Abstract
This study was conducted to evaluate the pharmacokinetic characteristics of vincristine and their correlation with its clinical effects in dogs with transmissible venereal tumor (TVT). Dogs with TVT were intravenously administered vincristine sulfate at a dose of 0.7 mg/m2 of body surface area. Blood samples were collected starting from 5 min to 48 hr after drug administration. The plasma concentration of vincristine was determined using liquid chromatography-tandem mass spectrometry (LC-MS/MS). The pharmacokinetic parameters of vincristine were characterized using a two-compartmental pharmacokinetic model. The volume of distribution, distribution half-life, elimination half-life and plasma clearance were 0.660 ± 0.210 l/kg, 21.5 ± 6.90 min, 47.6 ± 14.2 min and 0.010 ± 0.001 l/min/kg, respectively. Tumor regression was determined at weekly interval by a physical examination and histopathological analysis. In our study, three to eight administrations of vincristine at a dose of 0.7 mg/m2 were able to induce a complete tumor regression without any evidence of gross lesion of disease. Therefore, this investigation provides the pharmacokinetic characteristics of vincristine in dogs with TVT, which may be used as an integration tool to gain a better understanding of the disposition properties of the drug and the correlation of these properties with the drug’s clinical effects. In addition, we validated the LC-MS/MS method and found that it is suitable for the pharmacokinetic study of vincristine in dog plasma.
Keywords: canine, LC-MS/MS, pharmacokinetics, transmissible venereal tumor (TVT), vincristine sulfate
Canine transmissible venereal tumor (TVT), also known as transmissible venereal sarcoma or Sticker’s sarcoma, is a contagious tumor that commonly occurs in dogs [7]. The incidence of this tumor has been reported in several areas around the world. However, it has mostly been found in tropical and subtropical urban areas with an environment with a large population of free-roaming dogs with poor mating control [6, 19, 24]. The etiology of the tumor is a transplantation of tumor cells from the affected area to the mucous membrane, particularly to membranes that have lost their integrity. Sexual intercourse is considered a major route of transmission. In addition, other social behaviors of dogs, such as licking and sniffing, are able to promote the transmission of TVT [22]. The lesions are most commonly located on the genital areas, but have also been observed in other locations, such as the skin, conjuntiva, and oral and nasal cavities [28]. The tumor diagnosis is based on a physical examination and a cytological or histological analysis. Several treatments, including surgery, radiotherapy, immunotherapy and chemotherapy, have been applied for TVT [6]. However, chemotherapy is considered the most effective and practical method for TVT treatment, and vincristine sulfate is the drug of choice that is commonly employed [6, 20, 25].
Vincristine, a plant alkaloid, is a chemotherapeutic agent that is widely used to treat various neoplastic disorders, such as lymphomas, leukemias and sarcomas in dogs and cats [8, 12]. This alkaloid exerts cytotoxic activity by disrupting cellular microtubule formation. This sequence induces the inhibition of cell replication, including the replication of the cancer cells [4]. The TVT treatment consists of the weekly administration of vincristine at a dosage of 0.5 to 0.7 mg/m2 of body surface area for a period of 4–8 weeks [2]. For the quantification of vincristine in biological samples, several techniques, including radioimmunoassay [13], high-performance liquid chromatography (HPLC) [9, 14] and liquid chromatography mass spectrophotometry (LC-MS) [26], have been reported. Nevertheless, the method with higher sensitivity and selectivity is still required for vincristine pharmacokinetic studies. Hence, liquid chromatography-tandem mass spectrometry (LC-MS/MS) is considered as the preferred method for determination of drug concentration, particularly vincristine in biological samples with the most sensitive and reliable qualitative and quantitative analyses. Recently, various LC-MS/MS methods for the quantification of vincristine in human plasma have been published [5, 11, 29]. However, clinical pharmacokinetic studies of vincristine in dogs with TVT have not yet been reported. Therefore, this study evaluated the clinical pharmacokinetic characteristics of vincristine sulfate and their correlation with its effects in dogs with TVT.
MATERIALS AND METHODS
Animals: Six crossbred dogs with naturally occurring TVT (three males and three females, 2–5 years of age and weighing 11–29 kg) were obtained from a community that promotes the welfare of dogs and dogs family planning (Dog Chance, Ratchaburi Province, Thailand). The dogs were individually housed in cages at the Laboratory Animal Facility of the Faculty of Veterinary Medicine at Kasetsart University and were acclimatized to the environment for one week. The animals were allowed access to a standard diet and drinking water ad libitum throughout the experimental period. A cytological examination and a subsequent histological examination of the biopsies were performed to confirm the diagnosis following standard protocols. The processes used to monitor the health status of all of the dogs, which included a physical examination, a complete blood cell count (CBC) and a serum biochemistry profile related to renal and hepatic function, were performed weekly throughout the studies. The tumor sizes were also measured using a vernier caliper at the beginning of the experiment and weekly before the administration of vincristine. This study was performed according to the Guidelines for Animal Experiments and approved by the Animal Ethics Research Committee of the Faculty of Veterinary Medicine at Kasetsart University.
Drug administration and sample collection: The dogs were treated with vincristine sulfate at a dosage of 0.7 mg/m2 of body surface area through intravenous administration. Blood samples for single-dose pharmacokinetic analysis were collected from the cephalic vein through IV catheter using EDTA tubes at 0, 5, 10, 15, 30 and 45 min and 1, 2, 4, 6, 8, 12, 24 and 48 hr after drug administration. The samples were centrifuged, and the plasma was separated and stored at −20°C until analysis. The treatments were performed weekly until the tumor had visibly disappeared up to a maximum of eight treatments. The response to treatment and toxicity were assessed prior to each administration until the end of the treatment period.
Chemicals and reagents: The reference standards of vincristine sulfate and vinblastine sulfate (internal standard, IS) were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Vincristine sulfate (V.C.S.®; 1 mg/ml) for treatment was purchased from Boryung Pharmaceutical Co., Ltd. (Ansan, Korea). Other chemicals used in this study were of analytical grade.
Sample preparation: The samples were prepared according to a published method [17] with slight modifications. Briefly, 500 µl of plasma was diluted with 500 µl of 4% phosphoric acid, and 20 µl of 500 ng/ml IS solution was then added to the mixture. After thorough mixing, the mixture was applied to an Oasis HLB cartridge (Water, Milford, MA, U.S.A.). The cartridge was washed with 5% methanol in water and then with 1 ml of methanol:water (50:50, v/v). The drugs were eluted with 1.2 ml of 100% methanol. The eluate was evaporated and reconstituted in 100 µl of a mixture of mobile phase A (5 mM ammonium acetate in water titrated to pH 3.5 with acetic acid and mobile phase B (acetonitrile) (50:50, v/v). The mixture was then vortexed and centrifuged for 10 min. The supernatant was transferred to micro insert vials, and 10 µl was injected into the LC-MS/MS system for analysis.
Chromatographic system: Chromatographic separation through an LC system was performed using a Poroshell 120 EC-C18, 3.0 × 50 mm, 2.7-µm column (Agilent Technologies, Palo Alto, CA, U.S.A.). The mobile phase A consisted of 5 mM ammonium acetate in water titrated to pH 3.5 with acetic acid, and mobile phase B was 100% acetonitrile. The gradient elution was performed as follows: 0 to 0.5 min, isocratic 90% mobile phase A; 0.5 to 3.0 min, 90% to 5% mobile phase A; 3.0 to 5.0 min, isocratic 5% mobile phase A. The flow rate was 0.5 ml/min. The autosampler temperature was maintained at 7°C. The mass spectrometric analysis was performed using a 6460 triple quadrupole mass spectrometer (Agilent Technologies) and programmed using the Agilent MassHunter B.06.00 software (Agilent Technologies). The electrospray ion source was run in the positive ionization mode. The ionization source parameters were optimized as follows: capillary voltage, 3500 V; gas temperature, 330°C; gas flow rate, 8 l/min; nebulizer, 50 psi. The fragment energy was set to 230 V. The multiple reaction monitoring (MRM) transitions were selected to be m/z 825.4 precursor ion to m/z 765.4 production ion for vincristine and m/z 811.4 precursor ion to m/z 751.3 product ion for vinblastine.
Validation procedures: In this study, the LC-MS/MS method was developed and validated for the identification and quantification of vincristine in the plasma of dogs with TVT. The calibration standard concentrations were prepared by spiking the working standard solution into blank plasma to yield final concentrations of 0.5, 1, 2.5, 5, 10, 50, 100 and 150 ng/ml. Five duplicates of the quality control (QC) sample at concentrations of 2, 50 and 100 ng/ml were prepared and used to determine the recoveries, intra-day and inter-day precision, and accuracy of the method. The procedure was repeated five times within the same day to gain an intra-day run precision and accuracy and five times of each concentration over five different days to obtain an inter-day run precision and accuracy. The precision of the assay was assessed by calculating the relative standard deviation (R.S.D.) for each concentration level. The accuracy was calculated by comparing the average measurements with the nominal values and is expressed as a percentage. A blank plasma sample, eight calibrated standard concentrations and five duplicates of the QC samples were included in each run. The quantification was obtained using the multiple reaction monitoring (MRM) transitions for vincristine (m/z 825.4 → 765.4) and IS (m/z 811.4 → 751.3). The retention times of vincristine and IS were 2.92 and 2.93 min, respectively. The calibration curve exhibited linearity (r2=0.990) over the concentration range of 0.5 to 150 ng/ml. The lower limit of quantification (LLOQ), defined as the lowest concentration yielding a signal-to-noise ratio (S/N) higher than 10, was 0.5 ng/ml.
Pharmacokinetic study: The determination of the pharmacokinetic parameters of vincristine was performed using a two-compartmental pharmacokinetic model with PK solution 2.0TM (Summit Research Services, Montrose, CO, U.S.A.). The Cp0 term was the extrapolated plasma concentration time curve at zero time, K12 and K21 were micro-rate constants, AUC was the area under the curve, t1/2α was the distribution half-life, t1/2β was the elimination half-life, Vdarea was the volume of distribution, Cl was the plasma clearance, and MRT was the mean residence time.
RESULTS
Method validation: The results of intra-day and inter-day precision and accuracy of the assay are presented in Table 1. The intra-day precision and accuracy ranged from 0.92% to 6.46% and from 99.7% to 102%, respectively. The inter-day precision and accuracy ranged from 0.902% to 4.39% and from 98.9% to 108%, respectively. The extraction recoveries were 86.3 ± 10.54, 77.3 ± 9.88 and 88.9 ± 5.49% for low, medium and high QC levels, respectively (Table 2). All of the data are within the acceptable range.
Table 1. Intra-day and inter-day accuracy and precision of vincristine sulfate in dog plasma (n=5).
| QC sample concentration (ng/ml) |
Mean ± SD (ng/ml) |
Accuracy (%) |
Precision (% R.S.D) |
|---|---|---|---|
| Intra-day (5 days) | |||
| 2.0 | 2.05 ± 0.20 | 102 | 6.46 |
| 50.0 | 49.9 ± 0.81 | 99.7 | 1.59 |
| 100.0 | 99.9 ± 0.99 | 99.9 | 0.92 |
| Inter-day (5 days) | |||
| 2.0 | 2.22 ± 0.18 | 108 | 4.28–4.39 |
| 50.0 | 49.2 ± 1.97 | 98.9 | 3.65–3.76 |
| 100.0 | 99.9 ± 0.92 | 99.9 | 0.902–0.904 |
Table 2. Recovery of vincristine following extraction process (n=5).
| QC sample concentration (ng/ml) |
Mean extraction recovery (%) |
Coefficient of variation (%) |
|---|---|---|
| 2.0 | 86.3 ± 10.54 | 12.3 |
| 50.0 | 77.3 ± 9.88 | 11.8 |
| 100.0 | 88.9 ± 5.49 | 6.18 |
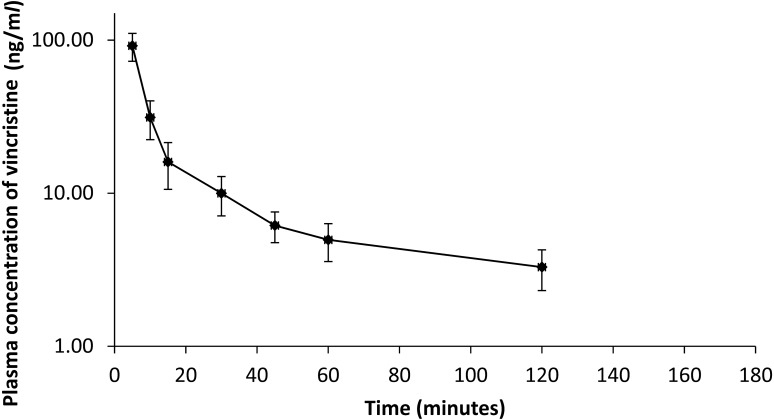
Pharmacokinetic analysis: The level of vincristine sulfate in plasma was detectable up to 120 min after drug administration. Figure 1 shows the mean plasma concentration of vincristine as a function of time after a single intravenous administration at a dose of 0.7 mg/m2 to dogs with TVT. The pharmacokinetic parameters for vincristine in dogs with TVT are presented in Table 3. The plasma vincristine concentration immediately after administration (Cp0) was 119 ± 18.0 ng/ml. The apparent volume of distribution (Vdarea) and mean residence time (MRT) were 0.660 ± 0.210 l/kg and 55.9 ± 19.3 min, respectively. The distribution half-life (t1/2α), elimination half-life (t1/2β) and plasma clearance were 21.5 ± 6.90 min, 47.6 ± 14.2 min and 0.010 ± 0.001 l/min/kg, respectively.
Fig. 1.
Time profile of vincristine plasma concentration after its intravenous administration to dogs with TVT at a dose of 0.7 mg/m2. The values represent the means ± SD (n=6).
Table 3. Pharmacokinetic parameters (mean ± SD) of vincristine sulfate after its intravenous administration at a dose of 0.7 mg/m2 to dogs with TVT (n=6).
| Pharmacokinetic parameters (units) | Average ± SD |
|---|---|
| K12 (min–1) | 0.094 ± 0.035 |
| K21 (min–1) | 0.029 ± 0.012 |
| Cp0 (ng/ml) | 119 ± 18.0 |
| t½α (min) | 21.5 ± 6.90 |
| t½β (min) | 47.6 ± 14.2 |
| Cl (l/min/kg) | 0.010 ± 0.001 |
| Vd(area) (l/kg) | 0.660 ± 0.210 |
| MRT (min) | 55.9 ± 19.3 |
| AUC (ng·min/ml) | 2,349 ± 317 |
K12, K21= micro-rate constants; Cp0= plasma concentration at initial time; t1/2α= distribution half-life; t1/2β = elimination half-life; Cl = clearance; Vd(area) = volume of distribution; MRT = mean residence time; AUC = area under the plasma concentration-time curve.
Clinical responses: Tumor regression was determined at weekly intervals by a physical examination and histopathological analysis. The three dimensions of each tumor were measured using vernier caliper and recorded. Complete remission or complete response means that all of the tumors completely disappeared, and partial remission means that the tumors regressed more than 50%, but less than 100%. In our study, three to eight administrations of vincristine at a dose of 0.7 mg/m2 were able to induce complete remission in five dogs (83.33%). In addition, these five dogs exhibited more than 50% remission after the first week of drug administration. In contrast, one dog exhibited only a partially response after the drug was administered eight times. The tumor mass (approximately 1 cm in diameter) in this dog continued to appear on the posterior portion of the gland penis. Gastro-intestinal side effects, such as vomiting, anorexia and diarrhea, were observed after the first drug administration in three dogs. Hematological side effects, such as leucopenia, neutropenia and thrombocytopenia, were observed in two dogs after the third drug administration, and anemia was observed in one dog after the fourth drug administration.
DISCUSSION
TVT is a contagious, sexually transmitted tumor that is commonly found in dogs. A high incidence of TVT is associated with a large population of free-roaming dogs with uncontrolled sexual activity. Chemotherapy, particularly vincristine sulfate, has been shown to be the most effective treatment and is thus frequently used. Several analytical methods for the determination of vincristine sulfate in biological samples derived from both humans and animals have been reported. Currently, LC-MS/MS is the method that provides the most sensitive and reliable qualitative and quantitative analyses. The present study was conducted to evaluate the pharmacokinetic parameters of vincristine sulfate in dogs with TVT and to determine their correlation with the clinical effects of the drug using the well-suited LC-MS/MS method to determine the concentration of vincristine sulfate in dog plasma. The results from our study show that three to eight weekly intravenous administrations of vincristine at a dose of 0.7 mg/m2 produced a good response. Five of six dogs achieved complete remission with a response rate of 83.33%. This response confirmed the efficacy of vincristine sulfate as a single agent for TVT treatment, which is in agreement with previous reports [1, 16, 20].
For the pharmacokinetic evaluation, the plasma concentration as a function of time was described by a two-compartment model. The concentration of vincristine in plasma markedly decreased starting 5 min after administration. The t1/2β indicates the overall rate of elimination and allows the prediction of vincristine accumulation with a value for vincristine of 47.6 min after i.v. administration in dogs with TVT, whereas the value of t1/2β was 22.8 hr in healthy dogs [30]. The MRT was 55.9 min. Thus, about 99% of the vincristine from plasma was cleared within 5 hr after i.v. administration. The distribution half-life (t1/2α, 21.5 min) of vincristine obtained in dogs with TVT was longer than in healthy dogs (0.14 hr) [30]. This may indicate that vincristine is distributed to the peripheral tissues of dogs with TVT, and this finding is further supported by the high uptake of the drug by tubulin-rich tissues [3, 21]. The above-described characteristics of the penetration of vincristine sulfate exhibited a good correlation with the clinical tumor response, which showed that more than 50% remission was obtained after the first drug injection. This may be due to the high drug distribution and tumor tissue uptake. The volume of distribution obtained in this study is in agreement with the results from previous investigations in mice [15], Tasmanian devils [23], children humans [27] and adult humans [18]. In previous studies, the accumulative concentration of the drug was found to be high in many organs, including the pancreas, spleen, thyroid, adrenal, intestinal mucosa, lung, liver, kidney and bone marrow [10], and the spleen was found to be the major organ in which the drug was accumulated [8]. To the best of our knowledge, this study provides the first demonstration of the clinical pharmacokinetics of vincristine sulfate in dogs with TVT using the LC-MS/MS method.
In conclusion, although pharmacokinetic parameters of vincristine sulfate in dogs with TVT showed rapid elimination and therefore no accumulation, it resulted in good clinical effects by dosing once a week. This suggests that the drug has long residence time in tumor tissue. In addition, it is well recognized that AUC is one of the most important PK parameters that have a relationship with an efficacy of antitumor drugs. Based on the value of AUC of vincristine in this study, it might indicate the penetration of the drug into the tumor mass, which increases the efficacy and antitumor activity of the drug. However, further investigation is needed to clarify the pharmacodynamics of the drug in TVT.
Acknowledgments
This work was supported by the Kasetsart Graduate Study Research Scholarship, Graduate School, Kasetsart University, Thailand.
REFERENCES
- 1.Amber E. I., Henderson R. A., Adeyanju J. B., Gyang E. O.1990. Single-drug chemotherapy of canine transmissible venereal tumor with cyclophosphamide, methotrexate, or vincristine. J. Vet. Intern. Med. 4: 144–147. doi: 10.1111/j.1939-1676.1990.tb00887.x [DOI] [PubMed] [Google Scholar]
- 2.Boscos C. M., Ververidis H. N.2004. Canine TVT-clinical findings, diagnosis and treatment. In proceedings of the 29th World Small Animal Veterinary Association, Oct 6–9. Rhodes, Greece. [Online]. Available from: http://www.vin.com/proceedings/ Proceedings.plx?CID=WSAVA2004&Category=&PID=8752&O=Generic.
- 3.Castle M. C., Margileth D. A., Oliverio V. T.1976. Distribution and excretion of (3H)vincristine in the rat and the dog. Cancer Res. 36: 3684–3689. [PubMed] [Google Scholar]
- 4.Coppoc G. L.2009. Chemotherapy of neoplastic diseases. pp. 1205–1231. In: Veterinary Pharmacology and Therapeutics. 9th ed. (Riviere J. E. and Papich. M. G. eds.), Willey-Blackwell, Ames. [Google Scholar]
- 5.Corona G., Casetta B., Sandron S., Vaccher E., Toffoli G.2008. Rapid and sensitive analysis of vincristine in human plasma using on-line extraction combined with liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 22: 519–525. doi: 10.1002/rcm.3390 [DOI] [PubMed] [Google Scholar]
- 6.Das U., Das A. K.2000. Review of canine transmissible venereal sarcoma. Vet. Res. Commun. 24: 545–556. doi: 10.1023/A:1006491918910 [DOI] [PubMed] [Google Scholar]
- 7.de Lorimier L. P., Fan T. M.2007. Canine Transmissible Venereal Tumor. pp. 799–803. In: Withrow and MacEwen’s Small Animal Clinical Oncology 4th ed. (Withrow, S. J. and Vail, D. M. eds.), Saunders Elsevier, St. Louis. [Google Scholar]
- 8.Dobson J. M., Hohenhaus A. E., Peaston A. E.2008. Cancer chemotherapy. pp. 330–366. In: Small Animal Clinical Pharmacology 2nd ed. (Maddison, J. E., Page, S. W. and Church, D. B. eds.), Saunders Elsevier, Edinburgh. [Google Scholar]
- 9.Embree L., Gelmon K. A., Tolcher A. W., Hudon N. J., Heggie J. R., Dedhar C., Webb M. S., Bally M. B., Mayer L. D.1997. Validation of a high-performance liquid chromatographic assay method for quantification of total vincristine sulfate in human plasma following administration of vincristine sulfate liposome injection. J. Pharm. Biomed. Anal. 16: 675–687. doi: 10.1016/S0731-7085(97)00087-3 [DOI] [PubMed] [Google Scholar]
- 10.Gidding C. E., Kellie S. J., Kamps W. A., de Graaf S. S.1999. Vincristine revisited. Crit. Rev. Oncol. Hematol. 29: 267–287. doi: 10.1016/S1040-8428(98)00023-7 [DOI] [PubMed] [Google Scholar]
- 11.Guilhaumou R., Solas C., Rome A., Giocanti M., Andre N., Lacarelle B.2010. Validation of an electrospray ionization LC/MS/MS method for quantitative analysis of vincristine in human plasma samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878: 423–427. doi: 10.1016/j.jchromb.2009.12.015 [DOI] [PubMed] [Google Scholar]
- 12.Hahn K. A.1990. Vincristine sulfate as single-agent chemotherapy in a dog and a cat with malignant neoplasms. J. Am. Vet. Med. Assoc. 197: 504–506. [PubMed] [Google Scholar]
- 13.Jackson D. V., Jr, Sethi V. S., Spurr C. L., McWhorter J. M.1981. Pharmacokinetics of vincristine in the cerebrospinal fluid of humans. Cancer Res. 41: 1466–1468. [PubMed] [Google Scholar]
- 14.Koopmans P., Gidding C. E., de Graaf S. S., Uges D. R.2001. An automated method for the bioanalysis of vincristine suitable for therapeutic drug monitoring and pharmacokinetic studies in young children. Ther. Drug Monit. 23: 406–409. doi: 10.1097/00007691-200108000-00014 [DOI] [PubMed] [Google Scholar]
- 15.Krishna R., Webb M. S., St Onge G., Mayer L. D.2001. Liposomal and nonliposomal drug pharmacokinetics after administration of liposome-encapsulated vincristine and their contribution to drug tissue distribution properties. J. Pharmacol. Exp. Ther. 298: 1206–1212. [PubMed] [Google Scholar]
- 16.Kunakornsawat S., Imsilp K., Yatbantoong N., Ratanapob N., Supsavhad W., Sreesampan S., Nunklang G.2009. Vincristine chemotherapeutic treatment for transmissible venereal tumor in 60 dogs. Kamphaengsean Acad. J. 8: 79–91. [Google Scholar]
- 17.Lee J. I., Skolnik J. M., Barrett J. S., Adamson P. C.2007. A sensitive and selective liquid chromatography-tandem mass spectrometry method for the simultaneous quantification of actinomycin-D and vincristine in children with cancer. J. Mass Spectrom. 42: 761–770. doi: 10.1002/jms.1211 [DOI] [PubMed] [Google Scholar]
- 18.Lönnerholm G., Frost B. M., Abrahamsson J., Behrendtz M., Castor A., Forestier E., Heyman M., Uges D. R., de Graaf S. S.2008. Vincristine pharmacokinetics is related to clinical outcome in children with standard risk acute lymphoblastic leukemia. Br. J. Haematol. 142: 616–621. doi: 10.1111/j.1365-2141.2008.07235.x [DOI] [PubMed] [Google Scholar]
- 19.Mello Martins M. I., Ferreira de Souza F., Gobello C.2005. The canine transmissible venereal tumor: etiology, pathology, diagnosis and treatment. In: Recent Advances in Small Animal Reproduction. (Concannon, P. W., England, G., Veretgegen, J. and Linde-Forsberg, C. eds.), International Veterinary Information Service, Ithaca NY (www.ivis.org) Apr 25, 2005: A1233.0405.
- 20.Nak D., Nak Y., Cangul I. T., Tuna B.2005. A Clinico-pathological study on the effect of vincristine on transmissible venereal tumour in dogs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 52: 366–370. doi: 10.1111/j.1439-0442.2005.00743.x [DOI] [PubMed] [Google Scholar]
- 21.Owellen R. J., Owens A. H., Jr, Donigian D. W.1972. The binding of vincristine, vinblastine and colchicine to tubulin. Biochem. Biophys. Res. Commun. 47: 685–691. doi: 10.1016/0006-291X(72)90546-3 [DOI] [PubMed] [Google Scholar]
- 22.Papazoglou L. G., Koutinas A. F., Plevraki A. G., Tontis D.2001. Primary intranasal transmissible venereal tumour in the dog: a retrospective study of six spontaneous cases. J. Vet. Med. A Physiol. Pathol. Clin. Med. 48: 391–400. doi: 10.1046/j.1439-0442.2001.00361.x [DOI] [PubMed] [Google Scholar]
- 23.Phalen D. N., Frimberger A., Pyecroft S., Peck S., Harmsen C., Lola S., de Mello Mattos B., Li K. M., McLachlan A. J., Moore A.2013. Vincristine chemotherapy trials and pharmacokinetics in tasmanian devils with tasmanian devil facial tumor disease. PLoS ONE 8: e65133. doi: 10.1371/journal.pone.0065133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers K. S., Walker M. A., Dillon H. B.1998. Transmissible venereal tumor: a retrospective study of 29 cases. J. Am. Anim. Hosp. Assoc. 34: 463–470. [DOI] [PubMed] [Google Scholar]
- 25.Singh J., Rana J. S., Sood N., Pangawkar G. R., Gupta P. P.1996. Clinico-pathological studies on the effect of different anti-neoplastic chemotherapy regimens on transmissible venereal tumours in dogs. Vet. Res. Commun. 20: 71–81. doi: 10.1007/BF00346579 [DOI] [PubMed] [Google Scholar]
- 26.Schmidt M. S., Huang R., Classon R. J., Murry D. J.2006. Determination of vincristine in infant plasma by liquid chromatography-atmospheric pressure chemical ionization-mass spectroscopy. J. Pharm. Biomed. Anal. 41: 540–543. doi: 10.1016/j.jpba.2005.11.039 [DOI] [PubMed] [Google Scholar]
- 27.Skolnik J. M., Barrett J. S., Shi H., Adamson P. C.2006. A liquid chromatography-tandem mass spectrometry method for the simultaneous quantification of actinomycin-D and vincristine in children with cancer. Cancer Chemother. Pharmacol. 57: 458–464. doi: 10.1007/s00280-005-0065-9 [DOI] [PubMed] [Google Scholar]
- 28.Tella M. A., Ajala O. O., Taiwo V. O.2004. Complete regression of Transmissible Venereal Tumour (TVT) in Nigerian Mongrel Dogs with Vincristine Sulphate Chemotherapy. ABNF J. 7: 133–138. [Google Scholar]
- 29.Yang F., Wang H., Liu M., Hu P., Jiang J.2013. Determination of free and total vincristine in human plasma after intravenous administration of vincristine sulfate liposome injection using ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 1275: 61–69. doi: 10.1016/j.chroma.2012.12.026 [DOI] [PubMed] [Google Scholar]
- 30.Zhong J., Mao W., Shi R., Jiang P., Wang Q., Zhu R., Wang T., Ma Y.2014. Pharmacokinetics of liposomal-encapsulated and un-encapsulated vincristine after injection of liposomal vincristine sulfate in beagle dogs. Cancer Chemother. Pharmacol. 73: 459–466. doi: 10.1007/s00280-013-2369-5 [DOI] [PubMed] [Google Scholar]