (3.0, 8.6) | |
| Kilifi, Kenya | |||||
| First 3 months of follow up | 94 | 15 | 16.0 | (9.6, 26.5) | <0.001 |
| >3 months of follow up | 829 | 43 | 5.2 | (3.8, 7.0) | |
| 2005–2008 | 304 | 25 | 8.2 | (5.6–12.2) | 0.49 |
| 2009–2012 | 692 | 48 | 6.9 | (5.2–9.2) | |
| At-risk women | |||||
| Cape Town, South Africa: Sexually active women | |||||
| First 3 months of follow up | 76 | 4 | 5.2 | (2.0, 14.0) | 0.18 |
| >3 months of follow up | 207 | 4 | 1.9 | (0.7, 5.1) | |
| Rustenburg, South Africa: Sexually active women | |||||
| First 3 months of follow up | 65 | 8 | 12.3 | (6.2, 24.7) | 0.28 |
| >3 months of follow up | 149 | 11 | 7.4 | (4.1, 13.4) | |
| Fishing Community members | |||||
| Masaka, Uganda (2012–2013 cohort) | |||||
| First 3 months of follow up | 119 | 9 | 7.6 | (3.9,14.6) | 0.25 |
| >3 months of follow up | 383 | 18 | 4.7 | (3.0,7.5) | |
| Masaka & Entebbe, Uganda (2009–2010 cohort) | |||||
| First 6 months of follow up ** | 417 | 23 | 5.5 | (3.7, 8.3) | 0.28 |
| >6 months of follow up | 803 | 33 | 4.1 | (2.9, 5.8) | |
* Comparing HIV incidence in the first three months to HIV incidence in subsequent months of follow up and HIV incidence in the first set of calendar years indicated to the subsequent set of years, where data were available
** First return visit for this cohort was at six months post enrollment
From 1995 (2004 in Ndola) to 2011, 3,800 HIV-discordant couples were enrolled at two CRCs in Zambia (Table 2). HIV incidence was similar across the two CRCs (p = 0.71) and was significantly higher in women compared to men in both Lusaka (p = 0.003) and Ndola (p = 0.01). HIV incidence in the first three months of study follow-up was significantly higher than in subsequent visits for women at both CRCs (Table 3), but the same was true only for men in Lusaka. With the exception of Lusaka women, HIV incidence did not vary significantly by year (Table 3), although a trend was also borderline significant among men in Ndola.
From 2006 through 2011, 1,574 couples were enrolled at both Ugandan CRCs, including 152 couples in Masaka who contributed follow-up while the HIV infected partner was on ART (Table 2). HIV incidence did not vary by sex (Table 2) but was significantly lower in Entebbe than in Masaka (p = 0.02). Masaka was the only CRC that enrolled discordant couples with the positive partner on ART, and HIV incidence was significantly lower in couples on ART compared to those not on ART (p = 0.009). In 2009, enrollment criteria changed in Masaka. There was no evidence of a drop in HIV incidence by time on study (Entebbe and Masaka) or by calendar year (Masaka) (Table 3).
2b. HIV incidence in men who have sex with men
In August 2005, the Kilifi, Kenya, CRC enrolled the first MSM into their HIV incidence cohort study. Through December 2012, 726 MSM have been enrolled in Kilifi and enrollment is ongoing; from January 2006-October 2009, 303 were enrolled in Nairobi; and from April 2009-November 2010, 29 in Rustenburg, South Africa. HIV incidence has been very high in these cohorts, from 5–10 cases/100 person-years (Table 2). Incidence dropped three-fold after the first three months on-study in Kilifi (Table 3, p<0.001); no similar drop was observed in Nairobi. No drop was observed in HIV incidence by calendar time (Kilifi).
2c. HIV incidence in Ugandan fishing communities
Between February and August 2009, 1,000 volunteers were enrolled from five fishing communities near Entebbe and Masaka, Uganda, into an 18-month prospective study. The overall HIV incidence was 4.9 cases/100 PY and did not vary by sex (Table 2) or by recruitment area. No evidence for a reduction in HIV incidence with time on study was observed (Table 3). Relatively low HIV incidence was observed among adolescents (ages 13–17: 2.8 cases/100 PY), however few enrolled (n = 26). Using updated screening criteria based on these results, a further 529 volunteers were enrolled in the Masaka district from January 2012; follow-up is ongoing and HIV incidence is high (6.7 cases/100 PY), particularly among women (Table 2).
2d. HIV incidence in other key populations
Other cohorts include female sex workers (FSW) in Kilifi [20], younger women in Cape Town [20], and women enrolled from the community and local clinics in Rustenburg [25] (Table 2). In 2010, the Rustenburg CRC shifted recruitment strategies into poorer, peripheral communities where HIV prevalence was higher. Eligibility criteria were also changed to include any woman reporting sexual activity or an STI. Over 300 women enrolled in Rustenburg with these eligibility criteria had an HIV incidence of nearly 9 cases/100PY (Table 2).
2. Epidemiologic linkages in the early HIV infection cohort
From February 2006 through December 2011, 613 volunteers with incident HIV infection were enrolled. The cohort comprised 255 (41.5%) women and 359 (58.5%) men, of whom 92 (25.6%) were MSM; all but three from Kenya. Most volunteers (460, 75.0%) were enrolled from HIV-discordant couples cohorts, 219 (47.6%) were women.
For 407 volunteers, suspected transmitting partners were enrolled, 393 (96.6%) of whom had sample to test HIV transmission linkages. The proportion of volunteers linked by HIV sequence similarity to their suspected transmitting partner ranged from 67.1% in the Copperbelt (Kitwe and Ndola) to 100% in Entebbe but these differences did not quite achieve significance (Table 4, p = 0.08, Fisher’s exact test), and they did not vary by the sex of the incident case (p = 0.27). In Kigali, a non-significant trend suggested women with incident HIV were more commonly linked to their partners than were men (36/41 [87.8%] vs. 38/52 [73.1%], respectively, p = 0.08).
Table 4. Number of volunteers with HIV sequence data who enrolled with their suspected partner and were epidemiologically linked.
| Enrolled | Linked | |||
|---|---|---|---|---|
| N | N | % | p value | |
| Total | 393 | 282 | 71.8 | |
| RC and country | ||||
| Kigali, Rwanda | 93 | 74 | 79.6 | 0.08 * |
| Lusaka, Zambia | 145 | 100 | 70.0 | |
| Copperbelt, Zambia | 76 | 51 | 67.1 | |
| Kilifi, Kenya | 4 | 3 | 75.0 | |
| Entebbe, Uganda | 12 | 12 | 100.0 | |
| Masaka, Uganda | 63 | 42 | 66.7 | |
| Sex of incident case | ||||
| Male | 220 | 153 | 69.5 | 0.27 |
| Female | 173 | 129 | 74.6 | |
RC: Research Center
*Fisher’s exact test
Discussion
From 2004 to present, IAVI has coordinated HIV observational epidemiology studies at nine CRCs in preparation for HIV prevention trials. Laboratory, clinical, and data management procedures were standardized to ensure comparability across CRCs. Research protocols typically began with 1) cross-sectional HIV prevalence research, followed by 2) HIV incidence cohorts, and then 3) early HIV infection cohorts. 4,428 volunteers were initially enrolled in Kenya and Uganda, and HIV prevalence was observed to be higher than the national average, even in the general population survey in Masaka. Nine CRCs enrolled over 10,000 HIV-negative volunteers in prospective studies of HIV incidence, which ranged from 2% to 10% annually. This work included the first prospective studies of HIV incidence in African MSM [20, 26]. Over 600 volunteers with incident infection were enrolled from these cohorts into the early HIV infection study. Among those with an identified HIV-positive regular sexual partner, about 28% of HIV transmissions were not from that partner.
Prospective studies remain the gold standard for obtaining reliable HIV incidence data [6] needed for informed decision making, from national level program planning to clinical trial design. However, prospective incidence studies are expensive, time consuming, and require real-time review of data to maintain focus on a study cohort that reflects true risk of HIV infection. While a goal of the 2004 prevalence studies was to guide selection of key populations, some populations with elevated HIV prevalence had low HIV incidence. This included data from rural villages in Masaka district [19] where a previous HIV prevention trial had been conducted [27], heterosexual men in Kenya [20], FSW in Nairobi [20] and HIV discordant couples in Kilifi (no observed HIV incidence, unpublished data). In Masaka, the investigative team shifted focus to HIV discordant couples and, later, to residents of fishing communities. In Kenya the focus changed to MSM when men came forward asking to enroll as sex workers. Among MSM along the coast, the Kilifi team recorded HIV incidence as high as 35 cases/100PY among those who report sex exclusively with other men [26] highlighting both the success of careful epidemiology and the extreme need for intervention. Collateral benefits from our work in MSM and sex workers include the development of health care provider training modules to strengthen service delivery to these marginalized groups [15], and the improvement of our outreach and educational activities within the larger community. Following an attack on our Kilifi clinic that took place in 2010 [28] an emphasis was placed on engaging a wide range of public officials and public health stake holders, from religious leaders to government and police officials, and a community liaison officer has been hired whose role is to manage this process of outreach and education. Each CRC successfully identified key populations both suitable for HIV prevention trials and in need of specialized program planning to reduce HIV transmission [15–17].
Among those cohorts where higher risk was observed, we recorded heterogeneity associated with volunteer sex, time on study, and calendar year. Women are frequently cited as at significant risk for HIV acquisition in Africa [29], and in five of the seven cohorts with men and women the incidence point estimate reflected this (Table 2), although this achieved statistical significance only in Zambia. Of the more than a dozen cohorts presented in Table 3, five (men in discordant couples at Lusaka, Ndola, and Kigali; women in Ndola; and MSM in Kilifi) recorded a reduction in HIV incidence after the first study follow-up visit. While pre-seroconversion HIV infection at enrollment may artificially inflate HIV incidence at the first follow-up visit, we employed the p24 antigen assay at every study visit thereby excluding some volunteers at enrollment prior to antibody seroconversion. Nine cohorts in table 3 had adequate follow-up time to consider HIV incidence as a function of calendar year. Only female members of DCs in Lusaka showed a statistically significant reduction in HIV incidence. Of the 16 cohorts reported in Table 3, 5 demonstrate a significant drop in HIV incidence, 9 show a non-significant drop in the incidence point estimate, 1 shows no change, and 1 shows a non-significant increase in the point estimate. We feel the most likely reason for the drop in HIV incidence was the regular counseling and testing and prevention messages, however, some cohorts were simply not powered to detect this drop (e.g., MSM in Nairobi, women in Cape Town and Rustenburg saw a non-significant drop of ~3 cases per 100 person years. This would otherwise represent a very large drop, but in our case, it remains non-significant in a 200–300 person cohort). Additionally, the different stages of the epidemic in different countries and cohorts may explain why we observe no significant changes in incidence, as perhaps some mature epidemics may be less susceptible to intervention. Cohort recruitment may change subtly over time as new clients seek HIV testing at the CRCs. Secular trends in the source populations such as increased awareness of HIV prevention techniques may also be prevalent. Counseling and prevention messages improve as health care providers get to know their stakeholders better. Treatment and care changes as antiretroviral therapy guidelines change, affecting risk of HIV acquisition in certain populations (e.g., HPTN 052 and the recommendation that ART be initiated in HIV-discordant couples). National level program planning requires incidence trends over time to best assess whether interventions are working or not. Clinical trial design (particularly sample size) should always factor in anticipated decline in incidence during the time of study as a result of national program interventions and that of the study itself. Taken as a whole, these data suggest that in our cohorts HIV incidence tends to be lower in men than women, declines with time on study, and may drop with calendar time. It is with caution that one should generalize findings in research cohorts to the broader community; however this is similar to overall trends in the African epidemic [2, 29].
Volunteers with incident HIV infection from our discordant couple cohorts provide an interesting lesson on risk that applies both to the clinical trialist and the public policy maker. Some of the highest HIV incidence was observed in these cohorts, with incidence estimates up to 10 cases /100PY. The HIV incidence among discordant couples who are not receiving quarterly behavioral counseling interventions is likely to be higher—possibly as high as 20–25% annually [30, 31]. Following the results of HPTN 052, which demonstrated a 96% reduction of HIV transmission within discordant couples when the volunteer with HIV infection received ART early [32], considerable attention has been focused on “test and treat” (i.e., putting newly diagnosed HIV-positive persons on ART as quickly as possible) as a means to reduce HIV transmissions [33, 34], and discordant couples represent a priority [35]. While others point out that real-world adherence, funding, and logistics all provide significant hurdles to this lofty goal [36], our data highlight another challenge: up to 30% of infections in our discordant couple cohorts originated from a partner other than the HIV infected steady partner. Indeed, in HPTN 052, 7 (17.9%) of the 39 transmission events were linked to someone other than the volunteer’s enrolled HIV-positive partner [32]. Although “test and treat” may eventually be a practical reality for much of the world’s population living with HIV, until that time discordant couples remain an important key population for public health interventions and for study.
We enrolled thousands of volunteers at risk for HIV infection, hundreds of volunteers with incident HIV infection, and identified key populations in need of public health interventions who might be suitable to participate in the next generation of HIV prevention trials. This work has spearheaded improvements to the health care delivery for marginalized groups such as sex workers and MSM [15] and reinforced the importance of testing and counseling couples together [30, 37, 38]. Specimens from these studies have also been the basis for dozens of ongoing collaborations ranging from estimating the seroprevalence of potential vaccine vectors [39] to characterizing the transmitted virus [40]and the host immune system following infection [41, 42]. IAVI has also tested 13 HIV vaccine products in 25 early-phase clinical trials in Africa [43–47], conducted a multi-site study to establish adult safety laboratory reference ranges against which to characterize adverse events in these clinical trials [48], and enrolled over 1,800 asymptomatic volunteers with prevalent HIV infection in a search for broadly neutralizing antibodies [49]. Collateral benefits extend beyond these CRCs as GCLP and assay-specific training are made available to laboratory technicians outside this partnership.
These large prospective observational epidemiology studies provide valuable data for prevention trial design and conduct, prevention planning, and service delivery. Over the past nine years, IAVI has identified and enrolled thousands of volunteers from key populations, observing a high HIV incidence despite regular HIV testing and counseling. The benefits to the public health, scientific and local communities are tremendous.
Acknowledgments
Assembling a research network of this size and scope is tremendously challenging and the authors thank all the staff, investigators, community members and study volunteers who participated. We are grateful to Family Health International which supported some of the cohort study work in Rustenburg [25] and to the European and Developing Countries Clinical Trials Partnership that helped fund some of the cohorts in the Ugandan fishing communities [50]. We also thank the staff at the Perinatal HIV Research Unit, Contract Laboratory Services, and IAVI’s Human Immunology Laboratory who worked very hard to assure these studies progressed efficiently and effectively. We also thank Wasima Rida for her help with advice on and review of the statistical analysis. This report was published with permission from KEMRI.
These data have been presented in part at The South African AIDS Conference in Durban, South Africa, 18–21 June 2013 (HIV incidence data).
The IAVI Africa HIV Prevention Partnership includes the following persons who contributed to the studies presented herein:
Kigali, Rwanda (Project San Francisco): Etienne Karita, Principal Investigator; Susan Allen, Principal Investigator; Roger Bayingana, Investigator; Kayitesi Kayitenkore, Investigator
Nairobi, Kenya (Kenya AIDS Vaccine Initiative): Omu Anzala, Principal Investigator; Gaudensia Mutua, Principal Investigator
Kilifi, Kenya (Center for Geographic Medicine Research—Coast & Kenya Medical Research Institute): Eduard J. Sanders, Principal Investigator; Peter Mugo, Investigator
Medical Research Council (MRC)/Uganda Virus Research Institute (UVRI) Uganda Research Unit on AIDS, Entebbe, Uganda: Anatoli Kamali, Principal Investigator; Rogers Twesigye, Study Coordinator, John Byabagambi, Study Physician; Florence Babirye, Nurse
Medical Research Council (MRC)/Uganda Virus Research Institute (UVRI) Uganda Research Unit on AIDS, Masaka, Uganda: Anatoli Kamali, Principal Investigator; Eugene Ruzagira, Study Coordinator, Agnes Bwanika, Ubaldo Bahemuka, and Freddie Mukasa Kibengo, Study Physicians; Peter Hughes, Laboratory Manager; Vincent Basajja, Community Liaison Officer
Lusaka, Ndola and Kitwe, Zambia (Zambia Emory HIV Research Project): William Kilembe, Principal Investigator; Susan Allen, Principal Investigator; Shabir Lakhi, Principal Investigator; Mubiano Inambao, investigator;
Rustenburg, South Africa (Aurum Institute): Mary H. Latka, Principal Investigator; Gavin J Churchyard, Investigator; Petra I Kruger, Investigator; Heeran Makkan, Study Coordinator; Candice M Chetty-Makkan, Study Coordinator; Ben Makhoana, Community Liaison Officer; Tiro Dinake, Nurse; Matsidi Malefo, Senior Research Assistant; Ireen Mosweu, Research Assistant
Cape Town, South Africa (Desmond Tutu HIV Foundation): Linda-Gail Bekker, Principal Investigator; Keren Middelkoop, Investigator; Surita Roux, Investigator
Data Availability
Data cannot be shared publicly due to ethical concerns. The authors are bound by requirements from the funders that all work stemming from these data be properly acknowledged and by the commitment to study volunteers to keep sensitive and potentially identifying information confidential. Data are available upon request for researchers who meet the requirements for access to confidential information. Requests to access data should be submitted to Matt Price (mprice@iavi.org).
Funding Statement
IAVI’s work is made possible by generous support from many donors including: the Bill & Melinda Gates Foundation; the Ministry of Foreign Affairs of Denmark; Irish Aid; the Ministry of Finance of Japan; the Ministry of Foreign Affairs of the Netherlands; the Norwegian Agency for Development Cooperation (NORAD); the United Kingdom Department for International Development (DFID), and the United States Agency for International Development (USAID). The full list of IAVI donors is available at www.iavi.org This work is made possible by the generous support of the American people through USAID. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government. Funding was also provided by the OPEC Fund for International Development (OFID), a development finance institution of the OPEC Member States, established to provide financial support for socio-economic development, particularly for low-income countries. Family Health International (now FHI360) supported some of the cohort study work in Rustenburg, and the European and Developing Countries Clinical Trials Partnership helped fund some of the cohorts in the Ugandan fishing communities. Several grants funded the HIV incidence and early infection studies, particularly in Rwanda and Zambia, including the Emory CFAR: P30 AI050409, NIH-FIC TW001042, NIH-NIAID R01 AI040951, NIH-NIMH R01 MH066767, NIH-NICHD R01 HD040125, NIH-NIAID R01 AI064060, and NIH-NIAID R37 AI51231. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. UNAIDS (2011) UNAIDS World AIDS Day Report. Geneva, Switzerland. [Google Scholar]
- 2. De Cock KM, Jaffe HW, Curran JW (2012) The evolving epidemiology of HIV/AIDS. AIDS 26: 1205–1213. 10.1097/QAD.0b013e328354622a [DOI] [PubMed] [Google Scholar]
- 3. Lihana RW, Ssemwanga D, Abimiku A, Ndembi N (2012) Update on HIV-1 diversity in Africa: a decade in review. AIDS Rev 14: 83–100. [PubMed] [Google Scholar]
- 4. Dunkle KL, Stephenson R, Karita E, Chomba E, Kayitenkore K, et al. (2008) New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet 371: 2183–2191. 10.1016/S0140-6736(08)60953-8 [DOI] [PubMed] [Google Scholar]
- 5. Smith A, Tapsoba P, Peshu N, Sanders E, Jaffe H (2009) Men who have sex with men and HIV/AIDS in sub-Saharan Africa. The Lancet 374: 416–422. 10.1016/S0140-6736(09)61118-1 [DOI] [PubMed] [Google Scholar]
- 6. Braunstein SL, van de Wijgert J, Nash D (2009) HIV Incidence in Sub-Saharan Africa: A Review of Available Data with Implications for Surveillance and Prevention Planning. Aids Reviews 11: 140–156. [PubMed] [Google Scholar]
- 7. Lyerla R, Murrill CS, Ghys PD, Calleja-Garcia JM, DeCock KM (2012) The use of epidemiological data to inform the PEPFAR response. J Acquir Immune Defic Syndr 60 Suppl 3: S57–62. 10.1097/QAI.0b013e31825d279a [DOI] [PubMed] [Google Scholar]
- 8. Fast PE, Kaleebu P (2010) HIV vaccines: current status worldwide and in Africa. AIDS 24 Suppl 4: S50–60. [DOI] [PubMed] [Google Scholar]
- 9. Hayes R, Kapiga S, Padian N, McCormack S, Wasserheit J (2010) HIV prevention research: taking stock and the way forward. AIDS 24 Suppl 4: S81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. UNAIDS Reference Group on Estimates Modelling and Projections (2002) Improved methods and assumptions for estimation of the HIV/AIDS epidemic and its impact: Recommendations of the UNAIDS Reference Group on Estimates, Modelling and Projections. AIDS 16: W1–14. 10.1097/00002030-200206140-00024 [DOI] [PubMed] [Google Scholar]
- 11. Busch MP, Pilcher CD, Mastro TD, Kaldor J, Vercauteren G, et al. (2010) Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS 24: 2763–2771. 10.1097/QAD.0b013e32833f1142 [DOI] [PubMed] [Google Scholar]
- 12. UNAIDS (2005) UNAIDS Reference Group on Estimates, Modelling and Projections’ statement on the use of the BED-assay for the estimation of HIV-1 incidence for surveillance or epidemic monitoring. [PubMed]
- 13. Kassanjee R, Pilcher CD, Keating SM, Facente SN, McKinney E, et al. (2014) Independent assessment of candidate HIV incidence assays on specimens in the CEPHIA repository. AIDS 28: 2439–2449. 10.1097/QAD.0000000000000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, et al. (2011) Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med 155: 209–216. 10.7326/0003-4819-155-4-201108160-00358 [DOI] [PubMed] [Google Scholar]
- 15. Brown B, Duby Z, Scheibe A, Sanders E (2011) Men Who Have Sex with Men: An Introductory Guide for Health Care Workers in Africa. Cape Town, South Africa: Desmond Tutu HIV Foundation. [Google Scholar]
- 16. WHO (2012) Guidance on couples HIV testing and counselling - including antiretroviral therapy for treatment and prevention in serodiscordant couples: recommendations for a public health approach. Geneva, Switzerland. [PubMed] [Google Scholar]
- 17. CDC (2007) Couples HIV Counseling and Testing (CHCT) - Trainer’s Manual.
- 18. Ngongo PB, Priddy F, Park H, Becker J, Bender B, et al. (2012) Developing standards of care for HIV prevention research in developing countries - a case study of 10 research centers in Eastern and Southern Africa. AIDS Care. 10.1080/09540121.2012.656572 [DOI] [PubMed]
- 19. Ruzagira E, Wandiembe S, Abaasa A, Levin J, Bwanika A, et al. (2011) Prevalence and incidence of HIV in a rural community-based HIV vaccine preparedness cohort in Masaka, Uganda. PLoS One 6: e20684 10.1371/journal.pone.0020684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Price MA, Rida W, Mwangome M, Mutua G, Middelkoop K, et al. (2012) Identifying at-risk populations in Kenya and South Africa: HIV incidence in cohorts of men who report sex with men, sex workers, and youth. J Acquir Immune Defic Syndr 59: 185–193. 10.1097/QAI.0b013e31823d8693 [DOI] [PubMed] [Google Scholar]
- 21. Ngongo PB, Priddy F, Park H, Becker J, Bender B, et al. (2012) Developing standards of care for HIV prevention research in developing countries -- a case study of 10 research centers in Eastern and Southern Africa. AIDS Care 24: 1277–1289. 10.1080/09540121.2012.656572 [DOI] [PubMed] [Google Scholar]
- 22. Price MA, Wallis CL, Lakhi S, Karita E, Kamali A, et al. (2011) Transmitted HIV Type 1 Drug Resistance Among Individuals with Recent HIV Infection in East and Southern Africa. AIDS Research and Human Retroviruses 27: 5–12. 10.1089/aid.2010.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amornkul PN, Karita E, Kamali A, Rida WN, Sanders EJ, et al. (2013) Disease progression by infecting HIV-1 subtype in a seroconverter cohort in sub-Saharan Africa. AIDS. 10.1097/QAD.0000000000000012 [DOI] [PMC free article] [PubMed]
- 24. Trask SA, Derdeyn CA, Fideli U, Chen Y, Meleth S, et al. (2002) Molecular epidemiology of human immunodeficiency virus type 1 transmission in a heterosexual cohort of discordant couples in Zambia. J Virol 76: 397–405. 10.1128/JVI.76.1.397-405.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feldblum PJ, Latka MH, Lombaard J, Chetty C, Chen PL, et al. (2012) HIV incidence and prevalence among cohorts of women with higher risk behaviour in Bloemfontein and Rustenburg, South Africa: a prospective study. BMJ Open 2: e000626 10.1136/bmjopen-2011-000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sanders EJ, Okuku HS, Smith AD, Mwangome M, Wahome E, et al. (2013) High HIV-1 incidence, correlates of HIV-1 acquisition, and high viral loads following seroconversion among MSM. AIDS 27: 437–446. 10.1097/QAD.0b013e32835b0f81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamali A, Kinsman J, Nalweyiso N, Mitchell K, Kanyesigye E, et al. (2002) A community randomized controlled trial to investigate impact of improved STD management and behavioural interventions on HIV incidence in rural Masaka, Uganda: trial design, methods and baseline findings. Trop Med Int Health 7: 1053–1063. 10.1046/j.1365-3156.2002.00963.x [DOI] [PubMed] [Google Scholar]
- 28. Nordling L (2014) Homophobia and HIV research: Under siege. Nature 509: 274–275. 10.1038/509274a [DOI] [PubMed] [Google Scholar]
- 29. Shetty AK (2013) Epidemiology of HIV Infection in Women and Children: A Global Perspective. Curr HIV Res 11: 81–92. 10.2174/1570162X11311020002 [DOI] [PubMed] [Google Scholar]
- 30. Allen S, Tice J, Van de Perre P, Serufilira A, Hudes E, et al. (1992) Effect of serotesting with counselling on condom use and seroconversion among HIV discordant couples in Africa. Bmj 304: 1605–1609. 10.1136/bmj.304.6842.1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guthrie BL, de Bruyn G, Farquhar C (2007) HIV-1-discordant couples in sub-Saharan Africa: explanations and implications for high rates of discordancy. Curr HIV Res 5: 416–429. 10.2174/157016207781023992 [DOI] [PubMed] [Google Scholar]
- 32. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. (2011) Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365: 493–505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohen MS, Mastro TD, Cates W Jr (2009) Universal voluntary HIV testing and immediate antiretroviral therapy. Lancet 373: 1077; author reply 1080–1071. [DOI] [PubMed] [Google Scholar]
- 34. Dieffenbach CW, Fauci AS (2009) Universal voluntary testing and treatment for prevention of HIV transmission. JAMA 301: 2380–2382. 10.1001/jama.2009.828 [DOI] [PubMed] [Google Scholar]
- 35. Curran K, Baeten JM, Coates TJ, Kurth A, Mugo NR, et al. (2012) HIV-1 prevention for HIV-1 serodiscordant couples. Curr HIV/AIDS Rep 9: 160–170. 10.1007/s11904-012-0114-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wagner BG, Blower S (2012) Universal access to HIV treatment versus universal ‘test and treat’: transmission, drug resistance & treatment costs. PLoS One 7: e41212 10.1371/journal.pone.0041212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roth DL, Stewart KE, Clay OJ, van Der Straten A, Karita E, et al. (2001) Sexual practices of HIV discordant and concordant couples in Rwanda: effects of a testing and counselling programme for men. Int J STD AIDS 12: 181–188. 10.1258/0956462011916992 [DOI] [PubMed] [Google Scholar]
- 38. Chomba E, Allen S, Kanweka W, Tichacek A, Cox G, et al. (2008) Evolution of couples’ voluntary counseling and testing for HIV in Lusaka, Zambia. J Acquir Immune Defic Syndr 47: 108–115. 10.1097/QAI.0b013e31815b2d67 [DOI] [PubMed] [Google Scholar]
- 39. Hara H, Hironaka T, Inoue M, Iida A, Shu T, et al. (2011) Prevalence of specific neutralizing antibodies against Sendai virus in populations from different geographic areas: implications for AIDS vaccine development using Sendai virus vectors. Hum Vaccin 7: 639–645. 10.4161/hv.7.6.15408 [DOI] [PubMed] [Google Scholar]
- 40. Baalwa J, Wang S, Parrish NF, Decker JM, Keele BF, et al. (2012) Molecular identification, cloning and characterization of transmitted/founder HIV-1 subtype A, D and A/D infectious molecular clones. Virology. 10.1016/j.virol.2012.10.009 [DOI] [PMC free article] [PubMed]
- 41. Addo MM, Altfeld M, Brainard DM, Rathod A, Piechocka-Trocha A, et al. (2011) Lack of detectable HIV-1-specific CD8(+) T cell responses in Zambian HIV-1-exposed seronegative partners of HIV-1-positive individuals. J Infect Dis 203: 258–262. 10.1093/infdis/jiq028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crawford H, Matthews PC, Schaefer M, Carlson JM, Leslie A, et al. (2011) The Hypervariable HIV-1 Capsid Protein Residues Comprise HLA-Driven CD8+ T-Cell Escape Mutations and Covarying HLA-Independent Polymorphisms. J Virol 85: 1384–1390. 10.1128/JVI.01879-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jaoko W, Nakwagala FN, Anzala O, Manyonyi GO, Birungi J, et al. (2008) Safety and immunogenicity of recombinant low-dosage HIV-1 A vaccine candidates vectored by plasmid pTHr DNA or modified vaccinia virus Ankara (MVA) in humans in East Africa. Vaccine 26: 2788–2795. 10.1016/j.vaccine.2008.02.071 [DOI] [PubMed] [Google Scholar]
- 44. Peters BS, Jaoko W, Vardas E, Panayotakopoulos G, Fast P, et al. (2007) Studies of a prophylactic HIV-1 vaccine candidate based on modified vaccinia virus Ankara (MVA) with and without DNA priming: effects of dosage and route on safety and immunogenicity. Vaccine 25: 2120–2127. 10.1016/j.vaccine.2006.11.016 [DOI] [PubMed] [Google Scholar]
- 45. Vardas E, Kaleebu P, Bekker LG, Hoosen A, Chomba E, et al. (2010) A phase 2 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 vaccine based on adeno-associated virus. AIDS Res Hum Retroviruses 26: 933–942. 10.1089/aid.2009.0242 [DOI] [PubMed] [Google Scholar]
- 46. Jaoko W, Karita E, Kayitenkore K, Omosa-Manyonyi G, Allen S, et al. (2010) Safety and immunogenicity study of Multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa. PLoS One 5: e12873 10.1371/journal.pone.0012873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hanke T, McMichael AJ, Dorrell L (2007) Clinical experience with plasmid DNA- and modified vaccinia virus Ankara-vectored human immunodeficiency virus type 1 clade A vaccine focusing on T-cell induction. J Gen Virol 88: 1–12. 10.1099/vir.0.82493-0 [DOI] [PubMed] [Google Scholar]
- 48. Karita E, Ketter N, Price MA, Kayitenkore K, Kaleebu P, et al. (2009) CLSI-derived hematology and biochemistry reference intervals for healthy adults in eastern and southern Africa. PLoS ONE 4: e4401 10.1371/journal.pone.0004401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, et al. (2009) Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326: 285–289. 10.1126/science.1178746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Seeley J, Nakiyingi-Miiro J, Kamali A, Mpendo J, Asiki G, et al. (2012) High HIV incidence and socio-behavioral risk patterns in fishing communities on the shores of Lake Victoria, Uganda. Sex Transm Dis 39: 433–439. 10.1097/OLQ.0b013e318251555d [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared publicly due to ethical concerns. The authors are bound by requirements from the funders that all work stemming from these data be properly acknowledged and by the commitment to study volunteers to keep sensitive and potentially identifying information confidential. Data are available upon request for researchers who meet the requirements for access to confidential information. Requests to access data should be submitted to Matt Price (mprice@iavi.org).


