Abstract
Background
Parkinson Disease (PD) patients treated with Dopamine Agonist therapy can develop maladaptive reward-driven behaviors, known as Impulse Control Disorder (ICD). In this study, we assessed if ICD patients have evidence of motor-impulsivity.
Methods
We used the stop-signal task in a cohort of patients with and without active symptoms of ICD to evaluate motor-impulsivity. Of those with PD, 12 were diagnosed with ICD symptoms (PD-ICD) and were assessed before clinical reduction of Dopamine Agonist medication; 12 were without symptoms of ICD [PD-control] and taking equivalent dosages of Dopamine Agonist. Levodopa, if present, was maintained in both settings. Groups were similar in age, duration, and severity of motor symptoms, levodopa co-therapy, and total levodopa daily dose. All were tested in the Dopamine Agonist medicated and acutely withdrawn (24 hours) state, in a counterbalanced manner. Primary outcome measures were mean reaction time to correct go trials (Go Reaction Time), and mean stop-signal reaction time (SSRT).
Results
ICD patients produce faster SSRT than both Healthy Controls, and PD Controls. Faster SSRT in ICD patients is apparent in both Dopamine Agonist medication states. Also, we show unique dopamine medication effects on GoRT. In Dopamine Agonist monotherapy patients, Dopamine Agonist administration speeds Go Reaction Time. Conversely, in those with levodopa co-therapy, Dopamine Agonist administration slows Go Reaction Time.
Discussion
PD patients with active ICD symptoms are significantly faster at stopping initiated motor actions, and this is not altered by acute Dopamine Agonist withdrawal. In addition, the effect of Dopamine Agonist on Go Reaction Time is strongly influenced by the presence or absence of levodopa, even though levodopa co-therapy does not appear to influence SSRT. We discuss these findings as they pertain to the multifaceted definition of ‘impulsivity,’ the lack of evidence for motor-impulsivity in PD-ICD, and dopamine effects on motor-control in PD.
Keywords: Dopamine Agonist, Parkinson Disease, Impulse Control Disorder, Inhibition, Motor impulsivity, Reaction Time
Introduction
“Impulsivity” describes a pattern of hastily made decisions or behaviors (Evenden 1999). The term itself invokes a negative connotation, although in certain circumstances, impulsive or spontaneous decisions can be quite functional (Dickman 1990). From a cognitive and behavioral perspective, impulsivity invites some confusion, as it describes a heterogeneous set of behaviors that manifest in distinct contexts and over distinct timescales (Evenden 1999). When recognized clinically, impulsivity is most often associated with maladaptive patterns of behavior. In recent years, a broad distinction has been made between ‘motor’ and ‘motivational’ impulsivity (Bari and Robbins 2013), where motor impulsivity describes inappropriate motor reactions to immediate circumstances or stimulus events on a millisecond timescale, and ‘motivational impulsivity’ characterizes decisions that lack reflection, forethought, patience, and consideration of long-term consequences and reward contingencies (Bari and Robbins 2013). In human and animal models, these two manifestations of impulsivity are linked to distinct neural mechanisms (Bechara 2005, Kenner, Mumford et al. 2010), and can be dissociated using germane cognitive tasks, thus providing a useful framework for classifying clinically observed forms of impulsive behavior.
Emergence of ‘impulsive behaviors’ as a consequence of medical therapy in Parkinson Disease (PD) is most often attributed to pharmacologic manipulations of dopamine, which include the use of the dopamine precursor levodopa and dopamine receptor agonists (DAAg)(Weintraub, Koester et al. 2010). The administration of DAAg (and to a much lesser extent, levodopa) has been linked to the development of Impulse Control Disorder (ICD) in approximately 15–20% of patients (Voon, Hassan et al. 2006, Weintraub, Koester et al. 2010). ICD describes excessive interest and participation in certain reward-driven behaviors, expressed in shopping, gambling, eating, sex, and hobbies (Ahlskog 2011). An understanding of the underlying neurocognitive processes that drive such marked behavioral changes is starting to emerge, but generally remains limited. Determining if ICD behaviors are linked to motor or motivational impulsivity would provide a significant advance in our understanding of the phenomenology of these behaviors. Some studies suggest that, compared to PD patients without ICD, individuals with a history of ICD prefer smaller immediate rewards over larger delayed rewards (i.e., show larger delay discounting effects) (Voon, Pessiglione et al. 2010), and those with active ICD symptoms pursue riskier choices (Claassen, van den Wildenberg et al. 2011). Neuroimaging studies highlight differences between patients with and without a history of ICD in mesocorticolimbic circuitry involved in risk decision-making, reward evaluation, and reward learning (Rao, Mamikonyan et al. 2010, van Eimeren, Pellecchia et al. 2010, Voon, Pessiglione et al. 2010, (Ray et al., 2012). Thus, ICD may represent an emergence of maladaptive ‘appetitive’ behaviors stemming from dopamine-mediated effects on the mesocorticolimbic network.
Few investigations have studied the role of motor impulsivity in ICD patients. We recently investigated differences between PD patients with and without active symptoms of ICD, in the susceptibility to acting on prepotent motor impulses and the proficiency of inhibiting interference from these impulses (Wylie, Claassen et al. 2012). Contrary to a motor impulsivity hypothesis, patients with active ICD showed a reduced tendency to act incorrectly on strong motor impulses compared to patients without ICD, irrespective of whether they performed under DAAg withdrawal or administration. Additionally, both groups showed similar proficiency in inhibiting interference from impulsive actions when tested withdrawn from DAAg and similar impairment to inhibitory control when tested On medication. These findings (Wylie, Claassen et al. 2012) provide the motivation to determine if PD-ICD patients have an enhanced susceptibility to acting on motor impulses or reduced ability to inhibit strong motor impulses.
To further investigate the role of motor impulsivity in PD patients with active ICD, we studied the speed at which patients are able to stop already-initiated movements. The gold standard for measuring stopping control is the stop-signal task, which requires participants to make speeded choice reactions to ‘go’ stimuli, but stop reactions upon the infrequent and unpredictable occurrence of a ‘stop’ stimulus, presented within a few hundred milliseconds after the onset of a ‘go’ stimulus (Logan 1994). The task measures the proficiency (i.e., latency) of interrupting or canceling the preparation of an initiated overt response. Prolonged stop signal reaction time (SSRT) is described in clinical populations characterized by impulsive behaviors and poor inhibitory control, including patients with attention-deficit hyperactivity disorder (Oosterlaan, Logan et al. 1998), substance abuse (Monterosso, Aron et al. 2005) (Fillmore and Rush 2002), obsessive-compulsive disorder (Krikorian, Zimmerman et al. 2004), and schizophrenia (Badcock, Michie et al. 2002). More so, individuals rating high on impulsive traits also have longer SSRTs (Logan, Schachar et al. 1997, van den Wildenberg and Christoffels 2010), thus reduced motor control is directly associated with impulsive behavior.
Here we assessed performance on the stop-signal task in PD patients with active ICD, patients without ICD, and healthy matched controls. All PD patients were taking DAAg, and groups were carefully matched for disease duration, duration of DAAg use, dose of DAAg and levodopa, and motor symptom severity. To determine if the presence of DAAg was critical to stopping effects, the stop-signal task was competed on optimal dopaminergic medication, and after withdrawing selectively from DAAg. Consistent with previous findings, we predicted that PD patients would show slower SSRTs when compared to healthy controls (Gauggel, Rieger et al. 2004). Support for the role of motor impulsivity in ICD was expected to manifest as exacerbated slowing of SSRT as compared to PD patients without ICD. Finally, we expected a role for DAAg in stopping control to be revealed by differences in stopping speed on versus temporarily withdrawn from DAAg medication.
Materials and Methods
Participants
Study participants included 24 PD patients and 12 healthy controls. All PD patients met diagnostic criteria based on the UK Brain Bank, and were diagnosed by a Movement Disorder Neurologist (D.C) (Hughes, Daniel et al. 1992). All participants were formally screened for global cognitive impairment (Mini-Mental Status Examination, MMSE; (Folstein, Folstein et al. 1975)) and depression (Center for Epidemiological Studies-Depression Scale, CESD; (Radloff 1977)). Motor symptom severity in the On medication state was graded using the UPDRS part III motor score ((Fahn, Elton et al. 1987)). All dopamine medications were converted to levodopa daily dose equivalent (LEDD) using previously reported formulas (Weintraub, Siderowf et al. 2006). See Table 1 for participant details. All participants had normal or corrected-to-normal vision. Participants were screened to ensure they did not have a history of any neurological condition other than PD, mood disorder such as major depression, history of bipolar affective disorder, schizophrenia, or other psychiatric condition with known effects on cognition, or an untreated or unstable medical condition known to interfere with cognition. Prior to study entry, all participants provided informed consent, which was compliant with standards of ethical conduct in human investigation as regulated by the institutional review board.
Table 1.
Participant Characteristics
| HC (n = 12) | PD-ICD (n = 12) | PD-Control (n=12) | |
|---|---|---|---|
| Age (years) | 58.5 (6.3) | 59.4 (5.5) | 60.8 (7.2) |
| Education (years) | 15.3 (2.9) | 17.1 (2.7) | 16.3 (2.8) |
| Gender (male:female) | 6:6 | 8:4 | 6:6 |
| MMSE** | 28 (1.7) | 29 (1.6) | 28.7 (1.6) |
| CES-Depression Score | 7.0 (6.2) | 11.8 (7.7) | 8.7 (5) |
| Disease Duration (years) | - | 6.5 (4.7) | 6.1 (3.8) |
| UPDRS Motor Score | - | 15.9 (6.6) | 15.7 (8.3) |
| Patients on DA Agonist Monotherapy | - | 5 | 5 |
| DA Agonist Duration (years) | - | 3.4 (3) | 2.7 (2) |
| Levodopa Dose (mg) | - | 408.2 (349.6) | 319.7 (318.9) |
| DA Agonist Dose in LEDD (mg) | - | 293.8 (167.4) | 200.6 (116.8) |
| Total LEDD (mg) | - | 618.7 (361.9) | 520.3 (314.9) |
Values represent mean scores with standard deviations reported in parentheses.
Comparisons between Parkinson Disease patients with ICD (PD-ICD) and PD patients without ICD (PD-Control) were not statistically significant (p>0.05).
ICD = impulse control disorder; MMSE = mini-mental state examination; CES = Center for Epidemiological Studies; DA= Dopamine; LEDD = levodopa equivalent daily dose.
Healthy controls completed the Montreal Cognitive Assessment (MoCA) in place of the MMSE.
All PD patients were taking DAAg, and about half were taking concomitant levodopa therapy. Both patients and a family member completed the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s disease to screen for the presence or absence of active ICD behaviors (Weintraub, Mamikonyan et al. 2012). All patients were interviewed by a neurologist (D.C.) and a neuropsychologist (S.W.) to confirm the presence or absence of ICD symptoms based on published criteria (McElroy, Keck et al. 1994, American Psychiatric Association 2000, Grant, Steinberg et al. 2004, Voon, Hassan et al. 2006). For those meeting ICD criteria, we confirmed the emergence of ICD symptoms subsequent to DAAg initiation. Behaviors included excessive participation, and heightened interest in sexual behaviors (5/12), shopping or buying (5/12), eating (6/12), and time spent on a hobby (9/12). Most patients endorsed at least two of the behaviors (11/12) listed above, and 2 patients endorsed three or more behaviors. PD controls (PD-C) did not meet criteria for any ICD behaviors based on screening and interview, and closely matched age, disease duration, UPDRS motor score, dose and duration of DAAg, and LEDD of the PD-ICD cohort.
PD participants completed two testing visits, once On, and once Off, DAAg therapy (i.e., after a 24 hour withdrawal). The order of sessions was counterbalanced, and levodopa therapy was not altered for either testing session. Healthy controls without PD completed a single testing session.
Stop Signal Task
We used a manual version of the Stop-Signal task requiring a speeded button press to a series of directional arrows presented one at a time in the center of a computer monitor. Following the display of a small fixation point, a green-colored arrow, pointing to the left or to the right, appeared on the screen, and participants were instructed to make a left or right hand button press based on the direction of the arrow (e.g., left pointing arrow = left button press). Responses were registered by depression of a button (using the thumbs) on the end of handheld grips. Participants were instructed to respond as quickly and as accurately as possible to green arrows (go trials). After a button press was issued or 1200 ms lapsed without a response, the arrow disappeared, and a random interstimulus interval ranging from 1250–1750 (in increments of 100 ms) transpired before the onset of the next green arrow. The fixation point remained on the screen during the interstimulus interval.
On 30% of the trials, the green arrow changed color to red shortly after its onset, and participants were instructed to try to stop their button press when the arrow turned red (stop trials). The timing of the delay between the onset of the green arrow and the onset of the color change (stop-signal delay, SSD) was set initially at 200 ms and then adjusted dynamically across stop trials using a staircase-tracking procedure that controlled for the success of stopping (i.e., inhibition probability; (Levitt 1971)). Following a successful stop, the SSD for the next stop trial was delayed by 50 ms, thus making it more difficult to stop. Following an unsuccessful stop, the SSD for the next stop trial was shortened by 50 ms, effectively making it easier to stop. These adjustments ensured that responses were successfully inhibited in approximately half of the stop trials, a requirement for estimating stop-signal reaction time that compensates for individual differences in choice reaction time to the go arrows (Band, van der Molen et al. 2003). SSRT was computed using the integration method described by Logan (Logan, Cowan et al. 1984). Participants first completed a block of 60 practice trials. Next, they completed 5 blocks of 60 experimental trials, yielding 90 total stop trials, which is more than adequate for producing a reliable estimate of SSRT (Band, van der Molen et al. 2003).
Statistical Techniques and Design
Extreme RT values, either excessively fast (so-called anticipatory errors; < 150 ms) or slow (> 3 standard deviations), were removed from the analysis using a combination of statistical procedures (e.g., value > 3 standard deviations above the mean) followed by visual inspection to ensure that only extreme outliers were excluded. On average, these procedures led to the exclusion of less than 0.5% of trials per subject. Three key dependent measures were computed: mean reaction time to correct go trials (GoRT), mean accuracy to go trials (GoAcc), and stop-signal reaction time (SSRT). The probability of successful inhibition on stop trials was computed to verify that the tracking algorithm approximated the targeted 50% stop success rate (Band, van der Molen et al. 2003). An additional measure, the mean RT for unsuccessfully inhibited responses on stop trials (i.e., signal-respond RT), was computed and compared to mean go RT to verify a key assumption of the race model regarding the independence of the go and stop processes that is required to estimate stopping latency (SSRT) reliably (Logan 1994); specifically, mean signal-respond RT should be shorter than mean GoRT.
We conducted three primary analyses. First, healthy controls without PD were compared to both PD groups in the On medication state. Previous work has shown that stopping is slowed in medicated PD patients (Gauggel, Rieger et al. 2004). This analysis included a single between-subjects factor of Group (PD-C, PD-ICD, HC). The dependent measures were analyzed separately using repeated-measures analysis of variance techniques (Huynh-Feldt adjustments for violations of sphericity) to determine the effect of Group on choice reaction time and accuracy (GoRT, GoAcc) and on speed of inhibition (SSRT). Next, we compared the two groups of PD patients On and Off DAAg. The primary design included one between-subjects factor, ICD Group (PD-C, PD-ICD), and one within-subjects factor, Agonist State (On, Off). The dependent measures were analyzed separately using repeated-measures analysis of variance techniques (Huynh-Feldt adjustments for violations of sphericity) to determine the main and interactive effects of Group and Agonist State on choice reaction time and accuracy (GoRT, GoAcc) and on stopping proficiency (SSRT). Because half of the PD patients were taking levodopa co-therapy, a third analysis included an additional between-subjects factor, Levodopa Status (sine L-Dopa, cum L-Dopa), to capture any influence of levodopa co-therapy on key dependent measures.
Results
Comparisons between healthy controls (HC) and medicated PD patients
Choice reaction performance (RT and accuracy on Go Trials): Mean RT and accuracy rates to go arrows (Figure 1a) did not differ among HC and PD subgroups, (Group: RT - F(2, 33)=.528, p=.594; Accuracy - F(2, 33)=.123, p=.885). All groups showed high accuracy rates to go arrows.
Stop-Signal Reaction Time (SSRT): The estimate of SSRT requires verification that (a) stopping accuracy approximated 50%, and (b) mean RT for failed stop trials is shorter than mean RT for go trials. Both conditions were satisfied across groups. Specifically, stopping accuracy was similar and near 50% for all groups (HC=49.2%, PD-C=50.8%, PD-ICD=50.5%), (Group, F(2,33)=.248, p=.782). Overall, mean signal-respond RTs was similar across groups, (Group, F(2,33)=.811, p=.453), and an average of 84 ms faster than mean RTs for go trials, (Trial Type (Go, Failed Stop), F(1,33)=79.149, p<.001), which did not differ among groups, (Group x Trial Type, F(2,33)=.637, p=.535). These analyses confirm the success of the tracking algorithm and the reliability of the estimate of stopping latency (SSRT) across groups. Mean SSRTs for each group are shown in Figure 1b, which reveals a significant effect of Group on SSRT, (Group, F(2,33)=4.411, p=.02). Post-hoc comparisons referenced to the HC group (using Dunnett’s post hoc test) revealed that PD-ICD patients stopped faster than HCs (p=.036), whereas PD-C patients showed similar SSRTs compared to HCs (p=.966). See Table 2 for illustration of these findings.
Figure 1.
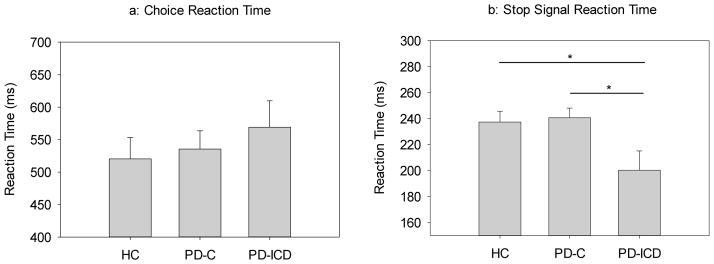
a) Mean reaction times (RT) to go arrows as a function of Group. All groups showed similar mean reaction times. Error bars reflect the standard error of the mean. b) Stop signal reaction times (SSRT) as a function of Group. PD-C patients showed similar SSRTs compared to HCs, with PD-ICD patients producing faster SSRTs than both PD-C patients and HCs. Error bars reflect the standard error of the mean.
Table 2.
Group differences in Stop-signal performance
| HC (n=12) | PD-Control (n=12) | PD-ICD (n=12) | |||
|---|---|---|---|---|---|
| ON | OFF | ON | OFF | ||
| Choice RT | 520 (113) | 535 (98) | 515 (133) | 569 (141) | 526 (118) |
| Choice Error | 1.8 (2.1) | 1.7 (1.3) | 2.3 (2.1) | 1.5 (1.2) | 1.4 (1.0) |
| Stop Probability | 49.2 (5.4) | 50.8 (3.8) | 49.2 (3.3) | 50.5 (7.8) | 48.9 (3.5) |
| Signal Respond RT | 450 (93) | 439 (42) | 447 (89) | 482 (109) | 455 (95) |
| Stop Signal RT | 237 (29) | 241 (26) | 227 (35) | 200 (52) | 194 (33) |
OHC= Older Healthy Controls; PD= Parkinson Disease; ICD= Impulse Control Disorder; RT= Reaction Time; ON= Testing 1 hour after Dopamine Agonist; OFF= Testing after 24 hour washout of Dopamine Agonist
Comparisons between PD-ICD and PD-C groups On and Off DAAg
Choice reaction performance (RT and accuracy on Go Trials): Mean RT and accuracy rates to go arrows (Figure 2a) did not differ between PD groups, (ICD Group: RT - F(1, 22)=.379, p=.545; Accuracy - F(1, 22)=1.300, p=.267), or between On and Off DAAg medication states (Agonist State: RT - F(1, 22)=.858, p=.364; Accuracy - F(1, 22)=.574, p=.457). Moreover, the groups showed similar patterns of mean RT and accuracy rates On and Off DAAg medication, (ICD Group x Agonist State: RT - F(1, 22)=.103, p=.751; Accuracy - F(1, 22)=.399, p=.534).
Stop-Signal Reaction Time (SSRT): We first verified the reliability of the estimate of SSRT. Specifically, stopping accuracy was similar and near 50% when On (50.6%) or Off (49.0%) DAAg, (Agonist State, F(1,22)=.858, p=.364), and both groups showed similar stopping accuracy that approximated 50% (PD-C: 50.0%, PD-ICD: 49.7%), (ICD Group, F(1,22)=.092, p=.764), irrespective of DAAg state, (ICD Group x Agonist State, F(1,22)=.000, p=1.00). Additionally, mean RTs for failed stop trials (456 ms) were 80 ms faster than mean RTs for go trials (536 ms), (Trial Type, F(1,22)=80.68, p<.001). This pattern was preserved On or Off DAAg, (Agonist State x Trial Type, F(1,22)=2.015, p=.170), and independent of ICD group status, (ICD Group x Trial Type, F(1,22)=.036, p=.852), irrespective of the DAAg state (ICD Group x Agonist State x Trial Type, F(1,22)=.149, p=.703). These analyses confirm the success of the tracking algorithm and the reliability of the estimate of stopping latency across PD subgroups and DAAg states (SSRT).
Figure 2.
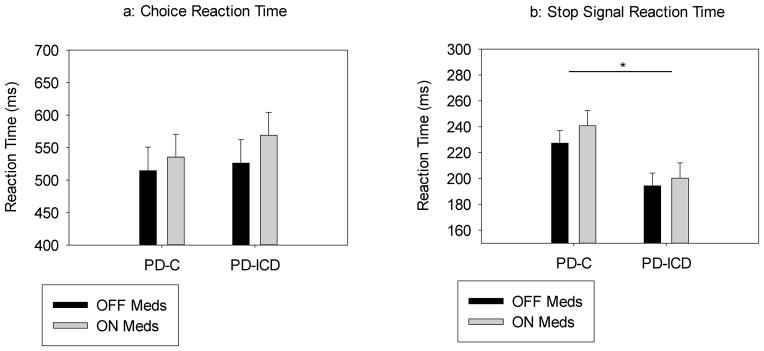
a) Mean reaction times (RT) to go arrows On and Off DAAg medications for PD groups (PD-C and PD-ICD). Mean reaction times were similar between groups and across medication states. Error bars reflect the standard error of the mean. b) Stop signal reaction times (SSRT) On and Off DAAg medications for PD groups (PD-C and PD-ICD). Mean SSRTs were comparable when patients were On or Off DAAg, however, the PD-ICD group showed faster SSRTs when compared to the PD-C group, independent of medication state. Error bars reflect the standard error of the mean.
Mean SSRTs for each group and medication state are presented in Figure 2b. Mean SSRT was similar when patients were On or Off DAAg, (Agonist State, F(1, 22)=1.243, p=.277). However, the PD-ICD group showed faster SSRT (i.e., more proficient inhibition) compared to the PD-C group, (ICD Group, F(1,22)=5.558, p=.008), a pattern that was preserved across DAAg states, (ICD Group x Agonist: F(1,22)=0.188, p=.669). See Table 2 for illustration of these findings.
Effects of levodopa co-therapy on Go and Stop measures
An equivalent, but slight, majority (58%) of patients in both groups were taking both levodopa and DAAg dual therapy and remained on their usual dose of levodopa for both testing sessions. The remaining 42% of patients, who were taking DAAg monotherapy, were tested On and Off medication. Thus, a difference in performance between these subgroups might relate to the role of levodopa. To examine this effect, we included an additional between-subjects factor, Levodopa Status (sine L-Dopa, cum L-Dopa), in the analysis comparing PD-ICD and PD-C subgroups. None of the aforementioned patterns regarding main or interactive effects of ICD Group and DAAg State on any of the dependent measures changed, thus we only describe results pertaining to the effects of Levodopa Status.
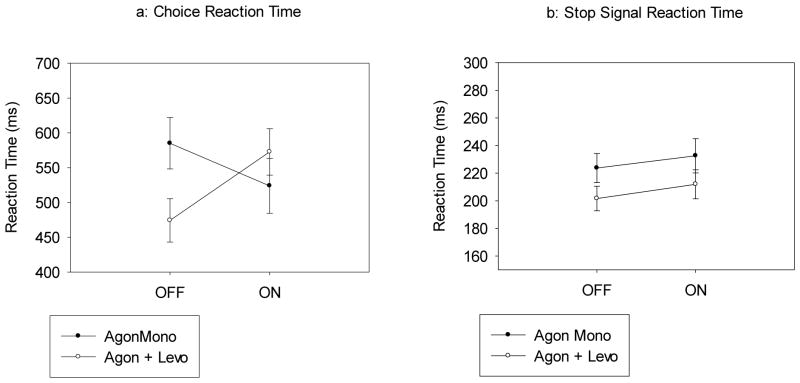
Choice reaction performance (RT and accuracy on Go Trials): Mean RT and accuracy rates on go trials did not differ between patients taking (523 ms, 98.1%) and not taking (554 ms, 98.5%) L-Dopa, (Levodopa Status: RT - F(1,20)=0.654, p=.428; Accuracy - F(1, 20)=.851, p=.367), irrespective of ICD status: (PD-ICD: sine L-Dopa =559 ms, 98.3%; cum L-Dopa=540 ms, 98.6%) (PD-C: sine L-Dopa =550 ms, 98.6%; cum L-Dopa=507 ms, 97.6%) (Levodopa Status x ICD Group: RT - F(1,20)=.093, p=.763; Accuracy - F(1, 20)=2.297, p=.145). However, the presence of levodopa significantly influenced the effect of DAAg on RTs, but not accuracy rate (Levodopa Status x Agonist State: RT - F(1,20)=6.211, p=.022; Accuracy - F(1,20)=.017, p=.898). The interaction on RT is illustrated in Figure 3a. Patients taking DAAg monotherapy showed a significant speeding of RT On compared to Off. The opposite effect was observed in dual-therapy patients; the addition of DAAg to levodopa caused a slowing of RT compared to when these patients performed only On levodopa. Notably, these patterns on RT and on accuracy rates did not vary with ICD status, (Levodopa Status x Agonist State x Group: RT - F(1,20)=.008, p=.928; Accuracy - F(1,20)=2.484, p=.131).
Stop-Signal Reaction Time (SSRT). Levodopa status did not alter the probability of stopping success nor the pattern of faster RTs for unsuccessful stop trials compared to RTs for go trials (all ps>.10), indicating that SSRT was estimated reliably and uniformly across all between-subject groups. Mean SSRT was marginally, but non-significantly, faster among patients taking (207 ms) compared to not taking (228 ms) levodopa dual therapy (Figure 3b), (Levodopa Status, F(1,20)=3.050, p=.096), a pattern that remained unchanged across ICD groups, (PD-ICD: sine L-Dopa=216 ms, cum L-Dopa=184 ms; PD-C: sine L-Dopa=240 ms, cum L-Dopa=230 ms)(Levodopa Status x ICD Group, F(1,20)=.819, p=.376), DAAg state, (Off Agonist: sine L-Dopa=224 ms, cum L-Dopa=202 ms; On Agonist: sine L-Dopa=233 ms, cum L-Dopa=212 ms)(Levodopa Status x Agonist State, F(1,20)=.006, p=.938), and the combination of these factors, (Levodopa Status x Agonist State x ICD Group, F(1,20)=2.059, p=.167). See Figure 3 and Table 3 for an illustration of these findings.
Figure 3.
a) Mean reaction times (RT) to go arrows as a function of Group (DAAg Monotherapy, DAAg with Levodopa cotherapy) and Medication State (on DAA, off DAA). Patients taking DAAg monotherapy showed a significant speeding of RT On compared to Off DAA medications. Patients taking both DAAg and Levodopa showed a reduction in RT when compared to task performance on Levodopa only. Error bars reflect the standard error of the mean. b) Stop signal reaction time (SSRT) as a function of Group (DAAg Monotherapy, DAAg with Levodopa cotherapy) and Medication State (on DAA, off DAA). Mean SSRT was marginally, but non-significantly, faster among patients on Levodopa cotherapy compared to those on DAAg Monotherapy, regardless of medication state. Error bars reflect standard error of the mean.
Table 3.
Stop-signal performance in patients on Dopamine Agonist Monotherpy versus Levodopa co-therapy
| All patients | PD-C | PD-ICD | |||
|---|---|---|---|---|---|
| ON | OFF | ON | OFF | ||
| DA Agonist Monotherapy (n=10) | |||||
| Choice RT | 530 (76) | 512 (45) | 588 (158) | 535 (102) | 582 (167) |
| Stop Signal RT | 213 (22) | 239 (32) | 241 (47) | 226 (35) | 206 (37) |
| DA Agonist + Levodopa (n=14) | |||||
| Choice RT | 548 (97) | 552 (124) | 462 (89) | 593 (168) | 486 (47) |
| Stop Signal RT | 222 (30) | 242 (23) | 217 (22) | 182 (56) | 185 (30) |
PD= Parkinson Disease; ICD= Impulse Control Disorder; RT= Reaction Time; ON= Testing 1 hour after Dopamine Agonist; OFF= Testing after 24 hour washout of Dopamine Agonist; DA= Dopamine
Discussion
The goal of this study was to directly test the motor impulsivity hypothesis of ICD in PD by investigating, for the first time, the speed with which patients with ICD inhibit initiated motor actions. The stop-signal task is a gold standard in measuring the speed of motor inhibition, and prolonged stopping has been directly linked to impulsive traits and patient groups. In PD-patients with moderate to severe disease severity, SSRT is typically delayed (Gauggel, Rieger et al. 2004). One study demonstrated that dopamine therapy has minimal influence on SSRT, compared to a dopamine withdrawn state, although levodopa and DAAg effects were treated collectively rather than separately (Obeso, Wilkinson et al. 2011). In contrast, bilateral subthalamic nucleus (STN) deep brain stimulation appears to improve stopping control (van den Wildenberg, van Boxtel et al. 2006). These studies indicate that changes in stopping control and, inferentially, susceptibility to motor impulsivity are vulnerable cognitive processes in PD.
Stopping RTs (SSRTs) and choice RTs were reliably measured in PD groups with and without ICD and in healthy controls. Choice RTs and accuracy to go stimuli did not differ among the ICD and non-ICD PD groups, suggesting that processes involved in the initiation and execution of speeded reactions were very similar across the groups. In striking contrast to the motor impulsivity hypothesis of ICD, patients with active ICD were significantly faster at stopping initiated motor actions compared to healthy controls and PD patients without ICD. The transient withdrawal from DAAg did not alter the SSRT advantage in inhibitory motor control for patients with ICD. In fact, SSRT did not vary whether PD patients, irrespective of ICD status, performed the stop-signal task on or withdrawn from DAAg. Finally, whether patients were or were not taking levodopa co-therapy had no influence on SSRTs, although the effect of DAAg on choice RT was strongly influenced by levodopa co-therapy.
Faster Stop-Signal Reaction Time in PD-ICD: Why?
These results expand evidence that ICD does not involve fundamental changes in the ability to inhibit motor behavior, a finding that also calls into question that PD-ICD involves fundamental deficits in motor impulsivity. Rather, PD-ICD patients show more proficient stopping control, a finding that is quite consistent with previous work showing that PD patients with ICD, compared to patients without ICD, showed reduced susceptibility to acting on strong motor impulses elicited in a response conflict task (Wylie, Claassen et al. 2012). Together, these findings suggest the contrary view that patients with active ICD are more proficient at inhibiting both intended and impulsive motor actions. How might this be explained? First, animal studies and human imaging work show that higher D2 -like receptor availability in the dorsal striatum is associated with faster SSRTs (Eagle and Robbins 2003, Ghahremani, Lee et al. 2012) and administration of agonists that target these receptors also speed SSRT (Nandam, Hester et al. 2013). In contrast, D2-like receptor antagonism slows SSRT, and self-reported impulsivity has been linked to slower SSRTs and reduced midbrain D2-like receptor availability (Lee, London et al. 2009)(Logan, Schachar et al. 1997, Buckholtz, Treadway et al. 2010). Thus, the finding that PD patients with active ICD show markedly faster SSRTs than PD and healthy controls may directly reflect a fundamental change or difference in D2-like receptor profiles in dorsal striatum, subsequent to chronic dopamine agonist use. Based on the aforementioned patterns, it could be hypothesized that PD patients with ICD have an increased D2 receptor availability that leads to faster, rather than slower, inhibitory motor control. Notably, no evidence to date has suggested differences in dopamine D2 receptor polymorphisms among those with and without ICD, even though specific variations in dopamine genetics have been linked to individual differences in SSRT in healthy adults (Cummins, Byrne et al., Congdon, Lesch et al. 2008), and see (Vallelunga, Flaibani et al. 2012) for study of dopamine genetics in PD patients with and without ICD). This will be an important area for future investigations.
A second explanation can be deduced from the assertion that dopamine activity at D2 receptors in dorsal striatum facilitates the braking of motor actions (Eagle, Wong et al. 2011). Moreover, a right lateralized network inclusive of specific prefrontal (right inferior cortex, pre-supplementary motor area) and basal ganglia (subthalamic nucleus, caudate nucleus) structures is proposed to mediate inhibitory action control (Ridderinkhof, van den Wildenberg et al. 2004). A recent study reported that, compared to non-ICD patients, PD patients with ICD show reduced dopamine transporter binding in the right striatum (Voon, Rizos et al. 2014). Thus, ICD patients may experience diminished dopamine clearance in right basal ganglia, the effect of which may be the facilitation of inhibitory control via D2 activation. Future studies might examine how striatal dopamine receptors and function, particularly in the right hemisphere, are differentially modified by chronic dopamine therapy, and the development of ICD.
Levodopa and Dopamine Agonist Effects on Motor Control
The acute administration of DAAg did not influence SSRTs compared to a temporarily withdrawn state. Animal studies of the stop task have found modulation of SSRT by dopamine D2 agonism (faster SSRT) and antagonism (slower SSRT). One difficulty in equating prior work with the current study is the fact that PD patients were treated with DAAg chronically, which likely produces different dopamine receptor and neurochemical effects. Our patients were also withdrawn for a minimum of 24 hours, which may not have been sufficient to fully eliminate DAAg. A more effective approach might consider the acute and chronic effects of DAAg medication on SSRT in de novo PD patients who are tracked longitudinally subsequent to initiating DAAg or levodopa therapy.
The acute administration of dopamine medication did impact choice RTs (i.e., go RTs) in PD patients, and the direction of the effect depended on concurrent levodopa use. Among patients taking only DAAg monotherapy, choice RTs were significantly faster when patients performed under the influence of DAAg compared to the withdrawn state. In contrast, in patients taking levodopa co-therapy, DAAg slowed choice RTs in patients taking levodopa. To the best of our knowledge, we have not seen this interaction reported previously, and very few studies have directly compared the levodopa and DAAg and their interactions on specific cognitive processes. Dopamine stimulation, via either mechanism, is typically associated with faster RTs, whereas dopamine antagonism usually slows RT (Eagle, Tufft et al. 2007). The current findings raise the possibility that levodopa (D1/D2 effects) and receptor agonists (D2/D3 effects) interact in complex ways, possibly by shifting the balance between the putative “go” pathway (i.e., D1-mediated direct pathway) and the “stop” pathway (i.e., D2-mediated indirect pathway). We suspect that the nature of this interaction is likely to depend on several factors, including an individual’s dopamine genetic polymorphism, baseline reaction time Off medications, and relative doses of DAAg and levodopa. It will be important for future work to understand these effects to optimize medication effects, and facilitate (rather than impede) RT.
Stop Signal Reaction time in Parkinson Disease
One notable finding in the current study is absence of SSRT differences between PD controls and healthy controls. Previous studies have generally confirmed a stopping speed deficit in PD patients (Gauggel, Rieger et al. 2004). The difference between this and the Gauggel et al study is the sample of PD patients--patients in our study were earlier in the disease course (6 versus 9 year duration of symptoms), and less severe (Hoehn and Yahr 1–2 versus 2.6). We speculate that global stopping speed deficits develop in more moderate stages of PD when dopamine degeneration is more likely to disrupt putative cognitive circuitries. Again, longitudinal studies to detect the emergence of inhibitory control deficits are desperately needed.
A second noteworthy issue is that PD patients with ICD included in the current investigation were all studied before any reduction or discontinuation of DAAg medication was initiated, and were actively symptomatic with ICD symptoms. This differs from many studies in the literature that investigated PD patients with a history of ICD, but who were no longer displaying active symptoms, or on the dosage of the offending medication. Few, if any, studies have investigated changes in cognitive functioning in ICD patients tested during active ICD and after DAAg discontinuation. Whether the SSRT advantage remains or dissipates following chronic withdrawal from DAAg may offer insights n
Are PD patients with Impulsive and Compulsive Behaviors really impulsive?
In light of past studies in PD ICD, the nature of impulsivity that develops in a subset of PD patients taking DAAg appears to weigh in favor of a ‘motivational,’ or ‘affective’ account of impulsivity rather than a motor impulsivity account. This distinguishes the nature of impulsivity in PD-ICD from that of other patient groups who show clear impulsive motor behavior (e.g., ADHD, OCD, substance abuse). Previous imaging and behavioral studies of ICD have focused on mesocorticolimbic-ventral striatal network in response to dopamine therapy. These studies link baseline differences in ventral striatal D2-like receptors, to exaggerated mesocorticolimbic dopamine release in patients with ICD (Rao, Mamikonyan et al. 2010, O’Sullivan, Wu et al. 2011). Previous work has also linked ICD with enhanced risk-taking and reward behavior emphasizing alterations to mesocorticolimbic function (Claassen, van den Wildenberg et al. 2011). Future work must reconcile the emergent dysfunction of risk-taking and reward and associated mesocorticolimbic circuitries and the apparent enhancement of motor inhibitory control.
Highlights.
The study assesses Impulsive and Compulsive Behaviors (PD-ICD) in Parkinson Disease.
Motor impulsivity was tested in the presence and absence of Dopamine Agonist therapy.
PD-ICD patients are significantly faster at stopping initiated motor actions.
Dopamine Agonist therapy does not alter stop signal reaction time.
Go Reaction Time is slower when Dopamine Agonist is added to levodopa therapy.
Acknowledgments
The authors would like to thank Dr. G. Frederick Wooten for his guidance on patient recruitment and study design.
Funding
Funding provided by NIH/NINDS K23 NS080988 and the American Academy of Neurology to Dr. Claassen, and NIH/NIA K23AG028750 to Dr. Wylie
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlskog JE. Pathological behaviors provoked by dopamine agonist therapy of Parkinson’s disease. Physiol Behav. 2011;104(1):168–172. doi: 10.1016/j.physbeh.2011.04.055. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, A. P. A. T. F. o. D. S. M. I. V. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Badcock JC, Michie PT, Johnson L, Combrinck J. Acts of control in schizophrenia: dissociating the components of inhibition. Psychological Medicine. 2002;32(2):287–297. doi: 10.1017/s0033291701005128. [DOI] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychol (Amst) 2003;112(2):105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic network differences in human impulsivity. Science. 2010;329(5991):532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen DO, van den Wildenberg WP, Ridderinkhof KR, Jessup CK, Harrison MB, Wooten GF, Wylie SA. The risky business of dopamine agonists in Parkinson disease and impulse control disorders. Behav Neurosci. 2011;125(4):492–500. doi: 10.1037/a0023795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Lesch KP, Canli T. Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: Implications for impulsivity. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B(1):27–32. doi: 10.1002/ajmg.b.30557. [DOI] [PubMed] [Google Scholar]
- Cummins TDR, Byrne M, Hawi Z, Bellgrove M. Dopamine gene variants are associated with EEG measures of error processing. Frontiers in Human Neuroscience [Google Scholar]
- Dickman SJ. Functional and dysfunctional impulsivity: personality and cognitive correlates. J Pers Soc Psychol. 1990;58(1):95–102. doi: 10.1037//0022-3514.58.1.95. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Inhibitory Control in Rats Performing a Stop-Signal Reaction-Time Task: Effects of Lesions of the Medial Striatum and d-Amphetamine. Behavioral Neuroscience. 2003;117(6):1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Tufft MR, Goodchild HL, Robbins TW. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology (Berl) 2007;192(2):193–206. doi: 10.1007/s00213-007-0701-7. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Wong JCK, Allan ME, Mar AC, Theobald DE, Robbins TW. Contrasting Roles for Dopamine D1 and D2 Receptor Subtypes in the Dorsomedial Striatum but Not the Nucleus Accumbens Core during Behavioral Inhibition in the Stop-Signal Task in Rats. The Journal of Neuroscience. 2011;31(20):7349–7356. doi: 10.1523/JNEUROSCI.6182-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146(4):348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, Committee UD. Unified Parkinson’s disease rating scale. Recent developments in Parkinson’s disease. 1987;2:153–163. [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug and Alcohol Dependence. 2002;66(3):265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gauggel S, Rieger M, Feghoff T-A. Inhibition of ongoing responses in patients with Parkinson’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75:539–544. doi: 10.1136/jnnp.2003.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, Brown AK, Monterosso JR, Aron AR, Mandelkern MA, Poldrack RA, London ED. Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci. 2012;32(21):7316–7324. doi: 10.1523/JNEUROSCI.4284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Steinberg MA, Kim SW, Rounsaville BJ, Potenza MN. Preliminary validity and reliability testing of a structured clinical interview for pathological gambling. Psychiatry Res. 2004;128(1):79–88. doi: 10.1016/j.psychres.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenner NM, Mumford JA, Hommer RE, Skup M, Leibenluft E, Poldrack RA. Inhibitory motor control in response stopping and response switching. J Neurosci. 2010;30(25):8512–8518. doi: 10.1523/JNEUROSCI.1096-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikorian R, Zimmerman ME, Fleck DE. Inhibitory control in Obsessive-Compulsive Disorder. Brain and Cognition. 2004;54(3):257–259. doi: 10.1016/j.bandc.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal Dopamine D2/D3 Receptor Availability is Reduced in Methamphetamine Dependence and is Linked to Impulsivity. J Neurosci. 2009;29(47):14734. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. Journal of the Acoustical Society of America. 1971;49(2 Pt 2):467–477. [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In: Carr DDTH, editor. Inhibitory processes in attention, memory, and language. San Diego, CA: US, Academic Press; 1994. pp. 189–239. [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10(2):276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and Inhibitory Control. Psychological Science. 1997;8(1):60–64. [Google Scholar]
- McElroy SL, Keck PE, Jr, Pope HG, Jr, Smith JM, Strakowski SM. Compulsive buying: a report of 20 cases. J Clin Psychiatry. 1994;55(6):242–248. [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu JS, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and Alcohol Dependence. 2005;79(2):273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Nandam LS, Hester R, Wagner J, Dean AJ, Messer C, Honeysett A, Nathan PJ, Bellgrove MA. Dopamine D(2) receptor modulation of human response inhibition and error awareness. J Cogn Neurosci. 2013;25(4):649–656. doi: 10.1162/jocn_a_00327. [DOI] [PubMed] [Google Scholar]
- Obeso I, Wilkinson L, Jahanshahi M. Levodopa medication does not influence motor inhibition or conflict resolution in a conditional stop-signal task in Parkinson’s disease. Experimental Brain Research. 2011;213(4):435–445. doi: 10.1007/s00221-011-2793-x. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Logan GD, Sergeant JA. Response inhibition in AD/HD, CD, comorbid AD/HD + CD, anxious, and control children: a meta-analysis of studies with the stop task. J Child Psychol Psychiatry. 1998;39(3):411–425. [PubMed] [Google Scholar]
- O’Sullivan SS, Wu K, Politis M, Lawrence AD, Evans AH, Bose SK, Djamshidian A, Lees AJ, Piccini P. Cue-induced striatal dopamine release in Parkinson’s disease-associated impulsive-compulsive behaviours. Brain. 2011;134(Pt 4):969–978. doi: 10.1093/brain/awr003. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Rao H, Mamikonyan E, Detre JA, Siderowf AD, Stern MB, Potenza MN, Weintraub D. Decreased ventral striatal activity with impulse control disorders in Parkinson’s disease. Mov Disord. 2010;25(11):1660–1669. doi: 10.1002/mds.23147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray NJ, Miyasaki JM, Zurowski M, Ko JH, Cho SS, Pellecchia G, et al. Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson’s patients with medication-induced pathological gambling: a [11C] FLB-457 and PET study. Neurobiology of disease. 2012;48(3):519–25. doi: 10.1016/j.nbd.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and cognition. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Vallelunga A, Flaibani R, Formento-Dojot P, Biundo R, Facchini S, Antonini A. Role of genetic polymorphisms of the dopaminergic system in Parkinson’s disease patients with impulse control disorders. Parkinsonism Relat Disord. 2012;18(4):397–399. doi: 10.1016/j.parkreldis.2011.10.019. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WPM, Christoffels IK. STOP TALKING! Inhibition of speech is affected by word frequency and dysfunctional impulsivity. Frontiers in Psychology. 2010;1(145) doi: 10.3389/fpsyg.2010.00145.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wildenberg WP, van Boxtel GJ, van der Molen MW, Bosch DA, Speelman JD, Brunia CH. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. Journal of Cognitive Neuroscience. 2006;18(4):626–636. doi: 10.1162/jocn.2006.18.4.626. [DOI] [PubMed] [Google Scholar]
- van Eimeren T, Pellecchia G, Cilia R, Ballanger B, Steeves TD, Houle S, Miyasaki JM, Zurowski M, Lang AE, Strafella AP. Drug-induced deactivation of inhibitory networks predicts pathological gambling in PD. Neurology. 2010;75(19):1711–1716. doi: 10.1212/WNL.0b013e3181fc27fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Hassan K, Zurowski M, de Souza M, Thomsen T, Fox S, Lang AE, Miyasaki J. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67(7):1254–1257. doi: 10.1212/01.wnl.0000238503.20816.13. [DOI] [PubMed] [Google Scholar]
- Voon V, Pessiglione M, Brezing C, Gallea C, Fernandez HH, Dolan RJ, Hallett M. Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron. 2010;65(1):135–142. doi: 10.1016/j.neuron.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Rizos A, Chakravartty R, Mulholland N, Robinson S, Howell NA, Harrison N, Vivian G, Ray Chaudhuri K. Impulse control disorders in Parkinson’s disease: decreased striatal dopamine transporter levels. J Neurol Neurosurg Psychiatry. 2014;85(2):148–152. doi: 10.1136/jnnp-2013-305395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Mamikonyan E, Papay K, Shea JA, Xie SX, Siderowf A. Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale. Mov Disord. 2012;27(2):242–247. doi: 10.1002/mds.24023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, Moberg PJ, Stern MB. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006;63(7):969–973. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, Claassen DO, Huizenga HM, Schewel KD, Ridderinkhof KR, Bashore TR, van den Wildenberg WP. Dopamine agonists and the suppression of impulsive motor actions in Parkinson disease. J Cogn Neurosci. 2012;24(8):1709–1724. doi: 10.1162/jocn_a_00241. [DOI] [PMC free article] [PubMed] [Google Scholar]