Abstract
FAM20C is an evolutionarily reserved molecule highly expressed in mineralized tissues. Previously we demonstrated that Sox2-Cre;Fam20Cfl/fl mice, in which Fam20C was ubiquitously inactivated, had dentin and enamel defects as well as hypophosphatemic rickets. We also showed that K14-Cre;Fam20Cfl/fl mice, in which Fam20C was specifically inactivated in the epithelium, had enamel defects but lacked hypophosphatemia and defects in the bone and dentin. These results indicated that the enamel defects in the Sox2-Cre;Fam20Cfl/fl mice were independent of dentin defects and hypophosphatemia. To determine if the dentin defects in the Sox2-Cre;Fam20Cfl/fl mice were associated with the enamel defects and hypophosphatemia, we crossed Fam20Cfl/fl mice with Wnt1-Cre and Osr2-Cre transgenic mice to inactivate Fam20C in the craniofacial mesenchymal cells that form dentin and alveolar bone. The resulting Wnt1-Cre;Fam20Cfl/fl and Osr2-Cre;Fam20Cfl/fl mice showed remarkable dentin and alveolar bone defects, while their enamel did not show apparent defects. The serum FGF23 levels in these mice were higher than normal but lower than those in the Sox2-Cre;Fam20Cfl/fl mice; they developed a mild type of hypophosphatemia that did not cause major defects in long bones. These results indicate that the dentin defects in the Sox2-Cre;Fam20Cfl/fl mice were independent of the enamel defects.
Keywords: FGF23, hypophosphatemia, neural crest cells, dentin, enamel, bone
Introduction
Family with sequence similarity 20-C (FAM20C) is an evolutionarily conserved molecule highly expressed in osteoblasts/osteocytes, ameloblasts, and odontoblasts (Wang et al. 2010). Our previous studies showed that ubiquitous inactivation of FAM20C in mice led to severe dentin and enamel defects and hypophosphatemic rickets (Wang, Wang, Li, et al. 2012; Wang, Wang, Lu, et al. 2012). Similar defects were identified in some human patients bearing loss-of-function mutations in FAM20C (Rafaelsen et al. 2013), while the majority of patients displayed osteosclerotic bone dysplasia (Raine Syndrome) (Simpson et al. 2007). In spite of the elusive mechanism underlying these controversial defects, the genetic findings highlighted the crucial role of FAM20C in mineralized tissues. Recent studies identified FAM20C as a Golgi-enriched kinase phosphorylating the secretory calcium-binding phospho- proteins (SCPP), which include the small-integrin-binding ligand, N-linked glycoproteins (SIBLINGs) and several enamel-matrix proteins (Ishikawa et al. 2012; Tagliabracci et al. 2012). These findings suggest that the mineralized-tissue defects in the FAM20C-deficient subjects may be associated with the phosphorylation failure of SCPP proteins.
Tooth development is governed by the interactions between the dental epithelium and dental mesenchyme (Thesleff and Nieminen 1996). Thus, interruption of the signaling in one tissue may cause abnormalities in the other. Analysis of our previous data indicated that FAM20C is expressed in both ameloblasts and odontoblasts (Wang et al. 2010); Sox2-Cre;Fam20Cfl/fl mice, in which Fam20C was ubiquitously inactivated, had both dentin and enamel defects, as well as hypophosphatemic rickets. It remains to be determined if the enamel and dentin defects in the Fam20C-cKO mice are correlated with, or independent of, each other, and if hypophosphatemia contributes to the dental defects, since phosphate composes the major content of hydroxylapatite in both dentin and enamel.
To address these questions, we first generated K14-Cre;Fam20Cfl/fl mice, in which Fam20C was specifically inactivated in epithelia, including the dental epithelium that forms enamel. These mice had enamel defects but lacked hypophosphatemia and defects in the bone and dentin, indicating that enamel defects in the Sox2-Cre;Fam20Cfl/fl mice were independent of the dentin defects and hypophosphatemia (Wang et al. 2013). In this study, we generated Wnt1-Cre;Fam20Cfl/fl mice to inactivate Fam20C in neural-crest-cell-derived craniofacial mesenchymal cells that form dentin and alveolar bone (Danielian et al. 1998; Lan et al. 2007). We also used Osr2-Cre transgenic mice, which have a relatively narrower expression breadth of the Cre recombinase in the craniofacial tissues than do Wnt1-Cre transgenic mice, to generate Osr2-Cre;Fam20Cfl/fl mice. The phenotypes of dentin and enamel, as well as the serum phosphate levels, in these cKO mice may help address whether the dentin defects were independent of the enamel defects, and whether the lower serum phosphate level contributed significantly to the dentin defects.
Materials and Methods
Protocol Approval
All animal procedures were approved by the Institutional Animal Care and Use Committee of Texas A&M University Baylor College of Dentistry (Dallas, TX, USA) and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Generation of Wnt1-Cre;Fam20Cfl/fl Mice, Osr2-Cre;Fam20Cfl/fl Mice, and Sox2-Cre;Fam20Cfl/fl Mice
Fam20Cfl/fl mice were crossbred with Wnt1-Cre and Osr2-Cre transgenic mice (The Jackson Laboratory, Bar Harbor, ME, USA). The resulting Wnt1-Cre;Fam20Cfl/+ and Osr2-Cre;Fam20Cfl/+ mice were inbred to generate Wnt1-Cre;Fam20Cfl/fl and Osr2-Cre;Fam20Cfl/fl mice, respectively. Sox2-Cre;Fam20Cfl/fl mice were generated as previously described (Wang, Wang, Li, et al. 2012). Genotyping was performed by PCR with primers specific for the Cre transgene and Fam20C floxed allele as previously described (Wang, Wang, Li, et al. 2012).
Plain X-ray, Micro-computed Tomography (Micro-CT), and Backscattered Scanning Electron Microscopy (SEM)
The mandibles and hind legs dissected from six 3-wk-old cKO mice and their wild-type (WT) littermates were analyzed under plain x-ray radiography at 24 kV for 6 s (Faxitron Bioptics, Tucson, AZ, USA). The mandibles and middle shaft of tibias from Wnt1-Cre;Fam20Cfl/fl and WT mice were subjected to quantitative micro-CT analysis in the Scanco micro-CT35 imaging system (Scanco Medical, Wayne, PA, USA) at high resolution of a 3.5-μm slice increment. The images were reconstructed with EVS Beam software at global thresholds of 200 Hounsfield units for bone and 240 Hounsfield units for dentin; parameters of dentin and alveolar bone were separately pulled from the mandibular scan. For SEM analyses, the mandibles from Wnt1-Cre;Fam20Cfl/fl and WT mice were fixed in 4% paraformaldehyde overnight. The specimens were then dehydrated through a gradient series of ethanol (70–100%) and embedded in methylmethacrylate (MMA) without prior decalcification. The frontal section at the first lower molar level was mounted, carbon-coated, and examined with field emission scanning electron microscopy at an acceleration voltage of 15 kV (Philips XL30, FEI Company, Eindhoven, Netherlands).
Histology and Histomorphometry
The mandibles from Wnt1-Cre;Fam20Cfl/fl and WT mice were fixed overnight at 4°C with 4% paraformaldehyde in 0.1% diethyl pyrocarbonate (DEPC)-treated PBS solution and then decalcified in 0.1% DEPC-treated 15% ethylene diamine tetraacetic acid (EDTA) (pH 7.4) at 4°C for 1~7 days. The samples were processed for paraffin embedding, and 5-μm serial sections were prepared for hematoxylin and eosin (H&E) staining, in situ hybridization staining, and tartrate-resistant acid phosphatase (TRAP) staining as described previously (Feng et al. 2006; Wang, Wang, Lu, et al. 2012). The thickness of the dentin and enamel matrixes in the first lower molar from six 6-d-old Wnt1-Cre;Fam20Cfl/fl mice and their WT littermates and the width of growth plates and bone cortex in tibias from 3-wk-old mice were measured at 3 points on each of 6 serial sections and subjected to statistical analysis.
Quantitative Real-time Polymerase Chain Reaction (PCR)
The lower incisors and alveolar bone dissected from the mandibles of six 6-d-old mice were crushed into powder in liquid nitrogen. The total RNAs were isolated in an Rneasy Mini Kit (QIAGEN, Valencia, CA, USA) and converted into cDNAs by means of a Reverse Transcription Kit (QIAGEN), according to the manufacturer’s instructions. The cDNAs derived from incisors were used for real-time PCR analysis of ameloblast markers including ameloblastin (AMBN), amelogenin (AMEL), and enamelin (ENAM), while the cDNAs obtained from alveolar bone were used for real-time PCR analysis of receptor activator of nuclear factor κ B (RANK) and RANK ligand (RANKL), on a Bio-Rad CFX96 system (Bio-Rad, Hercules, CA, USA) with SYBR Green Master Mix (Stratagene, La Jolla, CA, USA), following the manufacturer’s instructions. The Ct values were normalized to the reference gene 18s rRNA (SABiosci-ences, Valencia, CA, USA), and expressed as fold-changes over the experimental controls. The primers for mouse 18s rRNA, AMBN, AMEL, ENAM, RANK, and RANKL were purchased from SABiosciences.
Serum Biochemistry
The blood from six 3-wk-old Wnt1-Cre;Fam20Cfl/fl and Osr2-Cre;Fam20Cfl/fl mice and their WT littermates was collected and prepared as previously described (Wang, Wang, Li, et al. 2012). The serum phosphate was measured by the phosphomolybdate-ascorbic acid method. A colorimetric calcium kit (Stanbio Laboratory, Boerne, TX, USA) was used for measuring the serum calcium, and a full-length FGF23 ELISA kit (Kainos Laboratories, Tokyo, Japan) was used for the serum FGF23, as we previously described (Wang, Wang, Li, et al. 2012).
Statistical Analyses
For statistical analyses, the data expressed as mean ± SD of 6 individual determinations were tested by one-way analysis of variance (ANOVA), followed by the post hoc test.
Results
Inactivation of FAM20C in Craniofacial Mesenchymal Cells Caused Remarkable Dentin and Alveolar Bone Defects, But No Apparent Enamel and Long-bone Defects
Plain x-ray showed that the 3-wk-old Wnt1-Cre;Fam20Cfl/fl mice and Osr2-Cre;Fam20Cfl/fl had smaller teeth and incomplete root edification compared with their WT littermates. The cKO mice had remarkable defects in the dentin and alveolar bone (Fig. 1A) but did not show major defects in long bones, in comparison with those in the Sox2-Cre;Fam20Cfl/fl mice and their WT littermates (Fig. 1B) (Wang, Wang, Li, et al. 2012).
Figure 1.
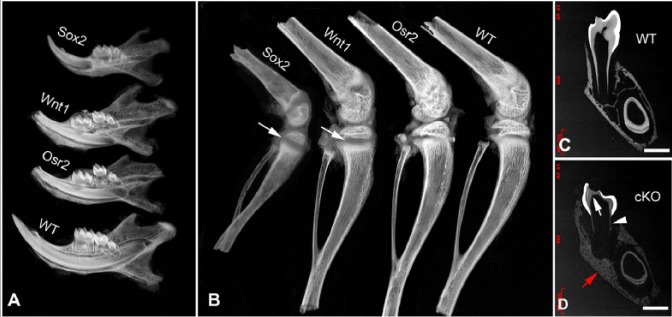
Plain x-ray and backscattered scanning electron microscopy (SEM) analyses of jaws and long bones. (A) Plain x-ray of the mandibles from 3-wk-old mice (from top to bottom, Sox2-Cre;Fam20Cfl/fl, Wnt1-Cre;Fam20Cfl/fl, Osr2-Cre;Fam20Cfl/fl, and wild-type [WT] mice). The jaws and teeth of the cKO mice showed similar severity of incomplete root edification and thinner dentin compared with the WT mice. (B) Plain x-ray of the long bones from 3-wk-old mice (from left to right, Sox2-Cre; Fam20Cfl/fl, Wnt1-Cre;Fam20Cfl/fl, Osr2-Cre;Fam20Cfl/fl, and WT mice). The long bones of the Sox2-Cre;Fam20Cfl/fl mice showed remarkably shorter length, enlarged growth plates (arrow), and lower mineralization than did the WT mice, while the Wnt1-Cre; Fam20Cfl/fl and Osr2-Cre;Fam20Cfl/fl mice showed nearly normal long bones except for an enlarged growth plate (arrow) in the Wnt1-Cre;Fam20Cfl/fl mice compared with that in the WT mice. (C, D) Backscattered SEM analyses of a frontal section at the first lower molar level of the mandible from 5-wk-old mice. The Wnt1-Cre-mediated cKO mice in (D) had thinner dentin (white arrow), incomplete root edification (arrowhead, note the extremely thin root dentin), enlarged pulp chamber, and malformed mandibular bone (red arrow) compared with those in their WT littermates in (C). Scale bars: 500 μm in C and D.
Backscattered SEM analyses showed that the 5-wk-old Wnt1-Cre;Fam20Cfl/fl mice had smaller teeth and thinner dentin, while their enamel did not show apparent defects (Fig. 1C, D). Quantitative micro-CT showed significant mineralization defects in dentin and alveolar bone but not in the cortex of the middle shaft of the long bones in the Wnt1-Cre;Fam20Cfl/fl mice (Appendix Table 1).
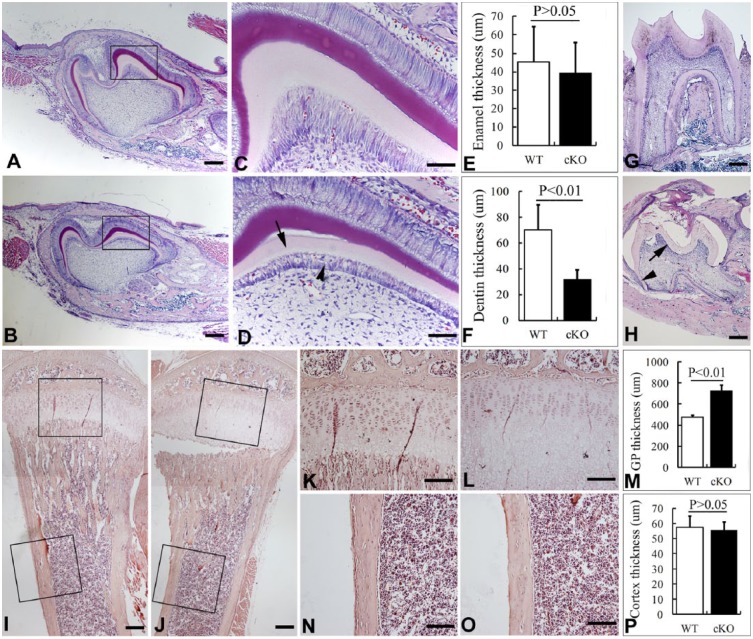
Histological analyses of the E18.5 Wnt1-Cre;Fam20Cfl/fl embryos did not show any significant defects in the odontoblasts (data not shown), while the odontoblasts of 1-wk-old Wnt1-Cre;Fam20Cfl/fl mice showed morphological alteration and formed abnormal dentin (Fig. 2A–D). Histomorphometric analysis showed significantly thinner dentin and enlarged growth plates in the cKO mice (Fig. 2A–F, I–M, O), while their enamel and tibia cortex had no significant alteration in thickness (Fig. 2A–F, O, P). At 3 wks, the Wnt1-Cre;Fam20Cfl/fl mice showed thinner dentin, incomplete root edification, and an eruption delay in the first lower molar compared with WT mice (Fig. 2G, H). TRAP staining on alveolar bone showed no differences between the Wnt1-Cre;Fam20Cfl/fl mice and their WT littermates (Appendix Fig.).
Figure 2.
Histomorphometry of bones and teeth. (A, B) Sagittal sections of the first lower molar from 6-day-old wild-type (WT) (upper) and Wnt1-Cre-mediated cKO mice (lower). (C, D) Higher magnification of the boxed areas in A and B shows that the malformed odontoblasts (arrowhead) of the cKO mice formed thinner dentin (arrow) than that in the WT mice. The ameloblasts of the cKO mice had normal morphology and formed slightly thinner enamel than normal. (E, F) Statistical analysis of the thickness of enamel and dentin in the Wnt1-Cre;Fam20Cfl/fl mice and their WT littermates. (G, H) Sagittal section of the first lower molar from 3-wk-old WT (G) and Wnt1-Cre-mediated cKO mice (H) showed thinner dentin (arrow) and incomplete root edification (arrowhead) in the cKO mice. (I, J) Sagittal section of the tibia from 3-wk-old WT (I) and Wnt1-Cre-mediated cKO mice (J) showed primarily normal bones in the cKO mice. (K–M) Higher magnification of the boxed growth plate (GP) areas in I and J shows significantly thicker growth plates in the cKO mice. (N–P) Higher magnification of the boxed cortex areas in I and J show no significant difference of the cortex thickness between WT and the cKO mice. Scale bars: 200 μm in A, B, G, H, I, and J; 100 μm in K, L, N, and O; and 50 μm in C and D.
Inactivation of Fam20C in Craniofacial Mesenchymal Cells Led to a Mild Type of Hypophosphatemia
The serum FGF23 levels in the Wnt1-Cre;Fam20Cfl/fl and Osr2-Cre;Fam20Cfl/fl mice were significantly higher than normal, but lower than those in the Sox2-Cre;Fam20Cfl/fl mice. Accordingly, their serum phosphate levels were significantly lower than normal but higher than those in the Sox2-Cre;Fam20Cfl/fl mice (Table).
Table.
Comparison of Serum Biochemistry among 3-wk-old Wild-type (WT), Osr2-Cre; Fam20Cfl/fl, Wnt1-Cre;Fam20Cfl/fl, and Sox2-Cre;Fam20Cfl/fl Mice.
| Serum Biochemistry (mean ± SD) | WT (n = 6) | Osr2-Cre; Fam20Cfl/fl (n = 6) | Wnt1-Cre; Fam20Cfl/fl (n = 6) | Sox2-Cre; Fam20Cfl/fl (n = 6) |
|---|---|---|---|---|
| Pi (mg/dl) | 12.42 ± 2.38 | 9.69 ± 1.57bcd | 8.38 ± 1.13bc | 5.96 ± 0.72b |
| Fgf23 (pg/ml) | 179.2 ± 66.5 | 2743.2 ± 527.6bcd | 4861.6 ± 2725.4bc | 13298.6 ± 2463.7b |
| Ca (mg/dl) | 15.22 ± 2.05 | 14.82 ± 1.84 | 13.87 ± 1.56a | 13.68 ± 1.43a |
Significant difference from WT (P < 0.05).
Significant difference from the WT mice (P < 0.01).
Significant difference from the Sox2-Cre;Fam20Cfl/fl group (P < 0.01).
Significant difference from the Wnt1-Cre;Fam20Cfl/fl group (P < 0.01).
DMP1 and DSPP Were Downregulated in the Odontoblasts of Wnt1-Cre;Fam20Cfl/fl Mice
In situ hybridization (ISH) analyses showed significant downregulation of dentin matrix protein 1 (DMP1) in the odontoblasts of 3-wk-old Wnt1-Cre;Fam20Cfl/fl mice (Fig. 3A, B). Dentin sialophosphoprotein (DSPP) was slightly downregulated in the coronal odontoblasts but was nearly abrogated in the odontoblasts localized in the dental roots in the cKO mice (Fig. 3C, D).
Figure 3.
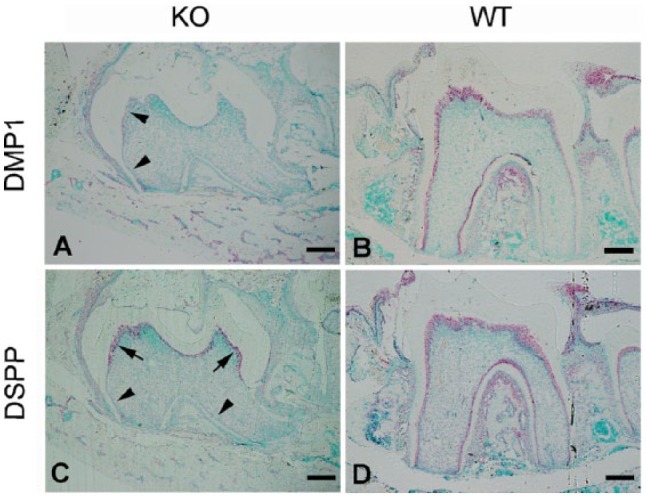
Dentin matrix protein 1 (DMP1) and dentin sialophosphoprotein (DSPP) were downregulated in Fam20C-deficient odontoblasts. (A, B) In situ hybridization (ISH) analysis of the sagittal section of the first lower molar from 3-wk-old Wnt1-Cre-mediated cKO mice in (A) showed significant downregulation of DMP1 in the odontoblasts (arrowheads) compared with that in the WT littermates in (B). (C, D) ISH on the sister section of (A) showed less DSPP expression in the coronal odontoblasts (arrows) and abrogation of DSPP in the root odontoblasts (arrowheads) compared with those in the WT littermates in (D). Scale bars are 200 μm.
Real-time PCR analysis indicated that the expression levels of AMBN, AMEL, and ENAM in the ameloblasts of the Wnt1-Cre;Fam20Cfl/fl mice did not differ from those in the WT mice (Appendix Table 2); the expression levels of RANK and RANKL in the alveolar bone did not differ between the cKO and WT mice (Appendix Table 2).
Discussion
FAM20C is essential to the formation and mineralization of bone, dentin, and enamel. In previous studies, we identified remarkable dentin and enamel defects in the Sox2-Cre;Fam20Cfl/fl mice (Wang, Wang, Lu, et al. 2012); these defects may have arisen from the phosphorylation failure of the SCPP proteins (Ishikawa et al. 2012; Tagliabracci et al. 2012) and/or the significant downregulation of these molecules in the odontoblasts and ameloblasts (Wang, Wang, Lu, et al. 2012). Although the bone defects in the Sox2-Cre;Fam20Cfl/fl mice were likely attributed to hypophosphatemia (Feng et al. 2006; Liu et al. 2006), it was unclear if the lower serum phosphate level contributed to the dental defects (Wang, Wang, Li, et al. 2012) and whether the enamel defects and dentin defects correlated with each other in these mice.
In a previous study, we specifically inactivated Fam20C in the epithelium by crossing the K14-Cre transgenic mice with the Fam20Cfl/fl mice. The resulting K14-Cre;Fam20Cfl/fl mice showed severe enamel defects but no dentin and bone defects, indicating that the enamel defects were independent of dentin defects and hypophosphatemia (Wang et al. 2013). To further investigate whether the dentin defects in the Sox2-Cre;Fam20Cfl/fl mice were associated with the enamel defects and the hypophosphatemia, we crossed Wnt1-Cre and Osr2-Cre transgenic mice with the Fam20Cfl/fl mice to specifically inactivate FAM20C in the craniofacial mesenchymal cells (including dental mesenchymal cells), a lineage known to form dentin and alveolar bone.
The odontoblasts of the Wnt1-Cre;Fam20Cfl/fl mice did not show obvious defects at embryonic (E) day E18.5, while they displayed morphological alteration and formed abnormal dentin 1 wk postnatally, indicating that odontoblast/dentin defects occurred during the initiation and mineralization of dentin matrices. Our previous study showed that the dentin defects in the Sox2-Cre;Fam20Cfl/fl mice might be a combined result of DMP1 and DSPP downregulation (Wang, Wang, Lu, et al. 2012) and the assumed phosphorylation failure of their proteins. Although the detailed mechanisms by which the transcription and phosphorylation of the dentin matrix proteins regulated the dentin formation were not defined in this study, we confirmed a significant downregulation of DMP1 and DSPP in the odontoblasts of the Wnt1-Cre;Fam20Cfl/fl mice. DSPP displayed more prominent downregulation in the roots, a pattern comparable with the more severe dentin defects in the root than in the coronal part. Future studies are warranted to examine the phosphorylation status of the SIBLING proteins in the dentin of Fam20C-deficient mice.
Although the Wnt1-Cre;Fam20Cfl/fl mice had dentin defects of severity similar to those of the Sox2-Cre;Fam20Cfl/fl mice, they did not display abnormal enamel and ameloblasts, indicating that the dentin/odontoblast defects in the Sox2-Cre;Fam20Cfl/fl mice were independent of the enamel/ameloblast defects. It is notable that the transections of incisors in Figure 1C and D, which were cut along the mesial roots of the first lower molars, showed different translucency of enamel between the WT and cKO mice. This variation may arise from the different lengths of incisors between the WT and KO mice, which may have changed the proportional location of the section relative to the whole lengths of incisors in the cKO mice.
The incomplete root edification and tooth eruption delay in the Wnt1-Cre;Fam20Cfl/fl and Osr2-Cre;Fam20Cfl/fl mice were similar to those in the Sox2-Cre;Fam20Cfl/fl mice, suggesting that the root edification in the Sox2-Cre;Fam20Cfl/fl mice was likely associated with abnormal dentin formation rather than with alterations in the Hertwig’s epithelial root sheath (Wang et al. 2013). The eruption of the first lower molar was delayed to 4 wks postnatally in both Sox2-Cre- and Wnt1-Cre-mediated Fam20C-cKO mice. We did not identify abnormality of osteoclasts in the Fam20C-cKO mice, indicating that the eruption delay may be associated with the incomplete root edification, but not with an osteoclast dysfunction.
Intriguingly, inactivation of FAM20C in the craniofacial mesenchymal cells led to a mild type of hypophosphatemia in the Wnt1-Cre;Fam20Cfl/fl and Osr2-Cre;Fam20Cfl/fl mice. In previous studies, we have proven that FAM20C is a regulator of Fgf23, a phosphaturic hormone-mediating phosphate homeostasis through the bone-kidney axis; inactivation of FAM20C, universally or specifically, in mineralized tissues led to a dramatic elevation of serum Fgf23 and a remarkable decrease of serum phosphate. Fgf23 is mainly produced in osteoblasts and osteocytes and is released into circulation, targeting the kidney to mediate phosphate homeostasis. The elevated Fgf23 targets renal Fgfr/Klotho coreceptors and inhibits NaPi2a/c transporters in the proximal tubule, thereby increasing phosphate excretion and reducing serum phosphate, eventually leading to hypomineralization in all bones, namely, hypophosphatemic rickets. Wnt1-Cre and Osr2-Cre removed floxed FAM20C gene from mesenchymal cells that form dentin and bone in the craniofacial complex. The craniofacial bone cells may have released more active Fgf23 into circulation and lowered the serum phosphate levels by increasing the phosphate excretion in the kidney. The elevation of Fgf23 in the Wnt1-Cre;Fam20Cfl/fl and Osr2-Cre;Fam20Cfl/fl mice was milder than that in the Sox2-Cre;Fam20Cfl/fl mice, which may be the reason for the milder reduction of serum phosphate in the former two than in the latter. The milder decrease of serum phosphate in the former two types of mice did not lead to major defects in the long bones except for an enlarged growth plate. It should be noted that Fam20C, Wnt1-Cre and Osr2-Cre are all expressed in the kidney. It remains to be clarified if the renal deletion of Fam20C was associated with the change of serum Pi and FGF23 in the Wnt1-Cre;Fam20Cfl/fl and Osr2-Cre;Fam20Cfl/fl mice.
Both Wnt1-Cre- and Osr2-Cre-mediated cKO mice showed remarkable dentin defects similar to those of the Sox2-Cre;Fam20Cfl/fl mice, although the reduction of serum phosphate levels in the former two was less remarkable than in the latter one. These observations indicate that the local effects of Fam20C inactivation might be the principal contributor to the dentin defects in the Fam20C-deficient mice. However, since hypophosphatemia is associated with the dentin defects in other mouse models, such as Fgf23 transgenic mice and Hyp mice (Chen et al. 2011; Ogawa et al. 2006), we cannot rule out the possibility that the dentin defects in the Fam20C-deficient mice may arise from both local effects of Fam20C inactivation and the systemic effects of hypophosphatemia.
Author Contributions
X. Wang, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; J. Wang, Y. Liu, B. Yuan, L.B. Ruest, J.Q. Feng, contributed to data acquisition and analysis, critically revised the manuscript; C. Qin, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
The authors are grateful to Jeanne Santa Cruz for her assistance with the editing of this article.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants DE022549 (to CQ) and DE23873-01 (to XW).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Chen L, Liu H, Sun W, Bai X, Karaplis AC, Goltzman D, Miao D. 2011. Fibroblast growth factor 23 overexpression impacts negatively on dentin mineralization and dentinogenesis in mice. Clin Exp Pharmacol Physiol. 38(6):395–402. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. 1998. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 8(24):1323–1326. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, et al. 2006. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 38(11):1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa HO, Xu A, Ogura E, Manning G, Irvine KD. 2012. The Raine syndrome protein FAM20C is a Golgi kinase that phosphorylates bio-mineralization proteins. PLoS One. 7(8):e42988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Wang Q, Ovitt CE, Jiang R. 2007. A unique mouse strain expressing Cre recombinase for tissue-specific analysis of gene function in palate and kidney development. Genesis. 45(10):618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Onishi T, Hayashibara T, Sakashita S, Okawa R, Ooshima T. 2006. Dentinal defects in Hyp mice not caused by hypophosphatemia alone. Arch Oral Biol. 51(1):58–63. [DOI] [PubMed] [Google Scholar]
- Rafaelsen SH, Raeder H, Fagerheim AK, Knappskog P, Carpenter TO, Johansson S, Bjerknes R. 2013. Exome sequencing reveals FAM20c mutations associated with fibroblast growth factor 23-related hypophosphatemia, dental anomalies, and ectopic calcification. J Bone Miner Res. 28(6):1378–1385. [DOI] [PubMed] [Google Scholar]
- Simpson MA, Hsu R, Keir LS, Hao J, Sivapalan G, Ernst LM, Zackai EH, Al-Gazali LI, Hulskamp G, Kingston HM, et al. 2007. Mutations in FAM20C are associated with lethal osteosclerotic bone dysplasia (Raine syndrome), highlighting a crucial molecule in bone development. Am J Hum Genet. 81(5):906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabracci VS, Engel JL, Wen J, Wiley SE, Worby CA, Kinch LN, Xiao J, Grishin NV, Dixon JE. 2012. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science. 336(6085):1150–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff I, Nieminen P. 1996. Tooth morphogenesis and cell differentiation. Curr Opin Cell Biol. 8(6):844–850. [DOI] [PubMed] [Google Scholar]
- Wang X, Hao J, Xie Y, Sun Y, Hernandez B, Yamoah AK, Prasad M, Zhu Q, Feng JQ, Qin C. 2010. Expression of FAM20C in the osteogenesis and odontogenesis of mouse. J Histochem Cytochem. 58(11):957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jung J, Liu Y, Yuan B, Lu Y, Feng JQ, Qin C. 2013. The specific role of FAM20C in amelogenesis. J Dent Res. 92(11):995–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang S, Li C, Gao T, Liu Y, Rangiani A, Sun Y, Hao J, George A, Lu Y, et al. 2012. Inactivation of a novel FGF23 regulator, FAM20C, leads to hypophosphatemic rickets in mice. PLoS Genet. 8(5):e1002708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang S, Lu Y, Gibson MP, Liu Y, Yuan B, Feng JQ, Qin C. 2012. FAM20C plays an essential role in the formation of murine teeth. J Biol Chem. 287(43):35934–35942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, Feng JQ. 2004. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 279(18):19141–19148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.