Abstract
Neuropsychological measures have been proposed as both a way to tap mechanisms and as endophenotypes for child ADHD. However, substantial evidence supporting heterogeneity in neuropsychological performance among youth with ADHD as well as apparent effect differences by sex, age, and comorbidity have slowed progress. To address this, it is important to understand sibling effects in relation to these moderators. 461 youth ages 6–17 years (54.8% male, including 251 youth with ADHD, 107 of their unaffected biological siblings, and 103 non-ADHD controls) completed diagnostic interviews and a theoretically informed battery of neuropsychological functioning. A structural equation model was used to consolidate neuropsychological domains. Group differences between unaffected siblings of youth with ADHD and controls across each domain were first examined as the primary endophenotype test for ADHD. Moderation of these effects was evaluated via investigation of interactions between diagnostic group and both proband and individual level characteristics, including sex, age, and comorbidity status. Unaffected siblings performed worse than control youth in the domains of inhibition, response time variability, and temporal information processing. Individual age moderated these effects, such that differences between controls and unaffected siblings were pronounced among younger children (ages 6–10 years) but absent among older youth (ages 11–17 years). Evidence for moderation of effects by proband sex and comorbidity status produced more variable and smaller effects. Results support the utility of inhibition, response time variability, and temporal processing as useful endophenotypes for ADHD in future genetic associations studies of the disorder, but suggest this value will vary by age among unaffected family members.
Keywords: attention-deficit hyperactivity disorder, neuropsychology, genetics-behavioral, endophenotype
INTRODUCTION
Attention-deficit hyperactivity disorder (ADHD) is a common, costly, and impairing psychiatric disorder characterized by developmentally-inappropriate symptoms of inattention-disorganization and hyperactivity-impulsivity. Despite much progress, knowledge of etiology as well as ability to make individualized clinical predictions remain unsatisfactory. It is known that genetic influences substantially contribute to the disorder throughout the lifespan (Chang, Lichtenstein, Asherson, & Larsson, 2013) and, crucially, that these vary based on the inattention-disorganization versus hyperactive-impulsive symptom domains (Nikolas & Burt, 2010), mirroring the clinical validity distinction of these two symptom domains (Willcutt et al., 2012).
To better address etiology and clinical prediction, consideration of intermediate phenotypes or endophenotypes has been often suggested in psychiatry as an alternative to diagnoses. We use the terms endophenotype and intermediate phenotype interchangeably herein, while recognizing that the former includes an expectation that the measure will have greater heritability than the disorder it underlies. Neuropsychological measures, particularly executive functioning, have been offered as a key intermediate phenotype domain for capturing underlying liability for ADHD (Doyle et al., 2005), enhancing etiological signal, improving clinical prediction (Arns, Conners, & Kraemer, 2013), or clarifying pathophysiology.
However, youth with ADHD demonstrate substantial heterogeneity in executive function performance (Nigg, 2005) and meta-analyses have indicated only moderate effect sizes (Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005), suggesting these measures capture one of multiple mechanistic pathways (Nigg, 2005) or that ADHD itself includes subgroups with different profiles (Fair, Batthula, Nikolas, & Nigg, 2012; Nikolas & Nigg, 2013). Past work has indicated that the association between measures of neuropsychological performance and ADHD may be further moderated by a variety of factors, including age, sex, and psychiatric comorbidity (Bidwell, Willcutt, Defries, & Pennington, 2007; Nigg, Blaskey, Stawicki, & Sachek, 2004). However, the potential moderation of effects and the consequences of such moderation on the utility of these measures as endophenotypes remain largely unexplored.
One of the several important features desired in endophenotypes is that they are present in unaffected family members of individuals with a disorder, so that they convey liability risk (Gottesman & Gould, 2003). Executive function deficits, particularly measures of inhibitory control, are observed among otherwise unaffected family members of youth with ADHD (Crosbie, Pérusse, Barr, & Schachar, 2008; Gau & Chang, 2010; Goos, Crosbie, Payne, & Schachar, 2009; Nigg et al., 2004) and common genes appear to influence the covariation between measures of inhibition and ADHD symptom dimensions (Crosbie et al., 2013). Past work has also identified working memory deficits (Gau & Chang, 2010), as well as deficits in motor timing (Rommelse et al., 2008) among unaffected siblings of youth with ADHD. Additionally, while it is now widely believed that domains of neuropsychological functioning other than executive functioning are relevant to ADHD (Sonuga-Barke, Bitsakou, & Thompson, 2010), most other domains have not been examined in family studies, particularly in the same sample or study (as an exception, see Bidwell et al., 2007).
Most crucially, however, moderation of these effects has scarcely been considered, despite evidence of potential moderation of associations between neuropsychological measures and ADHD (Bidwell et al., 2007; Nigg et al., 2004; Rucklidge & Tannock, 2002). Specifically, the moderating effects of age, sex, and comorbid problems (both within the probands as well as among individual family members) are particularly common suggestions but rarely studied (or are often covaried in analyses rather than explored as moderators). There is ample neurobiological reason to suspect age, particularly individual age, as a moderator of effects. Non-linear maturation during adolescence of prefrontal circuitry as well as subcortical and cerebellar regions implicated in ADHD may modulate the extent to which different cognitive domains are impaired among unaffected siblings or correlate with ADHD. Indeed, recent work has suggested that impairments documented among unaffected siblings of ADHD probands during childhood may not persist into adolescence (Thissen et al., 2014). Additionally, sex differences in the association between various neuropsychological measures and ADHD have been reported among probands (Bidwell et al., 2007; Martel, 2013; O’Brien, Dowell, Mostofsky, Denckla, & Mahone, 2010) but few studies have formally examined moderation of endophenotype effects by either proband sex or by individual sex. Such effects may be possible, given past work indicating potential sex differences among youth with ADHD in regard to family history of the disorder (Faraone et al., 2000).
Similarly, few family-sibling studies of endophenotypes of ADHD have considered the impact of comorbid problems, despite evidence that neuropsychological function may differ based on presence or absence of comorbid behavioral problems, as well as findings suggesting that reading ability may attenuate some performance differences among unaffected siblings (Bidwell et al., 2007; Nigg et al. 2004). Given work implicating neuropsychological impairments among youth ADHD and comorbid reading disorder (Wang & Gathercole, 2013), anxiety (Bloemsma et al., 2013), and conduct problems (Dolan & Lenox, 2013) as well as some evidence that comorbidity may impact the severity of neuropsychological impairment among children with ADHD (Qian, Shuai, Cao, Chan, & Wang, 2010), evaluation of such moderation, both by proband comorbidity status as well as by individual-level comorbidity status is clearly needed.
A final key concern is that most research in this area has focused on single measures of these constructs. This is a crucial limitation as many neuropsychological measures are plagued by low or poorly documented reliability (Frazier, Youngstrom, Chelune, Naugle, & Lineweaver, 2004), which can artificially constrain effect sizes. Indeed, correlations among individual measures have rarely been taken into account in endophenotype studies. Even when prior studies have considered multiple domains in samples of unaffacted siblings of children with ADHD, they generally have not considered both shared and distinct EF components. Evaluation of multiple domains of neuropsychological functioning using a structural equation modeling approach may therefore be useful for identifying which domains, rather than individual measures, may be the best candidates for further use in genetic analysis while also improving on construct reliability and hence validity.
The current study therefore evaluated multiple domains of neuropsychological functioning as potential endophenotypes within a well-characterized sample of youth with ADHD, their unaffected siblings, and non-ADHD controls. Based on prior work, we predicted that response inhibition would emerge as potential endophenotype for ADHD, such that unaffected siblings would show deficits relative to controls. However, we also tested effects among other executive and non-executive domains (e.g., response variability, arousal, temporal information processing). Lastly, a critical goal of the current study was to formally examine moderation of group differences by sex, age, and comorbid disorders, both at the level of the proband as well as at the level of the individual.
METHODS
Participants
Participants were 461 youth ages 6–17 years (M=10.7 years, SD=2.3 years, 54.8% male), providing the age range and statistical power needed for our aims. This sample included 185 sibling pairs and 91 singletons. Singletons were included to retain generalizability as they were easily included in statistical models, paralleling prior studies in the field. Participants were recruited using mass mailings to parents in the local school districts, public advertisements, and community outreach to local clinics in order to recruit as broad a range of volunteers for the study as possible and also in order to avoid potential biases inherent in a purely clinic-referred sample while also including clinic cases. A multi-stage, multi-informant screening process was used to identify cases and non-cases meeting research criteria. 902 individual children from 762 families completed the initial telephone screen. Of these, 724 individual children from 588 families were invited to complete the stage 2 diagnostic assessment. This included parents and teacher ratings on the DSM-IV ADHD Rating Scale (DuPaul, Power, Anastopoulos, & Reid, 1998), and the Conners’ Rating Scale – Revised Short Form (Conners, 1997). One parent completed the Kiddie Schedule for Affective Disorders and Schizophrenia-E (Puig-Antich & Ryan, 1986) for each child with a trained master’s level clinical interviewer. A best estimate diagnostic procedure was implemented in which all available clinical data were reviewed by a team consisting of a board-certified child psychiatrist and a licensed child clinical psychologist. Symptom counts were computed using a modified or algorithm based on parent and teacher ratings that was constructed to ensure cross-situational presence of symptoms and impairment. To be considered for an ADHD diagnosis, parents and teachers had to both report a minimum of three symptoms in one dimension and T-scores for both parent and teacher ratings on the Conners subscales of Cognitive Problems or Hyperactivity Problems had to be 60 or higher (indicative of one standard deviation above the mean of the measure norms). Using this information, both professionals arrived independently at a clinical decision regarding ADHD, ADHD subtype, and comorbid diagnoses (disruptive behavior disorders, internalizing disorders, and learning disorders) using full DSM-IV-TR criteria. Agreement rates were acceptable for all ADHD subtype diagnoses (kappa >.88) and all anxiety, mood, and disruptive behavior disorders occurring at a 5% or more base rate in the sample (all kappa>.70). Youth were excluded based upon the following criteria: intellectual disability (based on having a full-scale IQ <75), head injury with a loss of consciousness, history of seizures as ascertained by parent report, autism spectrum disorder by parent report, current major depressive episode, lifetime bipolar disorder, lifetime psychosis, or current substance abuse or dependence.
Of the 724 youth completing the diagnostic screen, 498 youth from 295 families completed the neuropsychological testing visit; the others were excluded for not meeting our inclusion criteria. We further excluded 34 youth with subthreshold/situational ADHD (n= five symptoms or failure to meet diagnostic criteria for age of onset and presence of symptoms in multiple contexts) and three control children prescribed stimulant medication, which resulted in the sample of n=461. Youth with all ADHD subtypes were included. All youth who were prescribed stimulant medication underwent a minimum washout period of 24 hours for short-acting preparations and 48 hours for long-acting preparations (washout range 24–152 hours, mean=58 hours) prior to completing the neuropsychological testing. Use of longer acting psychoactive prescription medications (including atomextine and guanfacine) was a rule-out to facilitate the washout, but only two percent of eligible youth were excluded on this basis. 37.4 percent of the ADHD cases were currently taking stimulant medications, consistent with expected treatment rates in a community ascertained, as opposed to clinic-ascertained samples (Jensen et al., 2001). All parents provided informed consent for themselves and their children, and all children and adolescents provided written assent. All procedures were reviewed and approved by the local Institutional Review Board.
The 461 youth were then subdivided into three groups: all youth classified as ADHD were assigned to an ADHD group, regardless of sibling status (n=251). Unaffected siblings of youth with ADHD were assigned to the unaffected sibling group (n=107). All remaining control youth were assigned to the control group (n=103).
Measures
The testing battery was designed to capture a variety of neuropsychological constructs hypothesized to be relevant to ADHD. These included measures of working memory (Martinussen & Tannock, 2006), memory span, response inhibition, (Barkley, 1997), processing speed, response variability, temporal information processing (Toplak & Tannock, 2005), and arousal (or activation) operationalized as signal detection sensitivity (Sergeant, 2005). A list of each construct, task, and measure is provided in Table 1. Internal consistencies for each of the measures were adequate (αs ranging from .83 to .97). Past research has established evidence of construct, convergent, and discriminant validity for several of the measures (Assesmany, McIntosh, Phelps, & Rizza, 2001; Delis, Kaplan, & Kramer, 2001; Logan, 1994; Martinussen & Tannock; Toplak & Tannock, 2005). Additionally, a number of validity checks were included for each measure (e.g., on spatial and digit span tasks, performance on forward trials had to be greater or equal to that of backward trials; on the Stop Task and continuous performance task, youth had to have at least one valid block of trials for their data to be included, see Nikolas & Nigg, 2013 for full description). In general, invalid data were rare (less than 5% on any one task) and presence of invalid data was unrelated to ADHD diagnostic status, age, and sex (all ps > .15).
Table 1.
Neuropsychological tasks and measures, constructs, and factor
| Task & Measure | Construct | First order factor |
|---|---|---|
| Spatial Span Forward Total Correct | Encoding, span | Memory span* |
| Spatial Span Backward Total Correct | Working memory | Working memory* |
| Digit Span Forward Total Correct | Encoding, span | Memory span* |
| Digit Span Backward Total Correct | Working memory | Working memory* |
| DKEFS Color-Word Color/Word Reading Time | Speeded naming | Processing speed |
| DKEFS Color-Word Inhibition Time | Interference control | Inhibition* |
| DKEFS Inhibition/Switching time | Switching speed | Inhibition* |
| DKEFS Trailmaking Number Sequencing Time | Sequencing speed | Processing speed |
| DKFES Trailmaking Number-Letter Sequencing Time | Switching speed | Working memory* |
| Stop Task Stop Signal Reaction Time | Response inhibition | Inhibition* |
| Stop Task Reaction Time Variability | Response time variability | Response variability |
| Continuous Performance Task d-prime | Arousal/activation | Arousal |
| Tapping Task Visual 400-ms detrended SD | Time reproduction | Temporal processing |
| Tapping Task Auditory400-ms detrended SD | Time reproduction | Temporal processing |
| Tapping Task Visual 1000-ms detrended SD | Time reproduction | Temporal processing |
| Tapping Task Auditory 1000-ms detrended SD | Time reproduction | Temporal processing |
Note.
factor significantly loaded on 2nd order cognitive control factor.
DKEFS=Delis-Kaplan Executive Function System, SD=standard deviation.
Data Analytic Strategy and Plan
Data Reduction
All scores were transformed such that higher scores were indicative of worse performance (i.e., slower reaction times, worse accuracy). As we did not wish to use an excessive number of redundant indicators in the analyses, we utilized a model derived from prior confirmatory factor analysis (Nikolas & Nigg, 2013) to construct a set of latent factors that captured variance common among measures that tapped into similar domains of neuropsychological functioning, while removing the error variance associated with each individual measure, so as to maximize our estimate of true effect sizes (see Nikolas & Nigg, 2013 for full description). As reported by us earlier (Nikolas & Nigg, 2013), we conducted a series of confirmatory factor analyses, which included evaluation of models comprised of one through eight factors as well as several second-order factor models. Our final second-order model provided a good fit to the data (χ2=94.88, df=60, CFI=.98, TLI=.98, RMSEA=.034). This model included seven lower-order factors we labeled as response inhibition, working memory, memory span, speed, response variability, arousal, and temporal processing as well as one second-order factor termed cognitive control. This second-order factor was comprised of the inhibition, working memory, and memory span factors (see Table 1 for breakdown of how each measure loaded on each respective factor). Factor scores from this model were retained for the study analyses, such that higher scores indicated worse performance in each domain.
Endophenotype Evaluation Analyses
Multiple linear regression analyses were first used to examine group differences in neuropsychological performance. Two non-orthogonal contrast codes were constructed to examine differences between (1) youth with ADHD and unaffected siblings and (2) unaffected siblings and control youth. The latter contrast code represents the key comparison in evaluating neuropsychological measures as endophenotypes, but inclusion of the first code allows simultaneous examination of within-sibling differences. Overall differences between youth with and without ADHD have been described in detail elsewhere (see Nikolas & Nigg, 2013). We also conducted follow-up analyses based on proband ADHD subtype in order to identify any areas in which unaffected siblings of ADHD-PI youth differed from unaffected siblings of ADHD-C youth.
Next, to evaluate sex as a moderator, we examined interactions between our contrast codes and proband sex (e.g., whether the sibling with ADHD was male or female) as well as by individual sex (i.e., whether the unaffected sibling was male or female). We chose to use evaluate both proband sex and individual sex due to findings indicating potential differential genetic loading by proband sex (Faraone et al., 2000) as well as findings indicating potential sex effects in neuropsychological performance among youth with and without ADHD (Bidwell et al., 2007; Nigg et al., 2004). Similarly, age moderation was examined by examining interactions between proband age (age of the ADHD proband) as well as by individual age (age of the unaffected sibling) and the two contrast codes (with sex interactions omitted), in order to determine if any impairments in neuropsychological functioning were present in all ages of unaffected siblings relative to controls. Lastly, specificity of effects to ADHD was evaluated by examining interactions between proband comorbidity (i.e., whether the identified youth with ADHD also meet criteria for a disruptive behavior disorder, internalizing disorder, or learning disorder) as well as individual comorbidity (i.e., whether the unaffected sibling met criteria for a disruptive behavior, internalizing, or learning disorder) and the contrast codes (with sex and age interactions omitted). Due to our use of non-orthogonal contrast codes, Bonferroni corrections were used to manage Type I error and to adjust p-values for each family of tests (eight measures per family of tests, alpha level set to .05/8=.006). All analyses, including CFA, were conducted in MPlus (Muthén & Muthén, 1998–2013) with the cluster option to enable appropriate parameter estimation while taking into account the non-independence of sibling data, as all youth (siblings and singletons) were included in regression analyses.
RESULTS
Demographic and Descriptive Statistics
Demographic and descriptive statistics are presented in Table 2. Examination of symptom counts, comorbid psychopathology, and rating scale scores indicated that our diagnostic procedures effectively discriminated children with ADHD from controls. The unaffected sibling and ADHD groups were similar in ethnicity and income, as would be expected given that many of these youth are from the same families. However, income was significantly different among control and families of children with ADHD, and was included as a covariate in all analyses. Crucially for our design, the control and unaffected sibling groups were similar in terms of ADHD and comorbid symptomatology as reported by parents and teachers, as well as in overall functioning as rated by the diagnostic team, supporting our designation of these siblings as unaffected by ADHD. The unaffected sibling and control groups significantly differed only in gender composition (more females in the unaffected sibling group), and comorbid oppositional defiant disorder diagnosis. Full scale IQ was also lower among unaffected siblings of youth with ADHD relative to controls, prompting two additional analytic checks. Although recent work has indicated that genetic influences contributing to the ADHD-IQ relationship are independent of those contributing to the ADHD-executive functioning relationship (Wood, Asherson, van der Meere, & Kuntsi, 2010; Wood et al., 2011), we conducted secondary analyses which included IQ as a covariate when predicting executive function domains (e.g., cognitive control, inhibition, working memory, and memory span). Secondly, we also examined sex, age, and comorbidity (proband and individual-level variables) as moderators of the difference in IQ between unaffected siblings of youth with ADHD and controls (see below).
Table 2.
Demographic and descriptive statistics.
| Control | Unaffected Sibs | ADHD | p | |
|---|---|---|---|---|
| N | 103 | 107 | 251 | |
| % Male | 54.4b | 31.8a | 66.9c | <.001 |
| % Caucasian | 82.5 | 72.0 | 72.1 | .26 |
| % African-American | 12.6 | 6.5 | 8.0 | .44 |
| % Latino | 2.1 | 4.3 | 6.0 | .63 |
| % Mixed/Biracial | 3.9a | 14.9b | 11.9ab | .05 |
| Age in years (SD) | 10.8 (2.3) | 11.3 (2.4) | 10.5 (2.3) | .08 |
| Income+ | 85.1 (51.0)a | 64.9 (34.3)b | 64.5 (68.7)b | .003 |
| % Stimulant Medication | 0a | 0a | 37.4b | <.001 |
| Full-Scale IQ | 110.5 (14.6)a | 104.5 (13.1)b | 102.5 (14.6)b | .044 |
| GAF Score | 78.4 (9.6)a | 77.8 (10.0)a | 65.5 (8.9)b | <.001 |
| Adaptive Functioning T Score | 50.7 (9.5)a | 51.4 (11.8)a | 43.1 (14.6)b | <.001 |
| KSAD Diagnostics | ||||
| Inattention Symptoms (SD) | .70 (1.4)a | .65 (1.2)a | 7.2 (1.9)b | <.001 |
| Hyperactive Symptoms (SD) | .55 (1.2)a | .65 (1.1)a | 4.3 (2.9)b | <.001 |
| % ODD (lifetime) | 9.7a | 17.8ab | 41.4b | <.001 |
| % CD (lifetime) | 0a | 0.9a | 7.6b | <.001 |
| % MDD (lifetime) | 4.8a | 8.4ab | 9.6ab | .05 |
| % Any Anxiety Disorder (lifetime) | 8.7a | 14.9ab | 29.1b | .082 |
| % LD (lifetime) | 1.9a | 4.7a | 21.9b | .002 |
| Parent Conners’ Rating Scale | ||||
| Cognitive Problems T Score | 48.9 (7.5)a | 48.6 (7.9)a | 70.7 (11.0)b | <.001 |
| Hyperactivity T Score | 49.2 (9.9)a | 48.9 (6.6)a | 65.2 (14.1)b | <.001 |
| Teacher Conners’ Rating Scale | ||||
| Cognitive Problems T Score | 47.9 (8.7)a | 50.4 (8.2)a | 69.4 (12.1)b | <.001 |
| Hyperactivity T Score | 48.6 (8.2)a | 49.5 (9.1)a | 68.0 (10.6)b | <.001 |
Note. p-values indicate 3-group significance test.
Superscript letters indicate significant pairwise differences at p<.05. Conners’ T scores and standard deviations provided reflect age and sex norms.
GAF=diagnostic team-rated global assessment of functioning score.
Income reported in thousands.
Tests of Endophenotype Effects
For the main analyses, individual-level gender, age, medication status, and family income were entered as covariates. Contrast code 1 (ADHD versus unaffected siblings) significantly statistically predicted all neuropsychological domains (after Bonferonni correction with adjusted p-value of p=.006) with the exception of temporal information processing, which was nominally significant but failed to meet multiple testing correction (β =.10, [02, .19], p=.05).
All results involving our key contrast of interest for evaluating endophenotype effects (contrast code 2, which examined differences between unaffected siblings and controls) are presented in Table 3. As can be seen there, contrast code 2 significantly predicted the second-order factor of cognitive control (β =.18, [.09, .28], p<.001). However, this difference was most pronounced for the lower-order factor of response inhibition specifically (β =.12, [.05, .18], p<.001), as unaffected siblings did not perform significantly worse than controls after correction for multiple testing on measures of working memory (β =.07, [.002, .14], p=.033) or memory span (β =.13, [.03, .23], p=.016). Additionally, unaffected siblings also evidenced poorer performance relative to controls on measures of response variability (β=.18, [.12, .25], p<.001) and temporal information processing (β =.17, [.09, .26], p<.001). Group differences were not statistically reliable for processing speed (β =.08, [−.03, .19], p=.19), and arousal (β =.10, [−.05, .25], p=.11) domains. Although ADHD symptoms did not significantly differ between the unaffected siblings and control youth, ADHD symptoms were covaried in repeat of the models as a precaution. Results were unchanged. Additionally, contrast code 2 remained a significant predictor (at p<.006) of cognitive control, inhibition, response variability, and temporal processing with IQ covaried.
Table 3.
Neuropsychological performance among unaffected siblings and non-ADHD controls: Summary of endophenotype effects
| Non-ADHD Control | Unaffected Siblings | β | d | Moderation | |
|---|---|---|---|---|---|
| Cognitive Control | −.26 (.60) | .13 (.69) | .18*** | .60 | Individual age |
| (.09, .28) | (.33, .88) | ||||
| Inhibition | −.28 (.62) | .05 (.72) | .12*** | .49 | Individual age |
| (.05, .18) | (.22, .77) | ||||
| Working Memory | −.29 (.71) | −.02 (.78) | .07+ | .36 | Individual age |
| (.002, .14) | (.09, .63) | Proband sex, Proband LD | |||
| Memory Span | −.32 (.87) | −.01 (.63) | .13+ | .41 | Individual age |
| (.03, .23) | (.13, .68) | ||||
| Processing Speed | −.35 (.59) | −.17 (.49) | .08 | .28 | Individual age |
| (−.03, .19) | (.004, .55) | Proband sex | |||
| Response Variability | −.36 (.79) | .28 (.61) | .18*** | .89 | Individual age |
| (.12, .25) | (.61, 1.2) | ||||
| Arousal | −.26 (.99) | −.03 (.87) | .10 | .25 | Individual age |
| (−.05, .25) | (−.02, .52) | Proband sex | |||
| Temporal Processing | −.34 (.80) | .03 (.57) | .17*** | .53 | Individual age |
| (.09, .26) | (.26, .81) |
Note.
indicates regression parameter for contrast code 2 significant at p<.001 following Bonferonni correction,
indicates p<.05, but not significant following Bonferonni correction. Cohen’s d represents mean-difference effect size comparing unaffected siblings and non-ADHD controls. 95% confidence intervals for each of the parameters are presented in parentheses.
Subtype Effects
Additional analyses were conducted to examine differences in performance between unaffected siblings of ADHD-PI and ADHD-C youth relative to controls. Analyses revealed that while all unaffected siblings of youth with ADHD performed worse on measures of cognitive control, inhibition, response variability and temporal processing relative to controls, siblings of probands with ADHD-PI and siblings of probands with ADHD-C did not perform significantly from each other (all ps>.12). Thus, it appears that the endophenotype effects conducted in the main analyses did not vary based upon subtype of the ADHD proband.
Moderation of Effects
Multiple regression analyses were used to examine moderation by proband and individual-level characteristics (sex, age, and comorbidity status), with key focus on contrast code 2 (unaffected siblings vs. control). All interactions between contrast code 2 and individual sex were not significant after correction for multiple tests. However, the contrast code 2 × proband sex interactions were significant in predicting speed (β =.14, [.03, .24], p<.001), working memory (β = .21, [.05, .37], p<.001) and arousal (β = −.13, [−.22, −.04], p<.001). Analyses of the group means revealed that unaffected siblings of male probands performed significantly worse on measures of arousal compared to the unaffected siblings of female probands. However, the opposite pattern emerged for working memory and speed, such that the unaffected siblings of female probands performed significantly worse in both domains than unaffected siblings of male probands.
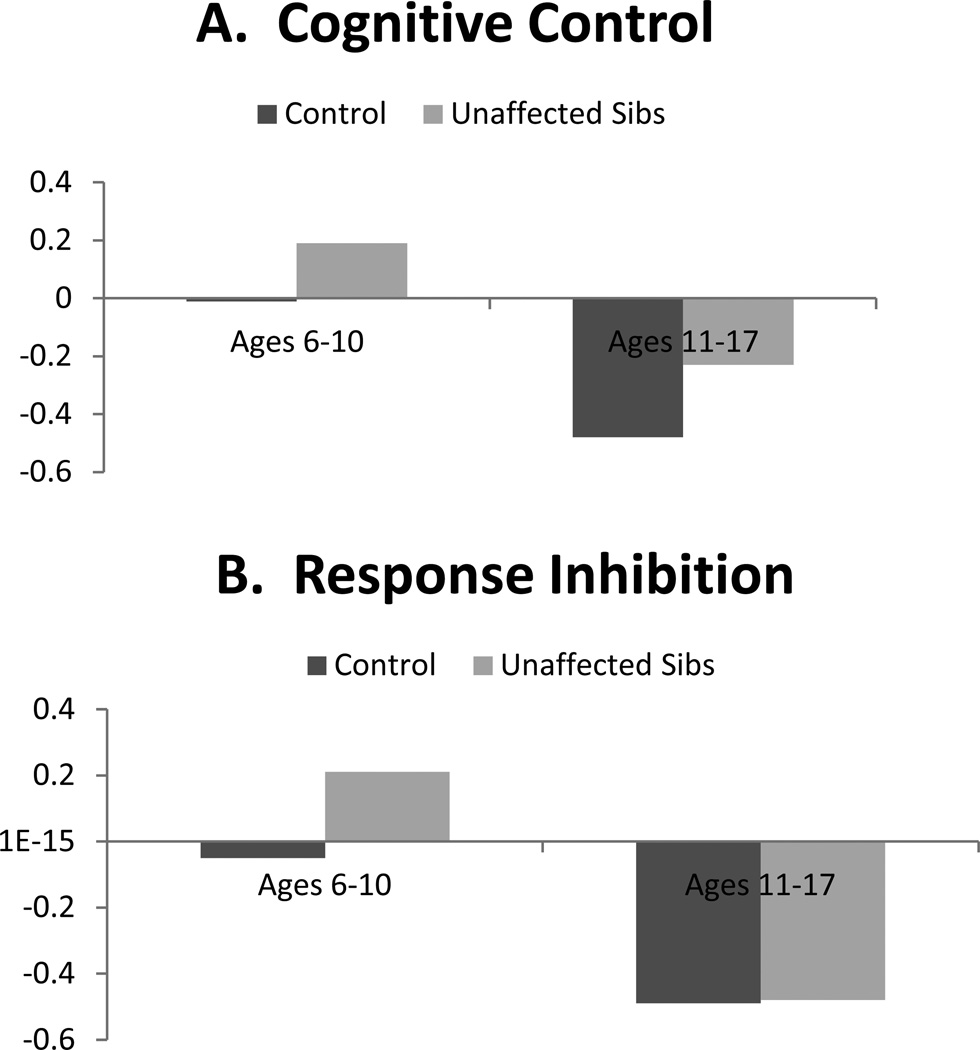
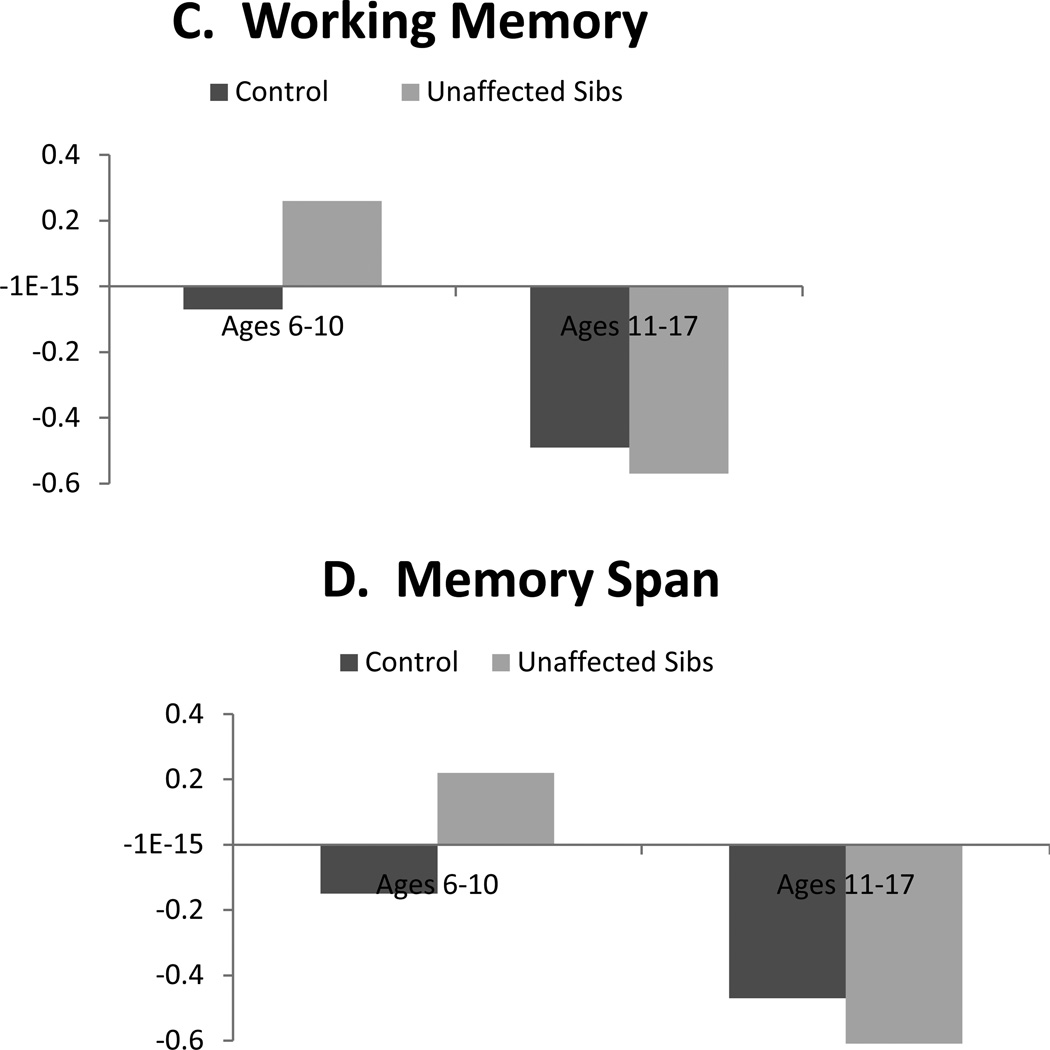
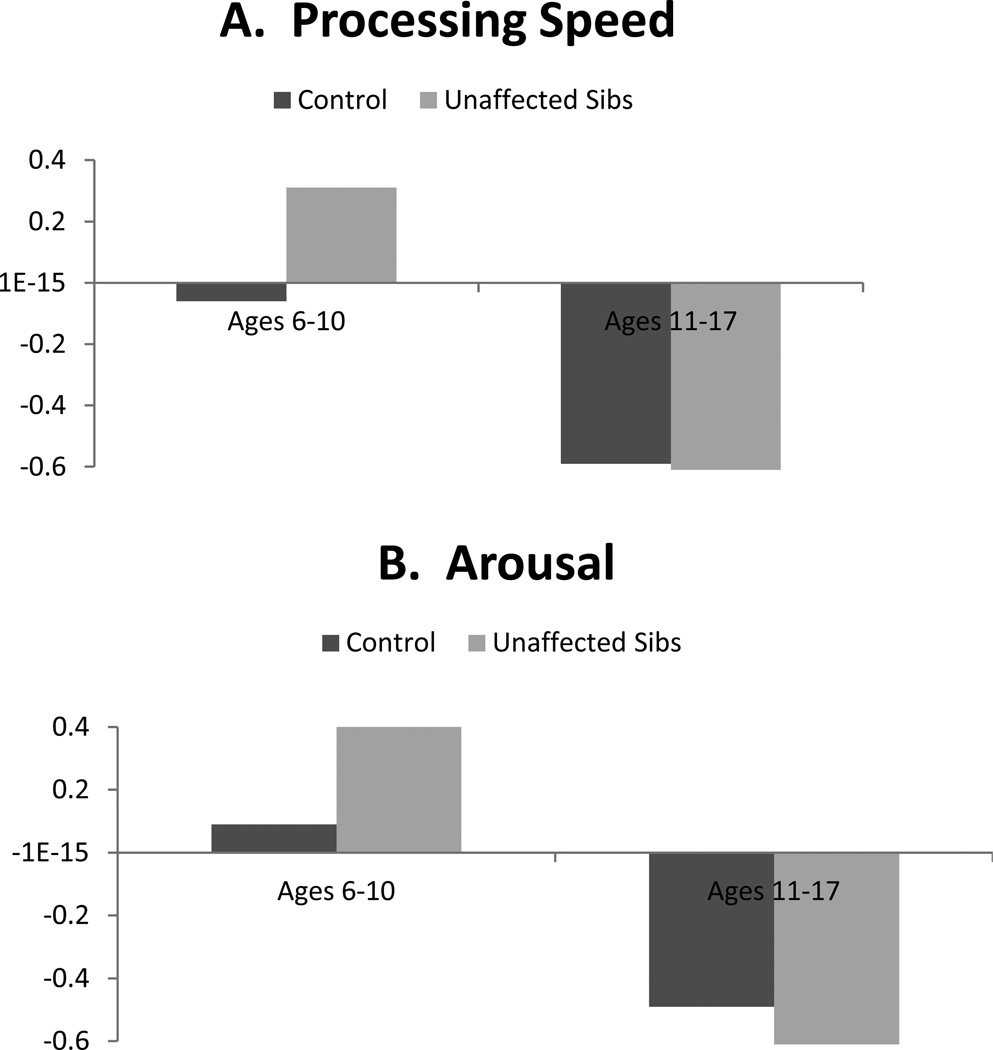
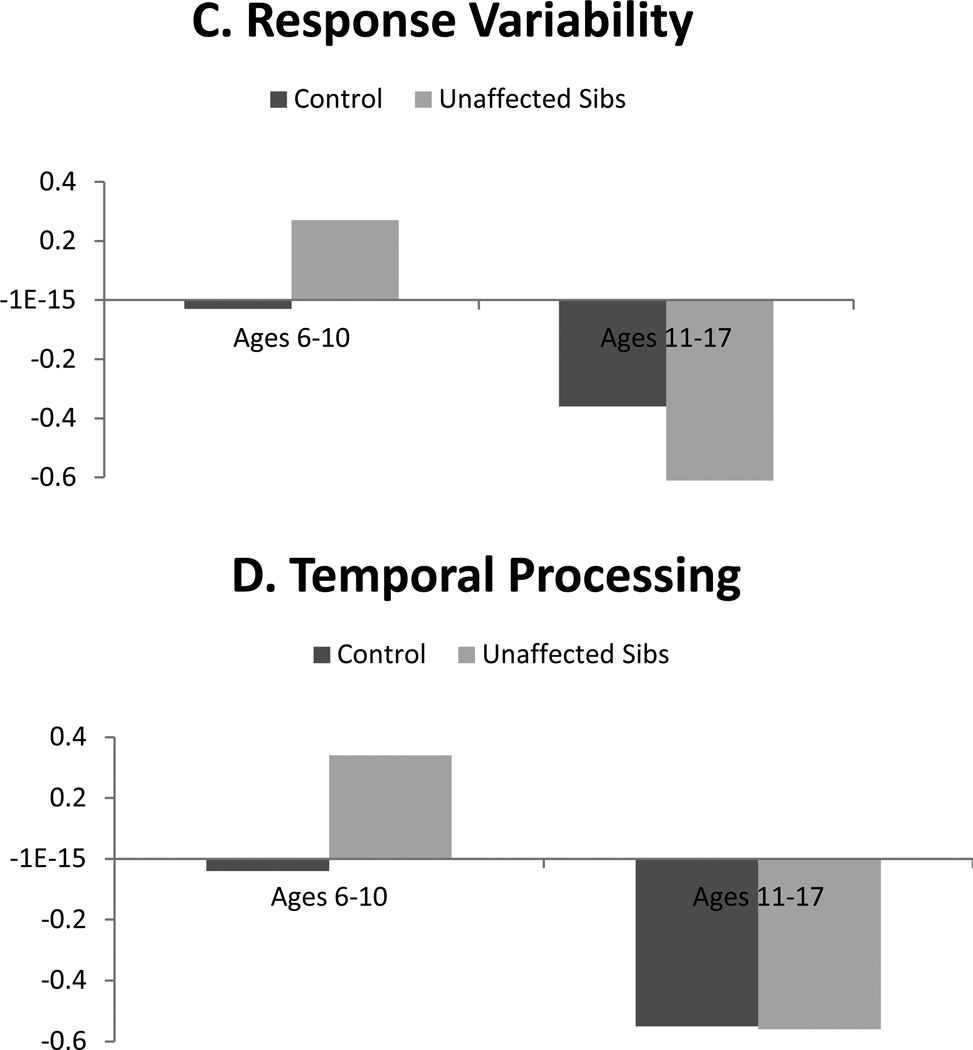
With regard to age, contrast code 2 × individual age interactions were significant for all neuropsychological domains (βs range from −.16 to −.28, all p<.001). Whereas age was included as a continuous moderator in the interaction terms in regression analyses, group differences are depicted categorically in order to facilitate interpretation in Figures 1 and 2. As seen there, differences between unaffected siblings and controls were more pronounced among younger children (ages 6–10 years) relative to older youth (ages 11–17 years) for all domains. Interactions between contrast code 2 and proband age were all not significant.
Figure 1.
Illustration of contrast (unaffected sibling versus control) × age interactions predicting cognitive control performance and its subdomains: Effects in younger but not older youth.
Note. Neuropsychological domains are standardized factor scores from structural equation model, such that higher scores index worse performance on all measures.
Figure 2.
Illustration of contrast (unaffected sibling versus control) × age interactions predicting processing speed, arousal, response inhibition, and temporal processing: Effects in younger but not older youth.
Note. Neuropsychological domains are standardized factor scores from structural equation model, such that higher scores index worse performance on all measures.
Lastly, we examined whether observed differences in performance between unaffected siblings and control could be accounted for by presence of comorbid disruptive behavior disorders, learning disorder and internalizing disorder (at the individual level and at the proband level). The contrast code 2 × proband learning disorder interaction was significant in predicting working memory (β =.24, [.07, .42], p<.001). Analyses of means indicated that unaffected siblings of youth with ADHD+LD had worse performance relative to controls compared to unaffected siblings of youth with ADHD alone, consistent with literature linking working memory to LD as well as ADHD (Martinussen & Tannock, 2006). No other contrast × proband comorbidity interactions were significant (all ps>.06) nor were any interactions between contrast code 2 and individual comorbidity (all ps>.07), indicating that impaired neuropsychological performance among unaffected siblings of youth with ADHD was not explained by learning, disruptive behavior, and internalizing disorder comorbidity either among probands or among the siblings themselves.
IQ as Endophenotype Measure
Because unaffected siblings and controls also significantly differed in IQ, we also conducted analyses exploring (1) whether contrast code 2 significantly predicted IQ when considering other covariates, and (2) whether this difference was also moderated by individual or proband sex, age, and comorbidity status. Contrast code 2 was significant in predicting IQ (β =−.17, [−.34, −.006], p=.042), but would not survive our correction for multiple tests. Analyses revealed that this difference was not moderated by proband or individual level sex, age, or comorbidity status (all ps>.29).
DISCUSSION
The current study evaluated age, sex, and comorbidity moderation of multiple domains of neuropsychological functioning as potential endophenotypes for ADHD using a sibling design. The first important finding was that results replicated and confirmed differences between unaffected siblings and non-ADHD control youth for several domains of neuropsychological functioning, suggesting that these measures do detect familial liability for ADHD and may be useful in future genetic studies. However, evaluation of moderators revealed several key qualifications to these findings that may help resolve prior ambiguities in the literature. We discuss each in turn below.
First, although executive functions have been proposed as a group of processes that may be useful as intermediate phenotypes for ADHD, our results highlight response inhibition as a specific executive domain of interest, in line with past theories regarding causes of ADHD (Barkley, 1997) as well as past empirical studies of unaffected family members of individuals with ADHD (Gau & Chang, 2010; Goos et al., 2009; Nigg et al., 2004).
The second, more novel finding here is that non-executive neuropsychological measures were also affected in non-ADHD siblings. Both response variability and temporal processing were impaired among unaffected siblings; those effects were not dependent upon individual or proband sex or comorbidity status. This is in line with past work indicating that variability in motor timing is impaired among unaffected siblings of youth with ADHD (Rommelse et al., 2008) as well as prior theories of neuropsychological heterogeneity in ADHD (Nigg, 2005), particularly those that include temporal information processing in such a model (Sonuga-Barke, Bitsakou, & Thompson, 2010).
Third, and most central to our study purpose, we reported here novel data regarding the dependence of many endophenotypes effects on proband comorbidity, sex, and in all cases, individual age. Importantly, these were found in the absence of such moderation in group differences between ADHD and controls, as interactions between overall diagnosis with sex, age, and comorbidity status were all not significant. Working memory differences were observed but were dependent upon both proband sex and ADHD−LD comorbidity, such that unaffected siblings of ADHD females as well as unaffected siblings of probands with ADHD+LD comorbid profiles performed worse relative to controls than did siblings of ADHD males and siblings of youth with ADHD but without LD. This may speak to the important overlap of ADHD and LD as having shared risk via working memory (Palladino & Ferrari, 2013). Proband sex moderated the effects of processing speed and arousal, but in opposite directions, making those sex effects difficult to interpret and suggesting that the overall nature of sex effects either varies by domain or is sensitive to sampling variation.
Perhaps most striking was that age robustly moderated all sibling effects. For all domains, moderation analyses revealed differences between controls and unaffected siblings were large and pronounced in younger children (when taking into account age moderation, Cohen’s d ranged .49 to 1.1), but markedly smaller and largely absent in older youth (ds for older youth ranged from −.09 to .17). This supports the claim by Thissen et al. (2014) that endophenotype effects may wane in adolescence. There are several potential explanations for this finding. First, moderation of effects by age may reflect real changes in neuropsychological functioning that correspond with brain maturation, particularly development of neural networks involving the prefrontal cortex and subcortical regions as well as the cerebellum (Hart et al., 2013; Mackie et al., 2007; Shaw et al., 2013). It may be the case that when neural networks are immature, differences in performance between unaffected siblings and controls are more readily detectible with neuropsychological measures. But, that as brain maturation continues throughout adolescence, the gap between unaffected family members and controls narrows somewhat, as the unaffected children form positive adaptation. This gap does not narrow for affected youth, who may fail to adapt as well.
Secondly, it may be the case that these endophenotype effects are only relevant in childhood because genes that contribute to neural circuit dysfunction influencing these measures are expressed earlier in development. Recent longitudinal work indicates the potential for additional genetic effects that come online during adolescence in particular (Chang et al., 2013; Larsson, Chang, D’Onofrio, & Lichtenstein, 2013). A final possibility is that the failure of detection is confined to adolescents, who show developmentally unique profiles of development of “hot” versus “cool” response control (Hare et al., 2008) that are not seen in children or adults. We did not examine “hot” decision making under emotional load here, although affective influences on processing may be relevant (Uebel et al., 2010), nor did we include adults in the age moderation test. It will be important for future work to test whether curvilinear age moderation exists in a sample with an even larger age range. However, our results regarding age moderation must be tempered given that these are cross-sectional data; future work should continue to explore these moderation effects within longitudinal designs. We are undertaking such studies now.
There are some limitations of the current work that were important to note. While our battery was extensive, as just alluded we did not measure emotional regulation, reward discounting, or delay aversion (Anokhin, Golosheykin, Grant, & Heath, 2011). Secondly, we excluded youth with ADHD taking long-acting stimulants as they could not do an appropriate wash-out, which may somewhat limit the generalizability of findings, although this effect was likely modest in view of the small number of youth thus excluded in the present instance (2% of all screened).
The current study was strengthened by the use of factor scores within the CFA framework, which allowed for removal of error variance not associated with the latent variables and creation of reliable latent factors (Bollen, 1989). Effect sizes observed in the current study regarding differences between unaffected siblings and controls were larger (Cohen’s d for the unmoderated differences between unaffected siblings and controls ranging from .25–.89) than reported in prior studies employing a large battery of single measures (effect sizes from Bidwell et al., 2007 ranging from .21–.53) or from studies of inhibition (ranging from .21–.40). This is crucial as measurement error of neuropsychological constructs is notably problematic (Kuntsi, Andreou, Ma, Borger, & van der Meere, 2005) and low reliability of single measures may artificially constrain effect sizes.
Overall, the current study provides additional evidence that inhibition, response variability, and temporal processing in particular may be relevant intermediate phenotypes for future genetic studies of ADHD. However, our findings also suggest that future study regarding the developmental trajectories of these measures as endophenotypes is needed and that genetic studies using these measures must carefully consider age effects.
ACKNOWLEDGEMENTS
This work was supported by R01-MH070004-01A2 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. The authors also thank all participating children and their families for making this work possible.
REFERENCES
- Anokhin AP, Golosheykin S, Grant JD, Heath AC. Heritability of delay discounting in adolescence: a longitudinal twin study. Behavior Genetics. 2011;41:175–183. doi: 10.1007/s10519-010-9384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arns M, Conners CK, Kraemer HC. A decade of EEG Theta/Beta Ratio Research in ADHD: a meta-analysis. Journal of Attention Disorders. 2013;17:374–383. doi: 10.1177/1087054712460087. [DOI] [PubMed] [Google Scholar]
- Assesmany A, McIntosh DE, Phelps L, Rizza MG. Discriminant validity of the WISC-III with children classified with ADHD. Journal of Psychoeducational Assessment. 2001;19:137–147. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHDP. sychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bidwell LC, Willcutt EG, Defries JC, Pennington BF. Testing for neuropsychological endophenotypes in siblings discordant for attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;62:991–998. doi: 10.1016/j.biopsych.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemsma JM, Boer F, Arnold R, Banaschewski T, Faraone SV, Buitelaar JK, Oosterlaan J. Comorbid anxiety and neurocognitive dysfunctions in children with ADHD. European Child & Adolescent Psychiatry. 2013;22:225–234. doi: 10.1007/s00787-012-0339-9. [DOI] [PubMed] [Google Scholar]
- Bollen KA. Structural models with latent variables. New York: Wiley; 1989. [Google Scholar]
- Chang Z, Lichtenstein P, Asherson PJ, Larsson H. Developmental twin study of attention problems: high heritabilities throughout development. JAMA Psychiatry. 2013;70:311–318. doi: 10.1001/jamapsychiatry.2013.287. [DOI] [PubMed] [Google Scholar]
- Connors CK. Connors’Rating Scales – Revised. Toronto, Ontario, Canada: Multi Health Systems, Inc.; 1997. [Google Scholar]
- Crosbie J, Arnold P, Paterson A, Swanson J, Dupuis A, Li X, Schachar RJ. Response inhibition and ADHD traits: correlates and heritability in a community sample. Journal of Abnormal Child Psychology. 2013;41:497–507. doi: 10.1007/s10802-012-9693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosbie J, Pérusse D, Barr CL, Schachar RJ. Validating psychiatric endophenotypes: inhibitory control and attention deficit hyperactivity disorder. Neuroscience and Biobehavioral Reviews. 2008;32:40–55. doi: 10.1016/j.neubiorev.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Delis CD, Kaplan E, Kramer JH. Delis-Kaplan executive function system (D-KEFS) technical manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Dolan M, Lennox C. Cool and hot executive function in conduct-disordered adolescents with and without co-morbid attention deficit hyperactivity disorder: relationships with externalizing behaviours. Psychological Medicine. 2013;43:2427–2436. doi: 10.1017/S0033291712003078. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Faraone SV, Seidman LJ, Willcutt EG, Nigg JT, Waldman ID, Biederman J. Are endophenotypes based on measures of executive functions useful for molecular genetic studies of ADHD? Journal of Child Psychology and Psychiatry. 2005;46:774–803. doi: 10.1111/j.1469-7610.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. The ADHD rating scale IV: Checklists, norms, and clinical interpretation. New York: Guilford Press; 1998. [Google Scholar]
- Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proceedings of the National Academy of Sciences. 2012;109:6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mick E, Williamson S, Wilens T, Spencer T, Zallen B. Family study of girls with attention deficit hyperactivity disorder. American Journal of Psychiatry. 2000;157(7):1077–1083. doi: 10.1176/appi.ajp.157.7.1077. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Youngstrom EA, Chelune GJ, Naugle RI, Lineweaver TT. Increasing the reliability of ipsative interpretations in neuropsychology: a comparison of reliable components analysis and other factor analytic methods. Journal of the International Neuropsychological Society. 2004;10:578–589. doi: 10.1017/S1355617704104049. [DOI] [PubMed] [Google Scholar]
- Gau SS-F, Shang C-Y. Executive functions as endophenotypes in ADHD: evidence from the Cambridge Neuropsychological Test Battery (CANTAB) Journal of Child Psychology and Psychiatry. 2010;51:838–849. doi: 10.1111/j.1469-7610.2010.02215.x. [DOI] [PubMed] [Google Scholar]
- Goos LM, Crosbie J, Payne S, Schachar R. Validation and extension of the endophenotype model in ADHD patterns of inheritance in a family study of inhibitory control. American Journal of Psychiatry. 2009;166:711–717. doi: 10.1176/appi.ajp.2009.08040621. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Hinshaw SP, Swanson JM, Greenhill LL, Conners CK, Arnold LE, Wigal T. Findings from the NIMH Multimodal Treatment Study of ADHD (MTA): implications and applications for primary care providers. Journal of Developmental and Behavioral Pediatrics. 2001;22:60–73. doi: 10.1097/00004703-200102000-00008. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Andreou P, Ma J, Börger NA, van der Meere JJ. Testing assumptions for endophenotype studies in ADHD: reliability and validity of tasks in a general population sample. BMC Psychiatry. 2005;5:40. doi: 10.1186/1471-244X-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H, Chang Z, D’Onofrio BM, Lichtenstein P. The heritability of clinically diagnosed attention deficit hyperactivity disorder across the lifespan. Psychological Medicine. 2013:1–7. doi: 10.1017/S0033291713002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. A user’s guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. San Diego, CA: Academic Press; 1994. pp. 189–239. [Google Scholar]
- Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, 3rd, Rapoport JL. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. American Journal of Psychiatry. 2007;164:647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- Martel MM. Individual differences in attention deficit hyperactivity disorder symptoms and associated executive dysfunction and traits: sex ethnicity, and family income. American Journal of Orthopsychiatry. 2013;83:165–175. doi: 10.1111/ajop.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinussen R, Tannock R. Working memory impairments in children with attention-deficit hyperactivity disorder with and without comorbid language learning disorders. Journal of Clinical and Experimental Neuropsychology. 2006;28:1073–1094. doi: 10.1080/13803390500205700. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Los Angeles, CA: Muthén & Muthén; 1998–2013. [Google Scholar]
- Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biological Psychiatry. 2005;57:1424–1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Stawicki JA, Sachek J. Evaluating the endophenotype model of ADHD neuropsychological deficit: results for parents and siblings of children with ADHD combined and inattentive subtypes. Journal of Abnormal Psychology. 2004;113:614–625. doi: 10.1037/0021-843X.113.4.614. [DOI] [PubMed] [Google Scholar]
- Nikolas MA, Burt SA. Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: a meta-analysis. Journal of Abnormal Psychology. 2010;119:1–17. doi: 10.1037/a0018010. [DOI] [PubMed] [Google Scholar]
- Nikolas MA, Nigg JT. Neuropsychological performance and attention-deficit hyperactivity disorder subtypes and symptom dimensions. Neuropsychology. 2013;27:107–120. doi: 10.1037/a0030685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JW, Dowell LR, Mostofsky SH, Denckla MB, Mahone EM. Neuropsychological profile of executive function in girls with attention-deficit/hyperactivity disorder. Archives of Clinical Neuropsychology. 2010;25:656–670. doi: 10.1093/arclin/acq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino P, Ferrari M. Interference control in working memory: comparing groups of children with atypical development. Child Neuropsychology. 2013;19:37–54. doi: 10.1080/09297049.2011.633505. [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, Ryan N. The Schedule for Affective Disorders and Schizophrenia for School-Age Children (Kiddie-SADS) Pittsburgh, Pennsylvania: Western Psychiatric Institute and Clinic; 1986. [Google Scholar]
- Qian Y, Shuai L, Cao Q, Chan RCK, Wang Y. Do executive function deficits differentiate between children with attention deficit hyperactivity disorder (ADHD) and ADHD comorbid with oppositional defiant disorder? A cross-cultural study using performance-based tests and the behavior rating inventory of executive function. The Clinical Neuropsychologist. 2010;24:793–810. doi: 10.1080/13854041003749342. [DOI] [PubMed] [Google Scholar]
- Rommelse NNJ, Altink ME, Oosterlaan J, Buschgens CJM, Buitelaar J, Sergeant JA. Support for an independent familial segregation of executive and intelligence endophenotypes in ADHD families. Psychological Medicine. 2008;38:1595–1606. doi: 10.1017/S0033291708002869. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ, Tannock R. Neuropsychological profiles of adolescents with ADHD: effects of reading difficulties and gender. Journal of Child Psychology and Psychiatry. 2002;43:988–1003. doi: 10.1111/1469-7610.00227. [DOI] [PubMed] [Google Scholar]
- Sergeant JA. Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biological Psychiatry. 2005;57:1248–1255. doi: 10.1016/j.biopsych.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W. Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2013;74:599–606. doi: 10.1016/j.biopsych.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke E, Bitsakou P, Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:345–355. doi: 10.1016/j.jaac.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Thissen AJAM, Rommelse NNJ, Hoekstra PJ, Hartman C, Heslenfeld D, Luman M, Buitelaar JK. Attention deficit hyperactivity disorder (ADHD) and executive functioning in affected and unaffected adolescents and their parents: challenging the endophenotype construct. Psychological Medicine. 2014;44:881–892. doi: 10.1017/S0033291713001153. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Tannock R. Tapping and anticipation performance in attention deficit hyperactivity disorder. Perceptual and Motor Skills. 2005;100:659–675. doi: 10.2466/pms.100.3.659-675. [DOI] [PubMed] [Google Scholar]
- Uebel H, Albrecht B, Asherson P, Börger NA, Butler L, Chen W, Banaschewski T. Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. Journal of Child Psychology and Psychiatry. 2010;51:210–218. doi: 10.1111/j.1469-7610.2009.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Gathercole SE. Working memory deficits in children with reading difficulties: memory span and dual task coordination. Journal of Experimental Child Psychology. 2013;115:188–197. doi: 10.1016/j.jecp.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, Lahey BB. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. Journal of Abnormal Psychology. 2012;121:991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AC, Asherson P, van der Meere JJ, Kuntsi J. Separation of genetic influences on attention deficit hyperactivity disorder symptoms and reaction time performance from those on IQ. Psychological Medicine. 2010;40:1027–1037. doi: 10.1017/S003329170999119X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B, Arias-Vasquez A, Kuntsi J. The relationship between ADHD and key cognitive phenotypes is not mediated by shared familial effects with IQ. Psychological Medicine. 2011;41:861–871. doi: 10.1017/S003329171000108X. [DOI] [PMC free article] [PubMed] [Google Scholar]