Abstract
AIMS
To assess change in glycemic control concurrent with increased clinic visits, HbA1c testing, and education. Rates of complications were also examined.
METHODS
A 1–2 year follow-up of 214 members of the Rwanda Life for a Child program (aged < 26 years) with a first HbA1c between June 2009 and November 2010 was conducted. Data were analyzed for the entire cohort and by age (< 18 years, ≥ 18 years). Trajectory analysis was performed to identify trends in HbA1c.
RESULTS
Mean overall HbA1c decreased significantly from baseline (11.2±2.7%; 99±30 mmol/mol) to one- (10.2±2.6%; 88±28 mmol/mol) and two- (9.8±26%; 84±25 mmol/mol) year follow up visits. The prevalence of microalbuminuria did not significantly change (21.0%, 18.8%, and 19.6%), nor did nephropathy (4.7%, 7.8%, and 5.4%). However, rates of hypertension (31.8%, 44.9%, and 40.3%) were higher than expected. Five HbA1c groups were identified by trajectory analysis, and those with the worst control monitored their glucose significantly fewer times per week.
CONCLUSIONS
The establishment of regular care, HbA1c testing, and increased education is associated with significant improvements in glycemic control in youth with type 1 diabetes (T1D) in sub-Saharan Africa, but the high prevalence of hypertension is of concern.
Keywords: Youth, Diabetes, Africa
INTRODUCTION
Diabetes is a non-communicable disease (NCD) of increasing global concern, especially for resource-limited developing countries. In sub-Saharan Africa, an estimated 18.7 million people will be affected by this disease by 2025.[1] Access to necessary treatment is often limited in these areas, preventing patients from achieving the level of glycemic control necessary for the prevention/delay of complications.[2–5]
In order to address this problem, outside support has been necessary. One program providing such help is the International Diabetes Federation’s Life For a Child (LFAC) program, which is managed in conjunction with the Australian Diabetes Council and HOPE worldwide. LFAC’s mission is to support the provision of the best possible healthcare, given local circumstances, for children and youth with diabetes (≤ 25 years) in developing countries. This is achieved by strengthening diabetes services through the provision of insulin, glucose monitoring supplies, HbA1c testing, diabetes education and expert advice and training. One organization receiving assistance from LFAC is the Association Rwandaise des Diabetiques (ARD) in Kigali, Rwanda – the major specialized care provider for diabetic patients in Rwanda.
The Rwanda LFAC program at the ARD was initiated in 2004 with 25 children receiving support and annual clinic visits. The program has expanded since then, and as of the end of 2011, 634 children and young adults were enrolled. The ARD has also been aided by the University of Pittsburgh Graduate School of Public Health (UPGSPH), which sends a Masters of Public Health (MPH) Student each year to assist with the annual assessment of the youth.
We previously reported on the first 286 children and youth with type 1 diabetes (T1D) in the LFAC Rwanda program, who had their first HbA1c test between June 2009-November 2010.[6] The overall level of glucose control was poor with a mean HbA1c of 11.1±2.8% (99±30 mmol/mol), and 30.9% (n=88) having HbA1c above 14%. Complications were also already present in this population, despite the mean diabetes duration of only 3.4±3.1 years. Since baseline, the care provided by the ARD has evolved, with the support of GSPH and LFAC, through the implementation of quarterly clinic visits with HbA1c testing and microalbuminuria (MA) assessment annually. Additionally, patient and provider education on daily diabetes management has increased. The primary objective of this report, therefore, is to assess the change in glucose control concurrent with this evolution of care with a 1–2 year follow up of the Rwanda LFAC 2009–2010 cohort. We will also examine patterns of HbA1c change, determine the characteristics of those developing complications, and those not returning for follow-up.
METHODS
This report is a quality improvement project of the LFAC Program in collaboration with the ARD and UPGSPH. The University of Pittsburgh’s IRB has determined that this project is exempt from review under the “Existing Data” category.
Study Population
All participants in this evaluation were registered members of the Rwanda LFAC program who had their first HbA1c measure between June 2009 and November 2010.[6] To be enrolled in the program, participants must be residents of Rwanda aged ≤ 25 years needing assistance with obtaining insulin and diabetes supplies. Participants either sought out care from the ARD or were referred by their physicians or healthcare providers.
Diabetes care for the LFAC participants in Rwanda has evolved over the last several years as outlined in table 1. This single-nurse led program started in 2004, but regular HbA1c testing was not available until 2009 after which more extensive quarterly visits were initiated. Since then, the program has expanded in size and scope through providing support to numerous district hospitals and development and execution of patient and care provider education sessions. While attempts have been made to improve provider care at hospitals, the quality of care still remains a problem due to factors including high staff rotation and relative unawareness of diabetes in children and youth.
Table 1.
Evolution of the LFAC program in Rwanda
| Year | Status |
|---|---|
| 2004–2007 |
|
| 2008 |
|
| 2009* |
|
| 2010* |
|
| 2011 |
|
| 2012 |
|
Baseline HbA1c measures were collected during these years
Data Collection
Baseline data were collected from June 2009 through November 2010. Baseline and follow up data were collected using the LFAC forms and protocol (previously described[6]) either at the ARD or at several district hospitals supported by the program. Follow up data were collected from baseline through April 30, 2012 by the ARD staff and UPGSPH students. Seventy nine of the 286 subjects were not yet eligible for a two- year follow up visit. All assisting University of Pittsburgh students and ARD clinical staff were trained by authors TO and DE.
No data were collected for research purposes and all data reported are routinely recorded for clinical care purposes.
Laboratory Data
Blood and urine samples were processed on the Siemens DCA Vantage™ by the MPH students or ARD staff. HbA1c and Albumin/Creatinine (A/C) ratio results were reported to the nearest tenth percent. The maximum HbA1c value for this machine is “>14 % (130 mmol/mol),” so for data analysis purposes these results were reported as “14.1.” Regular quality control testing resulted in a coefficient of variation for the DCA of 2.1% to 3.8% during data collection.
Anthropometric and Blood Pressure Percentiles for Youth
Height and weight were measured with a stadiometer and floor scale, respectively. Height was recorded to the nearest 0.1 cm and weight to the nearest 0.5 Kg. Blood pressure (BP) was assessed with a manual cuff for a portion of 2009 and then by an automatic BP machine (Omron Healthcare, Inc.) and cuff for the duration of follow-up. Cross-over studies showed no significant differences between methods, and post hoc analysis showed no trends in data due to this change. For those under age 18 years of age, height for age,[7] systolic and diastolic BP for height percentile and age,[8] and BMI for age[9] percentiles and z-scores were calculated. It should be noted that the percentiles and z-scores are based on US standards as no appropriate Rwanda data are available. Short stature was defined as those under the 5th percentile.[7] Those under the 5th percentile for BMI were considered to be underweight, those from the 5th to 84th were normal weight, 85th to 94th were overweight, and those over the 95th percentile were obese.[9] For systolic and diastolic blood pressure, those over the 95th percentile were considered hypertensive.[8]
For those over 18 years of age, a BMI under 18.5 was considered underweight, 18.5–<25 normal weight, 25–30 overweight, and over BMI of 30 obese.[10] Hypertension was defined as a systolic BP ≥130 mmHg and/or diastolic BP ≥80 mmHg or a history of BP medication (only 2 patients used BP medications). Sensitivity analyses with differing cut points were also performed.
Patient and Provider Education
Initially (2008–2010), education for patients focused mostly on proper injection of insulin, when to monitor glucose (minimal goal twice daily (pre-prandial) before morning and evening insulin doses) how to adjust insulin doses appropriately based on food availability and glucose monitoring results, when available, and recognizing hypoglycemia and the appropriate actions to take. Additional education materials and information were later (2011) introduced on: relevance of HbA1c, what to do when blood sugar levels are very low (hypoglycemia), when to call the clinic, possible complications from diabetes, proper nutrition, how to account for exercise, and what to do when sick with an infection. Education for care providers focused mainly on the different insulins available, how to properly prescribe and adjust insulin doses, and how to handle hypo- and hyper-glycemia.
Complication Assessment
Neuropathy
Neuropathy was defined as failure to feel a 10g monofilament (<7 of 10 correct responses) on the dorsum of the great toe and/or failure to feel vibration from a 128Hz tuning fork on the dorsum of the great toe for longer than 10 seconds.[11]
Microalbuminuria
Microalbuminuria (MA) was defined as an albumin/creatinine (A/C) ratio of 30–299 mg/g in a spot urine sample, and overt nephropathy as an A/C ratio ≥ 300 mg/g.
Data Analysis
Descriptive statistics including mean, standard deviation and frequencies were calculated for all variables. Two-sample and paired t-tests, chi squared test and Fishers Exact tests were used as appropriate for comparisons. Spearman correlation coefficients were used to assess the association between HbA1c and other continuous variables of interest at each visit. When examining changes in anthropometric data over time, only subjects who were either ≥18 years or <18 years for the entirety of follow up were included, thus excluding 9 from baseline to visit-1 (V1) and 20 for baseline to visit-2 (V2). Analysis of variance was used to assess differences in BP by HbA1c control group and Tukey’s HSD was used for post-hoc analyses.
Logistic regression modeling was used to identify factors that predict MA. Univariate associations were first examined to identify contributors and those with significance of p ≤ 0.2 were then considered for inclusion in the final model. Backwards stepwise regression was then completed using a significance of p <0.05 for inclusion. Age was retained as a potential confounder.
Trajectory analysis was performed using the PROC TRAJ macro (found at http://www.andrew.cmu.edu/user/bjones/) to determine if there were distinctive HbA1c trajectory profiles within the overall population using group-based semiparametric mixture modeling. This macro uses longitudinally collected data to define trajectories and then categorize participants into those groups based on a posterior probability.[12] We used data that were collected at baseline and at 3-month intervals up to 24 months. Given the censored distribution of our data, we used a censored normal model. To determine the number of trajectories, we used the Bayesian information criterion (BIC) log Bayes factor approximation:
where ΔBIC is the BIC of the more complex model less the BIC of the simpler model.[12] After participants were classified into trajectory groups, we examined differences in other factors using the PROC Mixed procedure in SAS.
The analysis for this paper was generated using SAS/STAT software, Version 9.3 of the SAS System for Windows, copyright © 2011 SAS Institute Inc.
RESULTS
A total of 214 youth out of 286 (75%) had an HbA1c measurement one year (V1) (11.7±2.3 months) after their baseline (BL) measurement and 144 out of 207 who were eligible (70%) had a follow up measurement two years (V2) (23.0±3.5 months) after baseline. 125 participants attended both V1 and V2 and therefore comprise a full compliance (FC) sub-group. Age specific measurements (<18 years/≥18 years) can be seen in supplemental tables 1 and 2 (Appendix A).
At baseline the mean age, diabetes duration and glucose monitoring frequency were 18.6±4.5 years, 3.4±3.1 years and 1.1±3.4 times per week, respectively (table 2). A high percent (48.2%) of those <18 years had short stature and 16.1% were underweight (table 2). Complications were already present in this cohort with MA (21.0%) and hypertension (31.8%) being the two most common (table 2).
Table 2.
Baseline characteristics of the 2009–2010 LFAC cohort overall, stratified by attendance
| Overall | Attendance at V1 | Attendance at V2 | |||
|---|---|---|---|---|---|
|
| |||||
| Yes | No | Yes | No& | ||
| N | 286 | 214 | 72 | 144 | 70 |
| Age (years) | 18.6±4.5 | 18.3±4.4ǂ | 19.4±4.7 | 17.5±4.7* | 19.9±4.3 |
| Male %(n) | 46.5 (133) | 44.9 (96) | 51.4 (37) | 35.4 (51)* | 67.1 (47) |
| Diagnosis Age (years) | 15.1±4.8 | 15.0±4.7 | 15.7±5.4 | 14.2±4.7* | 15.8±5.3 |
| Seen in 2009 %(n) | 46.2 (132) | 44.9 (96) | 50.0 (36) | 69.4 (100)* | 45.7 (32) |
| Duration (years) | 3.4±3.1 | 3.3±2.9 | 3.5±3.8 | 3.3±2.8 | 4.0±3.4 |
| HbA1c (%) | 11.2±2.7 | 11.3±2.7 | 11.0±2.6 | 11.4±2.5 | 11.2±2.6 |
| HbA1c (mmol/mol) | 99±30 | 100±30 | 97±28 | 101±27 | 99±28 |
| HbA1c >14% %(n) | 30.8 (88) | 32.7 (70) | 25.0 (18) | 30.6 (44) | 27.1 (19) |
| HbA1c <8% %(n) | 15.7 (45) | 16.4 (35) | 13.9 (10) | 11.1 (16) | 18.6 (13) |
| Glucose Monitor / wk | 1.1±3.4 | 1.0±3.2 | 1.4±4.2 | 1.7±4.4ǂ | 0.66±2.1 |
| Insulin units/kg | 0.73±0.36 | 0.75±0.39ǂ | 0.66±0.28 | 0.75±0.41 | 0.74±0.36 |
| Height (cm) | 154.2±14.7 | 153.6±14.6 | 155.8±15.1 | 151.6±14.5 | 156.9±17.3 |
| Height Z-score$ | −1.6±1.8 | −1.8±1.8ǂ | −1.0±1.7 | −1.7±1.8 | −1.3±1.7 |
| Short Stature %(n)$ | 48.2 (42) | 52.3 (34) | 36.4 (8) | 46.2 (24) | 42.9 (6) |
| Weight (kg) | 48.1±12.7 | 47.6±13.0 | 49.3±11.9 | 46.7±14.0 | 50.5±12.2 |
| BMI (kg/m2) | 20.2±4.0 | 20.1±3.9 | 20.2±4.3 | 20.2±4.1 | 20.5±4.8 |
| Underweight %(n) | 18.6 (53) | 19.6 (42) | 15.5 (11) | 16.7 (24) | 21.4 (15) |
| Healthy Weight %(n) | 69.5 (198) | 66.8 (143) | 77.5 (55) | 66.0 (95) | 34.0 (49) |
| Overweight %(n) | 5.0 (14) | 5.1 (11) | 4.2 (3) | 6.9 (10) | 1.4 (2) |
| Obese %(n) | 2.1 (6) | 2.3 (5) | 1.4 (1) | 2.8 (4) | 1.4 (2) |
| BMI Z-score$ | −0.7±1.4 | −0.7±1.4 | −0.7±1.1 | −0.6±1.6 | −0.6±0.8 |
| Systolic BP (mmHg) | 112±15 | 112±14 | 114±18 | 111±16ǂ | 115±15 |
| Systolic BP Z-score$ | −0.1±1.0 | −0.04±1.1 | −0.4±0.7 | −0.2±1.0 | −0.1±0.8 |
| Diastolic BP (mmHg) | 72±11 | 72±11.0 | 72.7±11 | 72±11 | 74±10 |
| Diastolic BP Z-score$ | 0.5±0.7 | 0.5±0.7 | 0.4±0.6 | 0.5±0.7 | 0.4±0.6 |
| Hypertension %(n)¥ | 31.8 (91) | 30.8 (66) | 34.7 (25) | 28.4 (41) | 38.6 (27) |
| Microalbuminuria %(n) | 21.0 (31)a | 20.5 (23)c | 21.6 (8)e | 20.4 (21)g | 21.2 (7)i |
| Nephropathy %(n) | 4.7 (7)a | 6.2 (7)c | 0.0 (0)e | 3.9 (4)g | 3.0 (1)i |
| Neuropathy %(n) | 2.1 (5)b | 2.3 (4)d | 1.6 (1)f | 1.8 (2)h | 5.1 (3)j |
|
|
|||||
| at V1 (one-year) and V2 (two- year) visits. | |||||
Includes only those who were eligible for a two-year follow –up
Indicates variable calculated for participants <18 years only
Rates were calculated by adding those who were <18 years and ≥95th percentile with those who were ≥18 years and met the definition of hypertension based on BP
Indicates significance at α < 0.05
Indicates borderline significance at α <0.1
=118 tested;
=180 tested;
=112 tested;
=174 tested;
=37 tested;
=62 tested,
=103 tested,
=5 tested,
=33 tested,
=59 tested
There were no significant differences in baseline characteristics for those who attended V1 and those who did not, although, those who attended V1 were somewhat younger at baseline (p=0.07) and took more insulin per kg (p=0.07) (table 2). Age specific height at baseline for those <18 years was the only characteristic that was borderline significant (p=0.07) by V1 attendance (table 2). Those who attended V2, however, were significantly younger at baseline (17.5±4.7 years vs 19.9±4.3 years) and had borderline lower systolic BP (p=0.08) and more frequently monitored their glucose levels (p=0.06) than those who were eligible for V2 but did not attend (table 2). At baseline, for those <18 years, HbA1c was negatively correlated with systolic BP z-score (r=−0.2, p=0.03) and for those ≥18 years it was negatively correlated with systolic BP (r=−0.2, p=0.007) and positively correlated with units of insulin/kg (r=0.2, p=0.002).
At V1 participants monitored their glucose more frequently (2.6±4.7 times per week vs 1.0±3.2 times per week; at BL 3.3% monitored 2+ times per week, at V1 = 8.0%) and had higher systolic (118±16 mmHg vs 112±14 mmHg) and diastolic (77±13 mmHg vs 72±11 mmHg) BP than at baseline (table 3). Similar patterns were seen for the FC sub-group (data not shown). For those <18 years, systolic and diastolic BP z-scores were higher at V1 than baseline as were rates of hypertension (table 3). For those ≥18 years, mean systolic (115±14 mmHg at BL vs 122±15 mmHg at V1) and diastolic (74±11 mmHg at BL vs 79±13 mmHg at V1) BP also significantly increased (table 3).
Table 3.
Clinical characteristics of one (V1) and two (V2) year follow up visits as compared to baseline and one-year data.
| V1 | V2 | ||||
|---|---|---|---|---|---|
|
| |||||
| Baseline | V1 | Baseline | V1 | V2 | |
| N | 214 | 214 | 144 | 126 | 144 |
| Male % (n) | 44.9 (96) | 44.9 (96) | 35.4 (51) | 36.5 (46) | 35.4 (51) |
| HbA1c (%) | 11.3±2.7 | 10.2±2.6* | 11.4±2.5 | 10.5±2.7 | 9.8±2.3◊* |
| HbA1c (mmol/mol) | 100±30 | 88±28* | 101±27 | 97±28 | 84±25◊* |
| HbA1c >14% %(n) | 32.7 (70) | 12.2 (26) | 30.6 (44) | 12.7 (16)* | 9.0 (13) |
| HbA1c <8% %(n) | 16.4 (35) | 24.8 (53) | 11.1 (16) | 20.6 (26)* | 23.6 (34) |
| Glucose Monitor / wk | 1.0±3.2 | 2.6±4.7* | 1.7±4.4 | 2.5±4.4 | 6.6±6.9◊* |
| Insulin units/kg | 0.75±0.39 | 0.72±0.31 | 0.75±0.41 | 0.72±0.29 | 0.76±0.34 |
| Height (cm) | 153.6±14.6 | 155.7±14.4 | 151.6±14.5 | 154.5±15.2 | 154.4±14.3 |
| Height Z-score$ | −1.8±1.9 | −1.8±1.9 | −1.3±1.6 | −1.8±1.8 | −1.4±2.0 |
| Short Stature %(n)$ | 55.4 (31) | 52.5 (31) | 39.4 (13) | 40.7 (11) | 37.8 (14) |
| Weight (kg) | 47.6±13.0 | 49.8±12.3 | 46.7±14.0 | 49.6±12.6 | 49.9±12.4 |
| BMI (kg/m2) | 20.1±3.9 | 20.2±3.1 | 20.2±4.1 | 20.4±3.2 | 20.6±3.3 |
| Underweight %(n) | 19.5 (40) | 18.8 (38) | 15.3 (19) | 13.9 (15) | 14.5 (18) |
| Healthy Weight %(n) | 66.8 (137) | 70.7 (145) | 66.9 (83) | 72.2 (78) | 75.0 (93) |
| Overweight %(n) | 4.9 (10) | 6.8 (14) | 6.4 (8) | 7.4 (8) | 8.9 (11) |
| Obese %(n) | 2.4 (5) | 0.5 (1) | 2.4 (3) | 0.9 (1) | 0.0 (0) |
| BMI Z-score $ | −0.7±1.4 | −0.6±1.4 | −0.4±1.4 | −0.4±1.6 | −0.4±1.1 |
| Systolic BP (mmHg) | 112±14 | 118±16* | 111±16 | 117±15* | 118±19◊ |
| Systolic BP Z-score$ | −0.07±1.0 | 0.3±1.3* | −0.3±0.9 | 0.7±1.3* | 0.6±1.2◊ |
| Diastolic BP (mmHg) | 72±11 | 77±13* | 72±11 | 76±13* | 80±19◊* |
| Diastolic BP Z-score$ | 0.4±0.7 | 0.8±1.0* | 0.5±0.7 | 0.8±1.0 | 1.4±1.0◊* |
| Hypertension (130/80) %(n) ¥ | 30.8 (66) | 44.9 (96)* | 27.8 (40) | 38.8 (49) | 40.3 (58) ◊ |
| Hypertension (130/85) %(n) ¥ | 16.4 (35) | 34.6 (74)* | 15.3 (22) | 28.6 (36)* | 31.3 (45) ◊ |
| Hypertension (130/90) %(n) ¥ | 15.4 (33) | 28.5 (61)* | 13.9(20) | 23.0 (29) | 25.7 (37) ◊ |
| Hypertension (140/80) %(n) ¥ | 29.4(63) | 41.6 (89)* | 27.8 (40) | 35.7 (47) | 39.6 (57) ◊ |
| Hypertension (140/90) %(n) ¥ | 10.7(23) | 21.0 (45)* | 10.4 (15) | 14.3 (18) | 25.0 (36) ◊* |
| Microalbuminuria %(n) | 20.5 (23)a | 18.8 (12)c | 20.4 (21)e | 18.8 (8)g | 19.6 (11)k |
| Nephropathy %(n) | 6.2 (7)a | 7.8 (5)c | 3.9 (4)e | 4.6 (2)g | 5.4 (3)k |
| Neuropathy %(n) | 2.3 (4)b | 1.2 (1)d | 1.8 (2)f | 1.4 (1)h | 0.0 (0)m |
|
|
|||||
Indicates significance at α = 0.05 to year before
Indicates significance at α = 0.05 to two -years before
- 112 tested;
- 174 tested;
- 64 tested;
- 85 tested;
- 103 tested;
- 112 tested;
- 44 tested;
- 69 tested;
- 56 tested;
- 5 tested.
Indicates variable calculated for participants <18 years only
Rates were calculated by adding those who were <18 years and ≥95th percentile with those who were ≥18 years and met the definition of hypertension based on BP
The prevalence of hypertension likewise increased considerably at both V1 and V2 no matter which set of definitions were used. At V1 HbA1c was negatively correlated with monitoring frequency (r= −0.4, p=0.004 <18 years; r=−0.3, p<0.0001 ≥18 years) and height z-score (r=−0.3, p=0.02).
At V2 mean glucose monitoring frequency (1.7±4.4 at BL, 2.5±4.4 at V1 and 6.6±6.9 at V2; at BL 6.3% monitored 2+ times per week, at V2=33.1%) per week was significantly higher than both previous visits. For those <18 years, mean systolic and diastolic BP z-scores were significantly higher at V2 than baseline. For those ≥18 years mean systolic (115±16 mmHg at BL vs 122±21 mmHg at V2) and diastolic (75±11 mmHg at BL vs 80±14 mmHg at V2) BP were higher than baseline but not V1 (table 3). At V2 HbA1c was negatively correlated with BMI z-score (r=−0.3, p=0.03) for those <18 years, and with glucose monitoring frequency (r=−0.3, p=0.04) for those ≥18 years.
There was a significant decrease in mean HbA1c for the entire cohort from 11.2±2.7% (100±30 mmol/mol) at baseline to 10.2±2.6% (88±28 mmol/mol) (P <0.0001) at V1, and to 9.8±2.3% (84±25 mmol/mol) at V2 (P <0.0001 from BL, P <0.0001 from V1) (table 3). Very similar changes (P <0.0001) were seen in the FC sub-group (data not shown). At V1, 56.1% (n=120) saw a 0.5% improvement or greater in HbA1c, and 66.7% (n=96) saw similar improvements at V2. In the overall cohort, at baseline, only 15.7% of participants had HbA1c <8%, but this increased to 23.6% at V2 (p=0.04). The most striking change was the decrease in the percentage of participants with HbA1c >14% from 30.8% at baseline to 12.2% at V1 (p<0.0001), and to 9.0% at V2 (p<0.0001 from BL, not significant from V1, table 3). Similar patterns were seen for those in the FC sub-group (data not shown). At baseline, 10.8% of participants met the ADA glucose control goals for their age, 13.1% met the goals at V1, and 12.5% at V2.
Trajectory Analysis
In order to identify factors that were associated with improved glucose control we used trajectory analysis to identify different groups of participants based on their HbA1c patterns over time. Of the 201 participants with sufficient data, five distinct groups were identified (Figure 1): Group 1 (N=16, 8.0%) – started low and stayed low, Group 2 (N=17, 8.4%) – started low then increased, Group 3 (N=54, 26.9%) – started intermediate then declined, Group 4 (N=64, 31.8%) – started high then declined, Group 5 (N=50, 24.9%) – started high and stayed high. There were no significant differences in age, age at diagnosis, or diabetes duration among the groups.
Figure 1.
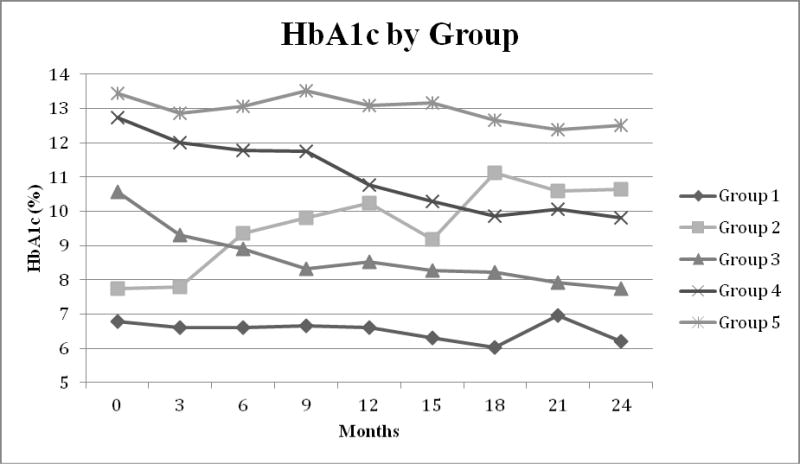
HbA1c Groups, as identified by trajectory analysis. A total of five different groups were identified. Group 1 N=16 (8.0%), Group 2 N=17 (8.4%), Group 3 N=54 (26.9%), Group 4 N=64 (31.8%), Group 5 N=50 (24.9%).
Repeated measures analysis was used to identify significant differences in clinical measures or behaviors by group. Only glucose monitoring per week was significant. Those in Group 5 (high-high) monitored their glucose on average fewer times per week (1.9±1.3 times/wk) than all other groups (averages over time: Group 1 = 4.2±2.8; Group 2 = 4.7±1.3; Group 3 = 5.3±3.0; Group 4= 3.0±1.8) [Group 1 to 5 p=0.006; Group 2 to 5 p=0.01; Group 3 to 5 p=0.002, Group 4 to 5 p=0.04], and those who were in Group 3 (intermediate-decline) monitored on average significantly more frequently than those in Group 4 (high-decline) (p=0.002).
Complications
The annual prevalence of MA remained fairly constant (21.0% at BL, 18.8% at V1, and 19.6% at V2), as did nephropathy (4.7%, 7.8%, and 5.4%) and neuropathy (2.1%, 1.2%, and 0.0%) (tables 6 and 8). Hypertension, rates, however, increased significantly over time (31.8% at BL, 44.9% at V1, and 40.3% at V2).
In the FC sub-cohort, eight cases of MA were noted at V1, comprising 4 new cases; 1 who had improved from nephropathy at baseline, and three cases with continued MA from baseline. Ten cases of MA were noted at V2, comprising 7 new cases and 3 cases with continuing MA. The tentative estimate of the annual incidence of MA was therefore 16.6% (95% CI 7.0–42%) and the annual regression rate was 23.5%. One new case of nephropathy was identified at V1, which had progressed from MA at baseline. At V2, there was 1 additional case that previously had MA at baseline. The annual incidence of nephropathy was, therefore, 4.9% (95% CI 0.8–17%). The total N for those with complications was too small to develop any meaningful models to identify predictors.
We examined weight, systolic BP, and diastolic BP by the HbA1c control groups in the FC subgroup to see if HbA1c control grouping impacted hypertension (table 4). While there were no overall significant differences, BP increased the most for Group 2 (low-increased) and the least for Group 1 (low-low), while BP for Group 3 (intermediate-low) remained fairly constant. Blood pressure also increased for Group 4 (high-declined) along with weight[6]. Our sample size was too small to examine the correlations between change in HbA1c and BP by HbA1c change group.
Table 4.
Weight, BP, and HbA1c stratified by HbA1c control group for those >18 years who had full compliance.
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|
|
| |||||
| Low-Low | Low-Increased | Intermediate-Decline | High-Decline | High-High | |
| N | 7 | 6 | 23 | 23 | 17 |
| Age at Baseline | 20.4±1.8 | 20.2±1.5 | 20.4±1.6 | 20.8±1.5 | 19.6±2.0 |
| Baseline systolic BP (mmHg) | 117±17 | 107±15 | 120±10 | 111±16 | 117±16 |
| Change BL-V1 | 6.7 | 24.0 | 0.2 | 6.3 | 4.6 |
| Change BL-V2 | −1.43 | 18.17 | 1.04 | 11.74 | 8.06 |
| Baseline diastolic BP (mmHg) | 79±13 | 70±9 | 78±10 | 72±11 | 74±15 |
| Change BL-V1 | −3.9 | 19.5$ | 1.4◊ | 3.4 | 3.2 |
| Change BL-V2 | −2.3 | 9.8 | −0.1 | 10.2 | 7.8 |
| Baseline weight (kg) | 52.6±8.5 | 51.8±8.9 | 53.0±7.3 | 50.9±10.5 | 53.2±12.0 |
| Change BL-V1 | 0.6 | 5.2 | 0.6 | 3.6 | 0.1 |
| Change BL-V2 | 0.3 | 2.9 | −0.2 | 2.7 | 2.9 |
| HbA1c (%) | 6.5±1.1 | 8.6±1.1 | 10.7±1.6 | 12.9±1.3 | 13.5±1.0 |
| Change BL-V1 | −0.3 | 0.6 | −2.1 | −2.4 | 0.1 |
| Change BL-V2 | −0.2 | 1.2 | −2.6 | −3.2 | −2.0 |
|
|
|||||
Indicates significantly different than Group 1
Indicates significantly different than Group 2
Indicates significantly different than Group 3
Indicates significantly different than Group 4
Indicates significantly different than Group 5
Rates of MA, neuropathy, and nephropathy did not differ significantly by trajectory group; however, the sample size and event N were too small for formal analysis.
DISCUSSION
In this follow up of children and youth (≤25 years) with diabetes in Rwanda after the introduction of systematic care, regular HbA1c testing, and enhanced education, we saw significant increases in glucose monitoring and blood pressure, and significant decreases in overall mean HbA1c (Table 3). The percentage of those meeting ADA glucose control goals for their age was substantially lower (11–13%) in Rwanda compared to that seen in a recent US study (32%) [13]. While we did not find any baseline predictors of V1 attendance, those who attended V2 were significantly younger at baseline than those who did not. Except for MA, prevalence rates of complications were low, and did not change significantly over the follow up period. However a major concern was the high, and increasing, prevalence of hypertension.
We also identified five distinct HbA1c control groups through trajectory analysis, and these groups differed by frequency of glucose monitoring per week, with those with the worst control (Group 5; high-high) measuring significantly fewer times per week than all other groups. As HbA1c decreased with each follow-up visit, it was negatively correlated with monitoring frequency. These findings support a higher emphasis on more frequent glucose monitoring to improve glycemic control. Severe hypoglycemia rates were reported with such low frequency and reliability that they were not included in these analyses. However these data are now being collected more rigorously and are a central feature of the quarterly visits. In support of our results, similar studies in sub-Saharan Africa report using education and HbA1c measurements to improve glucose control. These studies of older diabetes patients – one in Eritrea (n=350, mean age 50.5±15.5 years and duration 8.6 years),[14] and another in Kwazulu Natal, South Africa (n=284 with 197 completing; mean age 56±11 years and mean duration 7±6 years)[15] showed that improving the availability of HbA1c measurements and implementation of educational programs for physicians and diabetes educators, led to significant decreases in HbA1c (from 9.2±2.5% to 8.7±2.3% in Eritrea with mean follow up of 153 days; and from 11.6±4.5% at baseline to 8.7±2.3% by 6 months and 7.7±2.0% at 18 months in South Africa).
A further study from Kenya also showed improvements in glucose control through increases in glucose monitoring and regular contact with community diabetes care workers. At baseline, 43 participants had a mean HbA1c of 13.2% (95% CI 12.8–13.5), but this had fallen to 10.5% (95% CI 9.8–11.1) by 3–6 months.[16] Though there were no demographic data presented for comparison to our cohort, this study in Kenya highlights the importance of not just HbA1c knowledge, but also use of regular glucose monitoring to adjust insulin doses. In our population, participants originally were instructed to monitor their glucose at least twice per day (first thing in the morning and before evening insulin), but as the participants’ knowledge of monitoring increased and supplies were more available, more frequent monitoring was encouraged, particularly pre- lunch and/or pre-bedtime to help guide morning and evening regular insulin dosing. In this analysis we saw a significant negative correlation between glucose monitoring and HbA1c at V1 and V2, suggesting that improvements in glucose monitoring frequency were associated with improved glucose control. Studies from the developed world, for example Germany and Austria, also support these findings, showing that each additional blood glucose check each day is associated with 0.20%[17] and 0.26%[18] reductions in HbA1c.
Several of these previous studies included both type 1 and 2 diabetes patients, thus limiting any direct comparisons. Only 59% in Eritrea were taking insulin and even fewer were in South Africa (4%).[14,15] In the current study we believe the vast majority of our patients are type 1, based on their age at onset, lack of obesity, and insulin dependency. However, formal antibody testing and c-peptide testing were not available in our, or the other studies. The current study would thus appear to be the first report of improved diabetes control with regular HbA1c testing and education in a predominantly T1D population in sub-Saharan Africa.
The incidence of MA and nephropathy were estimated to be 16.6% and 3.3%, respectively. However, with the high rates of missing A/C data, patterns are uncertain. The incidence rates in our population appear quite high, especially in a young population with such short diabetes duration, and are considerably higher than seen in Denmark (MA=1.9%)[19] and recently in Australia (MA=4.6/1,000 person years)[20], despite a shorter duration (~ 4 years for Rwanda vs 12.2 for Denmark and 6.7 years for Australia). However, HbA1c in Rwanda, 11.2% (99 mmol/mol), was higher than in Denmark, 9.7% (83 mmol/mol), and a likely driving factor for the higher rates. [2,21,22] The prevalence of MA in Rwanda was slightly lower than an earlier report from the late 1980’s of a Pittsburgh cohort aged 6–21 years with 5 years of diabetes duration (similarly aged Rwanda cohort MA=15.1%; Pittsburgh=21.0%),[23] suggesting that current Rwanda rates may be similar to those seen in the US 25 years ago.
This population was lean and short based on US standards. Rates of obesity and overweight in those <18 years were very low (0.0–3.2% obese; 3.9%–7.2% overweight) in comparison to US youth with T1D in the SEARCH study (13.0% obese; 21.2% overweight).[24] Although the low rates are likely partly due to insulin deficiency and uncontrolled diabetes, many of these children and youth were born during or just after the Rwandan genocide, which could also have contributed to their small stature.
While the mean values for SBP and DBP (112±14 and 72±11 mmHg) at baseline were similar to those for African American (AA) youth with T1D (112±11 and 73±11), the rates of hypertension at baseline were significantly higher in Rwanda (AA=9.8%, Rwanda 30.8%).[25] An additional study of T1D in youth in the US (aged 6–21 years, 5 years duration) also showed significantly lower rates of hypertension than seen in Rwanda (%SBP ≥120 mmHg Pittsburgh USA 11.9% Rwanda=28.6%; % DBP ≥80 mmHg Pittsburgh 10.2%, Rwanda 42.8%).[23] The increased rate of hypertension may partly reflect the definitions used as a high number of Rwandans had a diastolic BP of exactly 80 mmHg (n=30; 20%). This, however, is not fully explained by “direct preference” as the majority of values were obtained by an automatic recorder. When hypertension was defined as 130/85 mmHg, the percent of hypertensive Rwandans (16.4%) (table 3) was closer to the afore mentioned rates in the US. Nonetheless the striking increase in BP at the subsequent one and two year follow-ups is of concern.
It is likely that glucose control was a driving force for much of the BP changes as evidenced by the increased BP in both those whose control worsened (Group 2 where SBP increased by 18mmHg at V2) and in those for whom control improved (Group 4 where SBP increased by 11mmHg at V2). The rise in the latter group appears to be associated with weight gain and thus likely may reflect the resolution of a dehydrated state of poor control. Previous work from a US cohort of youth with T1D (mean age 12.5±4.4 years, mean duration 4.5±3.3 years) showed a significant positive correlation between HbA1c and diastolic BP [26] further substantiating an association between glycemia and blood pressure in youth with T1D.
It is possible that other factors also contributed to the excess hypertension in this population. Previous studies found that the prevalence of hypertension in those under age 45 years was higher in sub-Saharan Africa (SSA) than the UK and the US (SSA=10.7%, UK=5.6%, US=8.2%).[27] Diet may be a factor, as salt is often used in food preparation and preservation in SSA.[27] Unfortunately, there are no comparable general population data for blood pressure with which to compare our results so it is not clear as to how much these high rates reflect T1D or a Rwandan effect. Unfortunately very few participants were taking BP medication due to prohibitive prices. This situation of increased rates of hypertension and low rates of treatment thus represents a critical issue that needs to be addressed urgently.
A major strength of this report is that it appears to be the first such study showing that improvements in glucose control can be obtained in children and adolescents with T1D in sub-Saharan Africa. We have also been able to provide preliminary estimates of the incidence and prevalence of MA and nephropathy in this population.
However, there are a number of limitations to this study. While 75% of our original cohort attended V1 and 70% of those eligible were seen at V2, these rates are lower than desired, and give rise to concern as to the current vital status of those who did not attend. Though several of the participants (N=10 at V1, N=16 at V2) would have been over age 25 at both visits, follow up of the remaining missing participants is a major focus of our current plans.
Complication assessment is limited, as reports of severe hypoglycemia were very limited, and only a small proportion have had their A/C measured (41.2% at BL, 29.9% at V1, and 38.9% at V2), which is a reflection of lack of supplies and examination logistic issues. Additionally, we were unable to assess retinopathy at this time, though we are currently working to address this for future care. Some clinic data were self-reported (monitoring frequency and units of insulin taken per day), and there is no way of monitoring compliance or the accuracy of these reports.
Though this cohort is representative of the LFAC program in Rwanda, it is possible that it does not reflect the true diabetes youth population. We believe, that due to poverty and lack of access to insulin, almost all cases are referred to the LFAC program for care and supplies, however, it is likely that we are missing undiagnosed cases as well as many who may have died before diagnosis. Thus our cohort likely represents, to some degree, a survivor cohort.
In summary, our data from the 1–2 year follow-up of the 2009–2010 LFAC cohort demonstrate that establishment of systematic care, regular HbA1c testing, and increased education may result in significant improvements in glycemic control in young (≤25 years) T1D patients in sub-Saharan Africa. Trajectory analysis allowed us to identify that glucose monitoring frequency is a potential specific area of intervention for improving glycemic control. Thus a future focus is to further increase access to testing supplies with the goal that all youth test at least twice daily. While we have reported improvements, it is clear that there is still a great need for further increases in glucose control in Rwandan youth and adolescents with T1D, as there are still several participants in this cohort with HbA1c >14%. Of major concern is the high prevalence of hypertension which represents a new major, and currently unmet, need for diabetic youth in Sub Saharan Africa.
Supplementary Material
Highlights.
We assessed improvement in glycemic control in Rwanda youth with diabetes.
2 yr after introduction of education and A1c testing mean A1c fell from 11.2 to 9.8%.
Hypertension was very common affecting over 40% of the population during follow up.
Different patterns of glycemic control over time were noted.
Those with worst control monitored their glucose less frequently.
Acknowledgments
We would like to thank the staff of the ARD in Kigali for all of the help and support they have given to us throughout this project. We would also like to acknowledge the LFAC program for providing us with the opportunity to work with them and the ARD. Finally we thank the wonderful children and youth with diabetes and their caregivers, for their willing acceptance of help and the dignity and courage they show in coping with diabetes in the most difficult circumstances.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors state that they have no conflicts of interest.
References
- 1.International Diabetes Federation. Diabetes Atlas, Fourth Edition. Brussels, Belgium: 2009. [Google Scholar]
- 2.Diabetes Control and Complications Trial (DCCT) Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. The Journal of pediatrics. 1994 Aug;125(2):177–88. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 3.Gill GV. Diabetes in Africa. Cambridge: FSG Limited; 1997. Outcome of Diabetes in Africa; pp. 65–71. [Google Scholar]
- 4.Beran D, Yudkin JS, De Courten M. Access to care for patients with insulin-requiring diabetes in developing countries: case studies of Mozambique and Zambia. Diabetes care. 2005 Sep;28(9):2136–40. doi: 10.2337/diacare.28.9.2136. [DOI] [PubMed] [Google Scholar]
- 5.Beran D, Yudkin JS. Diabetes care in sub-Saharan Africa. Lancet. 2006 Nov 11;368(9548):1689–95. doi: 10.1016/S0140-6736(06)69704-3. [DOI] [PubMed] [Google Scholar]
- 6.Marshall SL, Edidin D, Sharma V, Ogle G, Arena VC, Orchard T. Current clinical status, glucose control, and complication rates of children and youth with type 1 diabetes in Rwanda. Pediatric diabetes. 2013 May;14(3):217–26. doi: 10.1111/pedi.12007. [DOI] [PubMed] [Google Scholar]
- 7.National Health and Nutrition Survey (NHANES) CDC/National Center for Health Statistics. LMS Parameters for Girls/Boys: Height for Age. 2013. [Google Scholar]
- 8.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004 Aug;114(2 Suppl 4th Report):555–76. [PubMed] [Google Scholar]
- 9.National Health and Nutrition Survey (NHANES) CDC/National Center for Health Statistics. LMS Parameters for Girls/Boys: BMI for Age. 2013. [Google Scholar]
- 10.Healthy Weight: Assessing Your Weight: BMI: About Adult BMI | DNPAO | CDC.; Available from: http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html#interpreted
- 11.Walsh MG, Zgibor J, Borch-Johnsen K, Orchard TJ. A multinational assessment of complications in type 1 diabetes: the DiaMond substudy of complications (DiaComp) Level 1. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2006;3(2):80–3. doi: 10.3132/dvdr.2006.018. [DOI] [PubMed] [Google Scholar]
- 12.Jones BL, Nagin DS, Roeder K. A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociological Methods and Research. 2001:374–93. Available from: http://www.andrew.cmu.edu/user/bjones/pdf/ref1.pdf.
- 13.Wood JR, Miller KM, Maahs DM, Beck RW, Dimeglio LA, Libman IM, Quinn M, Tamborlane WV, Woerner SE. Most Youth With Type 1 Diabetes in the T1D Exchange Clinic Registry Do Not Meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes Clinical Guidelines. Diabetes care. 2013 Jul;36(7):2035–7. doi: 10.2337/dc12-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Windus DW, Ladenson JH, Merrins CK, Seyoum M, Windus D, Morin S, Tewelde B, Parvin CA, Scott MG, Goldfeder J. Impact of a multidisciplinary intervention for diabetes in Eritrea. Clinical chemistry. 2007 Nov;53(11):1954–9. doi: 10.1373/clinchem.2007.095067. [DOI] [PubMed] [Google Scholar]
- 15.Gill GV, Price C, Shandu D, Dedicoat M, Wilkinson D. An effective system of nurse-led diabetes care in rural Africa. Diabetic medicine : a journal of the British Diabetic Association. 2008 May;25(5):606–11. doi: 10.1111/j.1464-5491.2008.02421.x. [DOI] [PubMed] [Google Scholar]
- 16.Pastakia SD, Karwa R, Kahn CB, Nyabundi JS. The evolution of diabetes care in the rural, resource-constrained setting of western Kenya. The Annals of pharmacotherapy. 2011 Jun;45(6):721–6. doi: 10.1345/aph.1P779. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler R, Heidtmann B, Hilgard D, Hofer S, Rosenbauer J, Holl R. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatric diabetes. 2011 Feb;12(1):11–7. doi: 10.1111/j.1399-5448.2010.00650.x. [DOI] [PubMed] [Google Scholar]
- 18.Schütt M, Kern W, Krause U, Busch P, Dapp A, Grziwotz R, Mayer I, Rosenbauer J, Wagner C, Zimmermann A, Kerner W, Holl RW. Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Experimental and clinical endocrinology & diabetes. 2006 Jul;114(7):384–8. doi: 10.1055/s-2006-924152. [DOI] [PubMed] [Google Scholar]
- 19.Olsen BS, Sjølie A, Hougaard P, Johannesen J, Borch-Johnsen K, Marinelli K, Thorsteinsson B, Pramming S, Mortensen HB. A 6-year nationwide cohort study of glycaemic control in young people with type 1 diabetes. Risk markers for the development of retinopathy, nephropathy and neuropathy. Danish Study Group of Diabetes in Childhood. Journal of diabetes and its complications. 2000;14(6):295–300. doi: 10.1016/s1056-8727(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 20.Stone ML, Craig ME, Chan AK, Lee JW, Verge CF, Donaghue KC. Natural history and risk factors for microalbuminuria in adolescents with type 1 diabetes: a longitudinal study. Diabetes care. 2006 Sep;29(9):2072–7. doi: 10.2337/dc06-0239. [DOI] [PubMed] [Google Scholar]
- 21.Microvascular and acute complications in IDDM patients: the EURODIAB IDDM Complications Study. Diabetologia. 1994 Mar;37(3):278–85. doi: 10.1007/BF00398055. [DOI] [PubMed] [Google Scholar]
- 22.Raile K, Galler A, Hofer S, Herbst A, Dunstheimer D, Busch P, Holl RW. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes care. 2007 Oct;30(10):2523–8. doi: 10.2337/dc07-0282. [DOI] [PubMed] [Google Scholar]
- 23.D’Antonio JA, Ellis D, Doft BH, Becker DJ, Drash AL, Kuller LH, Orchard TJ. Diabetes complications and glycemic control. The Pittsburgh Prospective Insulin-Dependent Diabetes Cohort Study Status Report after 5 yr of IDDM. Diabetes care. 1989;12(10):694–70. doi: 10.2337/diacare.12.10.694. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez BL, Dabelea D, Liese AD, Fujimoto W, Waitzfelder B, Liu L, Bell R, Talton J, Snively BM, Kershnar A, Urbina E, Daniels S, Imperatore G. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. The Journal of pediatrics. 2010 Aug;157(2):245–251.e1. doi: 10.1016/j.jpeds.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Mayer-Davis EJ, Beyer J, Bell RA, Dabelea D, D’Agostino R, Imperatore G, Lawrence JM, Liese AD, Liu L, Marcovina S, Rodriguez B. Diabetes in African American youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes care. 2009 Mar;32(Suppl 2):S112–22. doi: 10.2337/dc09-S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torchinsky MY, Gomez R, Rao J, Vargas A, Mercante DE, Chalew SA. Poor glycemic control is associated with increased diastolic blood pressure and heart rate in children with Type 1 diabetes. Journal of diabetes and its complications. 2004;18(4):220–3. doi: 10.1016/S1056-8727(03)00031-X. [DOI] [PubMed] [Google Scholar]
- 27.Twagirumukiza M, Van Bortel LM. Management of hypertension at the community level in sub-Saharan Africa (SSA): towards a rational use of available resources. Journal of human hypertension. 2011 Jan;25(1):47–56. doi: 10.1038/jhh.2010.32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


