Abstract
The basal ganglia nuclei are critical for a variety of cognitive and motor functions. Much work has shown age-related structural changes of the basal ganglia. Yet less is known about how the functional interactions of these regions with the cerebral cortex and the cerebellum change throughout the lifespan. Here, we took advantage of a convenient sample and examined resting state functional magnetic resonance imaging data from 250 adults 18 to 49 years of age, focusing specifically on the caudate nucleus, pallidum, putamen, and ventral tegmental area/substantia nigra (VTA/SN). There are a few main findings to report. First, with age, caudate head connectivity increased with a large region of ventromedial prefrontal/medial orbitofrontal cortex. Second, across all subjects, pallidum and putamen showed negative connectivity with default mode network (DMN) regions such as the ventromedial prefrontal cortex and posterior cingulate cortex, in support of anticorrelation of the “task-positive” network (TPN) and DMN. This negative connectivity was reduced with age. Furthermore, pallidum, posterior putamen and VTA/SN connectivity to other TPN regions, such as somatomotor cortex, decreased with age. These results highlight a distinct effect of age on cerebral functional connectivity of the dorsal striatum and VTA/SN from young to middle adulthood and may help research investigating the etiologies or monitoring outcomes of neuropsychiatric conditions that implicate dopaminergic dysfunction.
Keywords: functional connectivity, fMRI, resting state, aging, basal ganglia, striatum
1.0 Introduction
The basal ganglia nuclei play a key role in a variety of cognitive and motor abilities throughout the human lifespan. Based on neurophysiology and anatomical data, it has been proposed that the caudate, putamen, and pallidum are organized into parallel and overlapping “loops” connecting to the cerebral cortex (Alexander et al., 1986, Joel & Weiner, 1997; Middleton & Strick 2000; Haber, 2003). Similar organization is implicated in humans using probabilistic tractography (Draganski et al., 2008). Modulated by dopaminergic input from the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc), these circuits are implicated in maintenance and updating of working memory (Hazy et al., 2006), control of goal-directed and habitual behavior (Redgrave et al., 2010), reward-based learning (Berridge & Robinson 1998; Schultz, 2002; Wise, 2004), control of posture and movement (DeLong, Crutcher, & Georgopoulos, 1983, Mink, 1996), and providing motivational signals to enhance attention and cognition (Pessoa & Engelmann, 2010).
Many of these cognitive and motor functions deteriorate with age (Mark & Rugg, 1998; Smith et al., 1999; Mattay et al., 2002), and co-occur with marked anatomical changes in the basal ganglia. Morphology studies consistently reveal declines in striatal/pallidal volume by 4–8% per decade, starting as early as age 20 (e.g., Brabec et al., 2003; Raz et al., 2003; Walhovd et al., 2011; Goodro et al., 2012). Postmortem studies have shown age-related neuronal loss and changes to basic cellular structure such as the myelin sheath in basal ganglia (for reviews, see Haug, 1985; Kemper, 1994; Peters, 2002). Diffusion tensor imaging demonstrated significant age-related reductions in fractional anisotropy and age-related increases in mean diffusivity in the SN and striatum (Cherubini et al., 2009; Vaillancourt et al., 2012). Accompanying findings of structural changes, age-related differences in functional activation of the basal ganglia nuclei have been consistently reported during cognitive and motor tasks (Mattay et al., 2002; Ward and Frackowiak, 2003; Wu and Hallett, 2005; Rubia et al., 2007; Langenecker et al., 2007). Despite an extensive literature on regional changes in brain activity, little is known about how functional connectivity of the basal ganglia with the cortex and cerebellum changes throughout the lifespan. The integrity of these circuits is likely crucial for healthy aging, given their role in a wide range of behaviors (for reviews, see Alexander & Crutcher, 1990; Haber 2003). The current study addressed this gap of research.
We examined the connectivity of the caudate, putamen, pallidum, and VTA/SN with other brain structures using resting state functional magnetic resonance imaging (rsfMRI) data in a large cohort of young and middle-aged adults. rsfMRI measures the correlations of spontaneous, low frequency blood oxygenation level dependent (BOLD) signals between brain regions (Biswal et al., 1995; Fox & Raichle, 2007). A proxy for the functional relatedness of neural circuits, resting state functional connectivity has gained wide appeal for its ease of use and reliability within and across individuals (Fox & Raichle, 2007). For example, rsfMRI has been used to delineate subregions of cortical structures (e.g., Mars et al., 2011; Zhang et al., 2012; Zhang & Li, 2012a), predict impulsive behaviors (Davis et al., 2013), and examine changes in developing neural circuits throughout adolescence (Stevens et al., 2009; Tomasi & Volkow, 2012a). Using rsfMRI, Tomasi & Volkow (2012b) demonstrated that from adolescence to young adulthood, VTA connectivity increased with structures in the DMN. Here, we extended this investigation to include seed regions in the dorsal striatum, areas that have received relatively little attention in the adult life-span rsfMRI literature. We posited that functional connectivity of the dorsal striatum and VTA/SN with other brain regions would be significantly altered with age and tested this hypothesis in a convenient sample of slightly older healthy adults (18 to 49 years of age) that we have analyzed extensively in previous work (Zhang et al., 2012; Zhang & Li, 2012a; Li et al., 2014; Zhang & Li; 2014).
2.0 Materials and Methods
2.1 Resting State Data
The resting state fMRI (rsfMRI) scans were pooled from three datasets (Leiden_2180/Leiden_2200, Newark, and Beijing_Zang, n=144) downloadable from the 1000 Functional Connectomes Project (Biswal et al., 2010) and our own data (n=106). Individual participants’ images were viewed one by one to ensure that the whole brain was covered. A total of 250 healthy participants’ resting state data (3-Tesla magnet; 18–49 (mean = 24.6 +/− 6.5) years of age; 104 men; one scan per participant; duration: 4.5–10 minutes; eyes closed during resting) were analyzed. Table 1 summarizes the scan characteristics and demographics of subjects of the data set.
Table 1.
Available demographic data and imaging parameters for the selected resting-state functional MRI datasets from the image repository for the 1000 Functional Connectomes Project and for our own dataset.
| Dataset | Subjects | Age (years) | Timepoints | TR (s) | Slice acquisition order |
|---|---|---|---|---|---|
| Beijing_Zang | 31 M/66 F | 18–26 | 225 | 2 | Interleaved ascending |
| Leiden_2180 | 10 M/0 F | 20–27 | 215 | 2.18 | Sequential descending |
| Leiden_2200 | 11 M/8 F | 18–28 | 215 | 2.22 | Sequential descending |
| Newark | 9 M/9 F | 21–39 | 135 | 2 | Interleaved ascending |
| Our own | 63 M/43 F | 19–49 | 295 | 2 | Interleaved ascending |
Note: M- Males; F- Females
2.2 Imaging Data Preprocessing
Standard image preprocessing was performed on the brain imaging data using Statistical Parametric Mapping (SPM 8, Wellcome Department of Imaging Neuroscience, University College London, U.K.), as described in our previous work (Zhang et al., 2012). Images of each individual participant were first realigned (motion corrected) and corrected for slice timing. Each individual’s structural image was coregistered to the mean EPI for each individual (i.e., the mean EPI was used as the reference image). Each individual structural image was then segmented and normalized to an MNI (Montreal Neurological Institute) EPI (echo- planar imaging) template with affine registration followed by nonlinear transformation (Ashburner and Friston, 1999, Friston et al., 1995). The normalization parameters determined for the structural volume were then applied to the corresponding functional image volumes for each participant. The voxels are 3×3×3 mm. Finally, the images were smoothed with a Gaussian kernel of 8 mm at full width at half maximum.
Additional preprocessing was applied to reduce spurious BOLD variances that were unlikely to reflect neuronal activity (Fair et al., 2007, Fox and Raichle, 2007, Fox et al., 2005, Rombouts et al., 2003). The sources of spurious variance were removed through linear regression by including the signal from the ventricular system, the white matter, and the whole brain, in addition to the six parameters obtained by rigid body head motion correction. First-order derivatives of the whole brain, ventricular and white matter signals were also included in the regression.
Cordes and colleagues suggested that BOLD fluctuations below a frequency of 0.1Hz contribute to regionally specific BOLD correlations (Cordes et al., 2001). The majority of resting state studies low-pass filtered BOLD signal at a cut-off of 0.08 or 0.1 Hz (Fox and Raichle, 2007). Thus, we applied a temporal band-pass filter (0.009Hz < f < 0.08Hz) to the time course in order to obtain low-frequency fluctuations (Fair et al., 2007, Fox and Raichle, 2007, Fox et al., 2005).
2.3 Head motion
As extensively investigated in Van Dijk et al., 2012, micro head motion (>0.1mm) is an important source of spurious correlations in resting state functional connectivity analysis. Therefore, we applied a “scrubbing” method proposed by Power and colleagues (Power et al., 2012) and successfully applied in previous studies (Smyser et al., 2010; Power et al., 2012; Tomasi and Volkow, 2012b) to remove time points affected by head motions. Briefly, for every time point t, we computed the framewise displacement given by FD(t) = |Δdx(t)| + |Δdy(t)| + |Δdz(t)| + r|α(t)| + r|β(t)| + r|γ(t)|, where (dx, dy, dz) and (α, β, γ) are the translational and rotational movements, respectively, and r (= 50mm) is a constant that approximates the mean distance between center of MNI space and the cortex and transform rotations into displacements (Power et al., 2012). The second head movement metric was the root mean square variance (DVARS) of the differences in % BOLD intensity I(t) between consecutive time points across brain voxels, computed as follows: , where the brackets indicate the mean across brain voxels. Finally, to compute each subject’s correlation map, we removed every time point that exceeded the head motion limit FD(t)>0.5mm or DVARS(t)>0.5% (Power et al., 2012; Tomasi and Volkow, 2012b). On average, 1% of the time points were removed across subjects.
2.4 Seed-based functional connectivity: linear correlations
We used the caudate, pallidum, and putamen templates from the Anatomical Automatic Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) and the maximum probability maps template for the VTA/SN seed (Hammers et al., 2003; Ahsan et al., 2007). The VTA/SN seed was derived from the structural MRIs of 30 healthy adults; after spatial normalization and averaging across subjects the size of the left VTA/SN seed was 561mm3 and the right VTA/SN seed was 545mm3 (Ahsan et al., 2007). The putamen and the caudate were bisected into anterior and posterior regions based on boundaries from previous studies; the putamen was divided along the coronal slice containing the anterior commissure (Postuma & Dagher, 2006; Helmich et al., 2010) and the caudate was divided along the coronal slice containing the interventricular foramina (Robinson et al., 2012). Bilateral masks were used for each seed region. We did not use a ventral striatum seed, as we reported the effects of age on the ventral striatum in a previous study (Li et al., 2014). The BOLD time courses were averaged spatially across all voxels for each of the seed regions. We computed the correlation coefficient between the averaged time course of each seed region and the time courses of individual voxels of the brain for individual participants. To assess and compare the resting state “correlograms,” we converted these image maps, which were not normally distributed, to z score maps by Fisher’s z transform (Jenkins and Watts, 1968; Berry and Mielke, 2000): z = 0.5loge[(1+r)/(1−r)]. The z maps were used in group analysis with multiple regression.
We used a multiple regression with three covariates: age, male gender (male = 1, female = 0) and female gender (female = 1, male = 0), to examine age and gender differences in functional connectivity and identified voxels that were significant at a corrected threshold. Investigators have argued that the corrected voxel peak threshold of p<0.05, based on the Gaussian random field theory, may be too restrictive, and suggested the use of a cluster threshold (Poline et al., 1997; Hayasaka and Nichols, 2003). Thus, we present results that satisfy both a p<.001 uncorrected threshold at the voxel level and a p<.05 family-wise error (FWE) corrected threshold, where the minimum cluster size = 10 voxels.
3.0 Results
Figure 1 shows the results of one-sample t-tests of whole-brain connectivity of each seed region across all 250 participants. Figures 2 and 3 show the results from the age regression analyses; Tables 2 and 3 show the significant “positive” (positive correlation with age) and “negative” (negative correlation with age) clusters, respectively, from the age regression analysis. Results from each set of analyses are discussed in detail below.
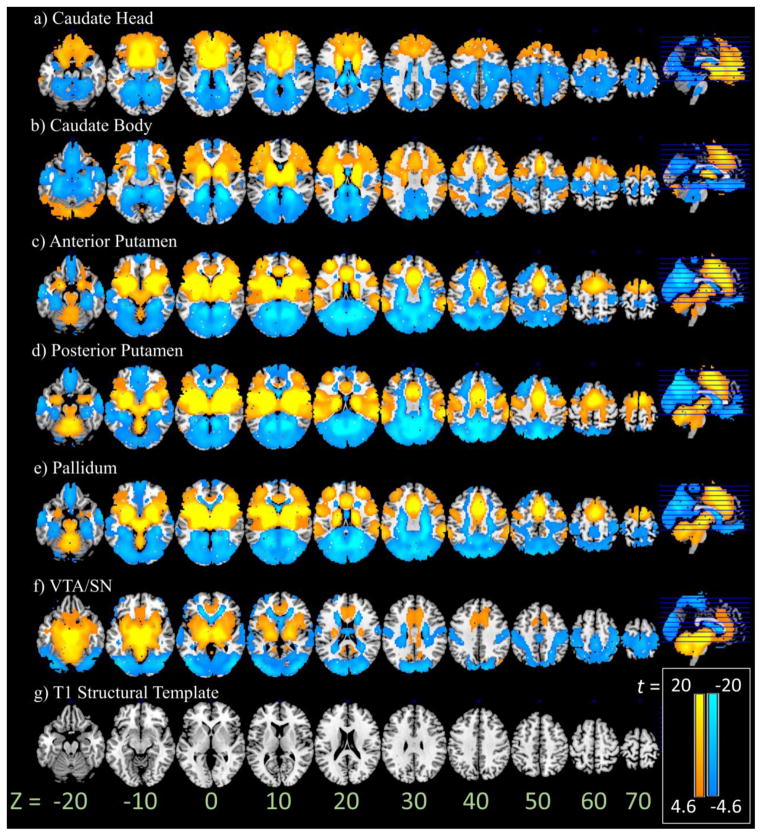
Figure 1.
One sample T-test (n = 250) of whole-brain functional connectivity with a) caudate head b) caudate body c) anterior putamen d) posterior putamen e) pallidum and f). VTA/SN p < .05, FWE corrected (scale: t =4.63 to 20), overlaid on a (g) T1 structural template.
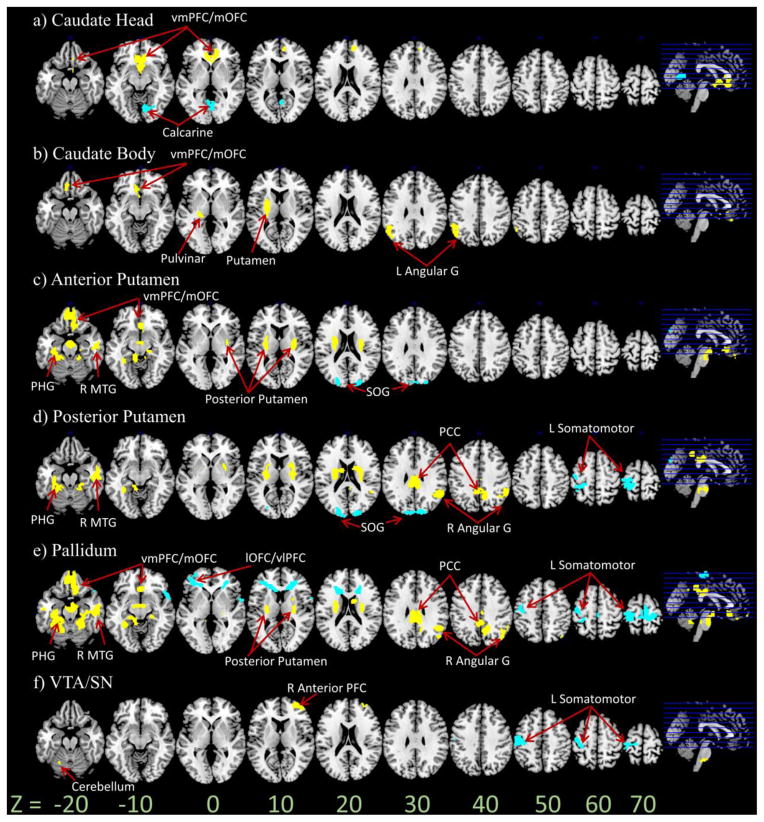
Figure 2.
Age regression on functional connectivity of the a) caudate head b) caudate body c) anterior putamen d) posterior putamen e) pallidum and f) VTA/SN seed regions. p < .001 uncorrected, peak voxel; < . 05 FWE, cluster (cluster size = 10). Yellow and blue color each indicates increases and decreases in functional connectivity with age. R = Right; L = Left; G = Gyrus; vmPFC/mOFC = Ventromedial Prefrontal Cortex/Medial Orbitofrontal Cortex; MTG = Middle Temporal Gyrus; PCC = Posterior Cingulate Cortex; PHG = Parahippocampal Gyrus; lOFC = Lateral Orbitofrontal Cortex; vlPFC = Ventrolateral Prefrontal Cortex; SOG = Superior Occipital Gyrus
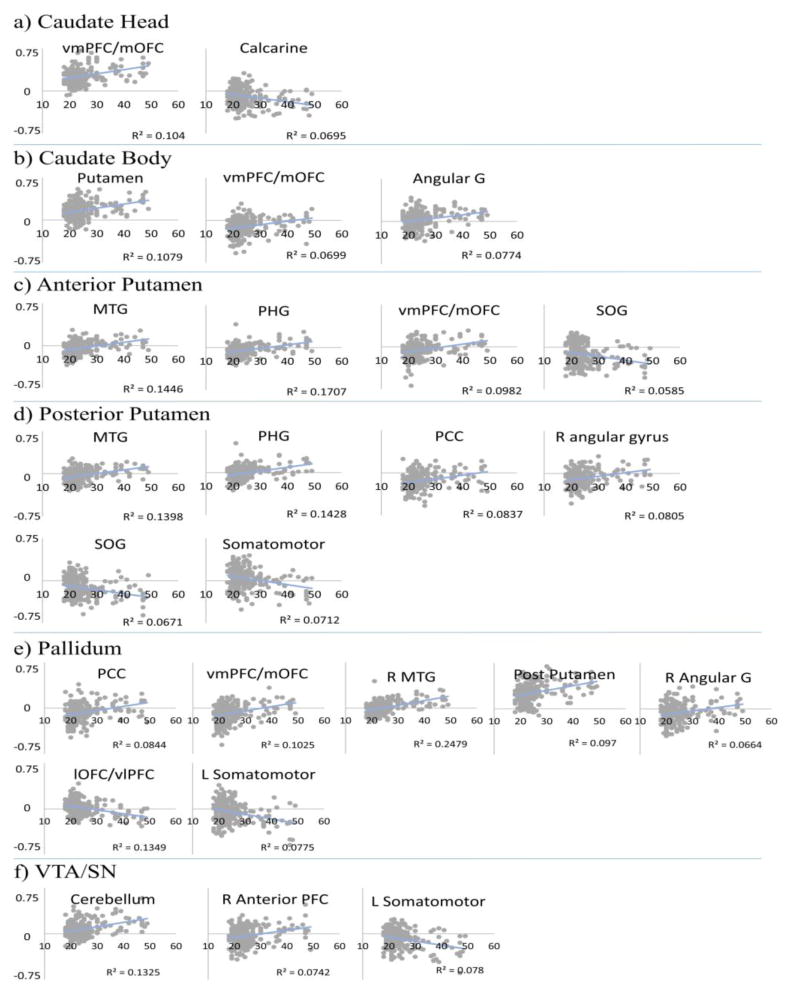
Figure 3.
Age regression on functional connectivity. Each scatterplot shows a region of interest whose connectivity significantly varied with age between a) caudate head b) caudate body c) anterior putamen d) posterior putamen e) pallidum and f) VTA/SN. The x-axis denotes the age of the participant, and the y-axis denotes the z-score for the correlation between the seed region and the region of interest. Each gray dot represents one subject and the blue lines show linear regression. Abbreviations: R = Right; L = Left; G = Gyrus; vmPFC/mOFC = Ventromedial Prefrontal Cortex/Medial Orbitofrontal Cortex; MTG = Middle Temporal Gyrus; PCC = Posterior Cingulate Cortex; PHG = Parahippocampal Gyrus; lOFC/vlPFC = Lateral Orbitofrontal Cortex/Ventrolateral Prefrontal Cortex; SOG = Superior Occipital Gyrus
Table 2.
Brain regions showing a significant positive relationship between seed-based functional connectivity and age; regression, at voxel p<0.001 uncorrected and cluster-level p<0.05, FWE corrected.
| Seed ROI | Cluster Size (mm3) | z-score | MNI Coordinates (mm)
|
Identified Region | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Caudate Head | 13122 | 4.3 | 12 | 50 | 19 | vmPFC/mOFC |
| 4.19 | −9 | 32 | 1 | vmPFC/mOFC | ||
|
| ||||||
| Caudate Body | 4617 | 5.08 | −24 | −4 | 13 | Putamen |
| 2646 | 4.25 | −3 | 35 | −23 | vmPFC/mOFC | |
| 5940 | 4.23 | −48 | −49 | 34 | Angular G | |
|
| ||||||
| Anterior Putamen | 19521 | 5.55 | 36 | 20 | −38 | Temporal Pole |
| 4.82 | 36 | −1 | −41 | Temporal Pole | ||
| 4.75 | 24 | −10 | −29 | MTG | ||
| 15228 | 5.03 | −12 | −43 | −14 | PHG | |
| 4.66 | −42 | −10 | −32 | MTG | ||
| 4.66 | −33 | −40 | −17 | PHG | ||
| 7614 | 4.73 | 6 | 47 | −23 | vmPFC/mOFC | |
|
| ||||||
| Posterior Putamen | 17955 | 5.35 | 30 | 14 | −38 | Temporal Pole |
| 4.93 | 48 | -16 | −23 | MTG | ||
| 4.67 | 48 | 11 | −32 | Temporal Pole | ||
| 9801 | 5.08 | −33 | −43 | −14 | PHG | |
| 10449 | 4.98 | −3 | −46 | 40 | PCC | |
| 5670 | 3.98 | 54 | −46 | 37 | R angular gyrus | |
|
| ||||||
| Pallidum | 80838 | 6.83 | 30 | 14 | −38 | R MTG |
| 6.16 | 39 | −4 | −38 | R Rostral Inferior Temporal | ||
| 5.90 | 24 | −10 | −29 | R Inferior Temporal | ||
| 12636 | 4.93 | −9 | −34 | 31 | PCC | |
| 4.51 | 9 | −34 | 31 | PCC | ||
| 4.44 | 12 | −58 | 40 | Precuneus | ||
| 3888 | 4.86 | 27 | −16 | 13 | Posterior Putamen | |
| 3564 | 4.75 | −27 | −16 | 16 | vmPFC/mOFC | |
| 8640 | 4.74 | 9 | 29 | −20 | R Angular G | |
|
| ||||||
| VTA/SN | 7776 | 4.67 | 39 | −46 | −50 | Cerebellum |
| 3051 | 4.22 | 30 | 56 | 13 | R Anterior PFC | |
Note: R = Right; L = Left; G = Gyrus; vmPFC/mOFC = Ventromedial Prefrontal Cortex/Medial Orbitofrontal Cortex; MTG = Middle Temporal Gyrus; PCC = Posterior Cingulate Cortex; PHG = Parahippocampal Gyrus
Table 3.
Brain regions showing a significant negative relationship between seed-based functional connectivity and age; regression, at voxel p<0.001 uncorrected and cluster-level p<0.05, FWE corrected.
| Seed ROI | Cluster Size (mm3) | z-score | MNI Coordinates (mm)
|
Identified Region | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Caudate Head | 5562 | 4.11 | 9 | −73 | −11 | Calcarine Sulcus |
|
| ||||||
| Caudate Body | N/A | |||||
|
| ||||||
| Anterior Putamen | 5481 | 3.87 | −21 | −94 | 25 | SOG |
|
| ||||||
| Posterior Putamen | 7587 | 4.56 | 6 | −91 | 31 | SOG |
| 8856 | 4.06 | −33 | −25 | 73 | L Somatomotor | |
|
| ||||||
| Pallidum | 8424 | 4.68 | −36 | 41 | 4 | lOFC/vlPFC |
| 13311 | 4.39 | −33 | −25 | 70 | L Somatomotor | |
|
| ||||||
| VTA/SN | 7749 | 4.29 | −51 | −16 | 49 | L Somatomotor |
Note: L = Left; lOFC = Lateral Orbitofrontal Cortex/Ventrolateral Prefrontal Cortex; SOG = Superior Occipital Gyrus
3.1 Seed-based functional connectivity: One-sample t-tests
The head of caudate (Figure 1a) showed positive connectivity with dorsomedial prefrontal cortex (PFC) including the anterior pre-supplementary motor area (pre-SMA) and rostral anterior cingulate cortex (rACC), anterior PFC, superior frontal gyri, a large area of the ventromedial PFC (vmPFC) and orbitofrontal cortex (OFC), angular gyrus, dorsal/anterior/medial thalamus, and basal ganglia, and negative connectivity with parietal, occipital, and somatomotor regions, as well as the midbrain, posterior insula, hippocampus, parahippocampal gyrus, posterior/ventral thalamus, amygdala, and the cerebellum. The body of caudate (Figure 1b) showed positive connectivity with a large area of the dorsomedial PFC including the posterior pre-SMA and SMA, superior frontal gyrus, ventrolateral PFC, supramarginal gyrus/superior temporal sulcus, dorsal/anterior/medial thalamus, anterior insula, basal ganglia, and posterior cerebellar cortex, and showed negative connectivity with occipital cortex, somatomotor cortex, vmPFC/mOFC, posterior/ventral thalamus, medial/superior temporal cortex, midbrain, and posterior insula.
The anterior putamen (Figure 1c) showed positive connectivity with medial superior frontal cortex including the pre-SMA, SMA, and ACC, lateral and anterior PFC, thalamus, midbrain, insula, supramarginal gyrus/superior temporal sulcus, superior/middle temporal structures, amygdala, and midline cerebellar structures including the vermis, and negative connectivity with occipital and parietal cortices, gyrus rectus and olfactory gyrus. The posterior putamen (Figure 1d) showed fairly similar patterns to the anterior putamen; it additionally showed positive connectivity with somatomotor cortex, premotor cortex, and negative connectivity to a larger region of vmPFC/mOFC. Pallidum (Figure 1e) showed patterns of functional connectivity largely similar to the anterior putamen, except in the mOFC where, as with the posterior putamen, the connectivity is negative. VTA/SN (Figure 1f) showed positive connectivity with SMA, ACC, vmPFC, superior temporal cortex, posterior cingulate cortex, thalamus, basal ganglia, insula and cerebellum, and negative connectivity with occipital, parietal and somatomotor cortices. These results largely replicate those of previous work (Di Martino et al., 2008; Barnes et al., 2010; Farr et al., 2014).
3.2 Age-related changes in functional connectivity: linear regression
Several distinct findings were associated with age. For the head of caudate, the strength of positive connectivity with vmPFC and mOFC increased with age, and connectivity to the calcarine sulcus decreased with age (Figure 2a, 3a; Tables 2 & 3). The body of caudate also showed age-related increases in connectivity to the vmPFC/mOFC, as well as putamen, pulvinar and left angular gyrus (Figure 2b, 3b; Table 2). The anterior putamen showed age-related increases in connectivity with the vmPFC/mOFC, parahippocampal gyrus, right middle temporal gyrus, and age-related decreases to superior occipital gyrus (Figures 2c, 3c; Tables 2 & 3). For the posterior putamen, the connectivity with parahippocampal gyrus, right middle temporal gyrus, posterior cingulate cortex (PCC) and right angular gyrus increased with age. Additionally, connectivity decreased with age to the superior occipital gyrus and somatomotor cortices (Figures 2d, 3d; Tables 2 & 3). Age-related results for the pallidum were similar to those for anterior and/or posterior putamen seeds; it additionally showed increased connectivity to posterior putamen and decreased connectivity to lateral OFC (lOFC)/ventrolateral PFC (vlPFC) (Figures 2e, 3e; Tables 2 & 3). Lastly, for the VTA/SN seed, connectivity with anterior lateral PFC and cerebellum increased with age. Additionally, connectivity decreased to somatomotor cortices (Figures 2f, 3f; Tables 2 & 3).
We also performed a supplementary analysis looking at age effects using the same linear regression in a smaller subset of the participants (data not shown here). For this analysis, only datasets from our own participant pool (n = 106) were included. This was a control analysis and its purpose was two-fold: first, while the larger data set had varying acquisition order and TR, our data set were homogeneous in these imaging parameters (see Table 1). Second, the total participant pool had a somewhat skewed age distribution; this smaller cohort of our own participants had a higher proportion of older individuals aged 30–49, and thus a less skewed age distribution. Hence, we ran this supplementary analysis to check if the general trend of age-related changes in functional connectivity still held when acquisition order/TR scanning parameters were controlled for and when there was a less skewed age distribution. Indeed, the results were largely similar for all six seed regions. For instance, we still observed significant age-related decreases in connectivity between pallidum/putamen/VTA/SN and somatomotor cortex and age-related increases in connectivity between pallidum/putamen and parahippocampal gyrus/middle temporal gyrus. However, the results were not identical, as may be expected in the analysis of a much smaller sample of the original cohort. Notably, the age-related increase in caudate head-vmPFC/OFC connectivity and decrease in putamen-superior occipital cortex connectivity now only reached significance with small volume correction for the cluster obtained from the original analyses.
Lastly, we performed one additional analysis to ensure that signal bleeding did not influence the results, which can occur because our striatal seed regions were adjacent to one another (e.g., Choi et al., 2012). We removed an outer layer, one voxel thick, from each striatal seed region, in the hope of removing any potential influence of signal mixing. This resulted in roughly a 50 to 75% reduction in volume, depending on the shape of each region. The size of each seed region before and after this procedure were: a) head of caudate 12496 mm3 reduced to 5880 mm3, b) body of caudate 3792 mm3 reduced to 976 mm3, c) anterior putamen 9144 mm3 reduced to 4314 mm3, d) posterior putamen 8592 mm3 reduced to 3136 mm3, and e) pallidum 4548 mm3 reduced to 1760 mm3. Hence, the seeds were significantly smaller and there was greater physical distance between them, decreasing the likelihood of signal bleeding. The results of age regression analysis on these smaller seeds were nearly identical to the main analysis (Supplementary Figure S2), and so only the results from the main analysis are presented here.
Tests for gender differences in functional connectivity yielded no significant results at the same statistical threshold whether age was controlled for or not.
4.0 Discussion
Our results demonstrate that cerebral functional connectivity of the basal ganglia nuclei and VTA/SN change from the ages of 18 to 49. With age, connectivity of the putamen and pallidum increased with default mode network (DMN) regions such as the vmPFC and PCC. We also observed that the putamen, pallidum, and VTA/SN showed decreased connectivity with regions in the “task-positive” network (TPN), such as somatomotor cortex. These results build on an emerging body of evidence supporting an anti-correlation between the DMN and the TPN and show that the strength of anti-correlation is reduced with age from young to middle adulthood.
4.1 Functional connectivity of the basal ganglia
Before discussing the age-related changes in functional connectivity in detail, we confirmed that cerebral connectivity of the striatum and VTA/SN are consistent with previous reports (Di Martino et al., 2008; Barnes et al., 2010; Farr et al., 2014). The caudate nucleus showed positive connectivity with a wide area of the frontal cortex and negative connectivity with somatomotor and occipital cortices. This is consistent with a role of the caudate nucleus in many forms of executive functions (e.g., Liston et al., 2006; Cools, 2008), in accord with known anatomy (Alexander et al., 1986; Middleton & Strick, 2000; Haber, 2003). The pallidum and putamen showed positive connectivity with the cerebellum, SMA/pre-SMA, and motor cortices, consistent with their large role in motor control (Alexander et al., 1990; Haber, 2003). Lastly, the VTA/SN seed showed positive connectivity with vmPFC and ACC, highlighting VTA/SN’s putative role in saliency processing (Horvitz, 2000; Ungless, 2004; Seeley et al., 2007; Ide et al., 2013). Overall, these findings replicate earlier studies and validate the use of rsfMRI in understanding functional brain networks throughout the lifespan.
4.2 Executive function and age-related changes in caudate-vmPFC connectivity
Reciprocally connected with a wide area of the prefrontal cortex (PFC) including the dorsolateral PFC and dorsal ACC (Yeterian & Van Hoesen, 1978; Selemon & Goldman-Rakic, 1985; Vogt & Pandya 1987; Haber, 2003; Zhang et al., 2012), the caudate nucleus is viewed as a subcortical hub for higher order cognitive functions (Cools, 2008). Our data showed that with age, connectivity increased between the caudate nucleus and the vmPFC/mOFC. Activity in the vmPFC is often found to be related to saliency and reward processing (Rolls, 2000; Elliot et al., 2000) and decision making such as during delayed discounting (McClure et al., 2007). Interestingly, Christakou et al. (2011) observed age-related increases from adolescence to the 30s in functional connectivity between vmPFC and a portion of ventral striatum including the caudate head. These increases in connectivity were associated with a reduction in delayed discounting, or a greater ability to wait for large delayed over small immediate rewards, a behavior that improves with normal aging (Simon et al., 2010). Further, fractional anisotropy of white matter in this region increased with age, from 9 to 23 years old, and reduced delay discounting (Olson et al., 2007). Hence, our findings show that functional connectivity between vmPFC and caudate increases throughout mid-adulthood and may support cognitive control of impulsive choices.
4.3 Somatomotor processing and age-related changes in pallidum/putamen connectivity
The basal ganglia nuclei have long been implicated in somatomotor processing (for reviews, see Alexander et al., 1986; Haber, 2003). Striatal neurons project to the output compartments of the pallidum and SN pars reticulata, which send projections to sensory and motor cortices via the thalamus (Kievit & Kuypers, 1977; Ueki, 1983; Middleton & Strick, 1994), in a circuit to process and gate sensory information and regulate motor output. As sensory and motor deficits are some of the most pronounced behavioral manifestations associated with aging, age-related changes in activity of the basal ganglia and somatomotor cortices is frequently observed in neuroimaging investigations (Sailer et al., 2000; Mattay et al., 2002; Wu & Hallett, 2005; Madden et al., 2007; Taniwaki et al., 2007). For instance, during a visual attention task, older subjects under-recruited occipital cortex but over-recruited fronto-parietal regions compared to young adults (Madden et al., 2007). Older subjects also showed higher activation of cerebellar, striatal, and motor cortical areas during simple motor tasks compared to young adults (Mattay et al., 2002; Wu & Hallett, 2005). Older subjects showed reduced functional connectivity between the nodes of the basal ganglia-cerebellar-motor cortical circuit during self-initiated movements (Taniwaki et al., 2007). Our data showed that posterior putamen, pallidum, and VTA/SN decreases in connectivity to left somatomotor cortices with age, consistent with findings of structural connectivity of bilateral putamen with left but not right somatomotor cortex in diffusion tensor imaging (Ystad et al., 2011). The decrease in functional connectivity to left somatomotor cortex may be mediated by age-related decline in dopaminergic signaling (Volkow et al., 2000), as drugs that augment (levodopa) or dampen (haloperidol) dopaminergic signaling each increased and decreased the connectivity between striatum and somatomotor cortex (Tost et al., 2010; Cole et al., 2013).
4.4 Default Mode and Task Positive Networks and age-related changes in connectivity
Much attention has been given to two functional networks in the brain: the DMN and the TPN (Fox et al., 2005). In healthy young adults in their early to mid 20s, these networks are robustly anticorrelated in activity; DMN regions such as vmPFC and PCC are more activated in the absence of a task or environmental stimulation, while TPN regions such as dorsolateral PFC, somatomotor cortex, and some basal ganglia nuclei including the putamen and pallidum are more activated during task performance (Fox et al., 2005; Fox et al., 2007; Robinson et al., 2009; Uddin et al., 2009). Our results show that this anticorrelation diminishes with age; while the connectivity within TPN regions (e.g., pallidum/posterior putamen to somatomotor cortex) decreases, connectivity between TPN and DMN regions (e.g., pallidum/posterior putamen to PCC) increases. As a result, TPN and DMN decreases in their contrasting pattern of activity with age.
The degree of anticorrelation in TPN and DMN activity is emerging as an important neural marker in the functional connectivity and aging literature. Kelly et al. (2008) and Grady et al. (2010) found in both young and older adults that weaker DMN-TPN anticorrelations were associated with more variable behavioral performance on cognitive tasks. Converging evidence has shown that the DMN-TPN anticorrelation diminishes across the lifespan. For instance, the degree of activation in TPN regions during cognitive tasks decreases with age (e.g., Andrews-Hanna et al., 2007; Esposito et al. 2008; Roski et al., 2013). Likewise, the degree of deactivation in DMN regions during tasks is reduced in older compared to younger adults (Lustig et al. 2003; Grady et al. 2006; Persson et al. 2007; Damoiseaux et al. 2008; Miller et al. 2008). Finally, Wu et al. (2011) showed that anticorrelations between DMN and TPN regions were reduced during resting state in old as compared to younger adults.
Thus, the present findings on the basal ganglia add to a growing body of work suggesting that the balance between the DMN and TPN may change during healthy aging, as early as from young to middle adulthood. This result also seems broadly consistent with recent work showing an age-related decrease in long-range cortical connectivity (Sala-Llonch et al., 2014). While the neurochemical substrates of this phenomenon have not been explored in detail, age-related alterations in dopaminergic signaling may be one mechanism behind this observation. Both dopamine transporters (Volkow et al., 1996; Troiano et al., 2010) and receptors (Wong et al., 1997; Volkow et al., 1998; Kaasinen et al., 2000) decrease with healthy aging throughout the brain, and age-related changes in dopaminergic signaling are associated with long-range changes in brain function (Volkow et al., 2000). Specifically, in healthy individuals, dopaminergic agonists strengthen positive correlations in activity between regions within the TPN such as caudate and frontal/parietal cortex (Cole et al., 2013). Likewise, unmedicated individuals with Parkinson’s disease, a population with striatal dopamine depletion, showed higher putamen-vmPFC connectivity than age-matched controls (i.e., a weaker TPN-DMN anti-correlation) and this alteration was remediated with L-DOPA (Kwak et al., 2010). Further, during a perceptual task unmedicated individuals with Parkinson’s disease failed to deactivate the posterior nodes of the DMN, but the typical deactivation pattern emerged after L-DOPA administration (van Eimeren et al., 2009; Delaveau et al., 2010). Thus, future studies are warranted to investigate whether dopaminergic signaling and TPN-DMN connectivity go in parallel during healthy aging and the clinical course of Parkinson’s disease.
Age-related changes in functional connectivity among cortico-basal ganglia circuits have also been observed in neurological conditions other than Parkinson’s disease. For instance, in individuals with Alzheimer’s disease, both the positive within-network default mode correlations and DMN-TNP anticorrelations diminished compared to controls, including reduced anticorrelations between PCC and basal ganglia nuclei (Wang et al., 2007; Zhang et al., 2009). Disruption of between-network activity has also been reported in individuals with amnestic mild cognitive impairment (Bai et al., 2008). These observations suggest that alternative mechanisms need to be considered to account for altered DMN-TNP anticorrelations during healthy aging and in neurological conditions.
4.5 A methodological consideration
Negative functional connectivity has been observed and reported since the very beginning of the resting state fMRI study (Biswal et al., 1995). Negative functional connectivity, also called anti-correlation, represents negative cross-correlation in spontaneous BOLD signals between two brain regions. It was suggested that the global signal regression, a common step of data preprocessing in seed region based functional connectivity analyses, is a likely cause of anti-correlated functional networks (Murphy et al., 2009; Weissenbacher et al., 2009). However, recent investigations demonstrated that the negative correlations are not an artifact but have biological origins (Fox et al., 2009; Chen et al., 2011; Chai et al., 2012). For instance, negative functional connectivity is associated predominantly with long range connections and correlates with the shortest path length in the human brain network (Scholvinck et al., 2010; Chen et al., 2011; Schwarz and McGonigle, 2011). Indeed, the negative correlations between brain regions with presumably opposing functional roles have been consistently observed in different studies (Greicius et al., 2003; Fox et al., 2005; Fransson, 2005; Kelly et al., 2008; Uddin et al., 2009; Chen et al., 2011), including those using independent component analysis, which does not involve global signal regression (Cole et al., 2010; Zuo et al., 2010; Zhang and Li, 2012b). Furthermore, the existence of the negative functional connectivity was also suggested by computational simulations of cerebral network activities in both monkeys and humans (Honey et al., 2007; Izhikevich and Edelman, 2008; Deco et al., 2009) and supported by simultaneous recording of unit activity and local field potential from task-positive and task-negative (default mode) networks in cats (Popa et al., 2009). Together, these earlier studies suggest functional significance of negative functional connectivity.
4.6 Limitations and conclusions
The current study had several limitations. Firstly, we did not account for cardiovascular and respiratory variables which depend on age and could potentially impact findings of resting state functional connectivity (although it is thought that significant age-related changes in these domains only begin to emerge in the fourth decade of life; see Farkas & Luiten, 2001). Second, the majority of subjects were under the age of 30. Ideally, the age distribution would have been centered around 35 for a study looking at ages between 18 and 49. These results thus need to be replicated both for the current age range and other developmental stages, as studied for other neural systems (Antonenko et al., 2013; Bernard et al., 2013; Damaraju et al., 2014; Gabard-Durnam et al., 2014). Furthermore, we did not observe age-related changes such as those of caudate – DLPFC connectivity that typically occur during cognitive aging. This is likely because our sample consists of participants in their young to middle adulthood and, thus, many of the age-related neural and cognitive changes reported for the elderly were not observed (Andrews-Hanna et al., 2007). Nonetheless, the current results demonstrate changes in cerebral functional connectivities for a chronological segment less explored in aging studies. Future work within a longitudinal setting will tell whether the current findings represent precursors to some of the more prominent changes or cerebral dysfunctions observed in the elderly. Third, we did not have information on education, cognitive reserve or neuropsychological performance of the participants. Thus, we cannot rule out the influence of these important factors on the results, nor draw any inferences regarding the relationship between these age-related changes and cognitive or affective functioning. Fourth, the pallidum and putamen functional connectivity maps were largely similar, raising the possibility that some signal mixing occurred between these spatially adjacent structures. After taking steps to remove the influence of signal mixing such as reducing the size of the seed regions and reducing our smoothing parameters, the patterns of connectivity remained highly overlapping, perhaps reflecting the close anatomical and functional link between these structures (e.g., Mink, 1996; Robinson et al., 2009). Nevertheless, the possibility remains that the pallidum results were influenced by signals from the putamen, particularly when considering inter-subject variability; therefore, the pallidum results in this study should be viewed with a lesser degree of certainty. Lastly, this study was based on a between-subject, cross-sectional analysis; thus, the present data on age-related differences are indirect and correlational. A longitudinal design would provide information on how functional connectivity changes with age on an individual basis.
Aging leads to broad changes in the structure and function of the brain. While much attention has been given to underlying neural changes from the cellular to the regional level, less is understood of how large-scale brain networks are altered throughout the lifespan. As more information emerges, it is becoming increasingly clear that the brain regions affected by aging should not be considered in isolation but in relation to their connections with other brain regions (Greene et al., 2014; Hugenschmidt et al., 2014; Schaefer et al., 2014). Here, we have reported results from a specific set of age-related changes in the cortico-basal ganglia circuitry, a network implicated in both healthy and disordered aging.
Supplementary Material
Acknowledgments
This study was supported by NIH grants DA023248, DA026990, AA018004, AA021449 to C-S.R.L. The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. We thank investigators of the 1000 Functional Connectomes Project and those who shared the data set for making this study possible
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahsan RL, Allom R, Gousias IS, Habib H, Turkheimer FE, Free S, Lemieux L, Myers R, Duncan JS, Brooks DJ, et al. Volumes, spatial extents and a probabilistic atlas of the human basal ganglia and thalamus. Neuroimage. 2007;38:261–70. doi: 10.1016/j.neuroimage.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Alexander G, DeLong M, Strick P. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–71. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–35. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenko D, Brauer J, Meinzer M, Fengler A, Kerti L, Friederici AD, Flöel A. Functional and structural syntax networks in aging. Neuroimage. 2013;83:513–23. doi: 10.1016/j.neuroimage.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;266:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Zhang Z, Yu H, Shi Y, Yuan Y, Zhu W, Zhang X, Qian Y. Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: a combined structural and resting-state functional MRI study. Neurosci Lett. 2008;438:111–5. doi: 10.1016/j.neulet.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Barnes KA, Cohen AL, Power JD, Nelson SM, Dosenbach YBL, Miezin FM, Petersen SE, Schlaggar BL. Identifying Basal Ganglia divisions in individuals using resting-state functional connectivity MRI. Front Syst Neurosci. 2010;4:1–10. doi: 10.3389/fnsys.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard Ja, Peltier SJ, Wiggins JL, Jaeggi SM, Buschkuehl M, Fling BW, Kwak Y, Jonides J, Monk CS, Seidler RD. Disrupted cortico-cerebellar connectivity in older adults. Neuroimage. 2013;83:103–19. doi: 10.1016/j.neuroimage.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berry KJ, Mielke PW. A Monte Carlo investigation of the Fisher Z transformation for normal and nonnormal distributions. Psychol Rep. 2000;87:1101–1114. doi: 10.2466/pr0.2000.87.3f.1101. [DOI] [PubMed] [Google Scholar]
- Biswal B. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal B, Mennes M. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabec J, Krásený J, Petrovický P. Volumetry of striatum and pallidum in man--anatomy, cytoarchitecture, connections, MRI and aging. Sb Lek. 2003;104:13–65. [PubMed] [Google Scholar]
- Chai X, Castañón A, Öngür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048.Anticorrelations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Chen G, Xie C, Li SJ. Negative functional connectivity and its dependence on the shortest path length of positive network in the resting-state human brain. Brain Connect. 2011;1:195–206. doi: 10.1089/brain.2011.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini A, Péran P, Caltagirone C, Sabatini U, Spalletta G. Aging of subcortical nuclei: microstructural, mineralization and atrophy modifications measured in vivo using MRI. Neuroimage. 2009;48:29–36. doi: 10.1016/j.neuroimage.2009.06.035. [DOI] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. Neuroimage. 2011;54:1344–54. doi: 10.1016/j.neuroimage.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Choi EY, Yeo BTT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–63. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010;52:590–9. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Searle GE, Plisson C, Tziortzi AC, Nichols TE, Gunn RN, Matthews PM, Rabiner Ea, Beaver JD. Orbitofrontal connectivity with resting-state networks is associated with midbrain dopamine D3 receptor availability. Cereb Cortex. 2012;22:2784–93. doi: 10.1093/cercor/bhr354. [DOI] [PubMed] [Google Scholar]
- Cole DM, Oei NYL, Soeter RP, Both S, van Gerven JMa, Rombouts SaRB, Beckmann CF. Dopamine-dependent architecture of cortico-subcortical network connectivity. Cereb Cortex. 2013;23:1509–16. doi: 10.1093/cercor/bhs136. [DOI] [PubMed] [Google Scholar]
- Cools R. Role of dopamine in the motivational and cognitive control of behavior. Neuroscientist. 2008;14:381–95. doi: 10.1177/1073858408317009. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski Pa, Moritz CH, Quigley Ma, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–33. [PMC free article] [PubMed] [Google Scholar]
- Damaraju E, Caprihan a, Lowe JR, Allen Ea, Calhoun VD, Phillips JP. Functional connectivity in the developing brain: a longitudinal study from 4 to 9months of age. Neuroimage. 2014;84:169–80. doi: 10.1016/j.neuroimage.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SaRB. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–64. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Davis FC, Knodt AR, Sporns O, Lahey BB, Zald DH, Brigidi BD, Hariri AR. Impulsivity and the modular organization of resting-state neural networks. Cereb Cortex. 2013;23:1444–52. doi: 10.1093/cercor/bhs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaveau P, Salgado-Pineda P, Fossati P, Witjas T, Azulay JP, Blin O. Dopaminergic modulation of the default mode network in Parkinson’s disease. Eur Neuropsychopharmacol. 2010;20:784–92. doi: 10.1016/j.euroneuro.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRa, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Klöppel S, Cook Pa, Alexander DC, Parker GJM, Deichmann R, Ashburner J, Frackowiak RSJ. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008;28:7143–52. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eimeren MT, Monchi O. Dysfunction of the Default Mode Network in Parkinson Disease. Arch Neurol. 2009;66:877–883. doi: 10.1001/archneurol.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10:308–17. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Esposito F, Aragri A, Pesaresi I, Cirillo S, Tedeschi G, Marciano E, Goebel R, Di Salle F. Independent component model of the default-mode brain function: combining individual-level and population-level analyses in resting-state fMRI. Magn Reson Imaging. 2008;26:905–13. doi: 10.1016/j.mri.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Fair D, Schlaggar B, Cohen A. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, Luiten PGM. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/S0301-0082(00)00068-X. [DOI] [PubMed] [Google Scholar]
- Farr OM, Zhang S, Hu S, Matuskey D, Abdelghany O, Malison RT, Li C-SR. The effects of methylphenidate on resting-state striatal, thalamic and global functional connectivity in healthy adults. Int J Neuropsychopharmacol. 2014:1–15. doi: 10.1017/S1461145714000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–84. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–83. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Snyder A, Vincent J, Corbetta M, Van Essen D, Raichle M. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Frith C, Frackowiak R, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995;2:166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Hare T, Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23years: A cross-sectional study. Neuroimage. 2014;95C:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodro M, Sameti M, Patenaude B, Fein G. Age effect on subcortical structures in healthy adults. Psychiatry Res. 2012;203:38–45. doi: 10.1016/j.pscychresns.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, Anderson JaE, Churchill N, McIntosh AR. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2010;20:1432–47. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DJ, Laumann TO, Dubis JW, Ihnen SK, Neta M, Power JD, Pruett JR, Black KJ, Schlaggar BL. Developmental Changes in the Organization of Functional Connections between the Basal Ganglia and Cerebral Cortex. J Neurosci. 2014;34:5842–54. doi: 10.1523/JNEUROSCI.3069-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hämäläinen A, Pihlajamäki M, Tanila H, Hänninen T, Niskanen E, Tervo S, Karjalainen Pa, Vanninen RL, Soininen H. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging. 2007;28:1889–903. doi: 10.1016/j.neurobiolaging.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Hammers A, Allom R, Koepp M. Three- dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;247:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Wang J, Zhao Z, Min B, Lu J, Li K, He Y, Jia J. Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: a resting-state fMRI study. Neuroimage. 2011;55:287–95. doi: 10.1016/j.neuroimage.2010.11.059. [DOI] [PubMed] [Google Scholar]
- Haug H. Are neurons of the human cerebral cortex really lost during aging? A morphometric examination. Sen Dement Alzheimer type. 1985:13–18. [Google Scholar]
- Hayasaka S, Nichols TE. Validating cluster size inference: Random field and permutation methods. Neuroimage. 2003;20:2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O’Reilly RC. Banishing the homunculus: making working memory work. Neuroscience. 2006;139:105–18. doi: 10.1016/j.neuroscience.2005.04.067. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I. Spatial remapping of cortico-striatal connectivity in Parkinson’s disease. Cereb Cortex. 2010;20:1175–86. doi: 10.1093/cercor/bhp178. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Kötter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci U S A. 2007;104:10240–5. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz J. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Hugenschmidt CE, Burdette JH, Morgan AR, Williamson JD, Kritchevsky SB, Laurienti PJ. Graph Theory Analysis of Functional Brain Networks and Mobility Disability in Older Adults. J Gerontol A Biol Sci Med Sci. 2014:1–8. doi: 10.1093/gerona/glu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Shenoy P, Yu AJ, Li CR. Bayesian prediction and evaluation in the anterior cingulate cortex. J Neurosci. 2013;33:2039–47. doi: 10.1523/JNEUROSCI.2201-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhikevich EM, Edelman GM. Large-scale model of mammalian thalamocortical systems. Proc Natl Acad Sci U S A. 2008;105:3593–8. doi: 10.1073/pnas.0712231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of the primate subthalamic nucleus: indirect pathways and the open-interconnected scheme of basal ganglia-thalamocortical circuitry. Brain Res Rev. 1997;23:62–78. doi: 10.1016/s0165-0173(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Någren K, Helenius H, Olsson H, Farde L, Rinne J. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000;21:683–8. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Kelly aMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–37. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kemper TL. Neuroanatomical and neuropathological changes during aging and dementia. In: Albert MLKJE, editor. Clinical neurology of aging. New York: Oxford University Press; 1994. pp. 3–67. [Google Scholar]
- Kievit J, Kuypers HGJM. Brain Organization of the Thalamo-Cortical Connexions to the Frontal Lobe in the Rhesus Monkey. Exp Brain Res. 1977;29:299–322. doi: 10.1007/BF00236173. [DOI] [PubMed] [Google Scholar]
- Kwak Y, Peltier S, Bohnen NI, Müller MLTM, Dayalu P, Seidler RD. Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson’s disease. Front Syst Neurosci. 2010;4:143. doi: 10.3389/fnsys.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker Sa, Briceno EM, Hamid NM, Nielson Ka. An evaluation of distinct volumetric and functional MRI contributions toward understanding age and task performance: a study in the basal ganglia. Brain Res. 2007;1135:58–68. doi: 10.1016/j.brainres.2006.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Ide JS, Zhang S, Hu S, Chao HH, Zaborszky L. Resting state functional connectivity of the basal nucleus of Meynert in humans: In comparison to the ventral striatum and the effects of age. Neuroimage. 2014 doi: 10.1016/j.neuroimage.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex. 2006;16:553–60. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100:14504–9. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel Sa. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol Aging. 2007;28:459–76. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark RE, Rugg MD. Age effects on brain activity associated with episodic memory retrieval. An electrophysiological study. Brain. 1998;121(Pt 5):861–73. doi: 10.1093/brain/121.5.861. [DOI] [PubMed] [Google Scholar]
- Mars RB, Jbabdi S, Sallet J, O’Reilly JX, Croxson PL, Olivier E, Noonan MP, Bergmann C, Mitchell AS, Baxter MG, et al. Diffusion-weighted imaging tractography-based parcellation of the human parietal cortex and comparison with human and macaque resting-state functional connectivity. J Neurosci. 2011;31:4087–100. doi: 10.1523/JNEUROSCI.5102-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino a, Scheres a, Margulies DS, Kelly aMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–47. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–635. doi: 10.1212/WNL.58.4.630. [DOI] [PubMed] [Google Scholar]
- Middleton Fa, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–61. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42:183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamäki M, Sperling Ra. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008;105:2181–6. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn R, Handwerker D, Jones T, Bandettini P. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson Ea, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: a diffusion tensor imaging study. J Cogn Neurosci. 2009;21:1406–21. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Hardingham GE. The Dichotomy of NMDA Receptor Signaling. Neurosci. 2007;13:572–579. doi: 10.1177/1073858407305833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini M, Krueger F, Raymont V, Grafman J. Ventromedial prefrontal cortex modulates fatigue after penetrating traumatic brain injury. Neurology. 2010;74:749–54. doi: 10.1212/WNL.0b013e3181d25b6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz Pa. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19:1021–32. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Engelmann JB. Embedding reward signals into perception and cognition. Front Neurosci. 2010;4:1–8. doi: 10.3389/fnins.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol. 2002;593:581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Popa D, Popescu AT, Paré D. Contrasting activity profile of two distributed cortical networks as a function of attentional demands. J Neurosci. 2009;29:1191–201. doi: 10.1523/JNEUROSCI.4867-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–21. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes Ka, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue K. Differential aging of the human striatum: longitudinal evidence. Am J Neuroradiol. 2003;24:1849–1856. [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso Ja. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11:760–72. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J, Laird A, Glahn D. The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage. 2012;60:117–129. doi: 10.1016/j.neuroimage.2011.12.010.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Basso G, Soldati N, Sailer U, Jovicich J, Bruzzone L, Kryspin-Exner I, Bauer H, Moser E. A resting state network in the motor control circuit of the basal ganglia. BMC Neurosci. 2009;10:1–14. doi: 10.1186/1471-2202-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–94. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rombouts SaRB, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: an fMRI study. Hum Brain Mapp. 2005;26:231–9. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts S, Stam C, Kuijer J. Identifying confounds to increase specificity during a “no task condition”: Evidence for hippocampal connectivity using fMRI. Neuroimage. 2003;20:1236–1245. doi: 10.1016/S1053-8119(03)00386-0. [DOI] [PubMed] [Google Scholar]
- Roski C, Caspers S, Langner R, Laird AR, Fox PT, Zilles K, Amunts K, Eickhoff SB. Adult age-dependent differences in resting-state connectivity within and between visual-attention and sensorimotor networks. Front Aging Neurosci. 2013;5:1–10. doi: 10.3389/fnagi.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28:1163–77. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer A, Dichgans J, Gerloff C. The influence of normal aging on the cortical processing of a simple motor task. Neurology. 2000;55:979–985. doi: 10.1212/WNL.55.7.979. [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R, Junqué C, Arenaza-Urquijo EM, Vidal-Piñeiro D, Valls-Pedret C, Palacios EM, Domènech S, Salvà A, Bargalló N, Bartrés-Faz D. Changes in whole-brain functional networks and memory performance in aging. Neurobiol Aging. 2014:1–10. doi: 10.1016/j.neurobiolaging.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Margulies DS, Lohmann G, Gorgolewski KJ, Smallwood J, Kiebel SJ, Villringer A. Dynamic network participation of functional connectivity hubs assessed by resting-state fMRI. Front Hum Neurosci. 2014;8:1–13. doi: 10.3389/fnhum.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schölvinck ML, Maier A, Ye FQ, Duyn JH, Leopold Da. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A. 2010;107:10238–43. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, McGonigle J. Negative edges and soft thresholding in complex network analysis of resting state functional connectivity data. Neuroimage. 2011;55:1132–46. doi: 10.1016/j.neuroimage.2010.12.047. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon L, Goldman-Rakic P. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, LaSarge CL, Montgomery KS, Williams MT, Mendez Ia, Setlow B, Bizon JL. Good things come to those who wait: attenuated discounting of delayed rewards in aged Fischer 344 rats. Neurobiol Aging. 2010;31:853–62. doi: 10.1016/j.neurobiolaging.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Umberger GH, Manning EL, Slevin JT, Wekstein DR, Schmitt FA, Markesbery WR, Zhang Z, Gerhardt GA, Kryscio RJ, et al. Critical decline in fine motor hand movements in human aging. Neurology. 1999;53:1458–1461. doi: 10.1212/WNL.53.7.1458. [DOI] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20:2852–62. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg C, Riedl V. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer’s disease. Ann N Y Acad Sci. 2007;1097:146–55. doi: 10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- Sporns O, Ko R, Montagna M, Menin C, Scaini MC, Bartel F, Pempe C, Gradhand E, Bond GL. Key role of coupling, delay, and noise in resting brain fluctuations. Proc Natl Acad Sci. 2009;106:12207–12208. doi: 10.1073/pnas.0906701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–66. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniwaki T, Okayama A, Yoshiura T, Togao O, Nakamura Y, Yamasaki T, Ogata K, Shigeto H, Ohyagi Y, Kira JI, et al. Age-related alterations of the functional interactions within the basal ganglia and cerebellar motor loops in vivo. Neuroimage. 2007;36:1263–76. doi: 10.1016/j.neuroimage.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow N. Aging and Functional Brain Networks. Mol Psychiatry. 2012a;17:549–558. doi: 10.1038/mp.2011.81.Aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Functional Connectivity of Substantia Nigra and Ventral Tegmental Area: Maturation During Adolescence and Effects of ADHD. Cereb Cortex. 2012b doi: 10.1093/cercor/bhs382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Braus DF, Hakimi S, Ruf M, Vollmert C, Hohn F, Meyer-Lindenberg A. Acute D2 receptor blockade induces rapid, reversible remodeling in human cortical-striatal circuits. Nat Neurosci. 2010;13:920–2. doi: 10.1038/nn.2572. [DOI] [PubMed] [Google Scholar]
- Troiano AR, Schulzer M, de la Fuente-Fernandez R, Mak E, McKenzie J, Sossi V, McCormick S, Ruth TJ, Stoessl aJ. Dopamine transporter PET in normal aging: dopamine transporter decline and its possible role in preservation of motor function. Synapse. 2010;64:146–51. doi: 10.1002/syn.20708. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly aM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–37. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki A. The mode of nigro-thalamic transmission investigated with intracellular recording in the cat. Exp Brain Res. 1983;49:116–124. doi: 10.1007/BF00235546. [DOI] [PubMed] [Google Scholar]
- Ungless Ma. Dopamine: the salient issue. Trends Neurosci. 2004;27:702–6. doi: 10.1016/j.tins.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Spraker MB, Prodoehl J, Zhou XJ, Little DM. Effects of aging on the ventral and dorsal substantia nigra using diffusion tensor imaging. Neurobiol Aging. 2012;33:35–42. doi: 10.1016/j.neurobiolaging.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt Ba, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262:271–89. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Logan J, Fowler JS, Wang GJ, Gur RC, Wong C, Felder C, Gatley SJ, Ding YS, Hitzemann R, et al. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry. 2000;157:75–80. doi: 10.1176/ajp.157.1.75. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Ding YS, Gur RC, Gatley J, Logan J, Moberg PJ, Hitzemann R, Smith G, et al. Parallel loss of presynaptic and postsynaptic dopamine markers in normal aging. Ann Neurol. 1998;44:143–7. doi: 10.1002/ana.410440125. [DOI] [PubMed] [Google Scholar]
- Volkow N, Ding Y, Fowler J, G-J W, Logan J, Gatley S, Hitzemann R, Smith G, Fields S, Gur R. Dopamine transporters decrease with age. J Nucl Med. 1996;37:554–559. [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, et al. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. 2011;32:916–32. doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Liang M, Wang L, Tian L, Zhang X, Li K, Jiang T. Altered functional connectivity in early Alzheimer’s disease: A resting-state fMRI study. Hum Brain Mapp. 2007;28:967–978. doi: 10.1002/hbm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS. Age-related changes in the neural correlates of motor performance. Brain. 2003;126:873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–16. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Wise Ra. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wong D, Young D, Wilson P, Meltzer C, Gjedde A. Quantification of neuroreceptors in the living human brain: III. D2-like dopamine receptors: theory, validation, and changes during normal aging. J Cereb Blood Flow Metab. 1997;17:316–330. doi: 10.1097/00004647-199703000-00009. [DOI] [PubMed] [Google Scholar]
- Wu JT, Wu HZ, Yan CG, Chen WX, Zhang HY, He Y, Yang HS. Aging-related changes in the default mode network and its anti-correlated networks: a resting-state fMRI study. Neurosci Lett. 2011;504:62–7. doi: 10.1016/j.neulet.2011.08.059. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. The influence of normal human ageing on automatic movements. J Physiol. 2005;562:605–15. doi: 10.1113/jphysiol.2004.076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian E, Van Hoesen G. Cortico-striate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain Res. 1978;139:43–63. doi: 10.1016/0006-8993(78)90059-8. [DOI] [PubMed] [Google Scholar]
- Ystad M, Hodneland E, Adolfsdottir S, Haász J, Lundervold AJ, Eichele T, Lundervold A. Cortico-striatal connectivity and cognition in normal aging: a combined DTI and resting state fMRI study. Neuroimage. 2011;55:24–31. doi: 10.1016/j.neuroimage.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Wang SJ, Xing J, Liu B, Ma ZL, Yang M, Zhang ZJ, Teng GJ. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer’s disease. Behav Brain Res. 2009;197:103–8. doi: 10.1016/j.bbr.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Zhang S, Ide JS, Li CR. Resting-state functional connectivity of the medial superior frontal cortex. Cereb Cortex. 2012;22:99–111. doi: 10.1093/cercor/bhr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li C. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012a;59:3548–3562. doi: 10.1016/j.neuroimage.2011.11.023.Functional. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CR. Functional networks for cognitive control in a stop signal task: independent component analysis. Hum Brain Mapp. 2012b;33:89–104. doi: 10.1002/hbm.21197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CSR. Functional clustering of the human inferior parietal lobule by whole-brain connectivity mapping of resting-state functional magnetic resonance imaging signals. Brain Connect. 2014;4:53–69. doi: 10.1089/brain.2013.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks : Test – retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49:2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.