Abstract
Human laboratory and animal models implicate variation in the μ-opioid receptor gene (OPRM1) as relevant for alcohol-related reward. OPRM1 is associated with alcohol self-administration in nonhuman primate studies, but the relevance of this finding to human models is unclear. This study used computer-assisted self-infusion of ethanol (CASE) to examine associations among OPRM1 A118G genotype, subjective responses to alcohol, and intravenous alcohol self-administration in young heavy drinkers (n = 40, mean age = 19.95 years, SD = 0.82). Participants completed a two-hour CASE session comprising a priming phase followed by ad libitum self-administration in a free-access paradigm. Participants achieved a mean peak breath alcohol concentration (BrAC) of 81.18 mg% (SD=24.96). Those with the OPRM1 118G variant (GA or GG genotypes) achieved significantly higher peak BrAC (M=94.90 mg%, SD=16.56) than those with the AA genotype (M=74.46 mg%, SD=25.36), reflecting a significantly greater number of alcohol requests among GA/GG participants. Eighty percent of GA/GG participants surpassed a threshold defining a laboratory analogue of heavy alcohol exposure (80mg%) compared to 46% of AA participants. Results indicated significant associations between subjective measures of alcohol sensitivity and CASE outcomes, although the pattern of findings differed across self-report measures. Subjective responses did not differ by OPRM1 status. These results offer further support for the feasibility of the CASE paradigm and provide initial evidence for an association of OPRM1 with alcohol self-administration in a human laboratory context.
Keywords: Asn40Asp, pharmacogenetics, phenotype, rs1799971, SNP, subjective responses to alcohol
Introduction
Human alcohol administration paradigms have proved critical for advancing knowledge of alcohol dependence etiology and mechanisms of treatment response (King et al., 2011a, 2011b; Plebani et al., 2012; Ray et al., 2010). While only a small proportion of these studies have modeled alcohol consumption as the dependent variable (Zimmermann et al., 2013), such paradigms are particularly important in that the progression from early use to addiction is ultimately contingent on repeated cycles of self-administration (Kalant, 2010). Early efforts to model self-administration under controlled conditions helped to characterize cognitive and motivational aspects of consumption in alcohol-dependent participants (Marlatt et al., 1973; Mello and Mendelson, 1965; Zimmermann et al., 2013). Subsequent evidence that self-administration varied based on reported stimulant and sedative effects of alcohol (de Wit et al., 1987, 1989) informed the conceptualization of subjective alcohol responses as candidate endophenotypes for alcohol dependence (Crabbe et al., 2010; Hines et al., 2005; King et al., 2011b; Morean and Corbin, 2010).
Human laboratory models have increasingly evaluated genetic correlates of subjective, neural and behavioral responses to alcohol (e.g., Kareken et al., 2010; Ramchandani et al., 2011; Ray and Hutchison, 2007). These studies reflect an “intermediate phenotype” approach that prioritizes laboratory-based measures over diagnostic outcomes, given expectations for improved phenotype precision and closer correspondence with neurobiological processes (Enoch et al., 2003; Hines et al., 2005). In the context of alcohol addiction, the advantages of this approach are illustrated by recent work implicating variation in the μ-opioid receptor gene (OPRM1) as relevant for individual variation in responses to alcohol. Most studies have focused on a common polymorphism (A118G, rs1799971) in exon 1 of the gene (Heilig et al., 2011; Mague and Blendy, 2010; Ray et al., 2012a). Notably, initial human laboratory studies found that participants with the minor (G) allele reported greater hedonic responses to alcohol (Ray and Hutchison, 2004, 2007), greater cue-elicited craving (van den Wildenberg et al., 2007), and enhanced mesolimbic activation during exposure to alcohol cues (Filbey et al., 2008) relative to participants homozygous for the common (A) allele.
A subsequent translational study found important evidence for a functional association of A118G with neurobiological responses to alcohol (Ramchandani et al., 2011). This study used positron emission tomography to confirm enhanced alcohol-induced striatal dopamine release in 118G carriers, also demonstrating a similar result in rodent lines with a humanized OPRM1 sequence in which the A118G SNP was experimentally manipulated (Ramchandani et al., 2011). Importantly, the latter finding can be interpreted as supporting a functional role of A118G in alcohol-induced dopamine response (Heilig et al., 2011). Despite the apparent association of OPRM1 with measures of alcohol-related reward, the relevance of these findings for alcohol use behavior remains controversial given mixed evidence in studies focusing on self-reported drinking or diagnostic status (Arias et al., 2006; Kranzler et al., 2013). Laboratory self-administration paradigms offer a viable context for addressing this question, but human studies are few. Initial studies examining OPRM1 in the context of oral self-administration paradigms found no genotype differences in self-administration (Anton et al., 2012; Setiawan et al., 2011).
One barrier for oral alcohol administration paradigms is large variability in blood alcohol concentration profiles, reflecting high inter-individual variability in alcohol absorption and first-pass metabolism (Ramchandani and O'Connor, 2006; Ramchandani et al., 2009). This variability restricts experimental precision and may complicate pharmacodynamic interpretations for individual differences in behavioral or neurobiological outcomes. A specific implication for self-administration paradigms is unwanted variability in speed and extent of incremental increases in arterial blood alcohol concentration (aBAC, reflecting brain alcohol exposure) following standardized doses (Zimmermann et al., 2008). Computer-assisted self-infusion of ethanol (CASE), a novel self-administration paradigm (Zimmermann et al., 2008, 2009, 2013), can obviate many of these concerns. CASE combines intravenous alcohol administration with physiologically-based pharmacokinetic (PBPK) modeling (Plawecki et al., 2008; Ramchandani et al., 1999), allowing uniform increments in aBAC across participants. The intravenous route of administration also enables close temporal contingencies between operant responses and aBAC increases. Coupled with a relatively rapid decline in aBAC (due to immediate blood-to-tissue distribution), this feature allows relatively better control over self-imposed aBAC compared to oral methods (Zimmermann et al., 2008). Additionally, the absence of conditioned sensory cues is presumed to isolate perceived alcohol effects (relative to desired effects) as the main determinant of self-administration (Zimmermann et al., 2008).
Two initial reports demonstrated the feasibility of the CASE paradigm. In the first study, nine adult social drinkers achieved a mean peak breath alcohol concentration (BrAC) of 76.5mg% across repeated free-access sessions (Zimmermann et al., 2008). In a subsequent study of 22 young adult social drinkers, participants with a family history of alcohol dependence attained higher mean peak BrAC (81.6mg%) compared to family history negative participants (50.5mg%) (Zimmermann et al., 2009). The present study sought to further evaluate the CASE paradigm in a sample of young heavy drinkers, with the specific aim of examining OPRM1 in relation to self-administration. Given evidence that OPRM1 relates to subjective responses to alcohol (Ray and Hutchison, 2004, 2007), and that subjective responses relate to laboratory self-administration (de Wit et al., 1989; de Wit et al., 1987), trait and state measures of alcohol sensitivity were also examined. We predicted that participants with the 118G variant would show higher self-administration and greater hedonic responses during self-administration relative to AA homozygous participants. We also anticipated that, irrespective of genotype, greater perceived stimulant effects and lower perceived sedative effects would correlate with greater self-administration. To provide a developmental context and reduce potential confounds related to drinking histories, we focused on a narrow age range (19–21 years) approximating the peak age of onset for alcohol use disorders (Li et al., 2004).
Materials and Methods
Participants
Participants included 40 heavy-drinking young adults (mean age: 19.95 years, SD = 0.82; 18 females). Recruitment consisted of advertisements on public and university websites and university campuses, which requested social drinkers for research involving alcohol administration. The sample was predominantly Caucasian (27 participants), with additional participants reporting Asian (3), East Indian (3), Black (2), Native North American (1), or mixed race (4). No participants were married, 24 reported current employment, and 28 were full-time students. Self-reported drinking behavior is depicted in Table 1. Eleven participants reported current cigarette use and most (31) reported cannabis use in the prior 90 days.
Table 1.
Sample characteristics
| Total Sample | OPRM1 AA | OPRM1 GA/GG | Test | p | |
|---|---|---|---|---|---|
| Age (years) | 19.95 (.82) | 19.93 (.81) | 20.00 (.94) | t (36) = −0.23 | .82 |
| Race (% Caucasian) | 65 | 64 | 67 | χ2(1) = 0.02 | .90 |
| Sex (% female) | 48 | 54 | 30 | χ2(1) = 1.64 | .20 |
| Percent drinking days | 24.81 (13.3) | 23.0 (11.9) | 28.3 (15.2) | t (36) = −1.13 | .27 |
| Drinks per drinking day | 5.41 (1.82) | 5.13 (1.84) | 5.82 (1.63) | t (36) = −1.04 | .31 |
| Heavy episodes | 15.33 (12.60) | 13.96 (11.77) | 17.80 (13.87) | t (36) = −0.84 | .40 |
| AUDIT score | 11.08 (5.42) | 9.92 (3.72) | 12.90 (7.70) | t (36) = −0.13 | .90 |
| ASQ stimulant scale (%) | 42.1 | 35.7 | 60.0 | χ2(1) = 1.78 | .18 |
| ASQ sedation scale (%) | 57.9 | 57.1 | 60.0 | χ2(1) = 0.03 | .88 |
Notes. Values reflect (M, SD) unless indicated. Drinking variables assessed for the prior 90 days, with heavy episodes reflecting 4+ drinks for women/5+ drinks for men. AUDIT = Alcohol Use Disorders Identification Test. ASQ = Alcohol Sensitivity Questionnaire. ASQ values reflect proportion classified as low sensitivity (i.e., higher ASQ scale scores). N = 40 for total sample, n = 28 for OPRM1 AA, n = 10 for OPRM1 GA/GG.
Procedures
Phone screens determined initial eligibility based on the following criteria: age 19–21; at least 1 heavy drinking episode (4+ drinks for women/5+ drinks for men) in the last month; a Brief Michigan Alcohol Screening Test (MAST) score <10, with no history of treatment for alcohol use or current attempts to reduce drinking; a Fagerstrom Test for Nicotine Dependence (FTND) score <6; no prior adverse reactions to venipuncture; no current illicit drug use other than cannabis; and no medical conditions or medications for which alcohol was contraindicated. Participants then completed an in-person visit that included informed consent, verification of eligibility criteria, height and weight measurements, baseline questionnaires, DNA collection via saliva sample, and assessment of 90-day alcohol and substance use. After a physician further confirmed medical eligibility, participants were scheduled for alcohol administration sessions.
Experimental sessions took place in a hospital under medical supervision. Participants arrived in the mid-morning or early afternoon (depending on availability), receiving instructions not to eat within 4 hours or consume alcohol within 24 hours of arrival. Cigarette use was not allowed after arrival. Participants provided a breath alcohol reading (all tests were negative) and received a standardized snack. Women also completed a pregnancy test. Participants were then escorted to a private room and seated in a recliner chair, where the study nurse placed an indwelling catheter. After receiving orientation and instructions from a research assistant, participants completed baseline subjective questionnaires.
CASE sessions consisted of a free-access paradigm, Freibier (German for “free beer”), comprising a priming phase followed by ad libitum self-infusion (Zimmermann et al., 2008, 2009). Participants were instructed to self-administer alcohol at their discretion to achieve a level of intoxication that was pleasurable, while avoiding unpleasant effects. They were instructed not to interact with staff unless necessary, but could listen to music through headphones during the session. Participants remained seated for the 2-hour session with the exception of a scheduled bathroom break. Experimenter-obtained BrAC readings were entered into the software regularly for online adjustment of model-projected aBAC profiles (participants remained blind to BrAC readings). Subjective questionnaires occurred once before the infusion, immediately after the priming phase, and at intervals of ~30 minutes during the ad libitum phase. After the session participants relocated to a private room, where they were monitored and released after BrAC fell below 30mg%.
Infusion parameters
The infusion consisted of 100% dehydrated ethanol and saline, mixed to a 6.0% ethanol (v/v) solution and delivered by a dual-channel infusion pump. Participants submitted “drink” requests by pressing an electronic button; each request triggered the pump to deliver a volume calculated to raise aBAC by 7.5mg% over 2.5 minutes. During alcohol delivery the electronic button deactivated and additional requests could not be made. The CASE software generated instructions on a computer screen to guide participants during the experiment, conveying whether the “bar” was open or closed at a given point. At the onset of the session, instructions prompted participants to self-administer four successive priming doses (raising aBAC to ~30mg% in 10 minutes). A mandatory 5-minute waiting period ensued, during which participants completed post-priming subjective questionnaires. The CASE software specified a descending aBAC slope rate of −1mg%/minute during wait periods (resulting in aBAC of ~25mg% at the conclusion of the initial priming/waiting period). The ad libitum phase began at 15 minutes and lasted until 120 minutes.
The software calculated projected aBAC in the background, adjusting projected aBAC profiles with each BrAC entry. A safety ceiling of 100mg% was imposed, such that drink requests were not possible if the next button press would yield a projected aBAC >100mg%. During these intervals the button deactivated until a drink could be delivered without aBAC exceeding 100mg% (yielding a maximum of ~7.5 min before the next request was allowed). Additional details on the Freibier paradigm are provided elsewhere (Zimmermann et al., 2008, 2009, 2013).
Measures
Alcohol Use
Recent (90-day) alcohol, tobacco and other substance use was assessed at the in-person screening with the Timeline Followback interview (Sobell and Sobell, 1992). Participants also completed the 10-item Alcohol Use Disorders Identification Test (AUDIT, Saunders et al., 1993) as an index of severity of drinking and alcohol-related problems.
Alcohol Sensitivity
Individual differences in alcohol sensitivity were assessed using 1) a baseline self-report measure and 2) subjective responses during the CASE session. Baseline alcohol sensitivity was measured with the Alcohol Sensitivity Questionnaire (Bartholow et al., 2007; Bartholow et al., 2003). The ASQ lists 16 alcohol effects; 10 focus on experiences thought to be typical of the ascending limb of intoxication (e.g., “relaxed,” “talkative,” “buzzed”) and 6 on sedative effects (e.g., nausea, blackouts). Participants indicate whether they have experienced each effect when drinking. For items endorsed, participants list the minimum number of standard drinks after which the effect is noticed (for ascending limb items) or the maximum number of drinks that could be consumed before noticing the effect (descending limb items). The ASQ can be differentiated from other self-report measures of alcohol sensitivity by the use of 16 items covering presumed ascending and descending limb effects and the use of a single (current) recall time frame (Bartholow et al., 2007).
ASQ subscales (stimulant/ascending limb, sedative/descending limb) were examined separately, given a theoretical basis for predicting differential effects for stimulation/sedation as a function of OPRM1 (Ray and Hutchison, 2004) and laboratory self-administration behavior (de Wit et al., 1987). This decision was supported by evidence of moderate correlation between subscales (r = 0.54, p < .001) and somewhat higher internal consistencies for the separate scales (stimulant: α = 0.91, sedative: α = 0.91) compared to the total scale scores (α = 0.83). Participants were grouped into high- or low-sensitivity on each subscale by computing median splits (note that higher scores denote lower sensitivity to reported alcohol effects). Given the tendency for men to endorse a higher number of drinks than women, ASQ group assignments occurred separately for men and women (Bartholow et al., 2007).
Subjective responses during the CASE session were assessed with measures of stimulation and sedation, craving, and drug liking/wanting. Craving was assessed with the Alcohol Urge Questionnaire (Bohn et al., 1995), including eight items on current desire for alcohol (e.g. ‘All I want to do now is have a drink’). The AUQ showed adequate internal consistency (α = 0.81 for the post-priming assessment). Perceived stimulant and sedative effects were assessed with the 14-item biphasic alcohol effects scale (BAES, Martin et al., 1993), covering stimulant (e.g., energized, stimulated) and sedative (sedated, sluggish) effects of alcohol. Internal consistencies for stimulation and sedation scales were α = 0.89 and α = 0.83, respectively, at the post-priming assessment. Items adapted from the Drug Effects Questionnaire (DEQ, see Morean et al., 2013) served as measures of drug liking (Do you like any of the effects you are feeling right now?) and wanting (How much do you want more of the infusion?) using an analogue scale (0–100). All measures were presented on a computer monitor. The present analyses focused on a) the assessment point immediately after priming, when aBAC was equivalent across participants, and b) peak scores obtained across the CASE session.
Laboratory Outcomes
Peak BrAC, reflecting the maximum BrAC value obtained by breathalyzer, served as the primary outcome. Area under the curve (AUC) served as a cumulative index of alcohol exposure. AUC values were based on model-projected aBAC values recorded at 30-second intervals over the session, excluding the priming/waiting period (when the number and timing of drink requests were standardized). AUC values were established with a trapezoidal formula, yielding an index of cumulative brain alcohol exposure in mg% * minutes. Finally, CASE sessions were classified based on whether self-administration resulted in alcohol exposure sufficient to qualify for a heavy episode, defined as achieving BrAC ≥ 80mg% within 2 hours (National Institute on Alcohol Abuse and Alcoholism, 2004). This outcome served as a laboratory analogue of heavy episodic exposure based on a clinically relevant threshold.
Genotyping
Saliva samples (~2 mL) were collected in an Oragene OG-500 DNA kit (DNA Genotek, Ottawa, ON). DNA was extracted per manufacturer’s instructions. The OPRM1 A118G variant (rs1799971) was genotyped using the C___8950074_1_ TaqMan pre-designed assay (LifeTechnologies, Burlington, ON). For each reaction, 20 ng genomic DNA were amplified as per manufacturer’s directions scaled to a total volume of 10 µL in an Applied Biosystems (AB) 2720 thermal cycler. Post-amplification products were analyzed on the ViiA™ 7 Real-Time PCR System. Genotype calls were determined manually by comparison to six No Template Controls. Genotyping of 10% of samples from each run were replicated for quality control. DNA was unavailable for two participants, leaving a sample of 38 participants for analyses involving OPRM1. Genotype frequencies did not diverge from expected distributions under Hardy-Weinberg equilibrium (AA = 28, GA = 9, GG = 1, (χ2[1]= 0.07, p = 0.79). The GG participant was grouped with GA participants to compare 118G carriers to 118A homozygotes. Genotype groups did not differ significantly by sex, race, or substance use (Table 1).
Analytic plan
Preliminary analyses evaluated genotype differences in demographic and substance use variables. Genotype and sex differences in self-administration outcomes were evaluated using analysis of variance and chi square difference tests. Descriptive summaries of aBAC profiles were generated on a per-participant basis and by OPRM1 group using model-projected aBAC values. Associations of ASQ variables and subjective alcohol responses with CASE outcomes were evaluated with bivariate correlations and chi square tests, respectively. Associations of OPRM1 with subjective responses were examined based on bivariate associations with post-priming and peak subjective scores, as well as with repeated-measures ANOVA. Because these two methods did not lead to substantive differences in the conclusions, bivariate correlations are reported for simplicity.
Results
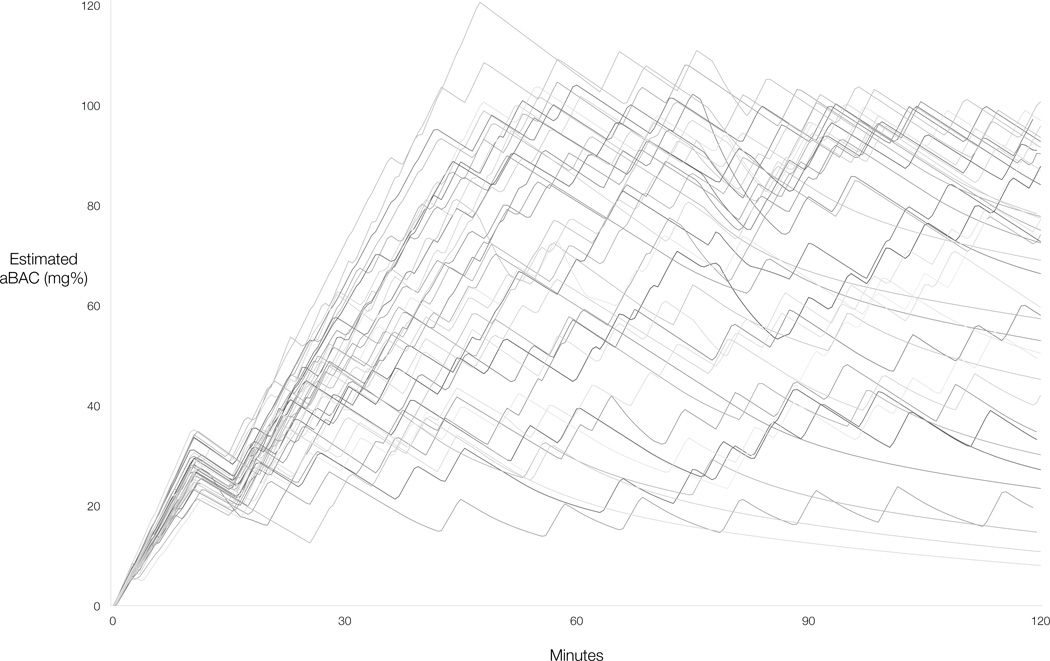
All CASE sessions were completed safely and all participants self-administered after the priming phase. There was considerable variability in self-administration, as evidenced by the aggregate model-projected aBAC profiles (Figure 1). The mean estimated quantity of ethanol administered was 0.57 g/kg (SD=.20, range = .21 – .96). BrAC exceeded 100mg% in some instances; most deviations fell within the expected error range of the breathalyzer unit (5mg%), although 5 participants recorded a reading above 105mg%. These deviations may be explained by the margin of error inherent to both breathalyzer-estimated and model-projected aBAC assessments at any given time. Of note, the descending slope of −1mg% / min generally allows quick recovery to the intended range. One participant registered an unusually high reading (121mg%). Because immediate follow-up readings suggested an error in the initial reading, this data point was removed and replaced with the participant’s next highest reading for the peak BrAC analysis.
Figure 1.
Composite self-administration profiles (N = 40) based on model-projected arterial blood alcohol concentration (aBAC).
Primary self-administration outcomes
Overall, participants achieved a mean peak BrAC of 81.18 mg% (SD=24.96). Sex differences were not significant (males: M=87.52 mg%, SD=22.62, females: M=74.16mg%, SD=26.12, F [1,38] = 3.00, p = .09). Estimated AUC values (reflecting cumulative alcohol exposure during the ad libitum phase in mg% * minutes), averaged 6307.37 (SD=2228.19) and did not differ significantly by sex (males: M=6864.76, SD=2058.69; females: M=5691.31, SD=2298.90, F [1,38] = 2.90, p = .10). Over half of participants (57.5%) surpassed the 80mg% threshold defining a heavy episode, including 66.7% of males and 47.4% of females, χ2[1]= 1.52, p = .22).
Associations of OPRM1 with self-administration outcomes
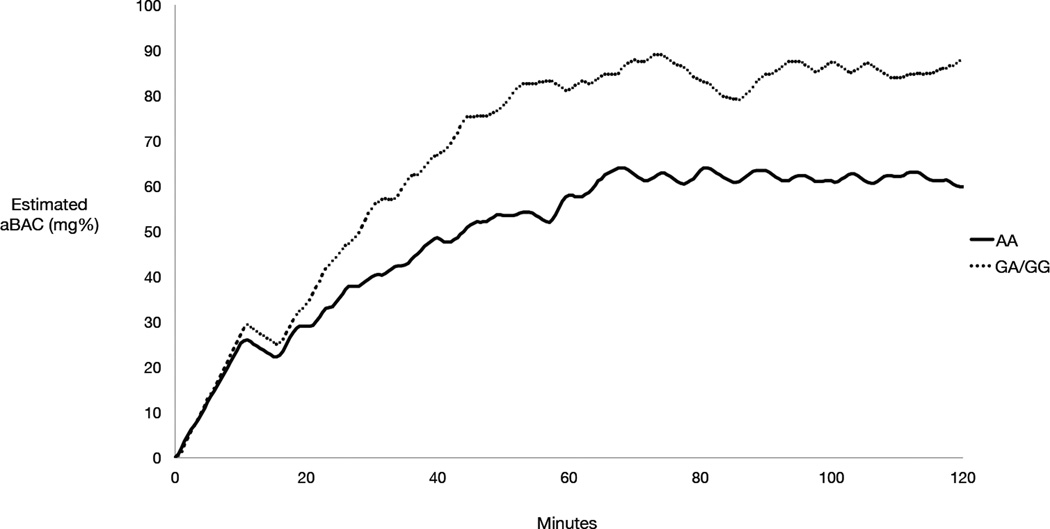
The mean time course of model-projected aBAC by genotype is shown in Figure 2. There was a significant association of OPRM1 with peak BrAC, with GA/GG participants achieving higher BrAC (M=94.90 mg%, SD=16.56) than AA participants (M=74.46 mg%, SD=25.36) (F [1,36] = 5.59, p = .02). Consistent with this finding, a supplemental analysis showed significantly more post-priming drink requests by GA/GG participants (M=16.90, SD=4.27) versus AA participants (M=13.14, SD=4.21, F [1,36] = 6.57, p = .02). Mean AUC estimates by genotype were 7212.02 (SD=1967.41) for GA/GG participants and 5827.82 (SD=2228.20) for AA participants (F [1,36] = 3.00, p = .09). Most GA/GG participants (80%) surpassed the 80mg% threshold, compared with 46% of AA participants (χ2[1] = 3.36, p = .07).
Figure 2.
Mean arterial blood alcohol concentration (aBAC) time course by OPRM1 genotype (AA: n = 28, GA/GG: n = 10).
Although genotype frequencies did not differ significantly by sex, supplementary analyses examined whether differences in peak BrAC could be attributed to the somewhat higher proportion of females in the AA group. Descriptive results showed similar genotype differences in BrAC in both sex groups, with the magnitude of genotype differences being somewhat greater for females (GA/GG: M=91.67mg%, SD=12.74, AA: M=68.20mg%, SD=25.57) than males (GA/GG: M=96.29mg%, SD=18.71, AA: M=81.69mg%, SD=24.06). Although limited by a small sample size, this pattern indicates that significant OPRM1 effects could not be attributed to a relatively higher proportion of females in the AA group. For further confirmation, a two-step linear regression model examined sex and OPRM1 as predictors of peak BrAC, estimating incremental variance in BrAC as a function of genotype. Sex accounted for an estimated 6% of variance in peak BrAC at Step 1 (β = −.30, t = −1.88, adjusted r2 = .06, stepwise F (1,36) = 3.52, p = .07). With the addition of OPRM1 (Step 2) the model accounted for 14% of the variance in peak BrAC (adjusted r2 = .14, stepwise F (2,35) = 4.00, p = .03), with genotype accounting for significant incremental variance in BrAC (r2Δ = .10, F change (1,35) = 4.16, p < .05). With sex and genotype estimated simultaneously, the parameter estimate for sex was not significant (β = −.23, t = −1.49, p = .15) but remained significant for OPRM1 (β = .32, t = 2.04, p < .05). The same findings were obtained in a model examining number of alcohol requests. Finally, a parallel set of regression models confirmed that the OPRM1 effect held while controlling for racial background (data not shown).
Given high peak BrAC values and a relatively smaller standard deviation in peak BrAC for GA/GG relative to AA participants, supplemental analyses examined whether the safety ceiling limited self-administration to a greater extent in GA/GG participants. GA/GG participants triggered the safety ceiling significantly more often during the session compared to AA participants (p = .02), indicating more frequent attempts to further increase aBAC when already approaching 100mg%. This result also implied that GA/GG participants spent a greater proportion of the session with access to alcohol blocked. This possibility was confirmed by analyzing the combined duration of “bar” closures during CASE sessions (GA/GG: M=28.90 [SD=26.50] minutes with access blocked, AA: M=10.85 [SD=19.21] minutes, F [1,36] = 5.31, p < .05). In a model including sex as a covariate, this effect fell short of significance (p = .054).
Associations of self-reported alcohol sensitivity and subjective responses with self-administration
Participants classified as low sensitivity on the ASQ stimulation scale (i.e., reporting more drinks to achieve ascending limb effects) achieved significantly higher peak BrAC (M=90.33, SD=22.63) than those classified as high sensitivity (M=73.68, SD=24.74), F (1,38) = 4.84, p = .03). Significant differences were not observed for peak BrAC or drink requests as a function of ASQ sedation score. AUC did not differ a function of ASQ stimulation or sedation scores (ps > .05). However, those classified as low sensitivity on ASQ stimulation were more likely to exceed 80mg% (77.8% of participants) compared to high sensitivity participants (40.9%), (χ2[1]= 5.51, p = .02). This finding was not observed for the ASQ sedation scale, with heavy episodes being as common in of low sensitivity (56.5%) and high sensitivity (58.8%) participants (χ2[1]= 0.02, ns).
Regarding subjective effects during self-administration, paired-samples t-tests confirmed significantly higher scores on all subjective rating scales (AUQ, BAES stimulation/sedation, DEQ liking/wanting) immediately after priming as compared to baseline (all ps < .01). OPRM1 groups did not differ significantly on any subjective effect subscales (Table 2). This pattern of findings did not change when evaluating partial correlations to control for group differences in peak BrAC. However, results did support significant associations of subjective effects with CASE outcomes (Table 3), the most consistent observation being greater self-administration as a function of lower BAES sedation and higher scores on DEQ liking/wanting scales.
Table 2.
Subjective effects by OPRM1 genotype
| Post-priming score | OPRM1 AA | OPRM1 GA/GG | t | p |
|---|---|---|---|---|
| BAES Stimulation | 48.0 (15.4) | 53.6 (16.4) | −.97 | .34 |
| BAES Sedation | 29.5 (15.7) | 22.3 (15.7) | 1.24 | .22 |
| AUQ | 32.9 (11.5) | 39.3 (18.6) | −1.27 | .21 |
| DEQ Liking | 56.0 (12.3) | 59.3 (18.9) | −.62 | .54 |
| DEQ Wanting | 49.0 (22.5) | 59.9 (21.0) | −1.55 | .13 |
| Maximum score | OPRM1 AA | OPRM1 GA/GG | ||
| BAES Stimulation | 61.0 (12.2) | 64.7 (17.4) | −.73 | .47 |
| BAES Sedation | 48.4 (14.2) | 46.6 (16.2) | .34 | .73 |
| AUQ | 45.4 (11.1) | 47.7 (21.1) | −.42 | .68 |
| DEQ Liking | 78.3 (15.7) | 79.4 (17.7) | −.19 | .85 |
| DEQ Wanting | 63.1 (19.6) | 70.0 (17.7) | −.98 | .34 |
Notes. Post-priming scores indicate subjective effects assessed immediately following priming (target blood alcohol concentration = 30mg%). Maximum score reflects the highest value obtained at any of five assessment points after the onset of alcohol administration. n = 28 for OPRM1 AA, n = 10 for OPRM1 GA/GG. AUQ = Alcohol Urge Questionnaire; BAES = Biphasic Alcohol Effects Questionnaire; DEQ = Drug Effects Questionnaire.
Table 3.
Associations of subjective effects with self-administration outcomes
| Post-priming score | M (SD) | Peak BrAC | AUC | BrAC > 80mg% |
| BAES Stimulation | 50.0 (15.4) | .01 | .06 | .03 |
| BAES Sedation | 26.7 (16.0) | −.44** | −.50** | −.33* |
| AUQ | 35.0 (13.6) | .09 | .17 | .08 |
| DEQ Liking | 64.1 (19.1) | .35* | .38* | .33* |
| DEQ Wanting | 50.0 (21.7) | .16 | .22 | .13 |
| Maximum score | M (SD) | Peak BrAC | AUC | BrAC > 80mg% |
| BAES Stimulation | 62.6 (13.6) | .27❖ | .29❖ | .13 |
| BAES Sedation | 46.4 (15.8) | −.45** | −.45** | −.40* |
| AUQ | 45.9 (14.5) | .22 | .30❖ | .15 |
| DEQ Liking | 76.6 (17.6) | .34* | .32* | .28❖ |
| DEQ Wanting | 59.6 (24.5) | .31* | .33* | .27❖ |
Notes. Post-priming scores indicate subjective effects assessed immediately following priming (target blood alcohol concentration = 30mg%). Maximum score reflects the highest value obtained at any of five assessment points after the onset of alcohol administration. Values reflect Pearson’s r with the exception of point biserial correlations for the column BrAC > 80mg%. BAES = Biphasic Alcohol Effects Scale. AUQ = Alcohol Urge Questionnaire. DEQ = Drug Effects Questionnaire. BrAC = breath alcohol concentration. n = 40.
p < .005
p ≤ .05.
p < .10
Discussion
The current study provides further evidence for the feasibility of the CASE paradigm, extending prior research (Zimmermann et al., 2008, 2009) to a sample of young heavy drinkers. Additionally, this study extends prior research on OPRM1 by providing initial evidence for genotype differences in alcohol self-administration using a paradigm characterized by high pharmacokinetic control. Heavy-drinking young adults with the 118G variant achieved higher peak brain alcohol exposure relative to 118A homozygotes, reflecting a greater number of alcohol requests. A similar pattern was observed for secondary self-administration outcomes, although group differences failed to reach significance. A secondary finding was that GA/GG participants triggered the 100mg% safety ceiling more frequently than AA participants, suggesting that the ceiling limited peak BrAC to a greater extent in the GA/GG group.
One advantage of the current paradigm is the potential for improved consilience with animal models, which often favor operant self-administration procedures (Kalant, 2010; Leeman et al., 2010; Zimmermann et al., 2013). From this perspective it is notable that the current findings converge with nonhuman primate studies examining the OPRM1 C77G polymorphism, considered functionally analogous to the human A118G variant. Specifically, rhesus monkeys with the 77G variant (proposed as a functional equivalent to 118G) displayed higher alcohol self-administration under simple operant and free access paradigms (Barr et al., 2007; Vallender et al., 2010). Jointly, these findings suggest a preference for higher self-imposed brain alcohol exposure among 118G/77G carriers under controlled conditions. The literature on OPRM1 includes other evidence for cross-species convergence, including associations with alcohol-induced striatal dopamine release (Heilig et al., 2011; Ramchandani et al., 2011) and differential reduction of alcohol consumption during treatment with the μ-opioid receptor antagonist naltrexone (Oslin et al., 2003; Vallender et al., 2010). These instances of cross-species replication are particularly important for inferring a functional role of OPRM1 for certain neurobiological and pharmacogenetic outcomes (Barr et al., 2010; Heilig et al., 2011).
Contrary to the hypotheses and some previous findings, OPRM1 did not relate to subjective effects during alcohol self-administration. Initial evidence for greater hedonic responses among 118G carriers came from studies using experimenter-determined infusion profiles designed to capture three BrAC levels on the ascending limb (Ray and Hutchison, 2004, 2007), whereas the current study included only one subjective assessment at which BrAC was standardized. At this point the target BrAC was relatively low (30mg%), and subsequent assessments occurred in the context of varying aBAC levels. Other laboratory studies examining OPRM1 and subjective responses have also found results that diverged from early reports (Anton et al., 2012; McGeary et al., 2006), perhaps reflecting differences in laboratory paradigms. Consistent with the study hypotheses we observed significant associations of subjective responses and CASE outcomes, adding to previous evidence that subjective responses relate to intravenous self-administration (Zimmermann et al., 2009). However, the nature of these associations differed based on measurement scales: when considering a baseline measure of alcohol sensitivity, participants who reported needing more drinks to achieve stimulant effects showed significantly higher self-administration. During self-administration, however, self-reported sedation appeared to be a more robust predictor of self-administration. These findings are not intuitive but may reflect complexities in studying alcohol sensitivity constructs, in part due to variability in measurement approaches (King et al., 2011b; Morean and Corbin, 2010).
Participants in the present study achieved somewhat higher peak alcohol exposure compared to prior CASE studies, in which BrAC ranged from 70–80mg% in the context of 100mg% (Zimmermann et al., 2008) and 120mg% (Zimmermann et al., 2009) safety ceilings. These differences likely reflect recruitment of heavier drinkers in the current study; for instance, mean AUDIT scores in this sample were considerably higher than in a prior study of young adults (Zimmermann et al., 2009). However, it is notable that initial CASE studies are consistent in showing considerably higher BrAC levels under free-access intravenous self-administration compared with oral self-administration. A review of oral self-administration studies reported average consumption in the range of .45 – .51g/kg, corresponding with peak BrAC levels of 40–50mg%, notwithstanding the inclusion of alcohol-dependent participants in some studies (Zimmermann et al., 2013). Higher alcohol exposure in intravenous studies could reflect differential demand characteristics, greater uncertainty of the quantity of alcohol consumed, differences in operant paradigms across studies, or undetermined factors (Zimmermann et al., 2008, 2013). Importantly, differences in behavior across paradigms could have implications for detecting genetic influences or the effects of experimental manipulations. For example, reduced variability and lower rates of self-administration in oral paradigms could limit sensitivity for detecting pharmacological manipulations, with potential implications for medication screening.
From a developmental perspective, the present results also support the utility of self-administration paradigms for studying phenomena that anticipate the onset of alcohol use disorders (Zimmermann et al., 2013). For example, the current results complement findings that adolescents with the 118G variant reported stronger enhancement motives for alcohol use (Miranda et al., 2010). Studying relatively younger cohorts has particular advantages in the context of alcohol administration. A focus on younger participants reduces (but does not eliminate) the influence of drinking histories on laboratory outcomes, improving the ability to establish temporal precedence of laboratory phenotypes in relation to drinking trajectories (e.g., King et al., 2011a). While ethical considerations preclude the types of studies that might eliminate confounds related to drinking history, including young cohorts is nonetheless important for studying developmental trajectories and evaluating laboratory responses to alcohol as candidate endophenotypes.
The current findings should be considered in the context of limitations and strengths of this study. The associations studied here are correlational and cannot be used to infer a causal influence of OPRM1. Although the sample size is equivalent to other alcohol administration studies of OPRM1 (Ray et al., 2012b; Ray and Hutchison, 2004, 2007; Setiawan et al., 2011), the relatively small sample is a limitation, particularly in the context of retrospective genotyping. The lack of a placebo control condition or an alternative reinforcer may also be considered a limitation. The current design cannot rule out the possibility that more frequent alcohol requests among GA/GG participants reflected motivational processes unrelated to desired level of intoxication; for example, one study found associations of OPRM1 with approach bias for alcohol stimuli as well as other appetitive stimuli (Wiers et al., 2009). Strengths of this study include the high degree of experimental control afforded by CASE and the focus on a young sample.
One obvious consideration is that CASE bears no resemblance to a naturalistic drinking episode. In addition to using intravenous delivery, no efforts are made to incorporate naturalistic cues or contingencies for self-administration—instead, efforts are made to eliminate these contingencies. This approach serves to isolate pharmacological effects to the extent possible, presumably bringing pharmacodynamic effects into clearer resolution. In this context, a relatively lower degree of external validity is intentional and acceptable. Intravenous paradigms are particularly well suited for studies concerned with manipulating pharmacological parameters (e.g., dose or exposure level, rate of aBAC change, blood alcohol limb). Alternatively, studies placing lower priority on these questions might opt for oral procedures, especially given the added cost and resources required for intravenous procedures.
Several directions can be proposed for future research involving variations of the CASE paradigm (e.g., Zimmermann et al., 2013). For example, self-administration paradigms can be important for evaluating mechanisms of pharmacotherapy response (O'Malley et al., 2002) and represent a valuable option for medication screening studies. Progressive ratio paradigms can be incorporated with CASE, perhaps facilitating pharmacotherapy and pharmacogenetic studies (Plawecki et al., 2013). Recent work also suggests the importance of self-administration paradigms for laboratory models of relapse (McKee, 2009). Because alcohol self-administration paradigms reflect a relatively small proportion of human laboratory studies overall (Zimmermann et al., 2013), greater use and refinement of these paradigms could inform novel approaches for studying addiction liability and treatment response.
Acknowledgements
The authors express thanks to Sean O’Connor and Victor Vitvitskiy at the Indiana Alcohol Research Center (NIH P60 AA007611) for software support. The authors also thank Ariel Graff, James Kennedy, Natalie Freeman, Peter Selby, Andriy Samokhvalov, Mike Markovich, Matthew McPhee, Vanessa Garofalo, Emma Ware, and David Greiss for their valuable assistance.
Funding
This study was supported by a grant from ABMRF/The Foundation for Alcohol Research to CH. The authors also acknowledge support from Canadian Institutes of Health Research (CIHR) grants 260418, 288905, 307742 (CH), NIH R21 AA020304 (EC, CH), NIAAA Division of Intramural Clinical and Biological Research (VAR), and NIH P60 AA007611.
Footnotes
Disclosure
The authors declare no conflicts of interest.
Author Contributions
CH carried out the study, drafted the manuscript, and acknowledges access to the data. EC and VAR contributed to study conception and methodology and provided critical review of the manuscript and results.
References
- Anton RF, Voronon KK, Randall PK, Myrick H, Tiffany A. Naltrexone modification of drinking effects in a subacute treatment and bar-lab paradigm: influence of OPRM1 and dopmapine transporter (SLC6A3) genes. Alcohol Clin Exp Res. 2012;36:2000–2007. doi: 10.1111/j.1530-0277.2012.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias A, Feinn R, Kranzler HR. Association of an Asn40Asp (A118G) polymorphism in the μ-opioid receptor gene with substance dependence: A meta-analysis. Drug Alcohol Depend. 2006;83(3):262–268. doi: 10.1016/j.drugalcdep.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Barr CS, Chen SA, Schwandt ML, Lindell SG, Sun H, Suomi SJ, et al. Suppression of alcohol preference by naltrexone in the rhesus macaque: A critical role of genetic variation at the micro-opioid receptor gene locus. Biol Psychiatry. 2010;67(1):78–80. doi: 10.1016/j.biopsych.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Schwandt M, Lindell SG, Chen SA, Goldman D, Suomi SJ, et al. Association of a functional polymorphism in the μ-opioid receptor gene with alcohol response and consumption in male rhesus macaques. Arch Gen Psychiatry. 2007;64(3):369–376. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Henry EA, Lust SA. Effects of alcohol sensitivity on P3 event-related potential reactivity to alcohol cues. Psychol Addict Behav. 2007;21:555–563. doi: 10.1037/0893-164X.21.4.555. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Pearson M, Sher KJ, Wieman LC, Fabiani M, Gratton G. Effects of alcohol consumption and alcohol susceptibility on cognition: A psychophysiological examination. Biol Psychol. 2003;64:167–190. doi: 10.1016/s0301-0511(03)00108-x. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19(3):600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Bell RL, Ehlers CL. Human and laboratory rodent low response to alcohol: is better consilience possible? Addict Biol. 2010;15(2):125–144. doi: 10.1111/j.1369-1600.2009.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Pierri J, Johanson CE. Assessing individual differences in ethanol preference using a cumulative dosing procedure. Psychopharmacology (Berl) 1989;98(1):113–119. doi: 10.1007/BF00442016. [DOI] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Pierri J, Johanson CE. Individual differences in behavioral and subjective responses to alcohol. Alcohol Clin Exp Res. 1987;11(1):52–59. doi: 10.1111/j.1530-0277.1987.tb01263.x. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Schuckit MA, Johnson BA, Goldman D. Genetics of alcoholism using intermediate phenotypes. Alcohol Clin Exp Res. 2003;27(2):169–176. doi: 10.1097/01.ALC.0000052702.77807.8C. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Ray L, Smolen A, Claus ED, Audette A, Hutchison KE. Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcohol Clin Exp Res. 2008;32(7):1113–1123. doi: 10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12(11):670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines LM, Ray L, Hutchison K, Tabakoff B. Alcoholism: The dissection for endophenotypes. Dialogues Clin Neurosci. 2005;7(2):153–163. doi: 10.31887/DCNS.2005.7.2/lhines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalant H. What neurobiology cannot tell us about addiction. Addiction. 2010;105(5):780–789. doi: 10.1111/j.1360-0443.2009.02739.x. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, et al. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage. 2010;50(1):267–276. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011a;68(4):389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Roche DJ, Rueger SY. Subjective responses to alcohol: A paradigm shift may be brewing. Alcohol Clin Exp Res. 2011b;35(10):1726–1728. doi: 10.1111/j.1530-0277.2011.01629.x. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Covault J, Tennen H. Variation in OPRM1 moderates the effect of desire to drink on subsequent drinking and its attenuation by naltrexone treatment. Addict Biol. 2013;18(1):193–201. doi: 10.1111/j.1369-1600.2012.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, O'Malley SS. Ethanol consumption: How should we measure it? Achieving consilience between human and animal phenotypes. Addict Biol. 2010;15(2):109–124. doi: 10.1111/j.1369-1600.2009.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Hewitt BG, Grant BF. Alcohol use disorders and mood disorders: A National Institute on Alcohol Abuse and Alcoholism perspective. Biol Psychiatry. 2004;56:718–720. doi: 10.1016/j.biopsych.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Mague SD, Blendy JA. OPRM1 SNP (A118G): Involvement in disease development, treatment response, and animal models. Drug Alcohol Depend. 2010;108(3):172–182. doi: 10.1016/j.drugalcdep.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Demming B, Reid JB. Loss of control drinking in alcoholics: An experimental analogue. J Abnorm Psychol. 1973;81(3):233–241. doi: 10.1037/h0034532. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res. 1993;17(1):140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McGeary JE, Monti PM, Rohsenow DJ, Tidey J, Swift R, Miranda R., Jr Genetic moderators of naltrexone's effects on alcohol cue reactivity. Alcohol Clin Exp Res. 2006;30:1288–1296. doi: 10.1111/j.1530-0277.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addict Biol. 2009;14(1):99–107. doi: 10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Operrant analysis of drinking patterns of chronic alcoholics. Nature. 1965;206:43–46. doi: 10.1038/206043a0. [DOI] [PubMed] [Google Scholar]
- Miranda R, Ray L, Justus A, Meyerson LA, Knopik VS, McGeary J, Monti PM. Initial evidence of an association between OPRM1 and adolescent alcohol misuse. Alcohol Clin Exp Res. 2010;34:112–122. doi: 10.1111/j.1530-0277.2009.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective response to alcohol: A critical review of the literature. Alcohol Clin Exp Res. 2010;34:385–395. doi: 10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O'Malley SS. The drug effects questionnaire: Psychometric support across three drug types. Psychopharmacology (Berl) 2013;227:177–192. doi: 10.1007/s00213-012-2954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) NIAAA Council Approves Definition of Binge Drinking. [Accessed 23 December, 2013];NIAAA Newsletter: Winter. 2004 Available at: http://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf.
- O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O'Brien CP. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Plawecki MH, Han JJ, Doerschuk PC, Ramchandani VA, O'Connor SJ. Physiologically based pharmacokinetic (PBPK) models for ethanol. IEEE Trans Biomed Eng. 2008;55:2691–2700. doi: 10.1109/TBME.2008.919132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki MH, Wetherill L, Vitvitskiy V, Kosobud A, Zimmermann US, Edenberg HJ, O'Connor S. Voluntary intravenous self-administration of alcohol detects an interaction between GABAergic manipulation and GABRG1 polymorphism genotype: A pilot study. Alcohol Clin Exp Res. 2013;37(Suppl 1):E152–E160. doi: 10.1111/j.1530-0277.2012.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebani JG, Ray LA, Morean ME, Corbin WR, MacKillop J, Amlung M, King AC. Human laboratory paradigms in alcohol research. Alcohol Clin Exp Res. 2012;36(6):972–983. doi: 10.1111/j.1530-0277.2011.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O'Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res. 1999;23:617–623. [PubMed] [Google Scholar]
- Ramchandani VA, O'Connor S. Studying alcohol elimination using the alcohol clamp method. Alcohol Res Health. 2006;29:286–290. [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Plawecki M, Li TK, O'Connor S. Intravenous ethanol infusions can mimic the time course of breath alcohol concentrations following oral alcohol administration in healthy volunteers. Alcohol Clin Exp Res. 2009;33:938–944. doi: 10.1111/j.1530-0277.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, Heilig M. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2011;16:809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Barr CS, Blendy JA, Oslin D, Goldman D, Anton RF. The role of the Asn40Asp polymorphism of the mu opioid receptor gene (OPRM1) on alcoholism etiology and treatment: a critical review. Alcohol Clin Exp Res. 2012a;36:385–394. doi: 10.1111/j.1530-0277.2011.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Chin PF, Miotto K. Pharmacogenetics of naltrexone in Asian Americans: A randomized placebo-controlled laboratory study. Neuropsychopharmacology. 2012b;37:445–455. doi: 10.1038/npp.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: A double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64:1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE, Tartter M. Application of human laboratory models to pharmacotherapy development for alcohol dependence. Curr Pharm Des. 2010;16:2149–2158. doi: 10.2174/138161210791516422. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Cox SM, Gianoulakis C, Palmour RM, Benkelfat C, Leyton M. The effect of naltrexone on alcohol's stimulant properties and self-administration behavior in social drinkers: Influence of gender and genotype. Alcohol Clin Exp Res. 2011;35:1134–1141. doi: 10.1111/j.1530-0277.2011.01446.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Vallender EJ, Ruedi-Bettschen D, Miller GM, Platt DM. A pharmacogenetic model of naltrexone-induced attenuation of alcohol consumption in rhesus monkeys. Drug Alcohol Depend. 2010;109:252–256. doi: 10.1016/j.drugalcdep.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wildenberg E, Wiers RW, Dessers J, Janssen RG, Lambrichs EH, Smeets HJ, van Breukelen GJ. A functional polymorphism of the mu-opioid receptor gene (OPRM1) influences cue-induced craving for alcohol in male heavy drinkers. Alcohol Clin Exp Res. 2007;31:1–10. doi: 10.1111/j.1530-0277.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Dictus M, Van den Wildenberg E. Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes Brain Behav. 2009;8:101–106. doi: 10.1111/j.1601-183X.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Laucht M, Vitvitskiy V, Plawecki MH, Mann KF, O'Connor S. Offspring of parents with an alcohol use disorder prefer higher levels of brain alcohol exposure in experiments involving computer-assisted self-infusion of ethanol (CASE) Psychopharmacology (Berl) 2009;202:689–697. doi: 10.1007/s00213-008-1349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Vitvitskyi V, Plawecki MH, Mann KF, O'Connor S. Development and pilot validation of computer-assisted self-infusion of ethanol (CASE): A new method to study alcohol self-administration in humans. Alcohol Clin Exp Res. 2008;32:1321–1328. doi: 10.1111/j.1530-0277.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, O'Connor S, Ramchandani VA. Modeling alcohol self-administration in the human laboratory. Curr Top Behav Neurosci. 2013;13:315–353. doi: 10.1007/7854_2011_149. [DOI] [PubMed] [Google Scholar]