Abstract
Naltrexone and bupropion, when administered alone in clinical trials, modestly reduce amphetamine use. Whether combining these drugs would result in greater reductions in methamphetamine taking relative to either drug alone is undetermined. This study examined the influence of naltrexone, bupropion and a naltrexone-bupropion combination on methamphetamine self-administration in humans. Seven subjects reporting recent illicit stimulant use completed a placebo-controlled, crossover, double-blind study in which the reinforcing, subject-rated and physiological effects of intranasal methamphetamine (0, 10 and 30 mg) were assessed during maintenance on placebo, naltrexone (50 mg), bupropion (300 mg/day), and naltrexone combined with bupropion. Methamphetamine maintained responding and produced prototypic subjective and physiological effects (e.g., increased ratings of Good Effects, elevated systolic blood pressure). Maintenance doses were well tolerated and generally devoid of effects. No maintenance condition reduced methamphetamine self-administration or systematically altered the subject-rated effects of methamphetamine. These outcomes demonstrate the robust behavioral effects of methamphetamine that could make it resistant to pharmacological manipulation. Although these outcomes indicate that this combination may be ineffective for managing methamphetamine use disorder, future work should evaluate longer maintenance dosing, individuals with different levels of amphetamine use, adding this combination to a behavioral platform and other pharmacotherapy combinations for reducing methamphetamine use.
Keywords: methamphetamine, bupropion, naltrexone, self-administration, subject-rated drug effects, physiological drug effects
1. Introduction
Methamphetamine use disorder is a significant problem in the United States. In 2012, 440,000 individuals over 12 years of age reported using methamphetamine in the last month and 535,000 individuals reported being dependent on non-cocaine stimulants including methamphetamine (Substance Abuse and Mental Health Services Administration [SAMHSA], 2013a). In 2011, approximately 6% of substance abuse treatment admissions reported methamphetamine or amphetamine as their primary substance of abuse (SAMHSA, 2013b). Methamphetamine use is associated with other drug use, physical and mental health problems and engagement in criminal activities (Stoops et al., 2007; 2005) and leads to an annual economic cost of $23 billion in the United States (Nicosia et al., 2009). Despite the problems posed by methamphetamine, a pharmacological adjunct for managing methamphetamine use disorder has yet to be approved.
Novel strategies are needed for the development of methamphetamine pharmacotherapies. One such strategy is combination treatment, especially with medications that display some efficacy when administered alone (Stoops and Rush, 2014). Naltrexone, a mu opioid receptor antagonist, and bupropion, a dopamine and norepinephrine reuptake inhibitor, are two candidate medications that have been evaluated for managing amphetamine use disorder from preclinical research to clinical trials. Naltrexone reduces drug primed reinstatement of amphetamine taking in rats (Häggkvist et al., 2009), as well as amphetamine self-administration in monkeys (Jimenez-Gomez et al., 2011). Bupropion pretreatment also reduces methamphetamine self-administration in both rats and monkeys (Reichel et al., 2009; Schindler et al., 2011). Human laboratory studies have shown that naltrexone and bupropion reduce the subject-rated effects of amphetamines (Comer et al., 2013; Jayaram-Lindström et al., 2004; 2008b; Marks et al., 2014; Newton et al., 2006).
Consistent with laboratory findings, both naltrexone and bupropion have demonstrated superiority to placebo for reducing amphetamine use in some patients, although neither of these medications completely eliminates drug taking when tested in randomized, double-blind, clinical trials (Jayaram-Lindström et al., 2008a; Elkashef et al., 2008; Shoptaw et al., 2008). In the first trial, treatment-seeking amphetamine-dependent patients received 50 mg/day naltrexone (N=40) or placebo (N=40) for 12 weeks (Jayaram-Lindström et al., 2008a). Patients attended the clinic twice weekly to provide urine samples that were screened for amphetamine use and to receive relapse prevention therapy. Patients maintained on naltrexone provided significantly more amphetamine-negative urine samples than their placebo-treated counterparts across the 12-week trial. Although statistically significant, the magnitude of the effect of naltrexone was small. The average percentage of amphetamine-negative urine samples across the trial was approximately 48% and 65% for the placebo- and naltrexone-treated patients, respectively.
In the second trial, methamphetamine-dependent patients were randomly assigned to receive sustained-release bupropion (150 mg, twice daily; N=79) or placebo (twice daily; N=72) (Elkashef et al., 2008). Thrice weekly drug urine tests were the primary outcome measure. The bupropion-treated patients provided fewer amphetamine-positive urine samples than the placebo-treated patients, although this effect did not attain significance according to traditional statistical standards. Secondary analyses showed that relative to placebo, bupropion produced a significant effect in patients that reported lighter methamphetamine use (i.e., ≤ 18 days out of the 30 prior to screening) at intake. Similar results were observed in the second trial with bupropion (Shoptaw et al., 2008). Whether combining naltrexone with bupropion would result in increased efficacy for reducing amphetamine use has yet to be determined.
The purpose of this study was to use human laboratory methods to screen the efficacy of combining naltrexone and bupropion for reducing methamphetamine use. Naltrexone and bupropion were selected for testing due to the supportive preclinical and clinical outcomes described above, as well as their distinct pharmacological mechanisms. Because self-administration measures generally have good predictive validity for clinical efficacy (Comer et al., 2008; Haney and Spealman, 2008), the primary outcome was the reinforcing effects of intranasal methamphetamine as a function of maintenance condition (i.e., placebo, naltrexone, bupropion and naltrexone plus bupropion). A battery of subject-rated and physiological measures was also included to more fully evaluate the pharmacodynamic effects of intranasal methamphetamine across maintenance conditions. We hypothesized that methamphetamine would produce prototypic stimulant effects (e.g., function as a reinforcer, increase positive subject-ratings, increase heart rate and blood pressure) and that maintenance on naltrexone, bupropion and the combination would attenuate these effects, with the greatest reductions observed during the combination condition. We also hypothesized that methamphetamine would be safe and tolerable during the active maintenance conditions.
2. Methods
2.1 Study Population, Inclusion/Exclusion Criteria and Screening
Seven non-treatment seeking adult subjects with recent histories of stimulant use who met criteria for stimulant abuse or dependence as determined by a computerized version of the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders – IV (SCID) completed this within-subjects, placebo-controlled, crossover study. All subjects admitted to the study completed the protocol. The Institutional Review Board of the University of Kentucky Medical Center approved this study and the subjects gave their written informed consent prior to participating. Subjects were informed that during the study they would be given medications including methamphetamine, naltrexone and bupropion, as well as placebo. Subjects were informed that the purpose of the study was to see how drugs affect mood and behavior and if they like the drugs administered and would be willing to take them again. Subjects were not informed of the specific drugs they received during the testing protocol, possible outcomes or performance expectations. Subjects were paid for their participation.
Prior to enrollment in the experimental protocol, all subjects underwent a comprehensive physical and mental health screening as described previously (Sevak et al., 2011). Subjects had to meet the following inclusion criteria: self-reported stimulant use, confirmation of recent stimulant use by a stimulant positive urine sample and fulfillment of the diagnostic criteria for stimulant abuse or dependence on a computerized version of the SCID that was reviewed by a psychologist or psychiatrist. Potential subjects with histories of serious physical disease or current physical disease, impaired cardiovascular functioning, chronic obstructive pulmonary disease, seizure, head trauma or central nervous system tumors, or current or past histories of serious psychiatric disorder (i.e., Axis I of DSM-IV) other than substance abuse or dependence, were excluded from participation. Female subjects who were pregnant, planning to become pregnant or lactating were also excluded from participation. All subjects were physically and psychologically healthy, as determined by the medical staff, with no contraindications to the drugs under study.
Subjects were 42 ± 1 (mean ± SEM) years old and weighed 75 ± 6 kg. Six subjects were male and one subject was female. Two subjects were black and five were white (one Hispanic). Subjects were primarily cocaine users (i.e., six met dependence criteria for cocaine, one met abuse criteria for cocaine), although a majority also had a history of using amphetamines. Subjects reported illicit stimulant use, including amphetamines and cocaine, on 10 ± 2 days in the month prior to screening. Six subjects were able to estimate the weight of the cocaine they used in the week prior to screening, averaging 4 ± 2 grams of cocaine. Six subjects reported smoking 10 ± 4 tobacco cigarettes daily, with a Fagerstrom Test of Nicotine Dependence score of 3 ± 1. Subjects also reported past use of a range of substances including alcohol, caffeine, marijuana, opioids, hallucinogens and sedatives, but did not meet diagnostic criteria for dependence on any of these substances that excluded them from participation in the opinion of the study physicians (PEG and LRH).
2.2 Study Procedures
Subjects resided at the University of Kentucky Medical Center Clinical Services Core (CSC) Inpatient Unit for approximately 31 days and completed one practice and twelve experimental sessions.
2.2.1 Practice Session
Subjects completed a practice session prior to beginning maintenance medication to familiarize them with the behavioral tasks and timeline of experimental sessions, as described in section 2.2.3. No medications were administered during the practice session.
2.2.2 Drug Maintenance Days
Drug maintenance began on the day immediately after the practice session and continued throughout the protocol. Placebo, naltrexone (25 mg) and/or sustained-release bupropion (150 mg) were administered orally at approximately 7:00 AM and 7:00 PM. There were four maintenance dose conditions: placebo, 50 mg naltrexone/day, 300 mg bupropion/day and 50 mg naltrexone plus 300 mg bupropion/day. The order of drug maintenance conditions was randomly assigned across subjects, with the exception that the combination of bupropion and naltrexone was not tested until both of those conditions were tested alone. After four days of maintenance on each condition, subjects completed three experimental sessions as described in the next section and began maintenance on the subsequent condition on the day immediately after completing those sessions. The duration of maintenance treatment necessary to reach steady-state plasma concentrations was based on the pharmacokinetic profile for naltrexone and bupropion and the need for drug to be administered for four to seven half-lives to attain steady state. The plasma half-life of naltrexone is about 4 hours while the half-life of its active metabolites (e.g., 2-hydroxy-3-methoxy-6β-naltrexol) is 12 hours (Meyer et al., 1984). The half-life of bupropion and its active metabolites (e.g., S,S-hydroxybupropion) is approximately 18–19 hours (Hsyu et al., 1997). Thus, a four-day maintenance period allowed both naltrexone, bupropion and and their metabolites to be present for more than four half-lives prior to methamphetamine testing.
2.2.3 Experimental Sessions
Experimental sessions were conducted in blocks of three consecutive days (e.g., Monday, Tuesday and Wednesday or Tuesday, Wednesday and Thursday). Subjects received the appropriate maintenance dose at 7:00 AM on the morning of all experimental sessions. All subjects who reported daily cigarette use were then allowed to smoke one cigarette, but were not allowed to smoke during sessions. Urine and breath samples were collected before each session to confirm drug and alcohol abstinence, respectively. Subjects occasionally tested positive for methamphetamine and amphetamine, which was likely attributable to the administration of the experimental medications. The urine samples were also used to test for pregnancy in the female subject and were negative throughout her participation.
Experimental sessions started at 9:00 AM and lasted approximately 8 hours. At 9:30 AM, subjects sampled the intranasal methamphetamine dose (0, 10 or 30 mg; administered in random order) that would be available later in session on the Modified Progressive Ratio Procedure (see below). In the afternoon, subjects completed the Modified Progressive Ratio Procedure and received the portion of the dose they earned on that task. The portion of the dose that the subjects earned was administered at 2:30 PM. Subjects had vital signs recorded and completed subject-rated drug effects questionnaires pre-methamphetamine administration and 0, 15, 30, 45, 60, 90 and 120 minutes following each methamphetamine administration. These measurement times were selected to capture the peak effects of intranasal methamphetamine, which occur at 5–15 minutes after dosing (Hart et al., 2008).
2.3 Outcome Variables
2.3.1 Modified Progressive-Ratio Procedure
During the self-administration portion of each experimental session, subjects had 10 opportunities to work to earn a portion of the drug sampled that morning (i.e., 0, 10 or 30 mg methamphetamine). Subjects were presented with the progressive-ratio task on a computer screen and they were instructed to use the computer mouse to click on a button to work to earn a portion of the drug. Each completed ratio earned 1/10th of the sampled dose. Subjects were instructed that they could choose to work to earn all, a portion of, or none of the sampled dose. To complete the first ratio, subjects were required to click 400 times and each additional ratio increased by 100 (i.e., 500, 600, 700, 800, 900, 1000, 1100, 1200 and 1300). The subject was allowed to terminate the task at any time if they clicked a button labeled stop. The portion of the dose earned (e.g., 50% if subjects completed 5 ratios) was administered after completion of this task. The primary outcome variable was number of ratios completed.
2.3.2 Subject-Rated Drug Effect Questionnaires
Two subject-rated drug effect questionnaires were administered using an Apple laptop computer with a mouse attached in a fixed order: the Adjective Rating Scale (Oliveto et al., 1992) rated on a Likert-type scale and the Drug Effect Questionnaire (Rush et al., 2003) rated on a visual analog scale.
2.3.4 Physiological Measures
Heart rate, blood pressure, temperature and heart rhythmicity (via ECG) were measured at regular intervals throughout session. If systolic blood pressure (mmHg), diastolic blood (mmHg) pressure, or heart rate (beats per minute [bpm]) exceeded 180, 120, or 130, respectively, or if clinically significant electrocardiographic changes occurred at any point after the administration of methamphetamine participation was discontinued. No subject was excluded from participation for exceeding these parameters. Sampling or self-administered doses were held if heart rate was 100 bpm or higher, systolic pressure was 150 mmHg or higher or diastolic pressure was 100 mmHg or higher.
2.4 Drug Administration
All medications were administered in a double-blind fashion. Maintenance medications were prepared by over-encapsulating commercially available naltrexone (25 mg) and sustained-release bupropion (150 mg) and loose filling the capsule with lactose monohydrate powder, N.F. Placebo capsules were prepared in the same way as the naltrexone and bupropion, but only contained lactose monohydrate powder, N.F. Methamphetamine doses were prepared by weighing out the appropriate dose (0, 10 and 30 mg) of methamphetamine provided by the National Institute on Drug Abuse Drug Supply Program (Research Triangle Institute, Research Triangle Park, NC), which was then mixed with lactose monohydrate powder, N.F. to make a total of 50 mg of powder. Subjects sampled the entire methamphetamine dose in the morning sampling portion of the session and had the opportunity to work for the sampled dose in the afternoon self-administration portion of the session.
2.5 Statistical Methods
For all statistical analyses, effects with p ≤ 0.05 were considered significant. Data from the progressive-ratio task were analyzed as number of ratios completed using a three-factor repeated-measures analysis of variance (ANOVA) (StatView, Cary, NC). The factors were Methamphetamine (0, 10 and 30 mg), Naltrexone (0 and 50 mg) and Bupropion (0 and 300 mg). If a main effect of Methamphetamine was observed, Fisher’s PLSD post hoc tests were used to compare all active doses to placebo (i.e., 0 mg methamphetamine during placebo maintenance). If a main effect of either maintenance condition or interaction was observed, Fisher’s PLSD post hoc tests were used to compare respective methamphetamine doses across all maintenance conditions (e.g., 30 mg methamphetamine during maintenance on placebo, naltrexone, bupropion and the combination). During the self-administration portion of sessions, subjects determined the amount of drug that they ingested. Thus, varying amounts of drug was administered to subjects during the self-administration session. Due to subjects ingesting varying amounts of drug in self-administration sessions, only data from subject-rated drug effects questionnaires and physiological measures following sampling doses were analyzed statistically as peak effect (i.e., the maximum response observed after dosing) in a fashion identical to that for self-administration data. Subject-rated data for one subject were not available from the 30 mg methamphetamine/300 mg bupropion alone condition because of a technical issue. The six other subjects’ responses to this dose were averaged and used to replace the missing values.
3. Results
3.1 Modified Progressive-Ratio Procedure
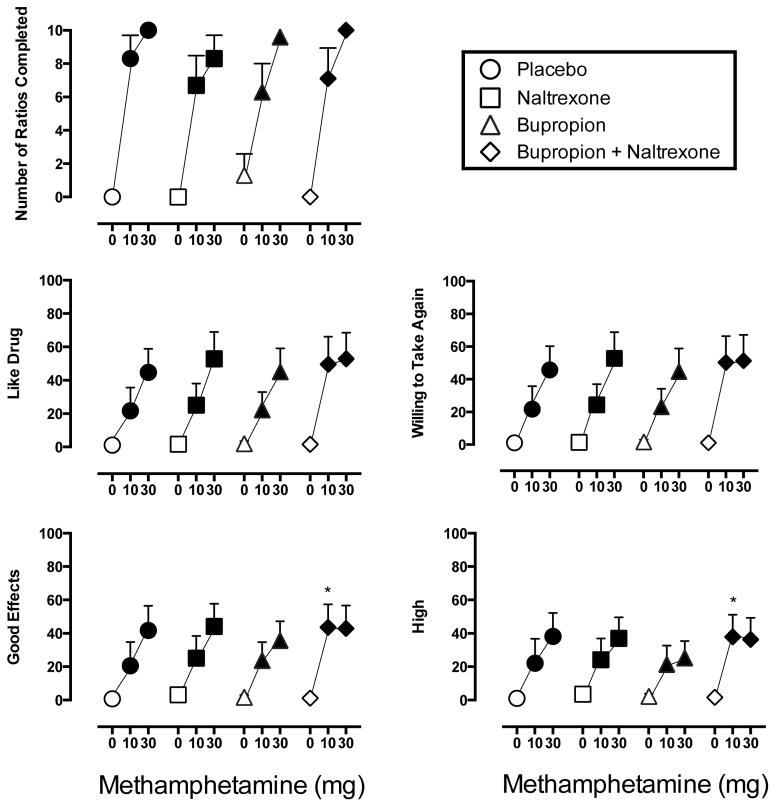
ANOVA revealed only a significant main effect of Methamphetamine on number of ratios completed (F2,12 = 43.7). All active doses increased the number of ratios completed relative to placebo regardless of the maintenance condition (Figure 1).
Figure 1.
Dose-response curves for Number of Doses Earned (Top Left), Subject Ratings of Like Drug (Middle Left), Willing to Take Again (Middle Right), Good Effects (Bottom Left) and High (Bottom Right) from the Drug Effect Questionnaire. Data from the Drug Effect Questionnaire are expressed as peak effect from the sampling session. For all panels circles represent placebo maintenance, squares represent 50 mg/day naltrexone, triangles represent 300 mg/day bupropion and diamonds represent naltrexone combined with bupropion. Filled symbols indicate a significant difference from placebo (0 mg methamphetamine during placebo maintenance). An asterisk indicates a significant difference between that dose condition and all other corresponding doses across other maintenance conditions. Brackets indicate 1 SEM.
3.2 Subject-Rated Drug Effect Questionnaires
3.2.1 Adjective Rating Scale
ANOVA revealed only a main effect of Methamphetamine for scores on the Stimulant Subscale of the Adjective Rating Scale (F2,12 = 15.7; data not shown). All active methamphetamine doses significantly increased these scores relative to placebo regardless of the maintenance condition. There were no significant effects on the Sedative Subscale of the Adjective Rating Scale (data not shown).
3.2.2 Drug-Effect Questionnaire
ANOVA revealed only a main effect of Methamphetamine for eight items from the Drug-Effect Questionnaire: Active/Alert/Energetic, Any Effect, Like Drug, Rush, Shaky/Jittery, Stimulated, Willing to Pay For and Willing to Take Again (F values 2,12 > 4.0). Figure 1 shows representative subject ratings of Like Drug and Willing to Take Again. In general, all active methamphetamine doses significantly increased ratings on these items relative to placebo regardless of the maintenance condition. Two exceptions were that 10 mg methamphetamine did not significantly increase ratings of Shaky/Jittery during placebo maintenance and Willing to Pay For during both placebo and naltrexone maintenance.
ANOVA revealed main effects of Methamphetamine and Naltrexone, but not an interaction of these factors, for ratings of Good Effects and High (F2,12 values > 5.7, F1,6 values > 6.9, respectively). As shown in Figure 1, all active methamphetamine doses significantly increased ratings on these items relative to placebo. In addition, subject ratings on these items for 10 mg methamphetamine were significantly higher during maintenance on the combination condition relative to all other conditions.
3.3 Physiological Measures
ANOVA revealed a significant interaction of Methamphetamine, Naltrexone and Bupropion on heart rate (F2,12 = 4.0; data not shown). During placebo maintenance, 10 mg methamphetamine significantly increased heart rate relative to placebo. During naltrexone maintenance, 30 mg methamphetamine significantly increased heart rate whereas 0 mg methamphetamine significantly decreased heart rate relative to placebo. Heart rate following 0 mg methamphetamine during maintenance on the combination was significantly higher than for that dose during naltrexone maintenance. Heart rate following 10 mg methamphetamine during naltrexone maintenance was significantly lower than heart rate following that dose during all other conditions.
ANOVA revealed main effects of Methamphetamine and Bupropion, but not an interaction of these factors, for systolic blood pressure (F2,12 = 8.2, F1,6 = 6.5, respectively; data not shown). The high dose of methamphetamine increased systolic blood pressure relative to placebo across all maintenance conditions. The low dose of methamphetamine increased systolic blood pressure relative to placebo during maintenance on bupropion and the combination. Despite a main effect of Bupropion, no corresponding methamphetamine doses were significantly different across maintenance conditions.
ANOVA revealed main effects of Naltrexone for diastolic blood pressure (F1,6 = 21.1, data not shown). No corresponding methamphetamine doses were significantly different across maintenance conditions for this measure, however.
4. Discussion
This experiment evaluated the reinforcing, subject-rated and physiological effects of methamphetamine during maintenance on placebo, naltrexone, bupropion and naltrexone combined with bupropion. Methamphetamine produced dose-related increases on both self-administration and subject-rated outcomes during placebo maintenance, with near maximal drug taking observed for the highest dose tested. Contrary to our hypotheses, the maintenance conditions generally failed to alter the reinforcing and subject-rated effects of intranasal methamphetamine, although the combination of naltrexone and bupropion enhanced ratings of Good Effects and High for the 10 mg dose. Methamphetamine doses increased heart rate and blood pressure, but not to a clinically significant degree, and all methamphetamine doses were safe and well tolerated across maintenance conditions.
The effects observed for methamphetamine alone are consistent with a number of human laboratory studies demonstrating its reinforcing and subject-rated effects across several routes of administration (De La Garza et al., 2012; Hart et al., 2008; Kirkpatrick et al., 2012; Stoops et al., 2013). It is possible that these robust effects contribute to the general resistance of methamphetamine use disorders to intervention. The results of these human laboratory studies also demonstrate that methamphetamine can be safely administered in controlled doses to human subjects for the purpose of screening putative behavioral and pharmacological interventions.
Previous research has indicated that self-administration outcomes from human laboratory studies have good predictive validity for screening putative pharmacotherapies for substance use disorders (Comer et al., 2008; Haney and Spealman, 2008). The self-administration outcomes observed here would thus suggest that naltrexone combined with bupropion does not hold promise for reducing methamphetamine use disorder. One caveat to that conclusion is that our results with each medication alone are not concordant with clinical trials demonstrating at least some efficacy for naltrexone and bupropion to reduce methamphetamine use (Jayaram-Lindström et al., 2008a; Elkashef et al., 2008; Heinzerling et al., 2014; Shoptaw et al., 2008). The reasons for this discrepancy are unknown, but could be due to the four-day dosing maintenance period. Four days of drug maintenance were selected to achieve steady state naltrexone and bupropion blood levels, but may not have been long enough to produce a full therapeutic effect, leading to the negative outcomes. Moreover, this study only evaluated a single dose of each medication, which may have also contributed to the negative results.
The discrepancy could also arise from the fact that this study enrolled non-treatment seekers whereas the clinical trials, by definition, enrolled individuals who wanted to stop using amphetamines. Previous research has shown that intervention efficacy is enhanced in those trying to stop using relative to those who are not (Perkins et al., 2010), leading to the possibility that human laboratory self-administration studies are prone to false negatives.
A third possibility is that this study evaluated the reinforcing effects of methamphetamine in a drug versus no-drug choice whereas the clinical trials included some form of supportive behavioral therapy (e.g., counseling, cognitive behavioral therapy, contingency management, relapse prevention; Jayaram-Lindström et al., 2008a; Elkashef et al., 2008; Heinzerling et al., 2014; Shoptaw et al., 2008). Given that behavioral interventions impact methamphetamine use in the human laboratory (e.g., Bennett et al., 2013) and in the clinic (Roll et al., 2006), it is also possible that combining behavioral and pharmacological treatments synergistically increased the efficacy in the clinical trials in a way that was not modeled in the drug versus no-drug choice in the current study. In order to better model clinical trials, human laboratory studies should include a model of behavioral treatments in self-administration outcomes (e.g., drug versus money choice; Stoops et al., 2012) when screening putative pharmacotherapies. In that study, bupropion reduced cocaine self-administration in a drug versus money choice procedure, comparable to effects observed clinically (Poling et al., 2006).
Like the self-administration outcomes, subject-rated and physiological effects were generally unaltered by any of the maintenance conditions. Subject ratings of Good Effects and High were increased during combination treatment for 10 mg methamphetamine relative to all other conditions, but this effect did not appear to influence self-administration outcomes and will not be discussed further. The physiological outcomes replicate earlier work showing that amphetamines can safely be combined with naltrexone and bupropion when administered as single entities (Comer et al., 2013; Jayaram-Lindström et al., 2004; 2008b; Marks et al., 2014; Newton et al., 2005). Several earlier studies have shown that acute pretreatment with or maintenance on 50 mg naltrexone attenuates the positive, abuse-related effects of amphetamine (Comer et al., 2013; Jayaram-Lindström et al., 2004; 2008b; Marks et al., 2014). The reasons for the discrepancy between the studies are unknown, but could be due to the fact that this study tested intranasal methamphetamine whereas the earlier research tested oral d-amphetamine. Other research indicates that 300 mg bupropion maintenance attenuates the positive, abuse-related effects of intravenous methamphetamine (Newton et al., 2006). Again, the reasons for the discrepancy between those outcomes and the present findings are unknown, but could be due to the longer dosing period for bupropion in the earlier study.
The relatively small sample size may be a limitation of the present study, which was terminated after 7 subjects completed. This decision was based on the observation of no difference between self-administration of the high methamphetamine doses and very little difference between self-administration of the low methamphetamine doses (i.e., Cohen’s d = 0.26) across the maintenance conditions of most interest (i.e., placebo and combination treatment). Using the effect size observed for the low methamphetamine dose, it was calculated that approximately 30 or more additional subjects would be needed to provide a conventional level of power to detect the statistical significance of this effect. We felt it was not ethically appropriate to expose more research subjects to methamphetamine, bupropion or naltrexone when our data indicated that the combination was ineffective in this smaller sample. A similar sample size was sufficient to detect an effect of d-amphetamine maintenance on cocaine self-administration (Rush et al., 2010), a clinically consistent outcome.
A final limitation of the study is that there was no washout period between maintenance dosing, resulting in the placebo condition for some subjects possibly being influenced by residual bupropion or naltrexone metabolites. This limitation does not seem significant, however, because of the similar responding for methamphetamine across dose conditions.
5. Conclusions
The overall results demonstrate that methamphetamine is safe and tolerable during maintenance on naltrexone, bupropion and combined naltrexone and bupropion, but that this combination did not alter the reinforcing or subject-rated effects of methamphetamine. Future research should address some of the limitations of this study noted above. Specifically, evaluating the reinforcing effects of methamphetamine in a drug versus alternative reinforcer choice procedure, using longer maintenance periods and multiple doses for the pharmacotherapies being tested as well as examining the effects of putative pharmacotherapies in individuals with different levels of amphetamine use should be considered. Preclinical research can contribute to the growing literature on combination treatment for methamphetamine use disorder by evaluating how different doses of the test compounds, both high and low, impact methamphetamine self-administration.
Highlights.
Methamphetamine functioned as reinforcer.
Methamphetamine produced prototypic subject-rated and physiological effects.
Maintenance conditions generally failed to alter the effects of methamphetamine.
Acknowledgments
The authors wish to thank the staff at the University of Kentucky Laboratory of Human Behavioral Pharmacology for their expert technical and medical assistance. The authors would like to gratefully acknowledge research support from the National Institute on Drug Abuse (R01DA025032) and from the National Center for Advancing Translational Sciences (UL1TR000117) of the National Institutes of Health. These funding agencies had no role in study design, data collection or analysis, or preparation and submission of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bennett JA, Stoops WW, Rush CR. Alternative reinforcer response cost impacts methamphetamine choice in humans. Pharmacol Biochem Behav. 2013;103:481–6. doi: 10.1016/j.pbb.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008;96:1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Mogali S, Saccone PA, Askalsky P, Martinez D, Walker EA, Jones JD, Vosburg SK, Cooper ZD, Roux P, Sullivan MA, Manubay JM, Rubin E, Pines A, Berkower EL, Haney M, Foltin RW. Effects of acute oral naltrexone on the subjective and physiological effects of oral D-amphetamine and smoked cocaine in cocaine abusers. Neuropsychopharmacology. 2013;38:2427–38. doi: 10.1038/npp.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, Newton TF, Haile CN, Yoon JH, Nerumalla CS, Mahoney JJ, Aziziyeh A. Rivastigmine reduces “Likely to use methamphetamine” in methamphetamine-dependent volunteers. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:141–6. doi: 10.1016/j.pnpbp.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinny J, Weis D. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33:1162–70. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Häggkvist J, Lindholm S, Franck J. The opioid receptor antagonist naltrexone attenuates reinstatement of amphetamine drug-seeking in the rat. Behav Brain Res. 2009;197:219–24. doi: 10.1016/j.bbr.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008;199:403–19. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Gunderson EW, Perez A, Kirkpatrick MG, Thurmond A, Comer SD, Foltin RW. Acute physiological and behavioral effects of intranasal methamphetamine in humans. Neuropsychopharmacology. 2008;33:1847–55. doi: 10.1038/sj.npp.1301578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson AN, Hall TM, Ba YY, Wu Y, Shoptaw SJ. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014 doi: 10.1111/add.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsyu PH, Singh A, Giargiari TD, Dunn JA, Ascher JA, Johnston JA. Pharmacokinetics of bupropion and its metabolites in cigarette smokers versus nonsmokers. J Clin Pharmacol. 1997;37:737–43. doi: 10.1002/j.1552-4604.1997.tb04361.x. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindström N, Hammarberg A, Beck O, Franck J. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008a;165:1442–8. doi: 10.1176/appi.ajp.2008.08020304. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindström N, Konstenius M, Eksborg S, Beck O, Hammarberg A, Franck J. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2008b;33:1856–63. doi: 10.1038/sj.npp.1301572. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindström N, Wennberg P, Hurd YL, Franck J. Effects of naltrexone on the subjective response to amphetamine in healthy volunteers. J Clin Psychopharmacol. 2004;24:665–9. doi: 10.1097/01.jcp.0000144893.29987.e5. [DOI] [PubMed] [Google Scholar]
- Jimenez-Gomez C, Winger G, Dean RL, Deaver DR, Woods JH. Naltrexone decreases D-amphetamine and ethanol self-administration in rhesus monkeys. Behav Pharmacol. 2011;22:87–90. doi: 10.1097/FBP.0b013e3283423d55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Johanson CE, Levin FR, Foltin RW, Hart CL. Comparison of intranasal methamphetamine and d-amphetamine self-administration by humans. Addiction. 2012;107:783–91. doi: 10.1111/j.1360-0443.2011.03706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks KR, Lile JA, Stoops WW, Rush CR. Separate and combined impact of acute naltrexone and alprazolam on subjective and physiological effects of oral d -amphetamine in stimulant users. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MC, Straughn AB, Lo MW, Schary WL, Whitney CC. Bioequivalence, dose-proportionality and pharmacokinetics of naltrexone after oral administration. J Clin Psychiatry. 1984;45:15–9. [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacology (Berl) 2005;182:426–35. doi: 10.1007/s00213-005-0102-8. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31:1537–44. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- Nicosia N, Pacula RL, Kilmer B, Lundberg R, Chiesa J. The Economic Cost of Methamphetamine Use in the United States, 2005. Rand Corporation; Santa Monica, CA, USA: 2009. [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. Caffeine drug discrimination in humans: acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther. 1992;261:885–94. [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Fonte CA, Mercincavage M, Stitzer ML, Chengappa KN, Jain A. Cross-validation of a new procedure for early screening of smoking cessation medications in humans. Clin Pharmacol Ther. 2010;88:109–14. doi: 10.1038/clpt.2010.65. [DOI] [PubMed] [Google Scholar]
- Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, Martell B, Kosten TR. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63:219–28. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Grant KM, Bevins RA. Bupropion attenuates methamphetamine self-administration in adult male rats. Drug Alcohol Depend. 2009;100:54–62. doi: 10.1016/j.drugalcdep.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Petry NM, Stitzer ML, Brecht ML, Peirce JM, McCann MJ, Blaine J, MacDonald M, DiMaria J, Lucero L, Kellogg S. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry. 2006;163:1993–9. doi: 10.1176/ajp.2006.163.11.1993. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PE, Hays LS. Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther. 2003;306:195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Sevak RJ, Hays LR. Cocaine choice in humans during d-amphetamine maintenance. J Clin Psychopharmacol. 2010;30:152–159. doi: 10.1097/JCP.0b013e3181d21967. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Gilman JP, Panlilio LV, McCann DJ, Goldberg SR. Comparison of the effects of methamphetamine, bupropion, and methylphenidate on the self -administration of methamphetamine by rhesus monkeys. Exp Clin Psychopharmacol. 2011;19:1–10. doi: 10.1037/a0022432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevak RJ, Vansickel AR, Stoops WW, Glaser PE, Hays LR, Rush CR. Discriminative-stimulus, subject-rated, and physiological effects of methamphetamine in humans pretreated with aripiprazole. J Clin Psychopharmacol. 2011;31:470–80. doi: 10.1097/JCP.0b013e318221b2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, De La Garza R, Newton T, Ling W. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96:222–32. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Bennett JA, Lile JA, Sevak RJ, Rush CR. Influence of aripiprazole pretreatment on the reinforcing effects of methamphetamine in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:111–7. doi: 10.1016/j.pnpbp.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR. Influence of acute bupropion pre -treatment on the effects of intranasal cocaine. Addiction. 2012;107:1140–7. doi: 10.1111/j.1360-0443.2011.03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Rush CR. Combination pharmacotherapies for stimulant use disorder: A review of clinical findings and recommendations for future research. Expert Rev Clin Pharmacol. 2014;7:363–74. doi: 10.1586/17512433.2014.909283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Tindall MS, Havens JR, Oser CB, Webster JM, Mateyoke-Scrivner A, Wright PB, Booth BM, Leukefeld CG. Kentucky rural stimulant use: a comparison of methamphetamine and other stimulant users. J Psychoactive Drugs. 2007;(Suppl 4):407–17. doi: 10.1080/02791072.2007.10399902. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Tindall MS, Mateyoke-Scrivner A, Leukefeld C. Methamphetamine use in nonurban and urban drug court clients. Int J Offender Ther Comp Criminol. 2005;49:260–76. doi: 10.1177/0306624X04273438. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-46, HHS Publication No. (SMA) 13–4795. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013a. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. BHSIS Series S-65, HHS Publication No. (SMA) 13-4772. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013b. Treatment Episode Data Set (TEDS): 2001–2011. National Admissions to Substance Abuse Treatment Services. [Google Scholar]