Abstract
Maternal behavior in the rabbit is restricted to a brief nursing period every day. Previously we demonstrated that this event induces daily rhythms of PER1 protein, the product of the clock gene Per1, in oxytocinergic and dopaminergic populations in the hypothalamus of lactating rabbit does. This is significant for the periodic production and ejection of milk, but the activation of other areas of the brain has not been explored. Here we hypothesized that daily suckling will induce a rhythm in the preoptic area, lateral septum and bed nucleus of the stria terminalis, which are important areas for the expression of maternal behavior in mammals including the rabbit. To this end, we analyzed PER1 expression in those areas through a complete 24-h cycle at lactation day 7. Does were scheduled to nurse during either the day at 10:00 (ZT03) or the night at 02:00 (ZT19) h. Non-pregnant, non-lactating females were used as controls. In contrast to control females, lactating does show a clear, significant rhythm of PER1 that shifts in parallel to timing of nursing in the preoptic area and lateral septum. We determined that the maximal expression of PER1 at 8 h after scheduled nursing decreased significantly at 24 and 48 h after the absence of suckling. This effect was more pronounced in the lateral septum than in the preoptic area. We conclude that daily suckling is a powerful stimulus that induces rhythmic activity in brain structures in the rabbit that appear to be part of a maternal entrainable circuit.
Keywords: Nursing, maternal behavior, Per1 protein, temperature rhythm
Introduction
In mammals, maternal behavior (MB) consists of a series of behaviors that contributes to the survival of the young by providing food, warmth, shelter, protection from predators and conspecifics and appropriate stimulation to the young (Olazábal et al., 2013). Most of our knowledge about the neural basis of MB comes from studies in rodents and sheep, and research in other species reveal that this behavior is diverse and expressed in different forms and modalities (González-Mariscal & Poindron, 2002). In this regard MB in the rabbit represents an exceptional example as mothers after parturition visit daily their pups only for nursing without providing any other care to their progeny (Zarrow, et al, 1965; Caba & González-Mariscal, 2009). More remarkably, this daily visit occurs at about the same hour every day (Jilge, 1995; González-Mariscal et al., 2013), which is unique among mammals. Previously we demonstrated daily rhythms of PER1 protein, product of the Per1 clock gene, that shift in parallel to the timing of daily nursing in hypothalamic oxytocinergic and dopaminergic cells of the lactating doe (Meza et al., 2008; 2011). This was not a general effect on neuronal activity in the doe's brain, as a dopaminergic population in the diencephalon was not affected by this stimulus (Meza et al., 2011). These results were important because they laid groundwork to explore the neuroendocrine mechanisms that control the periodic production and ejection of milk. However, the neural basis of the periodic maternal behavior remains poorly explored. Specifically, it is unknown whether other areas in the brain, such as the preoptic area (POA), lateral septum (LS), and the bed nuclei of the stria terminalis (BNST), important for the expression of MB in several species, including the rabbit (Numan & Insel, 2003; Olazábal et al., 2013) display rhythmic activity related to timed nursing. We considered it important to explore these regions to gain further insight into the possible neural substrates mediating control of MB in the rabbit, taking into account the fact that the suprachiasmatic nucleus (SCN), the master circadian clock, is not entrained by timing of nursing (Meza et al., 2008). Here, we hypothesized that the POA, LS and BNST will show rhythmic activity associated with timing of nursing (Meza et al., 2008). To this aim we explored the expression of PER1 in those prosencephalic areas in lactating does scheduled to nurse either during the day or the night.
Materials and Methods
Animals and housing
New Zealand white female rabbits bred in the colony in Xalapa, México, were housed under a LD cycle (12:12 h; lights on at 07:00= ZT0). Rabbits were provided with food pellets (Purina, México D.F., México) and water ad libitum. Females were mated and housed individually in stainless steel cages at room temperature of 23 ± 1°C and were monitored daily from day 28 of pregnancy until delivery. Each cage had two compartments, one for the mother and one for the nest (0.60 m wide × 0.50 m long × 0.40 m high) linked by a tunnel (0.25 m wide × 0.50 m long × 0.40 m high) between them. The tunnel had a sliding door on each end, permitting the experimenter to control the mother's access to the tunnel and nest. Before parturition, does had free access to the nest compartment and they were provided with approximately 100 g of straw to build a nest. Pups were born in the nest. On the day of parturition, litters were adjusted to four to five pups. Experiments and animal handling were conducted according to the national guide for care and use of animal experimentation approved by the Ethics Committee of Universidad Veracruzana, in accordance with the procedures of the National Guide for the Production, Care and Use of Laboratory Animals (Norma Oficial Mexicana NOM-062-ZOO-1999), which complies with international guidelines laid down by the Society for Neuroscience and were approved and conducted according to the Statement of Assurance with Standards for Humane Care and Use of Laboratory Animals approved by the National Institutes of Health (NIH) to the Universidad Veracruzana.
Experimental design
On the day of parturition (PP0), the door between mothers’ compartments and the tunnel was closed. Starting the next day (PP1), the door was opened for nursing at either ZT03 (Nursing ZT03 group) or ZT19 (Nursing ZT19 group). The doe entered the nest immediately after the door was opened to nurse. The mean daily duration of nursing was 235±8 s (Mean±S.E.) and 224±3 s for pups nursed at ZT03 and ZT19, respectively. Does were sacrificed at PP7 starting at their scheduled time of nursing (ZT03 or ZT19) and then at 4 h intervals at ZT03, ZT07, ZT11, ZT15, ZT19 and ZT23 (n=4 at each time point). We also determined the maintenance of the PER1-ir cells in nursing deprived does. Following the nursing schedules above, does were deprived of one (Nursing-Dep 24 h) or two (Nursing-Dep 48 h) cycles in both nursing groups. They were sacrificed 8 h after their previous scheduled time of nursing (n=4 at time point) at the time of expected maximum expression of PER1-ir cells in the POA, LS and BNST of nursing does. Non-pregnant, non-lactating adult females were used as controls (Control group, n=4 at each time point). Control females were handled as the lactating ones and sacrificed at 4 h intervals. For the present experiment, we used the same animals in which we previously demonstrated synchronization of locomotor behavior and rhythms in oxytocin cells, but not suprachiasmatic nucleus cells, in the hypothalami of lactating female rabbits (Meza et al., 2008).
Immunohistochemistry
Rabbits were anesthetized with an overdose of sodium pentobarbital (60 mg/kg, i.v.) and were perfused transcardially with saline solution (0.9%), followed by 4% paraformaldehyde in phosphate buffer (PB, pH 7.4). Brains were removed immediately after perfusion, cryoprotected successively in 10, 20 and 30% sucrose in PB and then sectioned coronally at 50 μm with a cryostat (Microm, Walldorf, Germany). Serial sections were collected in PB from the level of the organum vasculosum of lamina terminalis to the mammillary bodies. The fourth section of each set was used for PER1 labeling as described below, following similar protocols previously established for nuclear proteins (Caba et al., 2008) in rabbit brain. Tissue was washed in PB four times, 5 min each, to remove excess aldehydes and then exposed for 10 min in 0.5% hydrogen peroxide solution to eliminate endogenous peroxidase activity. Nonspecific antibody reactions were blocked by placing the sections in 3% normal horse serum (Vector Labs; Burlingame, CA) for 1 h at room temperature. Sections were then incubated for 48 h at 4 °C in polyclonal PER1 antibody (sc-7724) diluted at 1:5000 (Santa Cruz Biotechnology, Santa Cruz, CA) in 3% normal horse serum with 0.3% Triton X-100 (Sigma). Tissue was then placed in biotinylated horse anti-goat serum (1:200, Vector Labs) for 1 h. After 3 washes in PB, sections were incubated in avidin-biotin complex (1:250, Vector Labs) for 1 h. PER1 antibody-peroxidase complex was stained with a solution of 0.05% diaminobenzidine (DAB; Polysciences) in the presence of nickel sulfate (10 mg/ml, Fisher Scientific, Fair Lawn, NJ), cobalt chloride (10 mg/ml, Fisher scientific) and 0.01% hydrogen peroxide, which produced a black-purple precipitate. After 10 minutes, tissue was transferred to PB to stop the reaction. Sections were mounted onto gelatin-subbed slides, dehydrated, and cleared in Hemo-De (Fisher Scientific), then coverslipped with Permount. In all cases, tissue sections from subjects at each time point were processed together. The sc7724 PER1 antibody has been previously characterized and validated in rabbit brain tissue (Caba et al., 2008).
Quantification of Immunostaining
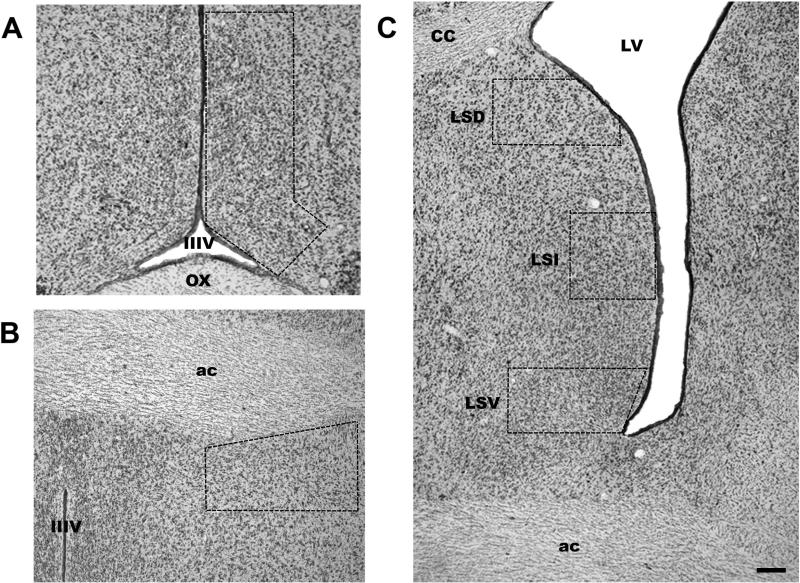
PER1-ir was identified as a black-purple precipitate from the DAB-nickel/cobalt reaction in the cell nucleus. To quantify immunoreactive nuclei staining, we determined the background optical density in a nearby region lacking immunoreactivity with the Image-Pro Plus program, v. 5 (Media cybernetics, Silver spring, MD). Immunoreactive cells that reached five times the optical density background level were considered positive. Cells below this staining level were considered negative. The numbers of PER1-ir positive cells were counted unilaterally by two observers blind to the experimental conditions with an Olympus BX41 (Tokyo, Japan) microscope using a grid according to a protocol previously published (Caba et al., 2008; Meza et al., 2008). The localization of brain structures was determined using terminology according to Girgis and Shi-Chang (1981) and our previous experience in studying the forebrain of this species (Caba et al., 2003). At the time of maximum expression, PER1-ir in the preoptic region was observed in the periventricular as well as in the medial preoptic area, being more abundant in the proximity to the third ventricle. Then we selected an area of 591,839 μm2 (Fig. 1) that included both PER1-ir cells in the periventricular and medial preoptic area (termed POA), similar to a previous study in the rabbit (Gonzalez-Mariscal et al., 2009). For the LS we analyzed the dorsal (LSD), intermediate (LSI) and ventral (LSV) subdivisions where we selected an area of 271 518, 227 320 and 220 440 μm2 respectively (Fig. 1). For the BNST, we analyzed its ventral subdivision (vBST; Numan & Insel, 2003), which lies dorsolateral to the POA, and selected an area of 370,416 μm2 (Fig. 1). In all cases cell counting was done in one representative sectioncorresponding to levels AP1 for the LS and BNST and AP2 for the POA from Bregma of the stereotaxic atlas of the rabbit (Girgis & Shi-Chang, 1981).
Figure 1.
Photomicrographs (Thionine stain) showing the location of the POA (A), BNST (B) and the LS (C). Dotted lines delimit the counted area. LSD = lateral septum dorsal; LSI = lateral septum intermediate; LSV = lateral septum ventral. IIIV = third ventricle; OX = optic chiasma; LV = lateral ventricle; CC = corpus callosum; ac = anterior commissure. Scale bar 50 μm.
Statistical analysis
Analysis of the number of PER1-ir cells was performed by one-way ANOVA to determine whether there were differences across different time points and between each group; this was followed by a post hoc analysis using the Tukey test (SigmaStat 3.5). Comparisons between groups at specific time points in deprived nursing does were performed using Student's t-test. Probability levels of P<0.05 were considered significant. Values are given as means ± S.E. Additionally, acrophases of the number of PER1-ir were evaluated by Cosinor analysis using Acro.exe software (version 3.5) described by Refinetti (2006), where a rhythm was considered to be significant when P<0.05.
Results
In control animals all studied structures expressed PER1 without a clear rhythm, however, nursing induced a robust and significant PER1 rhythm in the POA, LSD and LSV, which shifted in parallel to timing of nursing. PER1 expression reached a peak 8 h after scheduled nursing in these structures, regardless of whether nursing occurred during day or the night. In the LSI and in BNST there was a completely different pattern. In the LSI, there was a significant rhythm in the Nursing ZT03 group where the maximum level was observed 16 h after nursing, whereas in the Nursing ZT19 group, no significant rhythm was expressed. In the BNST, no significant rhythm was observed in any group. The absence of suckling significantly decreased PER1 expression at peak time in both POA and LSD and LSV but the effect was more pronounced in the LS than in the POA.
PER1-ir in the POA
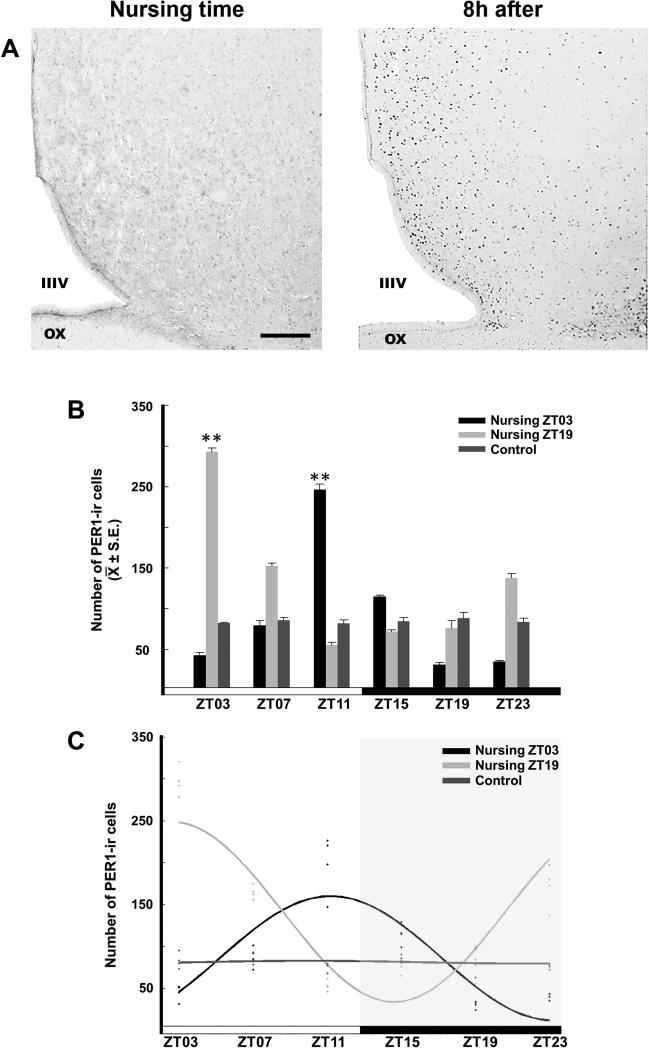
The POA showed a rhythm of PER1-ir in the nursing groups, with a peak at 8 h after nursing. No rhythm was detected in the Control group. The photomicrographs in Figure 2A show the POA of a representative section of a doe at the time of nursing and 8 h later. ANOVA revealed a significant difference across time in the Nursing ZT03 (F5,18 = 56.848, P < 0.001) and the Nursing ZT19 (F5,18 = 131.537, P < 0.001) groups. No significant differences across time were seen in the control group (F5,18 = 1.051, P = 0.419). For the Nursing ZT03 group, the highest value at ZT11 was significantly different from the remaining time point values (P < 0.001 in all cases). In the Nursing ZT19 group, the maximal value at ZT03 was significantly different from the remaining time point values (P < 0.001 in all cases; Fig. 2B). The cosinor analysis revealed significant rhythmicity of PER1-ir in both Nursing ZT03 (amplitude 101.0; acrophase 17.0h; P < 0.001) and Nursing ZT19 (amplitude 137.0; acrophase 9.0h; P < 0.001), but not in the Control group (amplitude 17.5 acrophase 17.0h; P > 0.05; Fig. 2C).
Figure 2.
Expression of PER1 in the POA. (A) Photomicrographs of representative sections at nursing time and at 8h after nursing in the Nursing ZT03 group. Note the density of PER1-ir cells 8h after nursing. IIIV, third ventricle, OX, optic chiasma. Scale bar 100 μm. (B) PER1-ir cells in Control, Nursing ZT03 and Nursing ZT19 groups; ** Denotes significant difference between higher and lower values within the same group. ** P < 0.001. Values are mean ± SEM. The black and white bar at bottom represents the LD condition. (C) Cosinor analysis indicates a significant rhythmicity of PER1-ir cells in Nursing ZT03 and Nursing ZT19, but not in Control group. See text for details.
PER1-ir in the LSD
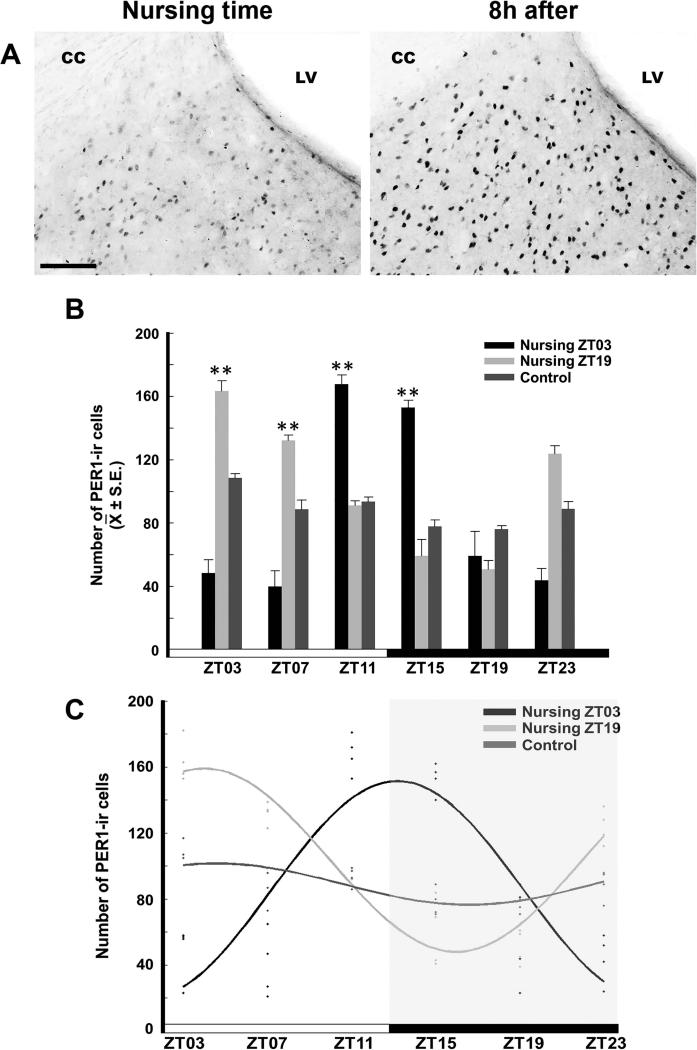
The LSD showed a rhythm of PER1-ir in the nursing groups, with peak values at 8 h after nursing. No rhythm was detected in the Control group. The photomicrographs in Figure 3A show the LSD in a representative section from a doe at the time of nursing and 8 h later. The ANOVA analysis revealed a significant difference across time in Control (F5,18 = 8.816, P < 0.001), Nursing ZT03 (F5,18 = 39.085, P < 0.001) and Nursing ZT19 (F5,18 = 11.303, P < 0.001) groups. In the Control group the peak value at ZT03 was significantly different from the ZT15 and ZT19 (P < 0.05 in both cases) values. In the Nursing ZT03 group, the higher values at ZT11 and ZT15 were significantly different from values at ZT03, ZT07, ZT19 and ZT23 (P < 0.001 in all cases). In the Nursing ZT19 group, the higher values at ZT03 and ZT07 were significantly different from remaining time point values (P < 0.001 for ZT15, ZT23 and P < 0.05 for ZT11 and ZT19; Fig. 3B). Cosinor analysis revealed significant rhythmicity of PER1-ir in both Nursing ZT03 (amplitude 80.0, acrophase 21.0h; P < 0.001) and Nursing ZT19 (amplitude 71.5; acrophase 9.0h; P < 0.001) groups, but not in the Control group (amplitude 23.0; acrophase 13h; P > 0.05; Fig. 3C).
Figure 3.
Expression of PER1 in the LSD. (A) Photomicrographs of representative sections at nursing time and at 8h after nursing in the Nursing ZT03 group. Note the density of PER1-ir cells 8h after nursing. LV, lateral ventricle, CC, corpus callosum. Scale bar 100 μm. (B) PER1-ir cells in Control, Nursing ZT03 and Nursing ZT19 groups; ** Denotes significant difference between higher and lower values within the same group.. ** P < 0.001. Values are mean ± SEM. The black and white bar at bottom represents the LD condition. (C) Cosinor analysis indicates a significant rhythmicity of PER1-ir cells in Nursing ZT03 and Nursing ZT19, but not in Control group. See text for details.
PER1-ir in the LSI
The LSI showed variations of PER1-ir in the nursing and control groups. The Nursing ZT03 group expressed a maximal value of PER1 at 16 h after nursing and was the only group with a significant rhythm. ANOVA revealed a significant difference across time in PER1-ir in the Control (F5,18 = 4.178, P < 0.05), Nursing ZT03 (F5,18 = 6.657, P < 0.001) and Nursing ZT19 (F5,18 = 7.870, P < 0.001) groups. For the Control group, the maximum value at ZT11 was only different from ZT03 (P < 0.05). For the Nursing ZT03 group, the maximal value at ZT19 was significantly different from the remaining time points (P < 0.001 in all cases). In the Nursing ZT19 group, maximal values at ZT11 and ZT19 were significantly different from ZT03, ZT07 and ZT23 (P < 0.001 for ZT03 and ZT07, P < 0.05 for ZT23). Cosinor analysis revealed significant rhythmicity of PER1-ir in Nursing ZT03 (amplitude 43.5; acrophase 1.0h; P < 0.05), but not in Nursing ZT19 (amplitude 35.0; acrophase 1.0h; P > 0.05) and control groups (amplitude 29.5; acrophase 21.0h; P > 0.05).
PER1-ir in the LSV
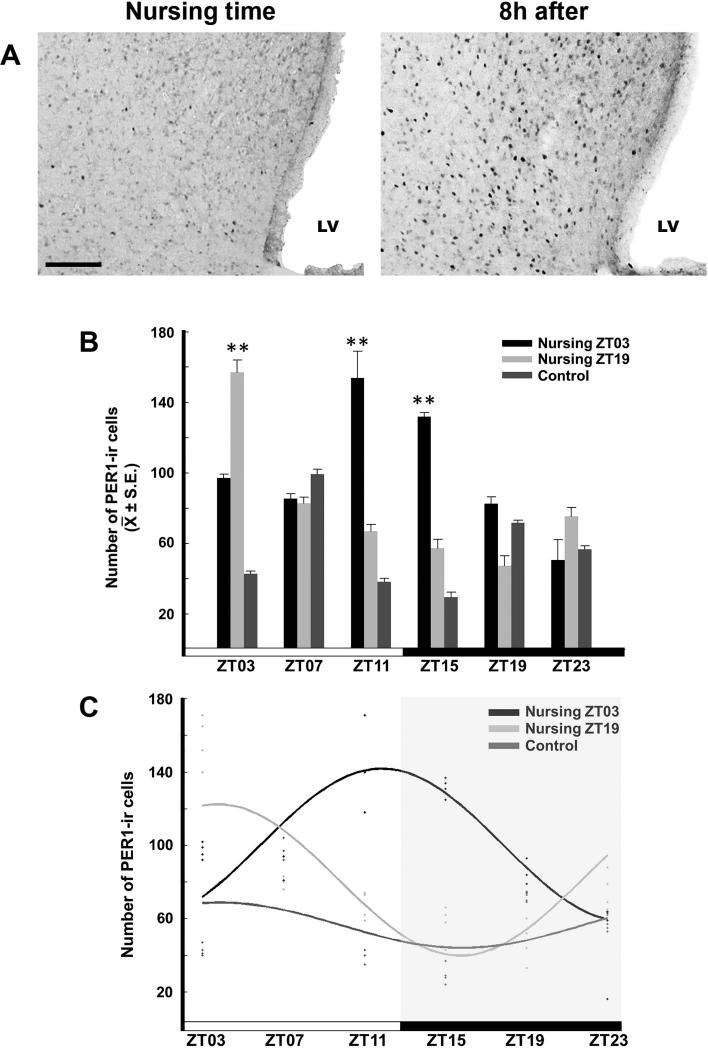
The LSV showed a rhythm of PER1-ir in the nursing groups, with maximal values at 8 h after of nursing. No rhythm was detected in the Control group. The photomicrographs in Figure 4A show the LSV in a representative section from a doe at the time of nursing and at 8 h later. ANOVA revealed a significant difference in PER-ir across time in the Control (F5,18 = 142.439, P < 0.001), Nursing ZT03 (F5,18 = 20.452, P < 0.001) and Nursing ZT19 (F5,18 = 22.918, P < 0.001) groups. For the Control group, the maximal value at ZT07 was significantly different from the remaining time point values (P < 0.001 in all cases). For the Nursing ZT03 group, the maximal values at ZT11 and ZT15 were significantly different from values at ZT03, ZT07, ZT19 and ZT23 (P < 0.001 in all cases). For the Nursing ZT19 group, the highest value at ZT03 was significantly different from the remaining time point values (P < 0.001 in all cases; Fig. 4B). Cosinor analysis revealed significant rhythmicity of PER1-ir in Nursing ZT03 (amplitude 85.0; acrophase 17.0h; P < 0.05) and Nursing ZT19 (amplitude 69.0; acrophase 9.0h; P < 0.01) groups, but not in Control group (amplitude 40.0; acrophase 9.0h; P > 0.05; Fig. 4C).
Figure 4.
Expression of PER1 in the LSV. (A) Photomicrographs of representative sections at nursing time and at 8h after nursing in the Nursing ZT03 group. Note the density of PER1-ir cells 8h after nursing. LV, lateral ventricle. Scale bar 100 μm. (B) PER1-ir cells in Control, Nursing ZT03 and Nursing ZT19 groups; ** P < 0.001 Denotes significant difference between higher and lower values within the same group. ** P < 0.001. Values are mean ± SEM. The black and white bar at botton represents the LD condition. (C) Cosinor analysis indicates a significant rhythmicity of PER1-ir cells in Nursing ZT03 and Nursing ZT19, but not in Control group. See text for details.
PER1-ir in the BNST
The BNST showed no variation in the number of PER1-ir cells in any group. ANOVA revealed no significant difference in PER1-ir across time in the Control (F5,18 = 1.051, P = 0.419), Nursing ZT03 (F5,18 = 1.966, P = 0.133) or Nursing ZT19 (F5,18 = 0.628, P = 0.680) groups. Cosinor analysis indicated no significant rhythmicity of PER1-ir in Control (amplitude 1.6; acrophase 21.40; P > 0.05), Nursing ZT03 (amplitude -6.5; acrophase 26.4; P > 0.05) and Nursing ZT19 (amplitude -8.5; acrophase 26.04; P > 0.05) groups.
PER1-ir in nursing deprived does
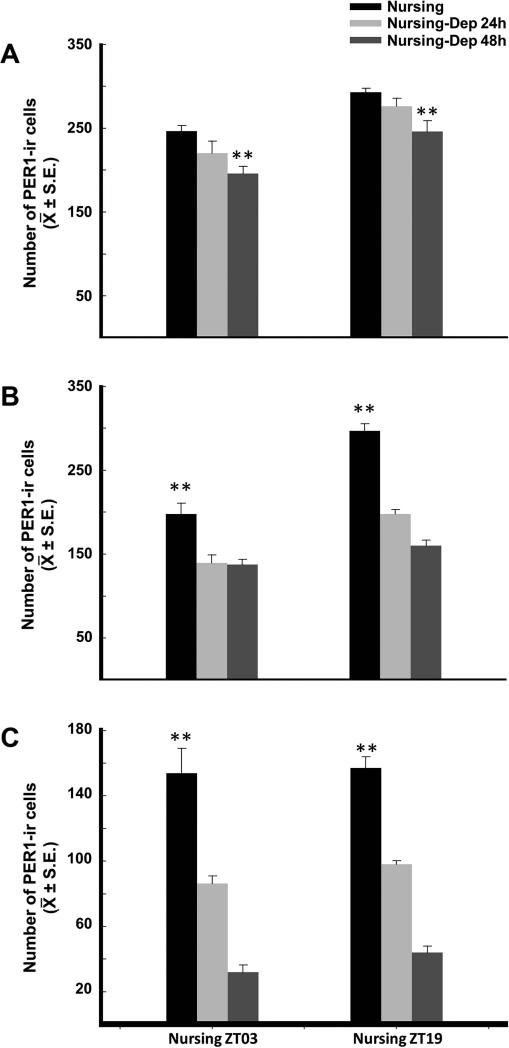
Figure 5 shows the number of PER1-ir cells 8 h after nursing compared to 8 h after the time of expected nursing in nursing-deprived does for 24 h and 48 h in POA, LSD and LSV. In the POA, quantitative analysis revealed a decrease of PER1-ir in Nursing-Dep 48h group in comparison to corresponding nursing group (P < 0.01 in both cases; Fig. 5A). In LSD (Fig. 5B) and LSV (Fig. 5C) a significant decrease was identified in Nursing-Dep 24h (P < 0.01, in both cases) and Nursing-Dep 48 h (P < 0.01; in both cases) groups in comparison to corresponding nursed does.
Figure 5.
Expression of PER1-ir cells in nursed does 8 h after nursing in comparison to nursed deprived females for 24 and 48 h, 8 h after expected nursing time in POA (A), LSD (B) and LSV (C). **Denotes significant differences between higher and lower values. See text for details. ** P < 0.001. Values are mean ± SEM.
Discussion
The results of this study confirm our hypothesis that timed nursing is associated with the rhythmic activity of areas in the forebrain critical for the expression of maternal behavior. Specifically we found that the PER1 protein rhythm shifts in parallel to the timing of nursing in the POA, and in the dorsal and ventral portion of LS, but not in the intermediate portion of this structure and the BNST.
The medial preoptic area in the rostral hypothalamus is critical for MB regulation in the rat and other species. In rodents, implants of estradiol, prolactin and placental lactogens in this area stimulate the onset of MB. Conversely, electrical lesions in the POA disrupt established MB or its onset at parturition but stimulation in this area induces the display of MB in sensitized virgin females (reviewed in Numan & Insel, 2003). In female rabbits treated with progesterone, estradiol implants in the POA elicited digging, and following progesterone withdrawal, straw carrying, two of the three components of maternal nest-building in this species (González-Mariscal et al., 2005). Moreover, after parturition, there is a significant three- to four-fold increase in the number of FOS expressing cells in the POA, including the periventricular preoptic region, in lactating rabbit does that express MB in comparison to virgins (González-Mariscal et al., 2009). Together, this evidence confirms the importance of POA for the establishment and expression of MB in the rabbit. In agreement, present results provide evidence that the POA can play an important role in the regulation of periodic rhythm of MB in this species as the rhythm of PER1 shifts in parallel to the timing of nursing and no rhythmic expression was observed in control animals. In this regard, the rhythmic activity of the POA is particularly interesting in light of the rhythm in body temperature that occurs during nursing. In pregnant rabbits, body temperature displays a rhythm that free runs with a period length greater than 24 h. But after parturition this rhythm is entrained by timing of scheduled nursing and the temperature peak coincides with the phase of nursing (Jilge et al., 2001). The POA contains temperature sensitive neurons, specifically located in the periventricular and medial POA, and together with other regions of the forebrain is considered the thermoregulatory center of the brain (Nakamura, 2011). We consider that this is an important point as perhaps temperature sensitive neurons in POA are important for the control of the daily rhythm of nursing in this species, and this possibility could be explored in future experiments.
Furthermore, our results in the POA are important as this area, together with mesolimbic circuitry that involves the ventral tegmental area and nucleus accumbens, promotes the activation or inhibition of brain pathways involved in maternal responses (Numan & Woodside, 2010). Thus the present results open the opportunity to explore the possible synchronization of this proposed pathway, as the POA may participate together with other structures as part of a multioscillatory mechanism to control the regulation of daily nursing. In this regard, it is interesting that the rhythm of locomotor behavior of lactating does shifts in parallel to timing of nursing, regardless of whether this is scheduled during the day or the night (Meza et al., 2008). Furthermore, females show an increase in locomotor behavior in the hours preceding nursing time (Meza et al., 2008). This behavioural pattern is similar to that observed in rodents under a food restriction schedule and which is known as food anticipatory activity (FAA; Mistlberger, 1994). FAA is present in rabbit pups (Caba et al., 2008) and it has been suggested that FAA is elicited by a multi-oscillatory system in which several central and peripheral structures interact (Mistlberger, 2011). In considering the present results with respect to the POA and LS (see below), we propose that these structures are part of an “entrainable maternal circuit” that supports regulation of daily maternal behavior in this species. Future studies are warranted to determine the identity and timing of hormonal factors during pregnancy or lactation that may be responsible for the establishment of this PER1 rhythm. Also, it will be important to determine the neurochemical phenotype of the PER1 expressing cells in order to get a better idea about neurotransmitters and/or peptides involved in the control of daily maternal behavior in this species.
The lateral septum is a complex structure in the telencephalon involved in the integration of several physiological and behavioral process related to higher cognitive functions, such as learning and memory, emotion, fear, aggression, and stress, among others (Jakab & Leranth, 1995). Additionally, this structure has been linked to the control of complex behaviors such as maternal behavior in rodents (Numan & Insel, 2003). Lesions of the septum disrupt MB, including deficiency of lactation and cannibalism, in mice (Carlson et al., 1968) and rats (Slotnick & Nigrosh, 1975). In female rabbits with lesions of the septum, pregnancy and parturition is normal, but females show marked disturbances in nest building and after parturition, lack of care of the young, unwillingness to nurse the litters and cannibalism (Cruz & Beyer, 1972). Together this evidence suggests that lesions of the septum affect both the neural control of this complex behavior and perhaps also the neuroendocrine system responsible for lactation. However, in a careful study of postpartum females with lesions in the septum, Fleischer and Slotnick (1978) demonstrated in the rat that failure to nurse their pups is not due to an interruption of neuroendocrine pathways necessary for lactation or milk ejection reflex. Nevertheless the septum massively projects to the limbic system, particularly the hippocampal formation, as well as to the diencephalon where several hypothalamic regions and nuclei such as the preoptic area are their main targets. In addition, many regions of the brain send projections to the LS, one of the heaviest being from the lateral preoptic area (Jakab & Leranth, 1995). Then, although is possible that the deficits in MB are due to the interruption of neural pathways to other areas related to the control of MB, such as those with the POA, it is possible that cells in the LS are indeed involved in the regulation of MB. The reason for this assumption comes from two sources. First, in lactating female rats expressing MB, there is a significant increase of FOS expressing cells in the LS, even when they are exposed to pups without suckling (Lonstein et al., 1998; Stack & Numan, 2000). In rabbits, the brief interaction with the pups for only one daily nursing episode on the day after parturition induces a two-fold increase in the number of FOS expressing cells in LS compared to lactating females that are not given pups for suckling on the corresponding day, or to virgin females (González-Mariscal et al., 2009). The second source of evidence comes from the present results. Nursing induces a clear rhythm of PER1 in the LSD and LSV that shifts in parallel to the timing of nursing either during the day or the night in comparison to non-lactating, non-pregnant females. Future experiments using excitotoxic axon-sparing lesions are warranted to investigate the specific importance of LS cells in MB (Numan et al., 1988). It is very important to take into account the massive afferent and efferent projections of the septum, which make it difficult to interpret the complex MB disturbances by lesions.
The area of the BNST analyzed, the vBST, lies dorsolateral to the mPOA, and shows activation, as indicated by FOS, during the expression of MB in the rat (Numan & Numan, 1994). The vBST, together with mPOA, are considered to form a functional system important for maternal behavior in rats (Numan & Insel, 2003). However, vBST lesions made by excitotoxic amino acids disrupt mainly retrieval behavior, and, in general, the deficit in MB is not as severe as that seen after lesions of the POA (Numan & Numan, 1996). Perhaps due to its importance in retrieval behavior, this region seems to be not activated in lactating rabbits (Gonzalez-Mariscal et al., 2009) and does not show a rhythm related to timing of nursing (present results) as the rabbit doe does not retrieve their pups during their only visit for nursing.
It is important to emphasize that the rhythms observed in the LS and POA are driven rhythms as they depend on suckling stimuli. As shown, skipping just one suckling bout is sufficient to significantly decrease the number of PER1 expressing cells in the LS. Missing two suckling bouts induces a further 3-4 fold decrease in comparison to animals that nursed. In contrast, the decrease of PER1 by nursing deprivation in the POA is less severe but is already significant after the missing of two suckling bouts. This PER1 decrease was also observed in neuroendocrine cells in the lactating doe (Meza et al., 2008; 2011) and confirms that suckling is a powerful stimuli that induce rhythmic activity in the female rabbit brain. Additionally, our results confirm that specific stimuli, other than light, are capable of inducing rhythms of clock genes and their protein products in brain regions outside the suprachiasmatic nucleus (Guilding & Piggins, 2007; Kriegsfeld & Silver, 2006).
We conclude that daily suckling of pups is a powerful stimulus that induces driven rhythms of PER1 in brain areas relevant for expression of daily maternal behavior in the rabbit, which perhaps are part of an entrainable maternal circuit.
Acknowledgements
We thank Sean Deats, Dr. Daniel Granados and Dr. Michael N. Lehman, for corrections and helpful comments. We also gratefully acknowledge Biol. Mercedes Acosta for her invaluable help with maintaining and caring for the rabbit colony. This study was partially supported by National Institutes of Health/Fogarty grant R01TW006636 (M.C.) and by grants from CONACYT: 1010/152/2014 C-133/2014 and 297440 (J.A.).
Abbreviations
- BNST
bed nuclei of stria terminalis
- DAB
3,3 diaminobenzidine
- FAA
food anticipatory activity
- ir
immunoreactive or immunoreactivity
- LS
lateral septum
- LSD
lateral septum dorsal
- LSI
lateral septum intermediate
- LSV
lateral septum ventral
- LD
light–dark
- MB
maternal behavior
- OT
oxytocin
- PB
phosphate buffer
- PER1
Period1 (protein)
- PP
postpartum
- POA
preoptic area
- ZT
zeitgeber time
Footnotes
The authors declare no conflicts of interest.
References
- Caba M, Beyer C, González-Mariscal G, Morrell JI. Immunocytochemical detection of estrogen receptor-α in the female rabbit forebrain: topography and regulation by estradiol. Neuroendocrinology. 2003;77:208–222. doi: 10.1159/000069508. [DOI] [PubMed] [Google Scholar]
- Caba M, Tovar A, Silver R, Morgado E, Meza E, Zavaleta Y, Juárez C. Nature's food anticipatory experiment: entrainment of locomotor behavior, suprachiasmatic and dorsomedial hypothalamic nuclei by suckling in rabbits pups. Eur. J. Neurosci. 2008;27:432–443. doi: 10.1111/j.1460-9568.2008.06017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caba M, González-Mariscal G. The rabbit pup, a natural model of nursing-anticipatory activity. Eur. J. Neurosci. 2009;30:1697–1706. doi: 10.1111/j.1460-9568.2009.06964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson NR, Thomas GJ. Maternal behavior of mice with limbic lesions. J. Comp. Physiol. Psychol. 1968;66:731–737. doi: 10.1037/h0026513. [DOI] [PubMed] [Google Scholar]
- Cruz ML, Beyer C. Effects of septal lesions on maternal behavior and lactation in the rabbit. Physiol. Behavior. 1972;9:361–365. doi: 10.1016/0031-9384(72)90160-6. [DOI] [PubMed] [Google Scholar]
- Fleischer S, Slotnick BN. Disruption of maternal behavior in rats with lesions of the septal area. Physiol. Behav. 1978;21:189–200. doi: 10.1016/0031-9384(78)90041-0. [DOI] [PubMed] [Google Scholar]
- Girgis M, Shi-Chang W. A New Stereotaxic Atlas of the Rabbit Brain. Warren H. Green Inc.; St Louis MO, USA: 1981. [Google Scholar]
- González-Mariscal G, Poindron P. Parental care in mammals: immediate, internal and sensory factors of control. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, brain and behavior. Academic press; Sn Diego, USA: 2002. pp. 215–298. [Google Scholar]
- González-Mariscal G, Chirino R, Rosenblatt JS, Beyer C. Forebrain implants of estradiol stimulate maternal nest-building in ovariectomized rabbits. Horm. Behav. 2005;47:272–279. doi: 10.1016/j.yhbeh.2004.11.004. [DOI] [PubMed] [Google Scholar]
- González-Mariscal G, Jiménez A, Chirino R, Beyer C. Motherhood and nursing stimulate c-Fos expression in the rabbit forebrain. Behav. Neuosci. 2009;123:731–739. doi: 10.1037/a0016487. [DOI] [PubMed] [Google Scholar]
- González-Mariscal G, Lemus AC, Vega-Gonzalez A, Aguilar-Roblero R. Litter size determines circadian periodicity of nursing in rabbits. Chronobiol. Int. 2013;30:711–718. doi: 10.3109/07420528.2013.784769. [DOI] [PubMed] [Google Scholar]
- Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur. J. Neurosci. 2007;25:3195–3216. doi: 10.1111/j.1460-9568.2007.05581.x. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Leranth C. Septum. In: Paxinos G, editor. The rat nervous system. Academic press; Sn. Diego, USA: 1995. pp. 405–442. [Google Scholar]
- Jilge B. Ontogeny of the rabbit's circadian rhythms without an external zeitgeber. Physiol. Behav. 1995;58:131–140. doi: 10.1016/0031-9384(95)00006-5. [DOI] [PubMed] [Google Scholar]
- Jilge B, Kuhnt B, Landerer W, Rest S. Circadian temperature rhythms in rabbit pups and in their does. Lab. Animal. 2001;35:364–373. doi: 10.1258/0023677011911831. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld L, Silver R. The regulation of neuroendocrine function: timing is everything. Hom. Behav. 2006;49:557–574. doi: 10.1016/j.yhbeh.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-Fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82:267–281. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- Meza E, Juárez C, Morgado E, Zavaleta Y, Caba M. Brief daily suckling shifts locomotor behavior and induces PER1 protein in paraventricular and supraoptic nuclei, but not in the suprachiasmatic nucleus, of rabbit does. Eur. J. Neurosci. 2008;28:1394–1403. doi: 10.1111/j.1460-9568.2008.06408.x. [DOI] [PubMed] [Google Scholar]
- Meza E, Waliszewski SM, Caba M. Circadian nursing induces PER1 protein in neuroendocrine tyrosine hydroxylase neurons in the rabbit doe. J. Neuroendocrinol. 2011;23:472–480. doi: 10.1111/j.1365-2826.2011.02138.x. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food anticipatory activity: formal models and physiological mechanisms. Neurosci. Biobehav. Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Mistlberber RE. Neurobiology of food anticipatory circadian rhythms. Physiol. Behav. 2011;104:535–545. doi: 10.1016/j.physbeh.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Nakamura K. Central circuitries for body temperature regulation and fever. Am J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R1207–R1228. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- Numan M, Corodimas KP, Numan M, Factor EM, Piers WD. Axon-sparing lesions of the preoptic region and substantia innominate disrupt maternal behavior in rats. Behav. Neurosci. 1988;102:381–396. doi: 10.1037//0735-7044.102.3.381. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ. Expression of Fos-like immunoreactivity in the preoptic area of maternally behaving virgin and postpartum rats. Behav. Neuosci. 1994;108:379–394. doi: 10.1037//0735-7044.108.2.379. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev. Psychobiol. 1996;29:23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. Springer; New York: 2003. p. 418. [Google Scholar]
- Numan M, Woodside B. Maternity: neural mechanisms, motivational processes, and physiological adaptations. Behav. Neurosci. 2010;124:715–741. doi: 10.1037/a0021548. [DOI] [PubMed] [Google Scholar]
- Olazábal DE, Pereira M, Agrati D, Ferreira A, Fleming AS, González-Mariscal G, Lévy F, Lucion AB, Morrell JI, Numan M, Uriarte N. Flexibility and adaptation of the neural substrate that supports maternal behavior in mammals. Neurosci. Biobehav. Rev. 2013;37:1875–1892. doi: 10.1016/j.neubiorev.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Refinetti R. Circadian physiology. Taylor & Francis group.; Boca Raton FL, USA: 2006. p. 27. [Google Scholar]
- Slotnick BM, Nigrosh BJ. Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. J. Comp. Physiol. Psychol. 1975;88:118–127. doi: 10.1037/h0076200. [DOI] [PubMed] [Google Scholar]
- Stack EC, Numan M. The temporal course of expression of c-Fos and Fos B within the medial preoptic area and other brain regions of postpartum female rats during prolonged mother-young interactions. Neuroscience. 2000;114:609–622. doi: 10.1037//0735-7044.114.3.609. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. The connections of the septal region in the rat. J. Comp. Neurol. 1979;186:621–655. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- Zarrow MX, Denenberg VH, Anderson C. Rabbit: frequency of suckling in the pup. Science. 1965;150:1835–1836. doi: 10.1126/science.150.3705.1835. [DOI] [PubMed] [Google Scholar]