Abstract
Disorders of human salivary glands resulting from therapeutic radiation treatment for head and neck cancers or from the autoimmune disease Sjögren syndrome (SS) frequently result in the reduction or complete loss of saliva secretion. Such irreversible dysfunction of the salivary glands is due to the impairment of acinar cells, the major glandular cells of protein, salt secretion, and fluid movement. Availability of primary epithelial cells from human salivary gland tissue is critical for studying the underlying mechanisms of these irreversible disorders. We applied 2 culture system techniques on human minor salivary gland epithelial cells (phmSG) and optimized the growth conditions to achieve the maintenance of phmSG in an acinar-like phenotype. These phmSG cells exhibited progenitor cell markers (keratin 5 and nanog) as well as acinar-specific markers—namely, α-amylase, cystatin C, TMEM16A, and NKCC1. Importantly, with an increase of the calcium concentration in the growth medium, these phmSG cells were further promoted to acinar-like cells in vitro, as indicated by an increase in AQP5 expression. In addition, these phmSG cells also demonstrated functional calcium mobilization, formation of epithelial monolayer with high transepithelial electrical resistance (TER), and polarized secretion of α-amylase secretion after β-adrenergic receptor stimulation. Taken together, suitable growth conditions have been established to isolate and support culture of acinar-like cells from the human salivary gland. These primary epithelial cells can be useful for study of molecular mechanisms involved in regulating the function of acinar cells and in the loss of salivary gland function in patients.
Keywords: acinar cells, calcium mobilization, primary epithelial cells, explant culture, primary cell culture, Sjögren’s syndrome
Introduction
Salivary glands are exocrine organs that produce saliva, a fluid composed of electrolytes and proteins, which is critical for maintenance of oral health and for mastication, ingestion, and initial digestion of food in the oral cavity (Miletich 2010). The main types of cells found in salivary glands are acinar and ductal cells. A major consequence of salivary gland dysfunction is loss of saliva secretion leading to dry mouth. Some conditions that lead to salivary dysfunction are Sjögren syndrome (SS), an autoimmune disease affecting exocrine glands, and radiotherapy for treatment of head and neck cancer. In both conditions, the irreversible loss of saliva secretion is associated, at least initially, with impairment of acinar cell function, caused either by destruction mediated by lymphocytic infiltration in SS or by DNA damage caused by irradiation. No adequate treatments are currently available for either condition.
A major roadblock in delineating the mechanisms underlying salivary dysfunction has been the lack of functional cultures of salivary gland cells. Few studies have reported the isolation and growth of primary epithelial cells from explants of salivary gland tissues (Sens et al. 1985; Sabatini et al. 1991; Okura et al. 1993; Dimitriou et al. 2002). However, maintenance of the acinar phenotype for long periods of time and preservation of the acinar cell functionality have not been demonstrated yet (Redman and Quissell 1993). Currently available immortalized human salivary cell lines, such as the human submandibular gland (HSG) cell line, are primarily of ductal origin (Shirasuna et al. 1981). When grown on extracellular matrix substrates, HSG cells can be induced to assume an acinar-like morphology (Hoffman et al. 1996; Lam et al. 2005). However, these cells are unable to form polarized monolayers and do not show transepithelial electrical resistance (TER) (Aframian et al. 2002; Tran et al. 2005).
Here, we aimed to establish conditions to maintain cultures of primary human minor salivary gland epithelial cells (referred as phmSG cells hereafter) and to shift them into a more acinar-like phenotype. Our data demonstrate that cultured phmSG cells express progenitor cell markers (keratin 5 and nanog) as well as several acinar-specific markers (α-amylase, cystatin C, TMEM16A, and NKCC1). Importantly, by raising the calcium concentration in the growth medium, phmSG cells function like acinar cells, as indicated by an increase in AQP5 expression, and demonstrate receptor-stimulated calcium mobilization, formation of epithelial monolayer with expression of the tight-junction protein ZO-1 with high TER, and polarized secretion of α-amylase secretion after β-adrenergic receptor stimulation.
Materials and Methods
Human Salivary Gland Tissue and Cell Cultures
Explant culture of human salivary gland biopsy and primary epithelial culture are described in the Appendix.
Western Blot and Quantitative Real-Time Polymerase Chain Reaction
Western blotting and quantitative real-time polymerase chain reaction (PCR) for the expression of cell type–specific markers are described in the Appendix.
Immunofluorescence and Confocal Microscopy Imaging
Immunocytostaining and confocal microscopy imaging for the expression of cell type–specific markers are described in the Appendix.
Measurement of TER and α-Amylase Activity
phmSG cells were plated at a density of 20 × 104 cells in 0.3 mL keratinocyte growth medium–low calcium, 0.05 mM (KGM-L) in the upper chamber and with 1.0 mL KGM-L in the lower chamber of a Transwell-COL (0.4 µm pore size, collagen-coated polyester insert; Corning, Corning, NY, USA). When the cultures were about 90% confluent, the culture medium was switched to keratinocyte growth medium–high calcium, 0.80 mM (KGM-H) for 3 d. TER measurements were conducted in 3 different locations in each insert using a Millicells-ERS device (Millipore, Billerica, MA, USA) following the manufacturer’s protocol. Each Transwell insert culture was prepared in triplicate. The readings from an insert without phmSG cells were used as a background control and subtracted from the average measurement from inserts seeded with phmSG cells.
To monitor α-amylase secretion, we plated the phmSG cells in Transwell inserts. Some of Transwell culture medium was changed to a KGM without epinephrine and incubated overnight (desensitization). The phmSG cultures were then stimulated with either epinephrine (10 µM) or isoproterenol (1 µM) in KGM-L or KGM-H for 45 min before collection of medium from the Transwell insert (top side) and from the microplate well (bottom side). Activity of α-amylase (mU/mL) was measured using the Amylase Activity Assay kit (BioVision, Milpitas, CA, USA) following the manufacturer’s protocol.
Measurement of Ca2+ Mobilization and Cell Volume Change in phmSG Cells
The phmSG cells were incubated with either Fura-2AM (10 µM; Invitrogen, Carlsbad, CA, USA) in standard extracellular solution (Ong et al. 2012) or Fluo-4AM (5 µM; Invitrogen) in its growth medium for 30 min at 37 °C. The cultures were washed twice with phosphate-buffered saline (PBS), and measurements were conducted in KGM without calcium. The agonist and antagonist (adenosine triphosphate [ATP], 100 µM [Sigma, St. Louis, MO, USA] or thapsigargin [Tg], 1 µM [Calbiochem, Billerica, MA, USA]) were added, followed by the addition of CaCl2 (1 mM). The Fura-2 signal was recorded using the Olympus IX51 microscope (Olympus, Tokyo, Japan) operated using the Metafluor imaging software (Molecular Devices, Sunnyvale, CA, USA) with a Till Photonics-Polychrome V spectrofluorometer (FEI, Hillsboro, OR, USA). The Fluo-4 AM signal was recorded by Fluostar Omega (BMG Labtech, Ortenberg, Germany) with the excitation and emission wavelengths set at 485 and 520 nm, respectively. Cell volume measurements in phmSG cells were performed as previously described (Liu et al. 2006).
Statistical Analysis
Results are presented as the mean ± standard error (SE). Statistical significance was determined by Student’s t test or analysis of variance (ANOVA). P values of less than 0.05 were considered statistically significant.
Results
Explant Culture of Human Minor Salivary Gland Tissue
The explant cell outgrowths were seen within 10 d (Appendix Fig. 1A(a) and (b)) and reached about 80% confluency in 3 wk. We observed 2 populations with differences in size and morphology in the outgrowth of phmSG cells. While there were some large cells exhibiting flat shape (Appendix Fig. 1A(c), arrows), most cells displayed cobblestone morphology. While the origin of the large cells is unclear, they decreased after the first passage. Fibroblast-like cells with spindle morphology were occasionally found in the initial outgrowth cultures (Appendix Fig. 1A(d), FL) but were separated from the epithelial cells (Appendix Fig. 1A(d), Epi) during the first passage through treatment with EDTA.
Two serum-free culture media were evaluated. For KGM containing 4 (bovine pituitary extract [BPE], human epidermal growth factor [hEGF], insulin [INS], and hydrocortisone [HC]) or 6 (BPE, hEGF, INS, HC, epinephrine, and transferrin) supplements, no apparent difference in morphology was observed (Appendix Fig. 1B, #1 vs. #3 or #2 vs. #4). Cells grown in KGM containing 6 supplements exhibited a more uniform population (#3, #4) compared with those in KGM with 4 supplements (#1, #2). Since [Ca2+] in media alters cell growth and differentiation (Hennings et al. 1980; Yuspa et al. 1989), we assessed the effect of [Ca2+] in the media of phmSG cultures. Cells maintained in various [Ca2+] media altered cell morphology and distribution (Appendix Fig. 1B). While phmSG cells grown in KGM with low [Ca2+] (0.05 mM; KGM-L) displayed a scattered distribution, cells in high [Ca2+] (0.8 mM; KGM-H) appeared smaller in size and aggregated together (Appendix Fig. 1B, #1 vs. #2, or #3 vs. #4).
The phmSG cells grown in mammalian epithelial basal medium (MEBM) with low [Ca2+] (0.05 mM) displayed large cell sizes with flat morphology (Appendix Fig. 1B, #5). Increasing [Ca2+] to 0.8 mM also induced changes similar to that seen with KGM-H (Appendix Fig. 1B, #6). In addition, the phmSG cells frozen for more than 3 mo were thawed and grown in KGM-L. The phmSG cells reached around 90% confluency within 3 d (Appendix Fig. 1C), suggesting that cell proliferation capacity was preserved after the freeze-thaw process. Importantly, when maintained in KGM-L, these cells were successfully cultured for over 10 passages.
Expression of Epithelial and Salivary Gland Markers in phmSG Cells
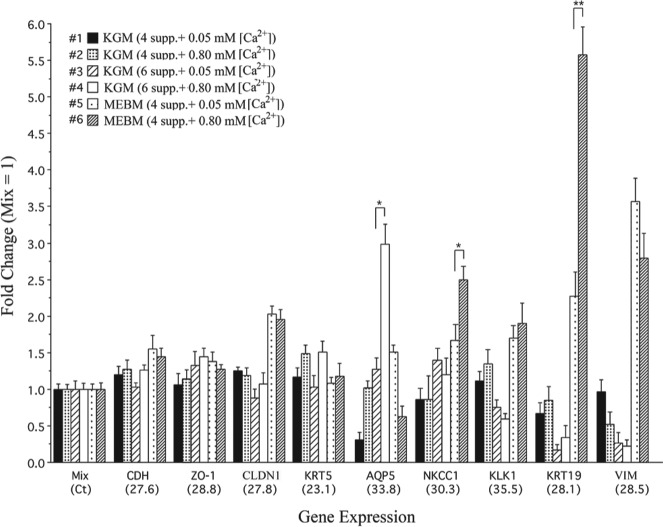
To characterize the cultured phmSG cells, we monitored the expression of acinar and ductal cell–specific markers. Expression of CDH (E-cadherin), ZO-1 (ZO1), and CLDN1 (claudin-1) was high in phmSG cells cultured in all types of media (Fig. 1). A relatively high expression level of AQP5 and SLC12A2 (Na+/K+/2Cl– cotransporter, NKCC1), 2 known markers of acinar cells, was observed in phmSG cells maintained in KGM containing 6 supplements with either low (0.05 mM) or high (0.80 mM) [Ca2+] (Fig. 1, #3 and #4). Expression levels of vimentin and the ductal cell markers, KLK1 (kallikrein 1) and KRT19 (keratin 19), were low. KRT5 (keratin 5), a progenitor cell marker (Knox et al. 2010), showed the highest expression among the transcripts examined. High expression of SLC12A2, KLK1, KRT19, and VIM (vimentin) was detected in phmSG cells grown in supplemented MEBM (Fig. 1, #5 and #6), suggesting that ductal characteristics were also enhanced with these media.
Figure 1.
Gene expression profiles in phmSG cells maintained in different growth medium. The phmSG cells were maintained in indicated supplemented growth medium (#1 to #6) for 3 d and the total RNA were isolated for the measurement of CDH, ZO1, CLDN1, KRT5, AQP5, SLC12A2, KLK1, KRT19, and VIM transcript by quantitative real-time reverse transcription (RT)–polymerase chain reaction. The data are from 3 independent experiments with triplicate samples for each transcript measurement. The relative quantitation of each transcript was calculated (see Appendix), and the results are presented as fold change (mean ± standard error) over each respective gene transcript measured in the mix control sample, which was generated by pooling an equal amount of complementary DNA from all 6 samples after RT reaction. The Ct value (cycle threshold, parenthesis at x-axis) represents each target gene expression level in the mix sample. *P < 0.05. **P < 0.005.
Expression of Simple Epithelial and Progenitor Cell Markers in phmSG Cells
Cytokeratin 18 (K18) exhibited a filamentous structure in the cellular cytoplasmic region (Appendix Fig. 2A). Expression of the progenitor cells marker of cytokeratin 5 (K5) displayed filament-like structures and distributed throughout the cytoplasm in phmSG cells (Appendix Fig. 2C), and nanog, a transcription factor of self-renewal marker in stem cells, was detected in the nucleus of most phmSG cells (Appendix Fig. 2E–G).
Expression of Salivary Acinar Marker Proteins in phmSG Cells
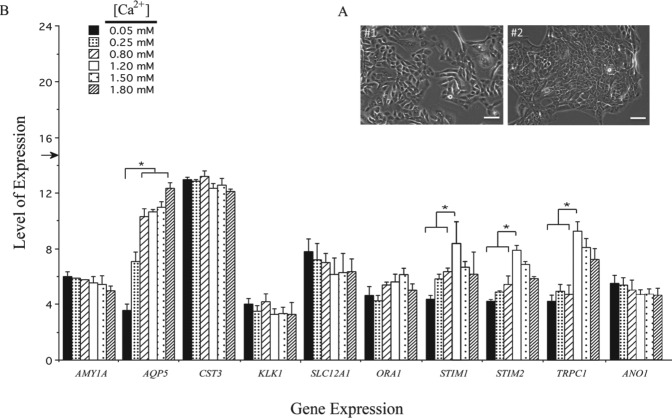
Since phmSG cells grown in KGM-H medium aggregated to a cluster pattern (Fig. 2A, #1 and #2), we investigated if the change of [Ca2+] in the KGM medium affects the expression of several cell type–specific markers. Most of the markers examined did not show significant changes in their expression when [Ca2+] was increased from 0.05 to 1.8 mM in the medium (Fig. 2B). Expression levels of CST3 (cystatin C), ORAI1, and SLC12A2 were relatively high and remained unchanged at all media tested. Modest levels of expression were found for α-amylase and ANO1, which were also unaffected by calcium changes. The expression levels of STIM1, STIM2, and TRPC1 were enhanced when [Ca2+] was increased from 0.05 mM to 1.2 mM, and the levels seen at 1.5 and 1.8 mM [Ca2+] were similar to that found at lower [Ca2+] (0.05, 0.25, and 0.8 mM), indicating that their expression is not [Ca2+] dependent, but instead, it is optimal at 1.2 mM [Ca2+]. Expression of AQP5 increased in a [Ca2+]-dependent manner in phmSG cells, with at least a 1.5-fold increase at [Ca2+] >0.05 mM, and reached a plateau after 0.8 mM. These data demonstrate that increasing [Ca2+] in KGM promotes AQP5 expression in phmSG cells.
Figure 2.
Gene expression profile of phmSG culture maintained in keratinocyte growth medium (KGM) containing different [Ca2+]. (A) Phase contrast images of phmSG cultures grown in the KGM with 0.05 mM (#1) or 0.8 mM (#2) calcium concentration. Bar = 50 µm. (B) Total RNAs were isolated from phmSG cultures grown under KGM containing indicated [Ca2+] for 3 d prior to total RNA isolation. Total RNAs were subjected to real-time reverse transcription–polymerase chain reaction. The results were from 3 separate experiments with triplicate samples for each transcript measurement. Data are shown as level of expression, which is calculated (as described in the Appendix) to represent the level of each gene expression and expressed as mean ± standard error for each transcript (AMY1A, AQP5, CST3, SLC12A1, ORA1, STIM1, STIM2, TRPC1, ANO1). (*P < 0.05, for AQP5: 0.05 mM vs. 0.8, 1.2, 1.5, and 1.8 mM calcium concentration, respectively; for STIM1, STIM2, and TRPC1: 1.2 mM vs. 0.05, 0.25 and 0.8 mM calcium concentration, respectively). Endogenous GAPDH expression was used to normalize the RNA input, and its expression level is indicated as an arrow shown on the y-axis.
Immunofluorescence staining for acinar cell markers such as SLC12A2 and CST3 (Ball 1993) showed a spotty distribution in the cytoplasmic region of SLC12A2 in phmSG cells (Appendix Fig. 3A), with more intense staining in the plasma membrane among cells with tight contact regions (arrowheads in Appendix Fig. 3A). CST3 also stained positively and distributed throughout the cytoplasmic region (Appendix Fig. 3B). AQP5 staining showed only a few positively stained phmSG cells when grown in KGM-L (Appendix Fig. 3C), whereas more positive cells with massive and intense staining were observed in phmSG cells grown in KGM-H (Appendix Fig. 3D). To monitor if the acinar cell markers were expressed after a few passages, the phmSG cells grown in either KGM-L or KGM-H were also stained positively with SLC12A2 and CST3 (Appendix Fig. 3E, F). Together, these results show that phmSG cells can maintain phenotypic characteristics after multiple passages and can be promoted into acinar-like epithelial cells when cells are grown in KGM-H (Wei et al. 2007; Patel et al. 2008).
Formation of Tight Monolayers and Polarized Regulated α-Amylase Secretion in phmSG Cell Cultures
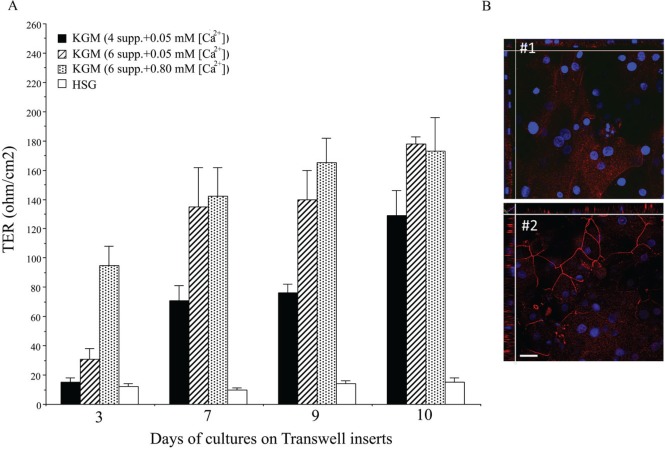
HSG and phmSG cells were seeded on collagen-coated Transwell inserts in KGM containing either 4 or 6 supplements in low or high [Ca2+] condition. The phmSG culture showed increased TER by day 3 and continued to increase until day 11, while HSG cells did not exhibit any TER (Fig. 3A). Cells in KGM-H showed the fastest rate of TER development. While cells in KGM-L with 6 supplements initially showed a lower rate of TER development on day 3, TER values for these cells increased to the same extent as those in KGM-H by day 7. Cells grown in KGM-L with 4 supplements also displayed increased TER but at a much slower rate and did not reach values similar to KGM-L with 6 supplements or in KGM-H at day 11. The tight junction protein ZO-1 was expressed in cells grown on the Transwell inserts and maintained in either KGM-L or KGM-H (Fig. 3B). While ZO-1 was loosely distributed in the cytoplasm of cells grown in KGM-L (day 3; Fig. 3B, #1), cells grown in KGM-H exhibited a contiguous expression in the plasma membrane, which showed the formation of mature cell-cell tight junctions. Shown in y-z-stack view (side view of Fig. 3B, #2), ZO-1 was localized at the basal-lateral region and toward the apical side of the cells. Together, the initial TER seemed calcium dependent in the day 3 Transwell insert culture, but TER showed no significant difference between KGM-L and KGM-H after 7 d.
Figure 3.
Transepithelial electrical resistance (TER) measurement and ZO-1 expression in phmSG cultures. Cells were seeded in Transwell inserts (as described in Materials and Methods) and maintained under indicated growth medium. (A) TER measurement was conducted at each indicated time (days after seeding). Three independent experiments with each duplicated samples and 3 separated readings were recorded, converted, and expressed as a mean ± S.E (ohm/cm2). (B) Immunofluorescence staining of ZO-1 in phmSG culture of Transwell insert maintained (day 3) under keratinocyte growth medium–low (KGM-L; #1) or keratinocyte growth medium–high (KGM-H; #2). DAPI was for nuclei staining. Image was taken on a confocal microscope with z-stacking starting from the basal layer toward the apical region. Bar = 50 µm.
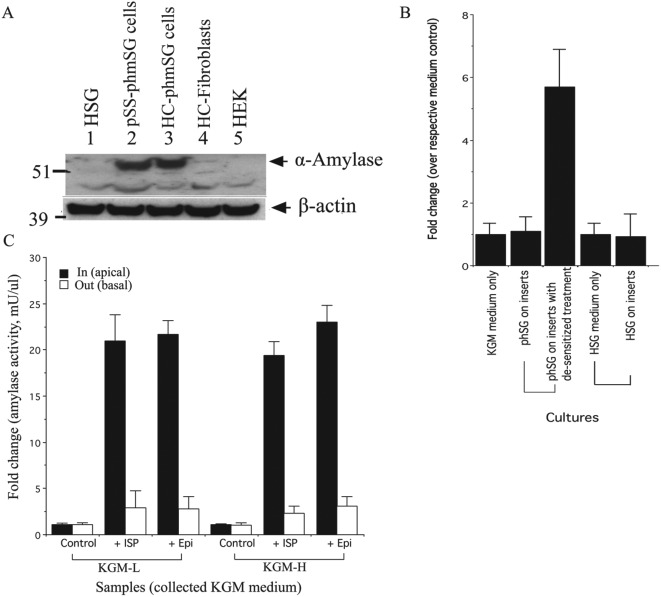
One distinct feature of salivary acinar cells is the secretion of α-amylase in response to stimulation of adrenergic receptors (Putney 1986). Western blotting confirmed the expression of α-amylase protein in phmSG cultures from 2 SS patients (#3090 and #3202) but not in HSG or HEK cells or in primary fibroblasts isolated from the salivary gland of a SS patient (#3202) (Fig. 4A). We also examined the capacity of the cells to secrete α-amylase in a polarized manner by plating phmSG cells on Transwell inserts through stimulation with adrenergic agonists. Nonstimulated levels of α-amylase activity were undetectable in the media obtained from both the apical and basal sides of a phmSG culture (Fig. 4B). However, when phmSG cells were first desensitized by incubation with KGM lacking epinephrine overnight, a high level of α-amylase activity was detected in the medium from the apical (but not the basal) side of the monolayer without agonist stimulation. Following stimulation by either isoproterenol (ISP) or epinephrine (Epi), a high level of α-amylase activity was detected in the apical media of a phmSG cell monolayer, with a 20-fold increase compared with control (Fig. 4C). No significant difference was found for α-amylase activity in the basal media between nonstimulated and stimulated conditions. Furthermore, agonist-induced α-amylase activity was unaffected by the [Ca2+] in the media.
Figure 4.
Expression and secretion of α-amylase in phmSG cultures. (A) Western blot analysis of α-amylase expression in immortalized human salivary gland cell line (HSG), pSS minor salivary gland epithelial cells (pSS-phmSG), healthy control minor salivary gland epithelial cells (HC-phmSG), healthy control minor salivary gland fibroblasts (HC-Fibroblasts), and human embryonic kidney cell line (HEK). The β-actin was used as a loading control. (B) Secretion of α-amylase in the keratinocyte growth medium (KGM) collected from the apical side of phmSG and HSG cultures grown on Transwell inserts. The desensitized treatment and measurement of α-amylase activity were performed as described in the Materials and Methods. Data are present as fold change over each respective medium control sample. (C) Effect of isoproterenol (ISP, 1 µM) and epinephrine (Epi, 10 µM) on α-amylase secretion in phmSG Transwell insert cultures. Cultures were treated with indicated agonists for 45 min before the culture medium (KGM-low [KGM-L] or KGM-high [KGM-H]) was collected from the top (apical side, in, black) or bottom (basal side, out, blank) of the inserts and tested for α-amylase activity. Results are from 3 separate experiments with duplicated samples and present as fold change (mean ± SE) over the untreated each respective control.
Volume Regulation and Agonist-Stimulated Calcium Mobilization in phmSG Cells
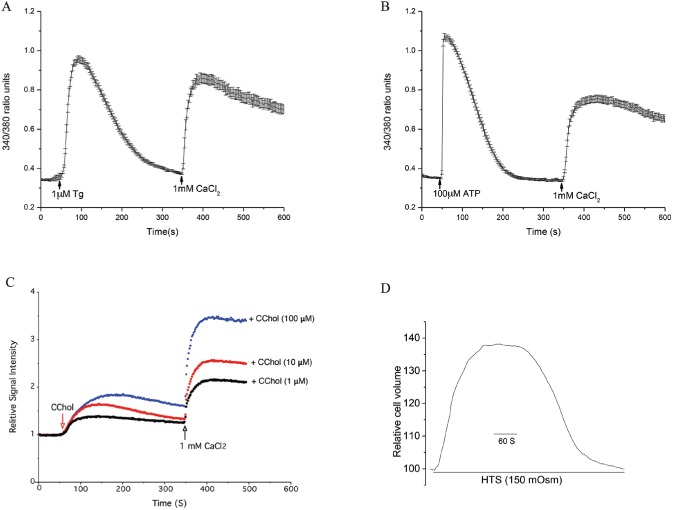
Until now, no study has examined agonist-induced [Ca2+]i (intracellular free calcium concentration) changes in primary salivary gland cells. Stimulation with Tg, a potent inhibitor of the sarco/endoplasmic reticulum calcium-ATPase (SERCA) pump, promoted an initial sharp transient increase of [Ca2+]i corresponding to a calcium release from the ER-Ca2+ stores. When 1 mM CaCl2 was added to the external medium, a second rise in [Ca2+]i was observed primarily due to the influx of Ca2+ into the cells (Fig. 5A). Similar changes in [Ca2+]i were observed when ATP, an agonist of purinergic receptors, was used (Fig. 5B).
Figure 5.
Measurement of calcium mobilization and volume change in phmSG cells. The phmSG cells were loaded with Fura2 (A, B) or Fluo4 (C) and treated with thapsigargin (Tg, 1 µM; A) or adenosine triphosphate (ATP, 100 µM; B) or the indicated concentration of carbachol (CChol; C) at 50 s in calcium-free media with subsequent add back of CaCl2 (1 mM) at 350 s. These data are representative of 3 separate experiments and presented as a ratio of 340/380 (arbitrary units; A, B) or as a relative signal intensity where each reading was normalized by the baseline (C). (D) Hypotonic solution (HTS)–induced cell volume change in phmSG cells maintained in keratinocyte growth medium–low (KGM-L) as described in the Materials and Methods.
Stimulation with carbachol (CChol) in calcium-free medium showed a biphasic response with an initial transient elevation of [Ca2+]i showing intracellular calcium release and a sustained extracellular calcium influx following CaCl2 addition (Fig. 5C). The degree of CChol-induced [Ca2+]i changes occurred in a dose-dependent manner, with higher concentrations of CChol producing higher levels of [Ca2+]i changes, which suggests a direct relationship between calcium release and subsequent influx with the degree of CChol stimulation.
Aquaporins (AQPs) play a critical role in mediating water permeability and regulatory volume changes acinar cells (Okura et al. 1993; Redman and Quissell 1993; Liu et al. 2006). When phmSG cells maintained in KGM-L were exposed to hypotonic solution (HTS), a sudden inflow of water into cells occurred mainly through water channel proteins, which in this assay was detected as an increase in cell volume (Fig. 5D). This was followed by a recovery phase whereby the cell volume decreased to preswelling levels due to the activation of ion channels and transporters that mediate effluxes of K+, Cl−, and H2O, resulting in cell shrinkage (a process known as regulatory volume decrease [RVD]) (Liu et al. 2006).
Discussion
Studies of human salivary gland physiology and pathology have been hampered by the inability to obtain long-term primary cultures of salivary gland cells that preserve phenotype and function. Acinar salivary cells are especially important, as they are the primary water, salt, and protein secreting cells. A key requirement for salivary secretion is the polarized arrangement of secretory components to achieve secretion via the apical region. Another critical process is the receptor-mediated Ca2+ signaling mechanisms for fluid secretion and cAMP signaling for protein secretion. Salivary acinar cells possess specific proteins that are involved in fluid and protein secretion.
Numerous studies have attempted to develop a suitable culture system for primary acinar cell cultures (Lamey et al. 1984; Sens et al. 1985; Oliver et al. 1987; Kurth et al. 1989; Sabatini et al. 1991; Chopra and Xue-Hu 1993; Okura et al. 1993), but thus far, no study has reported optimal conditions for growing cultures of human salivary gland acinar cells that retain their proliferative and functional abilities.
Here, we have developed techniques to obtain explant outgrowth using salivary gland biopsy specimens, as well as optimal conditions to maintain primary cultures of phmSG cells and “guide” them into an acinar-like phenotype. While supplemented basal epithelial medium (s-BEM) supported the outgrowth of epithelial cells from tissue explants, KGM contained supplements that promoted expression of acinar-specific proteins (AQP5, NKCC1, CST3) but not ductal cell markers (KLK1, KRT19). The supplemented MEBM appeared to promote expression of ductal-specific proteins in phmSG cells. More importantly, we have found a critical role for [Ca2+] in the culture medium for optimal growth of phmSG cells from explants of salivary gland biopsy specimens. Supplemented KGM with 0.05 mM [Ca2+] (KGM-L) provided the best growth conditions, allowing for multiple passages of phmSG cultures (8–10 passages) while retaining their phenotypic characteristics. The high expression of epithelial markers in cultured phmSG cells when maintained in KGM-L medium confirms their epithelial origins, whereas detection of keratin 5 and nanog suggests that most cells have some progenitor cell characteristics. The positive staining of AQP5, cystatin C, and NKCC1 of phmSG culture is in agreement with these acinar marker staining in the human salivary gland tissue section (Ball 1993; Barka and van der Noen 1994).
We also found that the [Ca2+] in the culture medium modulates the morphology of and gene expression in phmSG cells. In the presence of relatively high [Ca2+] (>0.8 mM) in KGM (6 supplements), phmSG cells are more tightly packed compared with those in a low [Ca2+] medium. This is accompanied by more than a 2-fold increase in AQP5 gene expression, suggesting that high [Ca2+] induces phmSG cells to a more acinar-like phenotype. Importantly, phmSG cells also display tight junction formation, as demonstrated by their growth as monolayers exhibiting a high TER value in Transwell inserts with polarization in the apical-basal orientation and ZO-1 expression (Tran et al. 2005). Another key feature of salivary gland acinar cells is the secretion of α-amylase. Our findings demonstrate that phmSG cells synthesize and secrete α-amylase via the apical membrane in response to β-adrenergic stimulation. In aggregate, our novel findings demonstrate functional recovery of cultured salivary acinar cells in the KGM medium with high [Ca2+]. To our knowledge, this is the first report showing polarized, regulated α-amylase secretion from primary cultures of human salivary gland cells.
Another critical function of salivary acinar cells is fluid secretion, which is controlled via cellular Ca2+ signaling mechanisms. We have provided strong evidence to demonstrate that Ca2+ mobilization events are functional in phmSG cell cultures. Both receptor and passive stimulation induced Ca2+ release and entry, demonstrating that both upstream and downstream mechanisms remain intact. These cells are also capable of undergoing volume changes in response to osmotic changes, exhibiting cell swelling and subsequent RVD when challenged with HTS.
Conclusively, we have developed a suitable culture system to isolate, maintain, and propagate primary epithelial cultures from human salivary gland tissue, and these primary cells can be further driven toward more acinar-like cells. The culture system we describe herein provides an excellent tool for studies of the molecular mechanisms involved in regulating the function of acinar cells from patients with salivary gland disorders. Finally, the acinar-like cells generated by this system can be used for high-throughput drug screening since they maintain their function and allow for exploration of therapeutic interventions for the pathologies that cause hyposalivation.
Author Contributions
S.I. Jang, contributed to conception, design, data acquisition, analysis, and interpretation, drafted the manuscript; H.L. Ong, A. Gallo, X. Liu, contributed to data acquisition and analysis, critically revised the manuscript; G. Illei, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript; I. Alevizos, contributed to conception, design, data analysis, and interpretation, drafted the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We are grateful to all healthy volunteers and the individuals with Sjögren syndrome who participated in this project. We thank Paola Perez-Riveros and William D. Swaim for technical assistance and Indu Ambudkar for valuable suggestions on this study and critical review of the manuscript.
Footnotes
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Dental and Craniofacial Research (NIDCR) .
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Aframian DJ, Tran SD, Cukierman E, Yamada KM, Baum BJ. 2002. Absence of tight junction formation in an allogeneic graft cell line used for developing an engineered artificial salivary gland. Tissue Eng. 8(5):871–878. [DOI] [PubMed] [Google Scholar]
- Ball WD. 1993. Cell-restricted secretory proteins as markers of cellular phenotype in salivary glands. Boca Raton (FL): CRC Press. [Google Scholar]
- Barka T, van der Noen H. 1994. Expressions of the genes for cysteine proteinase inhibitors cystatin C and cystatin S in rat submandibular salivary gland. Arch Oral Biol. 39(4):307–314. [DOI] [PubMed] [Google Scholar]
- Chopra DP, Xue-Hu IC. 1993. Secretion of α-amylase in human parotid gland epithelial cell culture. J Cell Physiol. 155(2):223–233. [DOI] [PubMed] [Google Scholar]
- Dimitriou ID, Kapsogeorgou EK, Abu-Helu RF, Moutsopoulos HM, Manoussakis MN. 2002. Establishment of a convenient system for the long-term culture and study of non-neoplastic human salivary gland epithelial cells. Eur J Oral Sci. 110(1):21–30. [DOI] [PubMed] [Google Scholar]
- Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. 1980. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 19(1):245–254. [DOI] [PubMed] [Google Scholar]
- Hoffman MP, Kibbey MC, Letterio JJ, Kleinman HK. 1996. Role of laminin-1 and TGF-beta 3 in acinar differentiation of a human submandibular gland cell line (HSG). J Cell Sci. 109(Pt 8):2013–2021. [DOI] [PubMed] [Google Scholar]
- Knox SM, Lombaert IM, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP. 2010. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science. 329(5999):1645–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth BE, Hazen-Martin DJ, Sens MA, DeChamplain RW, Sens DA. 1989. Cell culture and characterization of human minor salivary gland duct cells. J Oral Pathol Med. 18(4):214–219. [DOI] [PubMed] [Google Scholar]
- Lam K, Zhang L, Bewick M, Lafrenie RM. 2005. HSG cells differentiated by culture on extracellular matrix involves induction of S-adenosylmethione decarboxylase and ornithine decarboxylase. J Cell Physiol. 203(2):353–361. [DOI] [PubMed] [Google Scholar]
- Lamey PJ, Ferguson MM, Marshall W, Wallace M, Wiesenfeld D. 1984. Study of the ductal epithelial cell component of human labial salivary glands in vitro. J Biol Buccale. 12(3):191–199. [PubMed] [Google Scholar]
- Liu X, Bandyopadhyay BC, Nakamoto T, Singh B, Liedtke W, Melvin JE, Ambudkar IS. 2006. A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J Biol Chem. 281(22):15485–15495. [DOI] [PubMed] [Google Scholar]
- Miletich I. 2010. Introduction to salivary glands: structure, function and embryonic development. Front Oral Biol. 14:1–20. [DOI] [PubMed] [Google Scholar]
- Okura M, Shirasuna K, Hiranuma T, Yoshioka H, Nakahara H, Aikawa T, Matsuya T. 1993. Characterization of growth and differentiation of normal human submandibular gland epithelial cells in a serum-free medium. Differentiation. 54:143–153. [DOI] [PubMed] [Google Scholar]
- Oliver C, Waters JF, Tolbert CL, Kleinman HK. 1987. Growth of exocrine acinar cells on a reconstituted basement membrane gel. In Vitro Cell Dev Biol. 23:465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HL, Jang SI, Ambudkar IS. 2012. Distinct contributions of Orai1 and TRPC1 to agonist-induced [Ca2+](i) signals determine specificity of Ca2+-dependent gene expression. PLoS One. 7(10):e47146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VN, Likar KM, Zisman-Rozen S, Cowherd SN, Lassiter KS, Sher I, Yates EA, Turnbull JE, Ron D, Hoffman MP. 2008. Specific heparan sulfate structures modulate FGF10-mediated submandibular gland epithelial morphogenesis and differentiation. J Biol Chem. 283(14):9308–9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr. 1986. Identification of cellular activation mechanisms associated with salivary secretion. Annu Rev Physiol. 48:75–88. [DOI] [PubMed] [Google Scholar]
- Redman RS, Quissell DO. 1993. Isolation and maintenance of submandibular gland cells. In: Dobrosielski-Vergona K, editor. Biology of the salivary glands. Boca Raton (FL): CRC Press; p. 285–306. [Google Scholar]
- Sabatini LM, Allen-Hoffmann BL, Warner TF, Azen EA. 1991. Serial cultivation of epithelial cells from human and macaque salivary glands. In Vitro Cell Dev Biol. 27A(9):939–948. [DOI] [PubMed] [Google Scholar]
- Sens DA, Hintz DS, Rudisill MT, Sens MA, Spicer SS. 1985. Explant culture of human submandibular gland epithelial cells: evidence of epithelial origin. Lab Invest. 52(5):559–567. [PubMed] [Google Scholar]
- Shirasuna K, Sato M, Miyazaki T. 1981. A neoplastic epithelial duct cell line established from an irradiated human salivary gland. Cancer. 48(3):745–752. [DOI] [PubMed] [Google Scholar]
- Tran SD, Wang J, Bandyopadhyay BC, Redman RS, Dutra A, Pak E, Swaim WD, Gerstenhaber JA, Bryant JM, Zheng C, et al. 2005. Primary culture of polarized human salivary epithelial cells for use in developing an artificial salivary gland. Tissue Eng. 11(1–2):172–181. [DOI] [PubMed] [Google Scholar]
- Wei C, Larsen M, Hoffman MP, Yamada KM. 2007. Self-organization and branching morphogenesis of primary salivary epithelial cells. Tissue Eng. 13(4):721–735. [DOI] [PubMed] [Google Scholar]
- Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. 1989. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 109(3):1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.