Abstract
Aim
While prevalent periodontal disease associates with cardiovascular risk, little is known about how incident periodontal disease influences future vascular risk. We compared effects of incident versus prevalent periodontal disease in developing major cardiovascular diseases (CVD), myocardial infarction (MI), ischemic stroke and total CVD.
Material and Methods
In a prospective cohort of 39863 predominantly white women, age ≥ 45 years and free of cardiovascular disease at baseline were followed for an average of 15.7 years. Cox proportional hazard models with time-varying periodontal status (prevalent [18%], incident [7.3%] vs. never [74.7%]) were used to assess future cardiovascular risks.
Results
Incidence rates of all CVD outcomes were higher in women with prevalent or incident periodontal disease. For women with incident periodontal disease, risk factor adjusted hazard ratios (HRs) were 1.42 (95% CI, 1.14–1.77) for major CVD, 1.72 (1.25–2.38) for MI, 1.41(1.02–1.95) for ischemic stroke, and 1.27(1.06–1.52) for total CVD. For women with prevalent periodontal disease, adjusted HRs were 1.14 (1.00–1.31) for major CVD, 1.27 (1.04–1.56) for MI, 1.12(0.91–1.37) for ischemic stroke, and 1.15(1.03–1.28) for total CVD.
Conclusion
New cases of periodontal disease, not just those that are pre-existing, place women at significantly elevated risks for future cardiovascular events.
Keywords: periodontal diseases, cardiovascular diseases, survival analyses, C-reactive protein, smoking, diabetes, family history of MI
INTRODUCTION
Evidence accumulating over the past decade demonstrate a significant association between prevalent periodontal disease and future cardiovascular events (Eke, et al., 2012; Heidenreich, et al., 2011; Lockhart, et al., 2012; Tonetti & Van Dyke, 2013). Prevalence studies, however, are limited as they typically include wide variation in terms of disease duration prior to study enrollment, a source of variation that can lead to considerable uncertainty with regard to the true magnitude of association between an exposure of interest and a future clinical outcome (de Oliveira, Watt, & Hamer, 2010; Dorn, et al., 2010; Holmlund, Holm, & Lind, 2010; Howell, et al., 2001; Hujoel, et al., 2000; Hung, et al., 2004; Joshipura, et al., 2003; Joshipura, et al., 1996b; Tu, et al., 2007; Tuominen, et al., 2003; Wu, et al., 2000). By contrast, analysis of incident events that accrue during the course of a prospective observational study often provides a better measure of exposure duration and hence a more accurate estimate of risk. Research efforts examining effects of periodontal therapy on subclinical cardiovascular disease (CVD) surrogates such as endothelial function and carotid artery intima-media thickness (Jung, et al., 2014; Tonetti, et al., 2007), or reducing inflammatory markers were abundant (Bokhari, et al., 2012; Caula, et al., 2014); however, evidence is still limited to support the relief of CVD burden after periodontal treatment (D'Aiuto, Orlandi, & Gunsolley, 2013). To date, analyses of incident periodontal disease as a determinant of future vascular risk have been sparse (Dietrich, et al., 2008; Jimenez, et al., 2009).
Common risk factors such as diabetes and smoking are shared between cardiovascular and periodontal diseases (Dietrich, et al., 2013; Lockhart et al., 2012; Tonetti & Van Dyke, 2013). Therefore, efforts have been made in prior investigations to examine the association between periodontal disease and cardiovascular outcomes in non-smokers (Dorn et al., 2010) and in non-diabetic patients (Kodovazenitis et al., 2013). However, it remains an open question as to how the inter-relationships between these risk factors and periodontal disease may modify the process of developing cardiovascular disease. Further, genetic studies(Ernst, et al., 2010; Mucci, et al., 2009; Schaefer, et al., 2009) have suggested shared links between periodontal disease and coronary heart disease. Thus, it is of additional interest to evaluate the interaction between family history of myocardial infarction (MI), periodontal disease, and future vascular risks.
To address these issues, we examined the relationships of both prevalent and incident periodontal disease to the occurrence of first ever cardiovascular events in a prospective cohort of 39,863 American women followed for an average period of 15.7 years. We further sought evidence of effect modification between periodontal disease, cardiovascular disease, and common exposures such as obesity, smoking, diabetes, and family history of MI.
METHODS
Study Population
The Women's Health Study (WHS) is an NIH-funded prospective cohort that was initiated as a randomized, placebo-controlled trial examining low-dose aspirin and vitamin E in a 2x2 factorial design for the primary prevention of cardiovascular disease and cancer in initially-healthy middle-aged women. Details of the study design have been described previously(Ridker, et al., 2005a). Between September 1992 and May 1995, 39 876 female healthcare professionals in the US, aged 45 years or older, free of cardiovascular disease and cancer were enrolled and have been followed prospectively since that time for incident cardiovascular events. Approximately 72% of WHS participants provided a blood sample at baseline that was stored in liquid nitrogen for subsequent measurement of blood-based biomarkers. The study was approved by the institutional review board of Brigham and Women's Hospital, Boston, Massachusetts.
Self-reported Periodontal Disease
At study entry, participants in the WHS were asked about whether they had prevalent periodontal disease. New incident periodontal disease cases were then assessed at 36, 48, 60, 72, 84, 96, 108, 120 months during the randomized trial, and then subsequently at the first follow-up (about one year after trial completion) that occurred during the observational extension study. The month and year of the periodontal diagnosis as well as the number of teeth loss during the study interval were requested for the participants reporting incident periodontal disease.
Cardiovascular Events
Participants were followed for cardiovascular endpoints of by annual follow-up questionnaires, letters, or telephone calls. Medical records were obtained and reviewed in a blinded fashion for reported endpoints. Cardiovascular deaths were confirmed by family members, postal authorities, autopsy reports, medical records, and the National Death Index. Myocardial infarction was assessed by physician review of medical records according to WHO criteria as well as associated abnormal levels of cardiac enzymes or diagnostic electrocardiograms. Stroke was confirmed if the participant had a new focal neurologic deficit of sudden onset that persisted for more than 24 hours. Clinical information, computed tomographic scan, and magnetic resonance images were used to distinguish hemorrhagic from ischemic events. Reports of coronary revascularization procedures (bypass surgery or percutaneous coronary angioplasty) were confirmed by record reviews. Composite cardiovascular outcomes were used in the analysis. Major cardiovascular disease (CVD) events included non-fatal myocardial infarction, ischemic stroke, or death from cardiovascular causes. Total CVD events were defined as major CVD as well as bypass surgery, or percutaneous coronary angioplasty.
Assessment of Covariates
Details of the study protocol have been described in the primary endpoint report of the randomized control trial (Ridker et al., 2005a). Covariates of interests were collected at the baseline by validated questionnaires and used in the analysis – age, body mass index, race/ethnicity, education, diabetes, hypertension, hypercholesterolemia, smoking, family history of myocardial infarction, and physical activities. Hypertension was defined as a systolic blood pressure of at least 140 mm Hg, a diastolic blood pressure of at least 90 mm Hg, or self-reported physician-diagnosed hypertension; hyperlipidemia was defined as a total cholesterol level of at least 240 mg per deciliter (6.2 mmol per liter) or self-reported physician-diagnosed high cholesterol levels; other covariates were self-reported diagnosis of diabetes or estimation of physical activities. In the subset of WHS participants who provided a blood sample at baseline, plasma lipid fractions and high-sensitivity C-reactive protein (hsCRP) were measured as described(Ridker, et al., 2005b).
Statistical Analyses
We used time-varying survival analysis to investigate the effects of prevalent as well as incident periodontal disease as determinants of future vascular events. Prevalent periodontal disease (PD) was defined as PD reported as having occurred at or before baseline; while incident periodontal disease, defined as PD having occurred during the follow-up and before incident CVD if any, was encoded as time-varying independent variable in the data structure for the survival analysis with Cox proportional hazard model. Similarly, ever-having had PD status was combined from the baseline prevalent PD as well as the time-varying incident PD. Other covariates used in the analysis were based on the baseline characteristics (Therneau & Crowson, 2014). All analyses were performed using the `survival' package of R software. Analyses were adjusted on an a priori basis for age alone as well as for age, race/ethnicity, body mass index, education, smoking, diabetes, hypertension, hypercholesterolemia, family history of myocardial infarction, and physical activity (model 1). Given that smoking is a strong risk indicator in both periodontal and cardiovascular disease, we adjusted for never- vs. current/past-smokers as has been shown to be more robust in recent reports (Dorn et al,, 2010; Jung et al., 2014). Estimates were also obtained after further adjustment for C-reactive protein, a biomarker of inflammation (model 2). To assess effect modification by relevant risk factors, analyses were stratified by the presence or absence of obesity, smoking, hypertension, hypercholesterolemia, a family history of myocardial infarction, and diabetes. Survival curves were generated separately for the component endpoints of myocardial infarction and ischemic stroke, as well as for the joint endpoints of major CVD and total CVD.
RESULTS
Baseline Characteristics
Women having either prevalent or incident periodontal diseases were older compared to those without periodontal disease (mean age: 54.5 and 53.3 vs. 52.5; Table 1), more likely to be over-weight or obese, more likely to be current or former smokers (63.6% and 51.2% vs 45.2%), and exercised less frequently. Serum levels of total cholesterol, LDL cholesterol, triglyceride, and hsCRP were higher in women with prevalent periodontal disease at the baseline, and a higher prevalence of hypertension, diabetes, and hypercholesterolemia were also noted in participants with periodontal disease. We also provide demographic information for women corresponding to the different statistical analyses in Supplementary Table 2. Women having blood samples tended to have more education and fewer cardiovascular events.
Table 1.
Baseline Characteristics of Study Participants According to the Periodontal Disease Status
| Characteristicsa | No periodontal disease | Baseline prevalent periodontal disease | Incident periodontal disease | P Valueb |
|---|---|---|---|---|
|
| ||||
| No. (%) of patients | n = 29787 (74.7) | n = 7185 (18.0) | n = 2891 (7.3) | |
| Age | 52.5 (48.7–58.4) | 54.5 (50.0–60.3) | 53.3 (49.1–59.2) | <.001 |
| Body mass index (Kg/m2) | 0.012 | |||
|
| ||||
| <25 | 14907 (51.1) | 3569 (50.7) | 1367 (48.1) | |
| 25 ~ <30 | 9020 (30.9) | 2159 (30.7) | 900 (31.7) | |
| ≥30 | 5240 (18.0) | 1308 (18.6) | 574 (20.2) | |
| Race/ethnicity | <.001 | |||
|
| ||||
| White | 28061 (95.0) | 6746 (94.8) | 2662 (92.8) | |
| Other | 1481 (5.0) | 368 (5.2) | 205 (7.2) | |
| Highest education level | <.001 | |||
|
| ||||
| <Bachelor's degree | 16879 (57.7) | 3978 (56.3) | 1586 (55.6) | |
| Bachelor's degree | 6800 (23.2) | 1572 (22.2) | 671 (23.5) | |
| Master's degree or doctorate | 5593 (19.1) | 1520 (21.5) | 593 (20.8) | |
| Smoking | <.001 | |||
|
| ||||
| Current | 3254(11.0) | 1501(20.9) | 474(16.4) | |
| Past | 10191(34.2) | 3067(42.7) | 1005(34.8) | |
| Never | 16313 (54.8) | 2612 (36.4) | 1410 (48.8) | |
| Hypertension | <.001 | |||
|
| ||||
| Yes | 7574 (25.4) | 1983 (27.6) | 756 (26.2) | |
| No | 22206 (74.6) | 5201 (72.4) | 2134 (73.8) | |
| Hypercholesterolemia | <.001 | |||
|
| ||||
| Yes | 8470 (28.4) | 2402 (33.4) | 868 (30.0) | |
| No | 21306 (71.6%) | 4780 (66.6) | 2021 (70.0) | |
| Family Hx MI | 0.003 | |||
|
| ||||
| Yes | 3375 (12.6) | 891 (13.9) | 366 (14.1) | |
| No | 23449 (87.4) | 5523 (86.1) | 2228 (85.9) | |
| Hx of diabetes | <.001 | |||
|
| ||||
| Yes | 780 (2.6) | 274 (3.8) | 88 (3.0) | |
| No | 29007 (97.4) | 6911 (96.2) | 2803 (97.0) | |
| Physical activities | <.001 | |||
|
| ||||
| Rarely / Never | 11218 (37.7) | 2879 (40.1) | 1179 (40.8) | |
| <1 time per week | 5899 (19.8) | 1454 (20.3) | 571 (19.8) | |
| 1–3 times per week | 9406 (31.6) | 2135 (29.7) | 864 (29.9) | |
| 4+ times per week | 3250 (10.9) | 712 (9.9) | 276 (9.6) | |
| Total cholesterol (mg/L) c | 207.0 (183.0–235.0) | 212.0 (187.0–238.0) | 209.0 (183.0–238.0) | <.001 |
|
| ||||
| HDL (mg/L) c | 52.0 (43.2–62.5) | 51.5 (42.7–62.0) | 51.4 (42.5–61.2) | 0.021 |
| LDL (mg/L) c | 120.7 (100.1–143.5) | 124.0 (102.4–147.1) | 123.0 (100.2–146.2) | <.001 |
| Triglyceride (mg/L) c | 118 (83.0–175.0) | 124 (87.0–178.0) | 117 (82.0–174.0) | <.001 |
|
| ||||
| hsCRP(mg/L) c | 2.0 (0.8–4.3) | 2.1 (0.9–4.7) | 2.0 (0.8–4.3) | <.001 |
Abbreviations: BMI, body mass index; hsCRP, high sensitivity C-reactive protein; HDL, high density lipoprotein; LDL, low density lipoprotein.
Data are expressed as median (interquartile range) for continuous variables and as number (percentage) of participants unless otherwise indicated. Number across categories may not sum to the given total because of the missing data.
P values are based on Kruskal-Wallis test for continuous variables and of the chi-square tests for categorical variables.
Number of women having blood samples collected at the baseline and tested were 27,939.
Periodontal Disease and Future Cardiovascular Events
Overall in the full cohort, compared to women without periodontal disease, age-adjusted hazard ratios for the major CVD events were higher in women with prevalent (hazard ratio [HR], 1.27 [95% CI, 1.12–1.43]; P<.001) or incident (HR, 1.40 [95% CI, 1.15–1.72]; P<.001) periodontal disease. These effects remained comparable after adjustment for traditional vascular risk factors (multivariate model 1, Table 2). For women with incident periodontal disease, hazard ratios (HRs) were 1.42 (95% CI, 1.14–1.77; P=0.002) for major CVD, 1.72 (95% CI, 1.25–2.38; P<.001) for MI, 1.41(95% CI, 1.02–1.95; P=0.04) for ischemic stroke, and 1.27(95% CI, 1.06–1.52; P=0.01) for total CVD after adjusting for established risk factors and physical activity. After further accounting for inflammation indices such as serum level of hsCRP, effects were attenuated but still significant for major CVD (HR, 1.31 [95% CI, 1.01–1.71]; P=0.045) and MI (HR, 1.65 [1.11–2.45]; P=0.01). Likewise, for women with prevalent periodontal disease at baseline, adjusted hazard ratios were 1.14 (95% CI, 1.00–1.31; P=0.05) for major CVD, 1.27 (95% CI, 1.04–1.56; P=0.02) for MI, 1.12(95% CI, 0.91–1.37; P=0.3) for ischemic stroke, and 1.15(95% CI, 1.03–1.28; P=0.01) for total CVD (Multivariate model 1, Table 2). Similarly, effects were attenuated after further adjusting for hsCRP, but remained statistically significant for MI (HR, 1.35 [95% CI, 1.05–1.73]; P=0.02) and total CVD (HR, 1.12 [95% CI, 1.05–1.36]; P=0.007). Further including aspirin vs. placebo allocation in the multivariate analysis had negligible effects on the hazard ratios (data not shown). The cumulative incidences of these events are presented graphically in Figure 1 for those with any evident (i.e. either prevalent or incident) periodontal disease. Supplementary analyses examining risks of cardiovascular events in women having either prevalent or incident periodontal disease vs. never are provided in Supplementary Table 1. As a sensitivity analysis, we also repeated the analyses restricted to women having blood samples (the reduced set). Results were similar that effects were slightly attenuated after further adjusting for hsCRP and that higher CVD risks were found in women having incident vs. prevalent periodontal disease.
Table 2.
Risk of Cardiovascular Events among Women with Penodontal Disease
| Outcome | No Periodontal Disease | Prevalent Periodontal Disease at Baseline | Incident Periodontal Disease during Follow-upa | |||
|---|---|---|---|---|---|---|
| Major CVD | N (Events) | |||||
|
| ||||||
| Incidence rate (95% CI)b | 39863 (1549) | 2.25 (2.11–2.38) | 3.24 (2.90–3.57) | 2.98 (2.49–3.48) | ||
|
| ||||||
| HR (95% CIs) | P | HR (95% CIs) | P | |||
|
|
||||||
| Age-adjustedc | 39863 (1549) | [Reference] | 1.27 (1.12–1.43) | <.001 | 1.40 (1.15–1.72) | 0.001 |
| Multivariate model 1 d | 34228 (1274) | [Reference] | 1.14 (1.00–1.31) | 0.05 | 1.42 (1.14–1.77) | 0.002 |
| Multivariate model 2 e | 24033 (883) | [Reference] | 1.17 (0.99–1.37) | 0.06 | 1.31 (1.01–1.71) | 0.045 |
|
| ||||||
| Myocardial Infarction (MI) | ||||||
|
| ||||||
| Incidence rate (95% CI)b | 39863 (642) | 0.91 (0.82–0.99) | 1.36 (1.15–1.58) | 1.33 (1.00–1.66) | ||
|
| ||||||
| HR (95% CIs) | P | HR (95% CIs) | P | |||
|
|
||||||
| Age-adjustedc | 39863 (642) | [Reference] | 1.34 (1.12–1.62) | 0.002 | 1.56 (1.15–2.12) | 0.005 |
| Multivariate model 1 d | 34228 (525) | [Reference] | 1.27 (1.04–1.56) | 0.02 | 1.72 (1.25–2.38) | <.001 |
| Multivariate model 2e | 24033 (353) | [Reference] | 1.35 (1.05–1.73) | 0.02 | 1.65 (1.11–2.45) | 0.01 |
|
| ||||||
| Ischemic stroke | ||||||
|
| ||||||
| Incidence rate (95% CI)b | 39863 (677) | 0.96 (0.87–1.05) | 1.43 (1.21–1.65) | 1.41 (1.07–1.76) | ||
|
| ||||||
| HR (95% CIs) | P | HR (95% CIs) | P | |||
|
|
||||||
| Age-adjustedc | 39863 (677) | [Reference] | 1.28(1.07–1.54) | 0.006 | 1.40(1.04–1.90) | 0.03 |
| Multivariate model 1 d | 34228 (558) | [Reference] | 1.12(0.91–1.37) | 0.3 | 1.41(1.02–1.95) | 0.04 |
| Multivariate model 2e | 24033 (394) | [Reference] | 1.05(0.82–1.35) | 0.7 | 1.28(0.87–1.88) | 0.2 |
|
| ||||||
| Total CVD | ||||||
|
| ||||||
| Incidence rate (95% CI)b | 39863 (2387) | 3.52 (3.35–3.69) | 5.03 (4.61–5.45) | 4.39 (3.78–5.00) | ||
|
| ||||||
| HR (95% CIs) | P | HR (95% CIs) | P | |||
|
|
||||||
| Age-adjustedc | 39863 (2387) | [Reference] | 1.27(1.15–1.4) | <.001 | 1.25(1.06–1.48) | 0.009 |
| Multivariate model 1 d | 34228 (1968) | [Reference] | 1.15(1.03–1.28) | 0.01 | 1.27(1.06–1.52) | 0.01 |
| Multivariate model 2e | 24033 (1388) | [Reference] | 1.12(1.05–1.36) | 0.007 | 1.2(0.97–1.49) | 0.1 |
Abbreviations: HR, hazard ratio; CI, confidence interval; CVD, cardiovascular disease.
Follow up time of incident periodontal disease ended on March 31, 2006 and follow up of cardiovascular outcomes ended on March 14,2012.
Per 1000 person-years of follow up.
Models were adjusted for the age at randomization . These models were based on 1549, 642, 677, 2387 events of major CVD, MI, ischemic stroke, and total CVD, respectively, among 39863women.
Additionally adjusted for race/ethnicity, body mass index, education, smoking, diabetes, hypertension, hypercholesterolemia, family history of myocardial infarction, and physical activities. These multivariable models were based on 1274, 525, 588, 1968 events of major CVD, MI, ischemic stroke, and total CVD, respectively, among 34228 women, due to missing data.
Additionally adjusted for natural-log-transformed serum levels of C-reactive protein. These multivariable models were based on 883, 353, 394, 1388 events of major CVD, MI, ischemic stroke, and total CVD, respectively, among 24033 women, due to missing data.
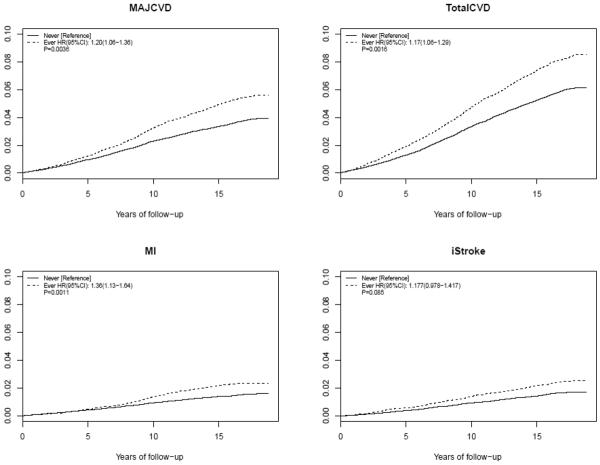
Figures 1.
Cumulative incidence rates of major cardiovascular disease (CVD), total CVD, myocardial infarction (MI), and ischemic stroke (iStroke) between women having either prevalent or incident periodontal disease versus never.
Hazard ratios and P-values were calculated after accounting for established cardiovascular risk factors, and physical activities (Multivariate model 1 in Supplementary Table 1).
As shown in Table 3, the magnitude of association between any evident periodontal disease and future vascular events was less prominent in women who were obese, diabetic, or did not smoke. However, none of these differential effects were statistically significant in analyses assessing for a multiplicative interaction (P>0.05), except smoking vs. total CVD (P=0.02); obesity vs. major CVD (P=0.03) and total CVD (P=0.02). Of note, confidence intervals still overlap between these stratified subsets of women as well as that care should be taken into the consideration of multiple testing given the stratifications (P <0.05/6=0.008).
Table 3.
Age-adjusted Risk of Cardiovascular Events among Subgroups of Women Ever Having Had Periodontal Diseases
| Outcomes | Major CVD | MI | Ischemic Stroke | Total CVD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Stratification a | N | E | HR (95% CIs)b | E | HR (95% CIs)a | E | HR (95% CIs)a | E | HR (95% CIs)a | |
| Obese | + | 7123 | 356 | 1.04 (0.82–1.32) | 149 | 1.22 (0.85–1.75) | 148 | 1.02 (0.7–1.47) | 587 | 1.05 (0.87–1.27) |
| -- | 31923 | 1147 | 1.38 (1.22–1.56)*** | 470 | 1.46 (1.2–1.78)*** | 515 | 1.39 (1.15–1.67) ** | 1727 | 1.34 (1.21–1.49)*** | |
|
| ||||||||||
| Smoking | + | 19493 | 897 | 1.33 (1.16–1.53)*** | 401 | 1.39 (1.13–1.7) ** | 368 | 1.34 (1.09–1.66) ** | 1348 | 1.33 (1.19–1.49)*** |
| -- | 20336 | 648 | 1.05 (0.87–1.27) | 239 | 1.08 (0.79–1.48) | 307 | 1.16 (0.88–1.51) | 1035 | 1.02 (0.88–1.19) | |
|
| ||||||||||
| Hypertension | + | 10314 | 737 | 1.21 (1.03–1.42) ** | 298 | 1.34 (1.05–1.72) * | 333 | 1.26 (1–1.59) | 1127 | 1.18 (1.04–1.34) * |
| -- | 29542 | 811 | 1.37 (1.18–1.59)*** | 344 | 1.41 (1.13–1.78) ** | 343 | 1.35 (1.07–1.7) * | 1259 | 1.33 (1.18–1.5)*** | |
|
| ||||||||||
| Hypercholesterolemia | + | 11741 | 632 | 1.43 (1.21–1.69)*** | 275 | 1.54 (1.2–1.97) ** | 286 | 1.23 (0.96–1.58) | 1063 | 1.35 (1.19–1.53)*** |
| -- | 28108 | 917 | 1.18 (1.02–1.37) * | 367 | 1.25 (1–1.57) | 391 | 1.35 (1.09–1.68) ** | 1324 | 1.17 (1.04–1.32) * | |
|
| ||||||||||
| Family Hx MI | + | 4633 | 204 | 1.28 (0.95–1.73) | 103 | 1.64 (1.1–2.45) * | 75 | 1.03 (0.62–1.71) | 355 | 1.14 (0.91–1.44) |
| -- | 31201 | 1148 | 1.28 (1.13–1.45)*** | 460 | 1.38 (1.13–1.69) ** | 514 | 1.3 (1.08–1.58) ** | 1727 | 1.25 (1.13–1.39)*** | |
|
| ||||||||||
| Hx of diabetes | + | 1142 | 175 | 1.21 (0.88–1.67) | 81 | 1.13 (0.71–1.82) | 68 | 1.44 (0.88–2.36) | 293 | 1.14 (0.89–1.46) |
| -- | 38721 | 1374 | 1.27 (1.13–1.43)*** | 561 | 1.39 (1.16–1.66)*** | 609 | 1.27 (1.06–1.51) ** | 2094 | 1.25 (1.14–1.37)*** | |
Abbreviations: N, number of women in the stratified subset; E, number of events; HR, hazard ratio; CVD, cardiovascular disease; MI, myocardial infarction; CHD, coronary heart disease.
*** P < 0.001;
P <0.01;
P <0.05
DISCUSSION
In this large, prospective cohort of middle-aged women, incident as well as prevalent periodontal disease (PD) was associated with statistically significant increased risks of developing future cardiovascular events. Moreover, in these data, the magnitude of vascular risk associated with incident periodontal disease was at least as large as that for prevalent periodontal disease. Thus, these data not only confirm prior work for prevalent periodontal disease, but importantly provide new evidence that incident periodontal disease is also associated with high vascular event rates. We believe these data likely to have relevance for the practice of periodontal medicine as they demonstrate that new cases of periodontal disease, not just those that have been present for long periods of time, put women at significantly elevated risks for vascular disease. It is possible that the lower hazard ratios shown in women having prevalent PD compared to women with incident PD may be due to treatment history or greater awareness of the oral hygiene care. However, this issue is complex and will require additional research. Lastly, our estimated cardiovascular risks among women having prevalent periodontal disease were similar to previous published population studies examining effects of existing periodontal disease on CVD risks (Helfand, et al., 2009).
In addition to providing incidence data, the current analysis also provides a comprehensive analysis of effect modification by established risk factors. In these data, risks of vascular events associated with periodontal disease were generally similar in most clinical subgroups, but tended to be preferentially elevated among women who smoked and who were not obese.
By contrast, the magnitude of association between periodontal disease and cardiovascular disease risk generally did not vary in our data according to family history of myocardial infarction, except for a slightly higher risk of MI among women ever having had periodontal disease as well as a family history of MI (Table 3 and Supplementary Table 4) although this enhanced effect did not meet statistical significance. While this observation is somewhat discordant with prior work (Ernst et al., 2010; Mucci et al., 2009; Schaefer et al., 2009), questionnaire data on family history is qualitative only and not equivalent to or representative of shared genetic risks. In this regard, recent genome wide association studies have suggested that some loci might have effects on periodontal risk, albeit most of the signals were below genome-wide significance level (Divaris, et al., 2012; Schaefer, et al., 2010; Teumer, et al., 2013). Further research of periodontal disease vs. cardiovascular risks in genomic medicine could lead to more insight of the underlying biology.
Strength and Limitation
Our study has several strengths including its prospective design, large sample size, and confirmation of all incident vascular events. Our approach using time-varying periodontal disease status in the survival analysis is an additional strength as it provides a more accurate assessment of future CVD risk associated with periodontal disease than available from retrospective case-control designs or studies that employ only prevalent periodontal disease. Nonetheless, the generalizability of our findings may be limited due to the study population of middle-aged female healthcare professionals. Previous reports from male healthcare professionals in the Physician Health Study(Howell et al, 2001) and the Healthy Professional Follow-Up Study(Joshipura et al., 1996b) did not identify self-reported periodontal disease status as a significant risk factor for cardiovascular disease. Second, while self-reported periodontal status has been widely used and previously validated for its discriminatory power among healthcare professionals(Joshipura, et al., 1996a) as well as for a high correlation with PD defined by clinical examination among the general population in the National Health and Nutrition Examination Survey (Eke, et al., 2013), it is a far less useful method than direct examination which can address disease severity. Given the design of the questionnaire, we unfortunately did not have information regarding tooth loss for the majority of women. Furthermore, history of periodontal treatment, unavailable in our dataset, could be a potential confounder in the association. Third, censored or misclassified periodontal disease status might have led to an underestimation of the magnitude of true effects. Such misclassification, however, would not have resulted in a false-positive finding.
CONCLUSION
Incident periodontal disease confers at least as high a risk of future cardiovascular events as prevalent periodontal disease in middle-aged women.
Supplementary Material
CLINICAL RELEVANCE.
Scientific rationale for the study
Does incident periodontal disease, recorded during a prospective observational study, support previously known associations between cardiovascular events and prevalent periodontal disease?
Principle finding
Among middle-aged women, having either incident or baseline prevalent periodontal disease imposed greater chances of developing future myocardial infarction, ischemic stroke, and vascular procedures when compared to women free of periodontal disease. Hazard ratios of women with incident periodontal disease were at least as high as those with baseline prevalent periodontal disease.
Practical implications
Women with either incident or prevalent periodontal disease are at elevated lifetime risk for life-threatening cardiovascular events.
Acknowledgment
None
Source of Funding Statement: The present study is supported by HL043851, HL080467, HL099355 from the National Heart, Lung, and Blood Institute, by CA047988 from the National Cancer Institute, and by the Donald W. Reynolds Foundation.
Footnotes
Conflict of Interests The authors declare no conflict of interest.
REFERENCES
- Bokhari SA, Khan AA, Butt AK, Azhar M, Hanif M, Izhar M, Tatakis DN. Non-surgical periodontal therapy reduces coronary heart disease risk markers: a randomized controlled trial. J Clin Periodontol. 2012;39:1065–1074. doi: 10.1111/j.1600-051X.2012.01942.x. [DOI] [PubMed] [Google Scholar]
- Caula AL, Lira-Junior R, Tinoco EM, Fischer RG. The effect of periodontal therapy on cardiovascular risk markers: a 6-month randomized clinical trial. J Clin Periodontol. 2014 doi: 10.1111/jcpe.12290. [DOI] [PubMed] [Google Scholar]
- D'Aiuto F, Orlandi M, Gunsolley JC. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J Clin Periodontol. 2013;40(Suppl 14):S85–105. doi: 10.1111/jcpe.12061. [DOI] [PubMed] [Google Scholar]
- de Oliveira C, Watt R, Hamer M. Toothbrushing, inflammation, and risk of cardiovascular disease: results from Scottish Health Survey. Bmj. 2010;340:c2451. doi: 10.1136/bmj.c2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich T, Jimenez M, Krall Kaye EA, Vokonas PS, Garcia RI. Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation. 2008;117:1668–1674. doi: 10.1161/CIRCULATIONAHA.107.711507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich T, Sharma P, Walter C, Weston P, Beck J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Clin Periodontol. 2013;40(Suppl 14):S70–84. doi: 10.1111/jcpe.12062. [DOI] [PubMed] [Google Scholar]
- Divaris K, Monda KL, North KE, Olshan AF, Lange EM, Moss K, Barros SP, Beck JD, Offenbacher S. Genome-wide association study of periodontal pathogen colonization. J Dent Res. 2012;91:21S–28S. doi: 10.1177/0022034512447951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn JM, Genco RJ, Grossi SG, Falkner KL, Hovey KM, Iacoviello L, Trevisan M. Periodontal disease and recurrent cardiovascular events in survivors of myocardial infarction (MI): the Western New York Acute MI Study. J Periodontol. 2010;81:502–511. doi: 10.1902/jop.2009.090499. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Beck JD, Taylor GW, Borgnakke WS, Page RC, Genco RJ. Self-reported measures for surveillance of periodontitis. J Dent Res. 2013;92:1041–1047. doi: 10.1177/0022034513505621. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Ernst FD, Uhr K, Teumer A, Fanghanel J, Schulz S, Noack B, Gonzales J, Reichert S, Eickholz P, Holtfreter B, Meisel P, Linden GJ, Homuth G, Kocher T. Replication of the association of chromosomal region 9p21.3 with generalized aggressive periodontitis (gAgP) using an independent case-control cohort. BMC Med Genet. 2010;11:119. doi: 10.1186/1471-2350-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- Helfand M, Buckley DI, Freeman M, Fu R, Rogers K, Fleming C, Humphrey LL. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:496–507. doi: 10.7326/0003-4819-151-7-200910060-00010. [DOI] [PubMed] [Google Scholar]
- Holmlund A, Holm G, Lind L. Number of teeth as a predictor of cardiovascular mortality in a cohort of 7,674 subjects followed for 12 years. J Periodontol. 2010;81:870–876. doi: 10.1902/jop.2010.090680. [DOI] [PubMed] [Google Scholar]
- Howell TH, Ridker PM, Ajani UA, Hennekens CH, Christen WG. Periodontal disease and risk of subsequent cardiovascular disease in U.S. male physicians. J Am Coll Cardiol. 2001;37:445–450. doi: 10.1016/s0735-1097(00)01130-x. [DOI] [PubMed] [Google Scholar]
- Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Periodontal disease and coronary heart disease risk. Jama. 2000;284:1406–1410. doi: 10.1001/jama.284.11.1406. [DOI] [PubMed] [Google Scholar]
- Hung HC, Joshipura KJ, Colditz G, Manson JE, Rimm EB, Speizer FE, Willett WC. The association between tooth loss and coronary heart disease in men and women. J Public Health Dent. 2004;64:209–215. doi: 10.1111/j.1752-7325.2004.tb02755.x. [DOI] [PubMed] [Google Scholar]
- Jimenez M, Krall EA, Garcia RI, Vokonas PS, Dietrich T. Periodontitis and incidence of cerebrovascular disease in men. Ann Neurol. 2009;66:505–512. doi: 10.1002/ana.21742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshipura KJ, Douglass CW, Garcia RI, Valachovic R, Willett WC. Validity of a self-reported periodontal disease measure. J Public Health Dent. 1996a;56:205–212. doi: 10.1111/j.1752-7325.1996.tb02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003;34:47–52. doi: 10.1161/01.str.0000052974.79428.0c. [DOI] [PubMed] [Google Scholar]
- Joshipura KJ, Rimm EB, Douglass CW, Trichopoulos D, Ascherio A, Willett WC. Poor oral health and coronary heart disease. J Dent Res. 1996b;75:1631–1636. doi: 10.1177/00220345960750090301. [DOI] [PubMed] [Google Scholar]
- Jung YS, Shin MH, Kim IS, Kweon SS, Lee YH, Kim OJ, Kim YJ, Chung HJ, Kim OS. Relationship between periodontal disease and subclinical atherosclerosis: the Dong-gu study. J Clin Periodontol. 2014;41:262–268. doi: 10.1111/jcpe.12204. [DOI] [PubMed] [Google Scholar]
- Kodovazenitis G, Pitsavos C, Papadimitriou L, Vrotsos IA, Stefanadis C, Madianos PN. Association between periodontitis and acute myocardial infarction: a case-control study of a nondiabetic population. J Periodontal Res. 2013 doi: 10.1111/jre.12101. [DOI] [PubMed] [Google Scholar]
- Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, Taubert KA, Newburger JW, Gornik HL, Gewitz MH, Wilson WR, Smith SC, Jr., Baddour LM. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. 2012;125:2520–2544. doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- Mucci LA, Hsieh CC, Williams PL, Arora M, Adami HO, de Faire U, Douglass CW, Pedersen NL. Do genetic factors explain the association between poor oral health and cardiovascular disease? A prospective study among Swedish twins. Am J Epidemiol. 2009;170:615–621. doi: 10.1093/aje/kwp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005a;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. Jama. 2005b;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- Schaefer AS, Richter GM, Groessner-Schreiber B, Noack B, Nothnagel M, El Mokhtari NE, Loos BG, Jepsen S, Schreiber S. Identification of a shared genetic susceptibility locus for coronary heart disease and periodontitis. PLoS Genet. 2009;5:e1000378. doi: 10.1371/journal.pgen.1000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AS, Richter GM, Nothnagel M, Manke T, Dommisch H, Jacobs G, Arlt A, Rosenstiel P, Noack B, Groessner-Schreiber B, Jepsen S, Loos BG, Schreiber S. A genome-wide association study identifies GLT6D1 as a susceptibility locus for periodontitis. Hum Mol Genet. 2010;19:553–562. doi: 10.1093/hmg/ddp508. [DOI] [PubMed] [Google Scholar]
- Teumer A, Holtfreter B, Volker U, Petersmann A, Nauck M, Biffar R, Volzke H, Kroemer HK, Meisel P, Homuth G, Kocher T. Genome-wide association study of chronic periodontitis in a general German population. J Clin Periodontol. 2013;40:977–985. doi: 10.1111/jcpe.12154. [DOI] [PubMed] [Google Scholar]
- Therneau T, Crowson C. Using Time Dependent Covariates and Time Dependent Coefficients in the Cox Model. Mayo Clinic; 2014. [Google Scholar]
- Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, Van Dyke TE. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013;40(Suppl 14):S24–29. doi: 10.1111/jcpe.12089. [DOI] [PubMed] [Google Scholar]
- Tu YK, Galobardes B, Smith GD, McCarron P, Jeffreys M, Gilthorpe MS. Associations between tooth loss and mortality patterns in the Glasgow Alumni Cohort. Heart. 2007;93:1098–1103. doi: 10.1136/hrt.2006.097410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen R, Reunanen A, Paunio M, Paunio I, Aromaa A. Oral health indicators poorly predict coronary heart disease deaths. J Dent Res. 2003;82:713–718. doi: 10.1177/154405910308200911. [DOI] [PubMed] [Google Scholar]
- Wu T, Trevisan M, Genco RJ, Dorn JP, Falkner KL, Sempos CT. Periodontal disease and risk of cerebrovascular disease: the first national health and nutrition examination survey and its follow-up study. Arch Intern Med. 2000;160:2749–2755. doi: 10.1001/archinte.160.18.2749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.