Abstract
Recent evidence suggests wheel running can abolish conditioned place preference (CPP) for cocaine in mice. Running significantly increases the number of new neurons in the hippocampus, and new neurons have been hypothesized to enhance plasticity and behavioral flexibility. Therefore, we tested the hypothesis that increased neurogenesis was necessary for exercise to abolish cocaine CPP. Male nestin thymidine kinase transgenic mice were conditioned with cocaine, and then housed with or without running wheels for 32 days. Half the animals were fed valganciclovir in their chow to induce apoptosis in newly divided neurons and the other half were fed standard chow. The first 10 days, mice received daily injections of bromodeoxyuridine (BrdU) to label dividing cells. The last 4 days mice were tested for CPP and then euthanized to measure adult hippocampal neurogenesis by counting the number of BrdU+ neurons in the dentate gyrus. Levels of running were similar in animals fed valganciclovir or normal chow. Valganciclovir significantly reduced numbers of neurons (BrdU+/NeuN+) in the dentate gyrus of both sedentary mice and runners. Valganciclovir-fed runners displayed similar levels of neurogenesis as sedentary normal-fed controls. However, valganciclovir-fed runners displayed the same abolishment of CPP as runners with intact neurogenesis. Results demonstrate that elevated adult hippocampal neurogenesis from running is not necessary for running to abolish cocaine CPP in mice.
Keywords: exercise, CPP, nestin, thymidine kinase, valganciclovir
INTRODUCTION
Relapse is a major obstacle to recovery from drug abuse, and is triggered, in part, by exposure to drug-associated cues (O’Brien et al., 1998; Volkow et al., 2006). In order to attenuate relapse, it would be useful to find interventions that extinguish drug-to-context associations. Recent evidence suggests exercise can reduce the incidence of relapse to drug use in humans (Brown et al., 2010). Rodent work supports the idea that exercise reduces strength of drug-context associations (Mustroph et al., 2011; Thanos et al., 2010). Taken together, human and rodent evidence suggest exercise may reduce relapse by facilitating extinction of drug-context associations, but the neurobiological mechanisms are unknown.
The hippocampus is a site in the brain where effects of exercise, associative learning and extinction converge. First, the hippocampus, specifically the dentate gyrus, plays a critical role in binding information from different sensory modalities into unique memories (Chauvet et al., 2011; Hernandez-Rabaza et al., 2008; Johnson et al., 2010; Meyers et al., 2003). fMRI studies show that drug users display bilateral activation of the hippocampus when shown images of drug paraphernalia (Ames et al., 2013; Michaelides et al., 2012). The hippocampus also plays an important role in extinction learning (Quirk and Mueller, 2008).
In addition to its role in associative learning and extinction, the hippocampus is also a major site of activation during an acute bout of exercise (Clark et al., 2011a), and a center for plasticity in response to exercise (Clark et al., 2009; Dietrich et al., 2008; Gomez-Pinilla et al., 2008; Neeper et al., 1995). Moreover, exercise-induced morphological changes in the hippocampus correlate with improvements in cognitive functions (Colcombe and Kramer, 2003; Creer et al., 2010; Griffin et al., 2009; O’Callaghan et al., 2009). One of the most remarkable features of hippocampal remodeling in response to exercise is the generation of new granule neurons in the dentate gyrus. New neurons may enhance associative learning (Drew et al., 2010; Hernandez-Rabaza et al., 2009; Winocur et al., 2006) because they are highly plastic units not yet fully integrated into existing circuitry (van Praag et al., 1999). When exercise is implemented after conditioning in the CPP paradigm, new neurons could be recruited during extinction and allow animals to rapidly acquire the new association, presented during testing, that context is no longer associated with drug (Mustroph et al., 2011). Computational modeling supports the idea that acquisition of a task in which novel aspects arise in a familiar context (i.e. learning that a previously drug-paired texture is no longer associated with drug) is enhanced by hippocampal neurogenesis (Garthe et al., 2009; Wiskott et al., 2006). Hence, it makes sense that new neurons might contribute to CPP extinction.
To the best of our knowledge, no previous study has directly tested the causal role of new neurons in extinction of CPP behavior. Therefore, the goal of this study is to directly test the hypothesis that intact neurogenesis is required for exercise to abolish cocaine CPP using the nestin-thymidine kinase transgenic mouse model, in which neurogenesis is selectively reduced.
MATERIALS AND METHODS
Animals
Eighty-two male nestin-thymidine kinase (nestin-TK) transgenic mice were used in this experiment. Nestin-TK transgenic mice were originally obtained from Steven G. Kernie (Columbia University, Department of Pathology and Cell Biology). The nestin-TK mice express a modified version of the herpes simplex virus thymidine kinase (HSV-TK) driven by the nestin promoter and its second intron regulatory element (Yu et al., 2008). This transgenic mouse line was generated by pronuclear injection into fertilized murine eggs in a C57BL/6J genetic background (Yu et al., 2008). The mouse line allows temporally regulated, inducible ablation of early dividing neural progenitors by systemic administration of the pro-drug ganciclovir (Yu et al., 2008). Mice were bred in the Beckman Institute’s animal facility, where a colony has been established. Genotype of each mouse was verified by tail snip followed by DNA extraction, PCR, and gel electrophoresis (with GADPDH used as a control gene). Mice were weaned at 3 weeks and housed 4 per cage in a climate-controlled environment on a 12 h light/dark cycle (lights off at 9:00 a.m.) for 4 weeks. Dimensions of cages without running wheels were 29 × 19 × 13 cm (L × W × H) (Harlan Tekland, Madison, Wisconsin, USA). Mice were individually housed for 2 weeks before starting the experimental procedures and remained singly housed throughout the experiment. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and adhered to NIH guidelines. All measures were taken to minimize the number of mice used as well as the pain and suffering of the animals.
Experimental design
At approximately 65 days of age (range: 59 days-71 days), all animals underwent habituation, pretesting, and cocaine CPP conditioning (see Conditioned place preference section below and Fig. 1). During the first 10 days after conditioning, all mice received daily injections (i.p.) of 50 mg/kg Bromodeoxyuridine (BrdU) to label dividing cells. The day after the four conditioning days, mice were placed in cages either without (Sedentary) or with running wheels (Runner) and received either a diet of normal (Control) or valganciclovir-infused (Val) rodent chow for 28 days. Sample sizes were as follows: Control Sedentary (n = 26), Control Runner (n = 18), Val Sedentary (n = 20), Val Runner (n = 18). After 28 days, mice underwent four consecutive days of CPP testing. Runners had continuous access to wheels, and animals continued to receive their designated diets during the days of testing.
Figure 1.
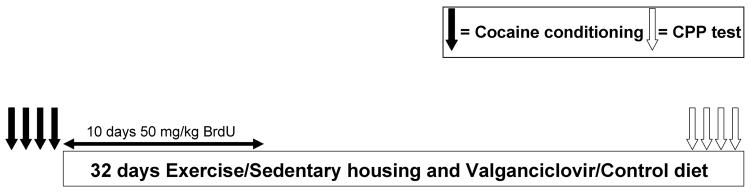
Schematic diagram of the experimental design. The black arrows indicate when CPP conditioning sessions were administered. The white arrows indicate when CPP testing took place. The boxes indicate when the runner/sedentary conditions and valganciclovir/control chow were administered relative to conditioning and CPP testing. The experiment contained 82 animals (20 Sedentary mice receiving Valganciclovir, 18 Runner mice receiving Valganciclovir, 26 Sedentary mice receiving Control Chow, and 18 Runner mice receiving Control Chow). Animals experienced 1 day of habituation to reduce novelty effects and, subsequently, 1 day of CPP pretesting to establish baseline texture preferences over the 2 days immediately preceding the conditioning. Animals experienced 28 days of uninterrupted running or sedentary treatment and a total of 4 days of CPP conditioning. Animals were returned to cages with or without running wheels immediately after conditioning and testing to avoid the potential confound of animals experiencing withdrawal from running during the testing procedures.
Running wheels and sedentary treatment
Dimensions of running wheel cages were 36 × 20 × 14 cm (L × W × H), with a 23 cm diameter wheel mounted in the cage top. Running wheel rotations were monitored continuously in 1 min increments throughout the experiment via magnetic switches interfaced to a computer. Mice assigned to the Sedentary group were deliberately not housed in cages with locked wheels because mice climb in locked wheels (Koteja et al., 1999; Rhodes et al., 2003; Rhodes et al., 2000) and we intended to keep physical activity to a minimum in the Sedentary group.
Valganciclovir administration
After the conditioning phase of the experiment was completed (see CPP section below), mice were switched from a diet of standard rodent chow (Harlan Teklad, Madison, Wisconsin, USA) provided ad libitum to a diet of rodent chow infused with valganciclovir (900 mg/kg dose) (Custom Animal Diets, Bangor, PA, USA), a valine ester pro-drug of ganciclovir (Pescovitz et al., 2000) or control chow (Custom Animal Diets, Bangor, PA, USA), also made available ad libitum. Upon ingestion, the vast majority of valganciclovir is rapidly converted to ganciclovir by hydrolysis (Jung and Dorr, 1999). Valganciclovir was used in this study because its bioavailability is 10-fold higher than that of ganciclovir (Pescovitz et al., 2000). Chow (valganciclovir-infused and control) was weighed every 7 days and replenished to maintain an ad libitum supply that would allow a suggested target valganciclovir consumption of 200 mg/kg (Blaiss et al., 2011).
Drugs
Cocaine hydrochloride (Sigma Aldrich, St. Louis, MO) was dissolved in 0.9% saline and was administered at a dose of 10 mg/kg via i.p. injections in a volume of 10 ml/kg. Dose was chosen based on the literature and was prepared according to the salt not the base form (Johnson et al., 2010; Mustroph et al., 2011; Zombeck et al., 2008).
Conditioned place preference
We used the same procedure as previously published by our lab (Johnson et al., 2010; Mustroph et al., 2011) based on Cunningham’s apparatus and experimental design (Cunningham et al., 2006).
Habituation
To familiarize the mice with the place conditioning chambers, animals were placed on a flat surface without a texture in the conditioning chambers in the morning (1000 h; for 30 min) and in the afternoon (1600 h; for 30 min) for one day without any injection treatment.
Pretesting
To determine individual biases in preference for the textures prior to drug pairing, animals were weighed, received a 10 ml/kg saline injection, and were immediately placed in the apparatus with HOLE/GRID floor in the morning (1000 h; for 30 min) and afternoon (1600 h; for 30 min).
Conditioning
Four conditioned stimulus (CS+) trials (i.e., cocaine paired with one floor texture: HOLE or GRID) and four CS− trials (i.e. vehicle paired with the alternate floor texture) were administered over four days. The assignment to HOLE or GRID was counterbalanced in each group. Each day, one CS+ trial and one CS− trial was administered in the morning and afternoon. The order of exposure to CS+ and CS− was counterbalanced within each group. Animals were weighed, received an injection of 10 mg/kg cocaine (CS+ trial) or vehicle (CS− trial), and were immediately placed on the appropriate floor texture in the morning (1000 h; for 30 min) and afternoon (1600 h; for 30 min).
Testing
Testing took place daily (morning and afternoon; 30 min) on days 29–32 after the last conditioning session. Prior to each testing session, each mouse was weighed, injected i.p. with 10 ml/kg saline, and placed into the center of the HOLE/GRID conditioning chamber. All testing was conducted by experimenters blinded to the group assignment of the animals.
Immunohistochemistry
Tissue preparation
Following behavioral testing, all the mice (n = 82) were anesthetized with 100 mg/kg sodium pentobarbital (i.p.) and then perfused transcardially with 4% paraformaldehyde in phosphate buffer solution (PBS; 0.287% sodium phosphate monobasic anhydrous, 1.102% sodium phosphate dibasic anhydrous, 0.9% sodium chloride in water). Brains were postfixed overnight and then transferred to 30% sucrose in PBS. Brains were sectioned using a cryostat in 40 μm thick coronal sections. Sections were placed into tissue cryoprotectant (30% ethylene glycol, 25% glycerin, 45% PBS) in 24 well plates and stored in −20° C. Two separate one-in-six series of these sections (i.e. series of sections throughout the rostro-caudal extent of the brain, with 240μm increments separating each section, approximately nine sections) were stained in the following ways.
BrdU-DAB
Purpose
To detect BrdU-positive (newly divided) cells in the dentate gyrus. Free-floating sections were washed in tissue buffering solution (TBS; 1.3% Trizma hydrochloride, 0.19% Trizma base, 0.9% sodium chloride) and then treated with 0.6% hydrogen peroxide in TBS for 30 min. To denature DNA, sections were treated for 120 min with a solution of 50% de-ionized formamide and 2X SSC buffer, rinsed for 15 min in 2X SSC buffer, then treated with 2 M hydrochloric acid for 30 min at 37 °C, then 0.1 M boric acid in TBS (ph 8.5) for 10 min at room temperature. Sections were then treated (blocked) with a solution of 0.3% Triton-X and 3% goat serum in TBS (TBS-X plus) for 30 min, and then incubated in primary antibody against BrdU made in rat (AbD Serotec, Raleigh, NC, USA, catalog number OB0030) at a dilution of 1:100 in TBS-X plus for 72 h at 4°C. Sections were then washed in TBS, blocked with TBS-X plus for 30 min, and then incubated in biotinylated secondary antibody against rat made in goat (Vector, Burlingame, CA, USA, catalog number BA-9400) at 1:250 in TBS-X plus for 100 min at room temperature. Sections were then treated using the ABC system (Vector, Burlingame, CA, USA, catalog number PK-6100) and stained using a diaminobenzidine kit (Sigma, St. Louis, MO, USA, catalog number D4418-505ET).
Double-fluorescent label
Purpose
To determine the proportion of BrdU-positive cells in the dentate gyrus that differentiated into neurons. Sections were treated as for BrdU-DAB except a cocktail was used for the primary antibody step. Rat anti-BrdU (1:100; AbD Serotec, Raleigh, NC, USA, catalog number OBT0030) was combined with mouse anti-neuronal nuclear protein (NeuN) (1:250; Millipore, Billerica, MA, USA, catalog number MAB377) for 48 at 4° C. Secondary goat antibodies were conjugated with fluorescent markers (Cy2-green anti-mouse and Cy3-red anti-rat; Jackson ImmunoResearch, West Grove, PA, USA, catalog numbers 115-225-166 and 112-165-167, respectively) at dilution of 1:250 and also delivered as a cocktail.
Image analysis
BrdU-DAB
All image analyses were conducted with experimenters blinded to the assignment group of the animals. The entire granule layer (bilateral), represented in the 1-in-6 series, was photographed by systematically advancing the field of view of the Zeiss brightfield light microscope and taking multiple photographs, via camera interfaced to a computer, under 10x (total 100x) magnification. Positively labeled cells in these photographs were counted to generate estimates of the total number of labeled cells. The total volume of the dentate gyrus represented in the series was measured so that the counts could be expressed per μm3 dentate gyrus sampled.
Double-label
All image analyses were conducted with experimenters blinded to the assignment group of the animals. A Leica SP2 laser scanning confocal microscope (using a 40x oil objective, pinhole size 81.35 μm diameter) was used to determine the proportion of dentate gyrus BrdU-positive cells that differentiated into neurons (NeuN+). Dentate gyrus BrdU-positive cells were identified as either co-expressing NeuN or not. Each BrdU-positive cell in the granule layer (represented in the 1-in-6 series) was analyzed by focusing through the tissue in the z-axis to establish co-labeling with NeuN. The number of new neurons per μm3 per mouse was calculated as the number of BrdU cells per μm3 multiplied by average proportion of BrdU cells co-expressing NeuN for the designated group.
Statistical analysis
Data were analyzed using SAS (version 9.3) statistical software. In all analyses, P <0.05 was considered statistically significant.
CPP
Conditioned place preference data were analyzed the following way: First, the duration spent on the HOLE texture was analyzed by 4-way repeated-measures ANOVA with conditioned stimulus (CS+HOLE versus CS+GRID; between-subjects), exercise history (Sedentary versus Runner; between-subjects), diet (Control versus Val; between-subjects), and day of testing (1–4; within-subjects) and all interactions entered as factors. Testing session, whether at 10:00 h or 16:00 h, was also included as a factor in initial models but was never significant and therefore was removed from the final linear models. Microanalysis of CPP on the initial test (day 1, a.m.) was analyzed by a similar repeated measures procedure, except the within-subjects factor was bin number (1–6, consisting of duration on HOLE in 5 minute bins over the 30 min test). Posthoc comparisons of CPP were conducted using unpaired t-tests comparing CS+HOLE versus CS+GRID within Runner/Sedentary-Val/Control treatment groups.
Body mass, food consumption, and wheel running
These variables (except wheel running) were analyzed by two-way ANOVA with exercise history (Sedentary versus Runner), diet (Control versus Val), and all interactions entered as factors. Total wheel running distance traveled over the course of the study was analyzed by an unpaired t-test comparing Control versus Val.
Neurogenesis
Total number of BrdU cells in the granule layer and total number of new neurons (BrdU cells co-labeled with NeuN) were analyzed by two-way ANOVA with exercise history (Sedentary versus Runner), diet (Control versus Val), and all interactions entered as factors. Total number of BrdU cells and new neurons in Runners were also analyzed using analysis of covariance with total distance run as the continuous predictor, and diet (Control versus Val) as the categorical factor. The proportion of BrdU-labeled cells in the granule cell layer that co-expressed NeuN was analyzed by logistic regression, where proportion (binomial response) was modeled as a linear function of exercise history (Sedentary versus Runner), diet (Control versus Val), and all interactions entered as factors.
RESULTS
Body mass
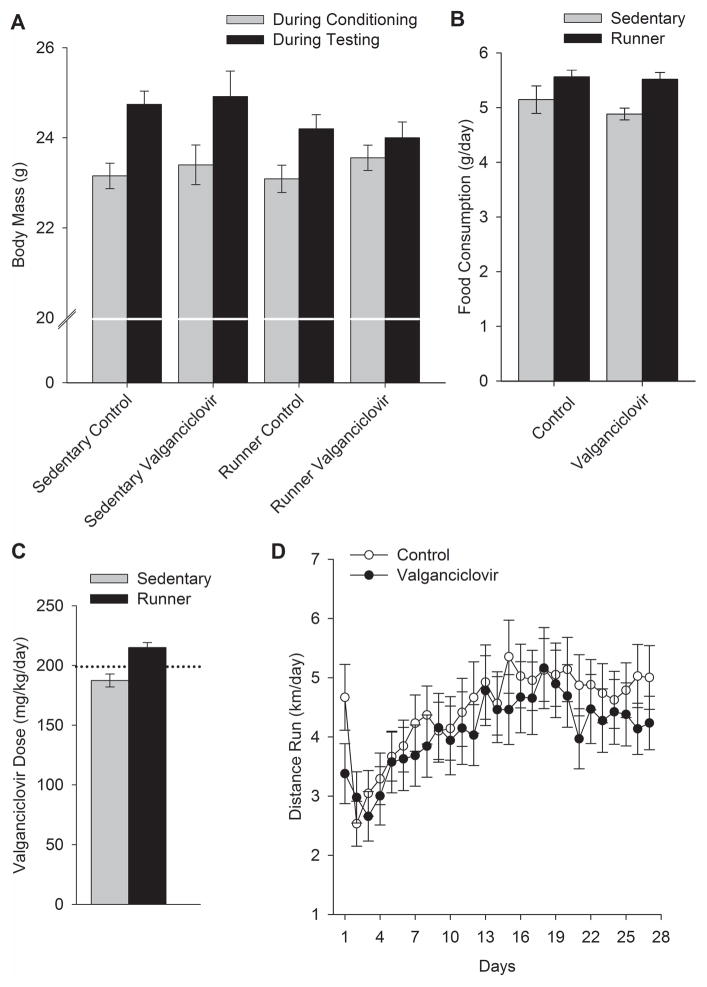
Average body masses of the animals during CPP training (at the beginning of the experiment) and testing (at the end of the experiment) are shown in Figure 1A. Body mass significantly increased over the duration of the experiment. However, runners gained significantly less weight than sedentary animals, a difference of approximately 1 gram (Fig. 1A). This was indicated by a significant main effect of age (beginning versus end of the experiment; F1,82 =102.23, P < 0.0001) and significant interaction between the exercise treatment (Sedentary versus Runner) and age (F1,82 =11.31, P = 0.001). No other main effects or interactions were significant.
Food consumption and dose of Valganciclovir received
Sedentary animals ate 91% as much chow as runners (F1,63 =3.43, P = 0.0011). There were no differences in consumption of the valganciclovir chow versus normal chow (Fig. 2B). Because they ate more chow, runners received an average daily dose of 215 mg/kg/day ± 4.2 SE, whereas sedentary animals received an average daily dose of 187 mg/kg/day ± 5.4 SE from ad libitum chow consumption (Fig. 2C).
Figure 2.
Body mass, chow consumption, valganciclovir dose received, and wheel running over the course of the study. A) Average body mass (g) (± SE) shown separately for each group during conditioning (i.e. before runner/sedentary and valganciclovir/control chow assignments were made) and during testing (i.e. at the end of runner/sedentary and valganciclovir/control chow phase of the experiment). Body weights slightly increased from conditioning to testing, significantly more so for sedentary mice than runners. B) Average food consumption (g/day) (± SE) shown separately for each group. Food consumption was higher among runners. C) Average valganciclovir dose received (mg/kg/day) (± SE) shown separately for sedentary and runner mice receiving valganciclovir chow. Runner mice exceeded the desired dose of 200 mg/kg/day of valganciclovir. D) Distance run (km/day) (± SE) shown separately for mice receiving control chow and mice receiving valganciclovir-infused chow. Valganciclovir did not affect running. Escalation of wheel running over the first 18 days is typical for mice.
Wheel running
Animals increased their wheel running over the first 2–3 weeks and thereafter maintained a plateau (Fig. 2D). No differences in running were observed between animals fed valganciclovir chow versus normal chow. Runner mice receiving regular rodent chow ran 4.3 km/day (± 0.54 SE), and Runner mice receiving valganciclovir-infused rodent chow ran 4.2 km/day (± 0.49 SE).
Hippocampal neurogenesis
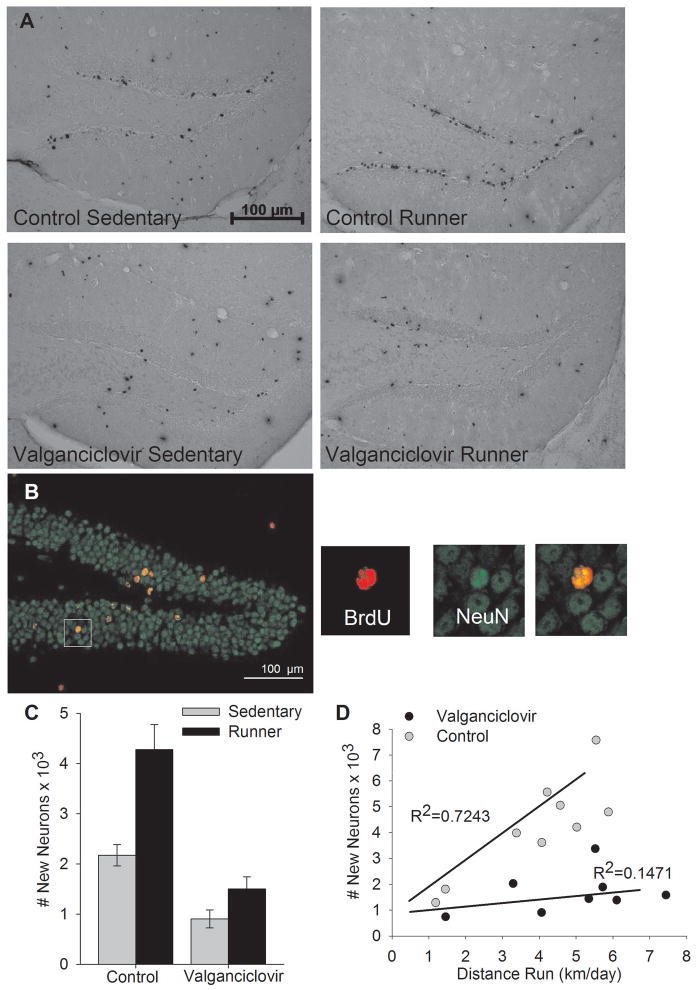
Running increased neurogenesis, as indicated by a significant main effect of exercise on total number of BrdU-positive cells (F1,32=22.5, P<0.0001) and total number of new neurons (F1,32=19.1, P=0.0001). Valganciclovir reduced neurogenesis, as indicated by a significant main effect of diet on total number of BrdU-positive cells (F1,32=40.99, P<0.0001) and total number of new neurons (F1,32=42.7.x, P<0.0001) (Fig. 3A, B). A significant interaction between diet and running on total number of BrdU-positive cells (F1,32=6.12, P=0.0189) and total number of new neurons (F1,32=5.9, P=0.0209) was observed (Fig. 3C). All posthoc pair-wise differences between groups were significant (P<0.05) except for Sedentary-Control vs. Runner-Val, and Sedentary-Val vs. Runner-Val (Fig. 3C). Levels of neurogenesis were reduced by a proportionally greater amount in runners versus sedentary animals. Among sedentary animals, the valganciclovir-fed mice displayed 42% as many total number of BrdU+ cells and 42% as many total number of new neurons cells as normal chow-fed mice. Among runners, valganciclovir-fed mice displayed 36% as many total number of BrdU+ cells and 35% as many total number of new neurons as normal chow-fed mice.
Figure 3.
Adult hippocampal neurogenesis. A) Photographs of the dentate gyrus stained for BrdU using DAB as the chromogen showing representative sections from each of the four groups. Black dots are nuclei stained positive for BrdU, indicating newly divided cells. B) Photographs of a representative section through the dentate gyrus of a runner mouse receiving control chow double-stained green for NeuN (mature neuronal marker) and red for BrdU. Panels to the right show the tissue illuminated for each color separately and combined zoomed in around the BrdU cell, indicating an episode of neurogenesis. C) Total number of new neurons (± SE) shown separately by exercise (sedentary versus runner) and diet treatment (control versus valganciclovir). Neurogenesis was significantly reduced in both runners and sedentary animals from valganciclovir. Running significantly increased neurogenesis in the control group but not the valganciclovir group. Importantly, runners treated with valganciclovir displayed similar levels of neurogenesis as sedentary untreated animals. D) Number of new neurons in runners plotted against average distance run (km/day) across the 28 days of uninterrupted running, graphed separately for runners receiving control chow and valganciclovir chow. A positive correlation between distance traveled and number of new neurons was observed in control runners, whereas the correlation was absent in valganciclovir-treated runners.
A significant correlation between distance traveled and number of new neurons was observed in the Runner-Control group. No correlation was observed in the Runner-Val group (Fig. 4D). This was indicated by a significant effect of distance traveled (F1,14=8.05, P=0.01), diet (F1,14=40.67, P<0.0001), and a significant interaction between distance traveled and diet (F1,14=11.06, P=0.005) by analysis of covariance. Results were the same for BrdU+ cells (distance traveled, F1,14=8.03, P=0.01; diet, F1,14=40.87, P<0.0001; interaction between distance and diet, F1,14=11.08, P=0.005).
Figure 4.
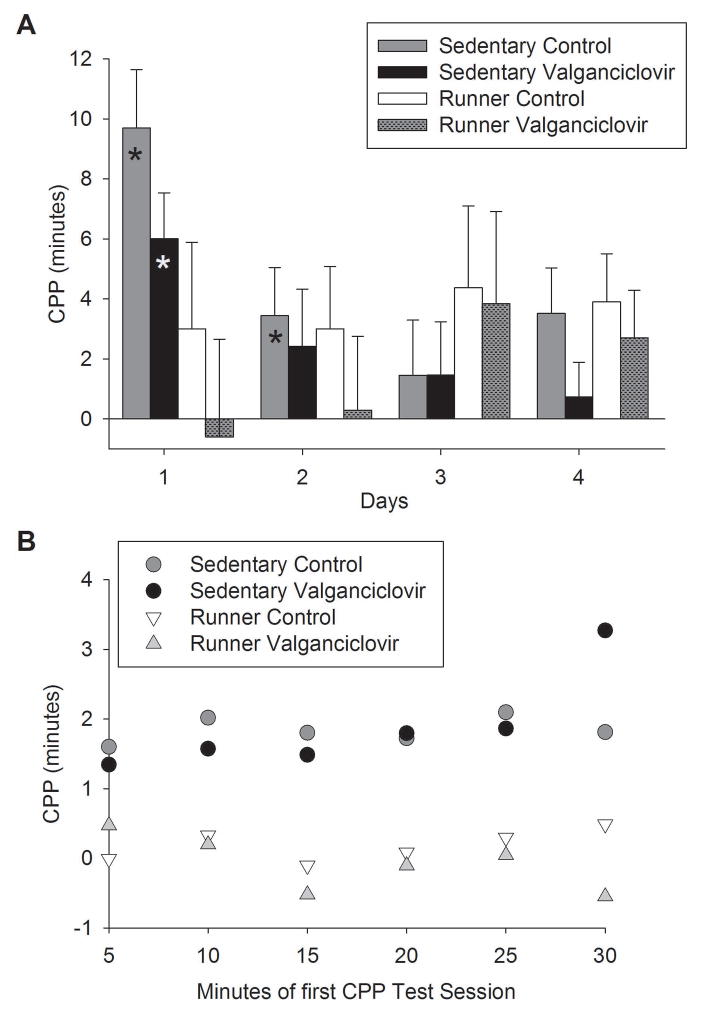
Conditioned place preference for cocaine during testing. A) Mean difference in duration (min) ± SE spent on the HOLE texture between animals receiving cocaine on HOLE texture (CS+HOLE) and animals receiving cocaine on GRID texture (CS+GRID) plotted separately for sedentary animals and runners receiving control chow or valganciclovir chow. Each bar represents data for the following animals: sedentary mice receiving control chow n = 13 CS+HOLE animals and n = 13 CS+GRID animals, sedentary mice receiving valganciclovir chow n = 10 CS+HOLE animals and n = 10 CS+GRID animals, runner mice receiving control chow n = 9 CS+HOLE animals and n = 9 CS+GRID animals, and runner mice receiving valganciclovir chow n = 10 CS+HOLE animals and n = 8 CS+GRID animals. The stars indicate significant place preference at P<0.05. B) Mean difference in duration (min) ± SE spent on the HOLE texture between animals receiving cocaine on HOLE texture (CS+HOLE) and animals receiving cocaine on GRID texture (CS+GRID) during the first CPP testing session plotted in 5-minute intervals separately for sedentary animals and runners receiving control chow or valganciclovir chow.
The percentage of BrdU-positive cells that differentiated into neurons, as indicated by co-expression of NeuN (mature neuronal nuclear marker) and BrdU, was: 83.5% (± 2.6), 81.3% (± 3.1), 87.8% (± 2.3), 88.0% (± 2.3) for the Sedentary-Control, Sedentary-Val, Runner-Control, and Runner-Val groups, respectively. Analysis of logistic regression revealed that running significantly increased the proportion of BrdU cells that differentiated into neurons (Deviance = 4.4, P = 0.04). Diet and the interaction between diet and running were not significant.
Neither running nor diet had a significant effect on volume of the dentate granule layer, nor were any significant interactions observed. Runner-Control animals displayed the largest volume (0.55 mm3 ± 0.020), followed by the other groups: Runner-Val (0.50 mm3 ± 0.020), Sedentary-Control (0.50 mm3 ± 0.020), and then Sedentary-Val (0.49 mm3 ± 0.020).
CPP
Baseline preference
During the pretest, before the animals ever experienced cocaine or valganciclovir, and before any of the animals ran on wheels, there was no significant difference in baseline preference observed between any of the groups.
Locomotor activity in CPP chambers
During testing, animals traveled an average of 24.4 meters (± 0.51 SE) per testing session. No differences between groups were detected.
CPP
Running completely abolished CPP in both animals fed control chow and valganciclovir-infused chow, whereas Sedentary animals fed either chow type displayed significant CPP that only extinguished by day 2 or 3 (Fig. 4A). This differential extinction of CPP, completely absent in Runners but present in Sedentary animals, was indicated by a significant interaction between texture group (whether conditioned with cocaine on HOLE or GRID), exercise history (Runner or Sedentary), and day of testing (1–4) (F2,144 = 9.51, P = 0.0001). Posthoc analyses revealed that sedentary animals fed normal chow displayed significant CPP on days 1 and 2, which was extinguished by day 3. Sedentary animals fed valganciclovir chow displayed significant CPP on day 1, which was extinguished by day 2. Runners never showed significant CPP on any day. There was no significant main effect or any significant interactions involving diet (Control or Val rodent chow) on CPP behavior.
Within-session extinction on test day 1
Microanalysis of the first CPP a.m. test session in 5-minute bins shows that sedentary animals start out with significant CPP, whereas runners do not, and that the level of preference is maintained across the 30 min session, as was indicated by a significant main effect of exercise history (Runner or Sedentary) (F1,20 = 106.04, P < 0.0001) and no effect of bin number, diet, or any interactions (Fig. 4B). This indicates that CPP was completely abolished in Runners, rather than extinguished during the first test.
DISCUSSION
The main finding of the study is that elevated neurogenesis is not required for running to abolish cocaine CPP. This result is important because the functional significance of new neurons in behavior is currently a highly contested topic, and the mechanisms underlying benefits of exercise for reducing relapse to drug use are not well understood. With respect to implications for drug abuse treatment regimens, results suggest that increases in neurogenesis are not necessary for a treatment to diminish the rewarding value of contextual cues.
In addition to establishing that increased neurogenesis is not required for wheel running to abolish CPP, our study also demonstrates that intact neurogenesis is not required for expression or extinction of cocaine CPP in sedentary mice. This is because the sedentary valganciclovir group displayed significantly reduced neurogenesis as compared to the sedentary normal-fed group (Fig 3) yet both displayed similar CPP on day 1, and extinction of CPP on subsequent days (Fig. 4). Note, that we believe it is wrong to conclude from these data that new neurons play no role in cocaine CPP. First, we did not completely eliminate neurogenesis, and it is possible that the remaining levels of neurogenesis were sufficient for expression and extinction of CPP. Also note that even if new neurons are not required for expression and extinction, it is still possible that they are required for acquisition of CPP. In our study, valganciclovir was administered after CPP training, because our intention was to eliminate exercise-induced neurogenesis. In order to determine whether intact neurogenesis is required for acquisition of CPP, future studies would need to administer valganciclovir for a period of time before CPP training.
There are several alternative explanations why eliminating exercise-induced neurogenesis had no significant influence on CPP behavior. We do not believe the cumulative evidence necessarily favors one over the other, and not all explanations are mutually exclusive. The first possibility is that exercise-induced neurogenesis plays no direct role in abolished CPP, i.e., wheel running affects CPP behavior via another mechanism. Alternative mechanisms include but are not limited to that exercise altered the perception of the reward associated with the conditioned stimulus, or that running caused the animals to forget about the drug-context association via mechanisms not involving neurogenesis. Another possibility is that while new neurons are not required for wheel running to abolish CPP, they may be preferentially involved, similar to the way the hippocampus is critically involved in contextual fear conditioning but not required. It is well established that if the hippocampus is removed (lesioned) before contextual fear conditioning, the animal still can remember that the context was associated with the foot shock and freeze. However, if the hippocampus is removed after the animal has acquired the task but before the animal is tested, then the animal cannot remember the association of foot shock with context and does not freeze. The interpretation is that the hippocampus is preferentially used if present, but that the brain can accomplish the task without the hippocampus if needed (Cho et al., 1999; Kim and Fanselow, 1992; Maren et al., 1997; Wiltgen et al., 2006).
Demonstration of CPP in our model requires that the animal forms a positive (rewarding) association between the CS+ (texture) and US (cocaine) during conditioning and that the animal remembers the association for 28 days between conditioning and testing. Therefore the task encompasses both reward and memory components (Cunningham et al., 2006). Exercise could have—independently of neurogenesis— altered the perception of the reward from cocaine-associated cues without affecting memory per se, or it could have affected memory without affecting reward, or some combination of both. After 28 days, the animal might remember that the cues were associated previously with cocaine but these cues might now be perceived as less valuable, possibly due to the substitution of the exercise reward for the cocaine reward (Fontes-Ribeiro et al., 2011; Lynch et al., 2013; Ozburn et al., 2008; Werme et al., 2000). In that case, changes in the brain underlying the exercise-induced abolishment of cocaine CPP might occur in brain regions that comprise the natural reward circuit such as the nucleus accumbens, lateral hypothalamus, extended amygdala, and ventral midbrain, not necessarily the hippocampus (De Chiara et al., 2010; Olsen, 2011; Shapiro et al., 2011; Werme et al., 2000; Zlebnik et al., 2014).
In the current study, the idea that running acts as a substitute reward is supported because runners lack CPP even on Day 1 of testing (Fig. 4A), suggesting that running blunted the rewarding effect of the cocaine cue. Moreover, the microanalysis of the first test indicated CPP was absent in runners even from the very beginning of the first test (Fig. 4B). There are many reasons to suppose exercise might have altered reward. In line with this theory are the findings that in humans, exercise diminishes sensitivity to monetary rewards (Bothe et al., 2013) and reduces neuronal responses in brain regions consistent with reduced pleasure of food (Evero et al., 2012), findings that support the idea that exercise blunts the salience of alternative rewards in general. There is also biological evidence that exercise produces effects that substitute for drug reward. Exercise increases circulating endocannabinoid levels (Ferreira-Vieira et al., 2014; Heyman et al., 2012; Raichlen et al., 2013; Rola et al., 2004; Sparling et al., 2003). Furthermore, running induces similar changes in the brain as cocaine. In Lewis rats, 30 days of running and 7 days of i.p. cocaine administration (10 mg/kg, identical to the dose used in our study) both upregulate mRNA of the endogenous opioid dynorphin in medial caudate putamen, part of the brain reward pathway, to comparable levels (Werme et al., 2000). This suggests a common mechanism of induction between running and cocaine and the possibility of common neuronal adaptations in brain regions to running and cocaine (Werme et al., 2000) that might allow running to substitute for drug reward.
The hypothesis that running serves as a substitute reward explains the abolished CPP we observed from running in the present study. Evidence from our previous paper, however, favors the learning hypothesis over the reward substitution hypothesis. In our previous study, runner mice exhibited robust CPP on Day 1 of testing, but CPP then extinguished more rapidly than the CPP of sedentary mice (Mustroph et al., 2011). The facilitated extinction of CPP from running in that study—rather than its complete absence from the start—suggests that an exercise-induced process promoted learning over the course of testing. Furthermore, in an experiment in which exercise was made available before conditioning, CPP was particularly robust, and did not extinguish even after 4 days, whereas CPP of sedentary animals extinguished by day 2 (Fig. 4A) (Mustroph et al., 2011). If exercise was a reward substitute, then running should have weakened CPP regardless of whether it had been implemented before or after conditioning. On the other hand, if exercise affected CPP by enhancing plasticity and learning, then it should strengthen CPP when it occurs before learning (conditioning), and facilitate CPP extinction when it occurs after learning which is what we in fact observed in our previous study (Mustroph et al., 2011).
The behavioral data from the current study suggesting that running reduced the salience of the drug reward cue prompted us to closely examine the behavioral results of our previous study (Mustroph et al., 2011). Microanalysis of the first CPP test session of that study revealed that CPP of runner animals started out at a higher magnitude than that of sedentary animals, but that CPP of runner animals rapidly extinguished, whereas that of sedentary animals remained significant. To us, this is compelling evidence that exercise promoted learning, i.e., acquisition of the new drug-cue association during testing, which manifested as accelerated within-session CPP extinction in the runner animals. This finding does not preclude the possibility that running in some situations can substitute as a drug reward. We speculate that in the present study exercise blunted the reward of the drug-associated cue on Day 1 to a large enough degree that the effect of running on learning during the subsequent testing days, in the form of accelerated CPP extinction, was not evident to us because there simply was no significant CPP to begin with.
Given the hippocampus’ central role in the conditioned associations thought to underlie drug addiction (Everitt and Robbins, 2005) and the growing understanding that it is a major locus in the brain for change induced from exercise (Carro et al., 2001; Clark et al., 2009; Dietrich et al., 2008; Gomez-Pinilla et al., 2008; Neeper et al., 1995), we believe that plasticity in the hippocampus contributes to the observed influences of exercise on CPP behavior. In addition to increasing adult neurogenesis, exercise increases extracellular brain-derived neurotrophic factor, insulin-like growth factor 1 (IGF-1), spine morphology, dendritic branching, and synaptogenesis any of which could account for the differences we observed between the sedentary and runner groups in our study (Dietrich et al., 2008; Eadie et al., 2005; Neeper et al., 1995; Neeper et al., 1996).
Our results demonstrate that exercise can abolish cocaine CPP without increased adult hippocampal neurogenesis, but it is still possible that new hippocampal neurons are preferentially involved. Our study and the more recent studies that are using highly specific methods to reduce neurogenesis are finding new neurons are not required for behavioral performance on a variety of hippocampus-involved tasks (Dupret et al., 2008; Groves et al., 2013; Saxe et al., 2006; Zhang et al., 2008). We believe the data obtained in our study along with the meta-analysis of other work (Groves et al., 2013) should be used to revise our thinking about the role of new neurons in behavioral performance. The revised view should consider the possibility that when animals are learning hippocampus-dependent tasks, that new neurons may be involved or even preferentially recruited if present, but if new neurons are not available, older, established neurons can compensate and display sufficient plasticity required for the learning.
In a CPP test, many cues (e.g., the walls of the apparatus, the size of the textured areas, the fact that the animals are moved into the testing room and handled right before the test among many other features) are identical in all trials. In order to display CPP, the animals must be able to differentiate a subtle feature of the apparatus, the texture of the floor, suggesting that the CPP test, like other tests of contextual learning, may have a pattern separation component (Nakashiba et al., 2008). This is important because the dentate gyrus plays a critical role in pattern separation (Groves et al., 2013). Moreover, adult neurogenesis in the dentate gyrus has been hypothesized to support pattern separation (Clelland et al., 2009; Scobie et al., 2009; Tronel et al., 2012). To the extent that CPP involves pattern separation, our results are consistent with the recent study and meta-analysis of the literature suggesting that adult hippocampal neurogenesis is not essential for pattern separation in rodents (Groves et al., 2013).
As with all approaches that intend to reduce neurogenesis by inducing the apoptosis of newly dividing cells, side effects of our method as well as compensatory mechanisms are critical to consider when interpreting results. One of the primary advantages of our method for ablating neurogenesis in comparison to many others in the literature is the specificity, and minimal level of invasiveness. We know from previous studies (Schloesser et al., 2009), and our own unpublished work, that valganciclovir alone fed to non-transgenic mice has no influence on neurogenesis or behavior. Previous work suggests that ganciclovir can induce toxicity related to the way it is administered (Singer et al., 2009). Hence, for the purposes of establishing an animal model for manipulating neurogenesis that is useful for behavioral testing, administering valganciclovir via chow appears to be the desired method, as opposed to i.p. or s.c. via minipump or i.c.v., administration techniques which can induce systemic toxicity or require implantation surgery, with risk of surgical complications (Singer et al., 2009). In our study, valganciclovir had no detectable influence on body mass (Fig. 2A), food intake (Fig. 2B), or wheel running behavior (Fig. 2D), and the mice displayed normal levels of locomotor activity (data not shown) and CPP behavior (Fig. 4A).
Hence, the reduction of adult neurogenesis in our experiment was quite specific, and our experimental design well-suited to determine the functional significance of the addition of new neurons from exercise in abolished CPP behavior. In our study, there were two possible outcomes. Outcome #1: runner mice with reduced neurogenesis display the same CPP as runner mice with intact neurogenesis. Outcome #2: runner mice with reduced neurogenesis display different CPP compared to runner mice with intact neurogenesis. We observed outcome #1, which is the strongest possible result we could have observed, because it establishes that the addition of new neurons from running are not necessary for running to abolish cocaine CPP. Note that if we observed #2, different CPP in runner mice with reduced as compared to intact neurogenesis then we could have only tentatively concluded that new neurons are necessary for the behavioral outcome. This is because possible unknown side effects or compensatory responses from the treatment used to reduce neurogenesis rather than the lost neurogenesis itself could have contributed to the behavioral outcomes. We speculate that many of the previous papers that used systemic administration of antimitotic agents (Doetsch et al., 1999; Shors et al., 2002) or focal irradiation (Mizumatsu et al., 2003) to reduce neurogenesis, including some of our own papers (Clark et al., 2008), may have found reduced task performance in treated animals because of enduring side effects related to inflammation or toxicity of the treatment used to reduce neurogenesis rather than because of the loss of neurogenesis (Singer et al., 2009).
Given the high incidence of relapse in drug abuse, effective treatment is likely going to require a large collection of life changes to be made. Nevertheless, any reduction in drug-cue strength may be helpful when recovering from drug use in an environment in which drug use occurred. Here, we provide evidence that exercise reduces drug-cue strength, and that it accomplishes this independently of new neurons. This finding is an important step in optimizing treatment regimens relevant for drug dependence, since it shows that maximizing neurogenesis from running will likely impart no additional benefit to treatment outcomes.
Acknowledgments
We wish to thank the Beckman Institute Animal Facility for expert animal care. We thank Claudia Lutz, Emelie Mies, and Stephanie Ceman for their critical review of the manuscript.
Funding support: Grant sponsor: NIH; Grant number: DA0270847 to J.S.R.
Grant sponsor: NIH; Grant number: F30DA034480-01A1 to M.L.M.
Grant sponsor: Erik Haferkamp Memorial Undergraduate Scholarship to A.L. H.
Footnotes
The authors declare no competing financial interests.
References
- Ames SL, Grenard JL, He Q, Stacy AW, Wong SW, Xiao L, Xue G, Bechara A. Functional imaging of an alcohol-Implicit Association Test (IAT) Addict Biol. 2013;19:467–81. doi: 10.1111/adb.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaiss CA, Yu TS, Zhang G, Chen J, Dimchev G, Parada LF, Powell CM, Kernie SG. Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J Neurosci. 2011;31:4906–16. doi: 10.1523/JNEUROSCI.5265-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothe N, Zschucke E, Dimeo F, Heinz A, Wustenberg T, Strohle A. Acute exercise influences reward processing in highly trained and untrained men. Med Sci Sports Exerc. 2013;45:583–91. doi: 10.1249/MSS.0b013e318275306f. [DOI] [PubMed] [Google Scholar]
- Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, Oakley JR, Ramsey SE, Kahler CW, Stuart GG, et al. A Pilot Study of Aerobic Exercise as an Adjunctive Treatment for Drug Dependence. Ment Health Phys Act. 2010;3:27–34. doi: 10.1016/j.mhpa.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21:5678–84. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Jaber M, Solinas M. Brain regions associated with the reversal of cocaine conditioned place preference by environmental enrichment. Neuroscience. 2011;184:88–96. doi: 10.1016/j.neuroscience.2011.03.068. [DOI] [PubMed] [Google Scholar]
- Cho YH, Friedman E, Silva AJ. Ibotenate lesions of the hippocampus impair spatial learning but not contextual fear conditioning in mice. Behav Brain Res. 1999;98:77–87. doi: 10.1016/s0166-4328(98)00054-0. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Bhattacharya TK, Miller DS, Rhodes JS. Induction of c-Fos, Zif268, and Arc from acute bouts of voluntary wheel running in new and pre-existing adult mouse hippocampal granule neurons. Neuroscience. 2011a;184:16–27. doi: 10.1016/j.neuroscience.2011.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19:937–50. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–58. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–3. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–72. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1(4):1662–70. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- De Chiara V, Errico F, Musella A, Rossi S, Mataluni G, Sacchetti L, Siracusano A, Castelli M, Cavasinni F, Bernardi G, et al. Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacology. 2010;35:374–87. doi: 10.1038/npp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Andrews ZB, Horvath TL. Exercise-induced synaptogenesis in the hippocampus is dependent on UCP2-regulated mitochondrial adaptation. J Neurosci. 2008;28:10766–71. doi: 10.1523/JNEUROSCI.2744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999;96:11619–24. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav Neurosci. 2010;124:446–54. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Evero N, Hackett LC, Clark RD, Phelan S, Hagobian TA. Aerobic exercise reduces neuronal responses in food reward brain regions. J Appl Physiol. 2012;112:1612–9. doi: 10.1152/japplphysiol.01365.2011. [DOI] [PubMed] [Google Scholar]
- Ferreira-Vieira TH, Bastos CP, Pereira GS, Moreira FA, Massensini AR. A role for the endocannabinoid system in exercise-induced spatial memory enhancement in mice. Hippocampus. 2014;24:79–88. doi: 10.1002/hipo.22206. [DOI] [PubMed] [Google Scholar]
- Fontes-Ribeiro CA, Marques E, Pereira FC, Silva AP, Macedo TR. May exercise prevent addiction? Curr Neuropharmacol. 2011;9:45–8. doi: 10.2174/157015911795017380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–87. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampal-dependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus. 2009;19:973–80. doi: 10.1002/hipo.20631. [DOI] [PubMed] [Google Scholar]
- Groves JO, Leslie I, Huang GJ, McHugh SB, Taylor A, Mott R, Munafo M, Bannerman DM, Flint J. Ablating adult neurogenesis in the rat has no effect on spatial processing: evidence from a novel pharmacogenetic model. PLoS Genet. 2013;9:e1003718. doi: 10.1371/journal.pgen.1003718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Hontecillas-Prieto L, Velazquez-Sanchez C, Ferragud A, Perez-Villaba A, Arcusa A, Barcia JA, Trejo JL, Canales JJ. The hippocampal dentate gyrus is essential for generating contextual memories of fear and drug-induced reward. Neurobiol Learn Mem. 2008;90:553–9. doi: 10.1016/j.nlm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Llorens-Martin M, Velazquez-Sanchez C, Ferragud A, Arcusa A, Gumus HG, Gomez-Pinedo U, Perez-Villalba A, Rosello J, Trejo JL, et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159:59–68. doi: 10.1016/j.neuroscience.2008.11.054. [DOI] [PubMed] [Google Scholar]
- Heyman E, Gamelin FX, Goekint M, Piscitelli F, Roelands B, Leclair E, Di Marzo V, Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans--possible implications for reward and depression. Psychoneuroendocrinology. 2012;37:844–51. doi: 10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Revis AA, Burdick MA, Rhodes JS. A similar pattern of neuronal Fos activation in 10 brain regions following exposure to reward- or aversion-associated contextual cues in mice. Physiol Behav. 2010;99:412–8. doi: 10.1016/j.physbeh.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Dorr A. Single-dose pharmacokinetics of valganciclovir in HIV- and CMV-seropositive subjects. J Clin Pharmacol. 1999;39:800–4. doi: 10.1177/00912709922008452. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Koteja P, Garland T, Jr, Sax JK, Swallow JG, Carter PA. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Anim Behav. 1999;58:1307–1318. doi: 10.1006/anbe.1999.1270. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37:1622–44. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–74. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–9. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Neisewander JL. Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport. 2003;14:2127–31. doi: 10.1097/00001756-200311140-00023. [DOI] [PubMed] [Google Scholar]
- Michaelides M, Thanos PK, Volkow ND, Wang GJ. Translational neuroimaging in drug addiction and obesity. ILAR J. 2012;53:59–68. doi: 10.1093/ilar.53.1.59. [DOI] [PubMed] [Google Scholar]
- Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–7. [PubMed] [Google Scholar]
- Mustroph ML, Stobaugh DJ, Miller DS, DeYoung EK, Rhodes JS. Wheel running can accelerate or delay extinction of conditioned place preference for cocaine in male C57BL/6J mice, depending on timing of wheel access. Eur J Neurosci. 2011;34:1161–9. doi: 10.1111/j.1460-9568.2011.07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–4. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- O’Callaghan RM, Griffin EW, Kelly AM. Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippocampus. Hippocampus. 2009;19:1019–29. doi: 10.1002/hipo.20591. [DOI] [PubMed] [Google Scholar]
- Olsen CM. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology. 2011;61:1109–22. doi: 10.1016/j.neuropharm.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Harris RA, Blednov YA. Wheel running, voluntary ethanol consumption, and hedonic substitution. Alcohol. 2008;42:417–24. doi: 10.1016/j.alcohol.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescovitz MD, Rabkin J, Merion RM, Paya CV, Pirsch J, Freeman RB, O’Grady J, Robinson C, To Z, Wren K, et al. Valganciclovir results in improved oral absorption of ganciclovir in liver transplant recipients. Antimicrob Agents Chemother. 2000;44:2811–5. doi: 10.1128/aac.44.10.2811-2815.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichlen DA, Foster AD, Seillier A, Giuffrida A, Gerdeman GL. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur J Appl Physiol. 2013;113:869–75. doi: 10.1007/s00421-012-2495-5. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Garland T, Jr, Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav Neurosci. 2003;117(6):1243–56. doi: 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Koteja P, Swallow JG, Carter PA, Garland T. Body temperatures of house mice artificially selected for high voluntary wheel-running behavior: repeatability and effect of genetic selection. J Therm Biol. 2000;25:391–400. doi: 10.1016/s0306-4565(99)00112-6. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–30. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser RJ, Manji HK, Martinowich K. Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response. Neuroreport. 2009;20:553–7. doi: 10.1097/WNR.0b013e3283293e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scobie KN, Hall BJ, Wilke SA, Klemenhagen KC, Fujii-Kuriyama Y, Ghosh A, Hen R, Sahay A. Kruppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis. J Neurosci. 2009;29:9875–87. doi: 10.1523/JNEUROSCI.2260-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A, Cheng KY, Gao Y, Seo DO, Anton S, Carter CS, Zhang Y, Tumer N, Scarpace PJ. The act of voluntary wheel running reverses dietary hyperphagia and increases leptin signaling in ventral tegmental area of aged obese rats. Gerontology. 2011;57:335–42. doi: 10.1159/000321343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–84. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BH, Jutkiewicz EM, Fuller CL, Lichtenwalner RJ, Zhang H, Velander AJ, Li X, Gnegy ME, Burant CF, Parent JM. Conditional ablation and recovery of forebrain neurogenesis in the mouse. J Comp Neurol. 2009;514:567–82. doi: 10.1002/cne.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M. Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci U S A. 2008;105:17145–50. doi: 10.1073/pnas.0806889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. Exercise activates the endocannabinoid system. Neuroreport. 2003;14:2209–11. doi: 10.1097/00001756-200312020-00015. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Tucci A, Stamos J, Robison L, Wang GJ, Anderson BJ, Volkow ND. Chronic forced exercise during adolescence decreases cocaine conditioned place preference in Lewis rats. Behav Brain Res. 2010;215:77–82. doi: 10.1016/j.bbr.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S, Belnoue L, Grosjean N, Revest JM, Piazza PV, Koehl M, Abrous DN. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2012;22:292–8. doi: 10.1002/hipo.20895. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M, Thoren P, Olson L, Brene S. Running and cocaine both upregulate dynorphin mRNA in medial caudate putamen. Eur J Neurosci. 2000;12:2967–74. doi: 10.1046/j.1460-9568.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26:5484–91. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Wiskott L, Rasch MJ, Kempermann G. A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus. 2006;16:329–43. doi: 10.1002/hipo.20167. [DOI] [PubMed] [Google Scholar]
- Yu TS, Zhang G, Liebl DJ, Kernie SG. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J Neurosci. 2008;28:12901–12. doi: 10.1523/JNEUROSCI.4629-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–7. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zlebnik NE, Hedges VL, Carroll ME, Meisel RL. Chronic wheel running affects cocaine-induced c-Fos expression in brain reward areas in rats. Behav Brain Res. 2014;261:71–8. doi: 10.1016/j.bbr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck JA, Chen GT, Johnson ZV, Rosenberg DM, Craig AB, Rhodes JS. Neuroanatomical specificity of conditioned responses to cocaine versus food in mice. Physiol Behav. 2008;93:637–50. doi: 10.1016/j.physbeh.2007.11.004. [DOI] [PubMed] [Google Scholar]