Abstract
Loperamide is a peripheral opiate agonist that can cause apoptosis and G2/M arrest in human cancer cell lines and may sensitize cells to chemotherapy. The objectives of this study were to investigate the effects of loperamide on viability, apoptosis and cell cycle kinetics in canine cancer cells and to establish whether the drug sensitizes cells to doxorubicin. Cell viability was assessed using Alamar Blue. Cell death and cell cycle were studied using flow cytometry with 7-Aminoactinomycin-D (7-AAD) and propidium iodide (PI), respectively. Loperamide decreased cell viability in a dose-dependent fashion and was most effective against canine osteosarcoma cells. In all cell lines, it induced a dose and time dependent apoptosis and resulted in accumulation in G0/G1. When co-incubated with doxorubicin, loperamide induced a synergistic cell kill in canine carcinoma cells. Investigation is warranted into the role of loperamide in the treatment of canine cancer.
Keywords: apoptosis, canine cancer, cell cycle, cell line, loperamide
Loperamide hydrochloride is a peripherally acting µ-opiate receptor agonist commonly recommended as a treatment for chemotherapy-related diarrhea in both canine and human cancer patients [3, 23]. In an effort to screen for affordable and well-tolerated anti-neoplastic medications, investigators have shown that various opiates have the ability to induce cytotoxicity in human cancer cell lines [6, 18]. The exact mechanism of action of cancer cell death in these studies is unknown, but theories include an interaction with somatostatin receptors [6] or potentially alternative splice variants of the opiate receptor [2]. Loperamide was recently shown to induce dose-dependent anti-proliferative and apoptotic effect on multiple human cancer cell lines, including breast, bone, lung and liver tumor cell lines [5]. The 50% inhibitory concentrations (IC50 values) obtained for most tumor lines tested varied from 23 µM to 41 µM, however, an osteosarcoma cell line was most sensitive with an IC50 of approximately 12 µM [5]. Additionally, loperamide caused a dose-dependent arrest in the G2/M phase of the cell cycle, suggesting that loperamide may function as a radiation sensitizer [5].
Separate from its ability to inhibit proliferation and induce apoptosis, loperamide acts as a P-glycoprotein substrate [14, 27]. Multidrug resistance of tumor cells is a significant problem in the use of cancer chemotherapy. The classical mechanism of development involves P-glycoprotein over-expression [4, 27]. Chemotherapy drugs kill many tumor cells, but may select for cells that overexpress P-glycoprotein; compounds that compete for P-glycoprotein, such as loperamide, may have the potential to enhance the efficacy of some anticancer agents [27]. Indeed, a recent study found that loperamide was able to reverse multidrug resistance in a doxorubicin-resistant breast cancer cell line due to its high affinity binding to the efflux pump, leading to intracellular doxorubicin accumulation [27].
In the present study, we investigated the effect of loperamide on various canine cancer cell lines. We hypothesized that, similar to the effects seen in human cancer cell lines, loperamide would cause a dose-dependent cytotoxicity, apoptosis and G2/M arrest, as well as increased cytotoxicity in doxorubicin-treated cells.
MATERIALS AND METHODS
Cell lines and culture conditions: Four cell lines (provided by the University of Wisconsin-Madison), CTAC (canine thyroid carcinoma, The Ohio State University), D-17 (canine osteosarcoma, American Type Culture Collection), CML-1 (canine melanoma, Auburn University) and CMT-12 (canine mammary gland carcinoma, Auburn University) were used. All cell lines were grown under standard culture conditions with Dulbecco’s Modified Eagle Medium (DMEM; Mediatech Masassas, Herndon, VA, U.S.A.) supplemented with 10% fetal bovine serum (FBS), penicillin-streptomycin and 5% carbon dioxide at 37°C. Confluent cells were subcultured every 3–7 days after detaching the cells with 0.1% trypsin and 0.02% ethylenediaminetetraacetic acid in phosphate buffered saline (PBS; Mediatech). All cell proliferation experiments were also performed with 1% FBS media. All experiments outlined were performed in triplicate.
Loperamide hydrochloride: Loperamide hydrochloride was purchased from Sigma (St. Louis, MO, U.S.A.). Stock solutions of 50 mM were prepared in dimethyl sulfoxide (DMSO) and stored at −20°C. Fresh dilutions of loperamide hydrochloride were made from the stock solutions in media supplemented with 10% FBS for each experiment such that the DMSO concentration did not exceed 0.2% as per standard experimental procedures, which has previously been shown to have no effect on growth of these cell lines [9, 11].
Inhibition of cellular proliferation assay: The effect of loperamide on cellular proliferation was evaluated using a bioreductive fluorometric assay (Alamar BlueTM, Molecular Probes, Invitrogen, Carlsbad, CA, U.S.A.). Between 1,500 and 6,000 cells per well were plated and grown in 10% FBS media in triplicate or quadruplicate in 96-well tissue culture treated microtiter plates and incubated for 12–24 hr at 37°C. Number of cells plated per well was dependent on the rate of growth of cell lines in the flask. Media were removed, and various concentrations of loperamide diluted in 10% FBS supplemented media were added. Control wells consisted of media alone.
Cell viability was determined at 24, 48 and 72 hr following addition of loperamide. At these time points, 20 µl of Alamar Blue was added to each well, and the plates were incubated at 37°C for 8 hr. After incubation, plates were read using a spectrophotometric microplate reader (Biotek Synergy 4; Biotek Instruments, Winooski, VT, U.S.A.). Relative viable cell percentage was standardized to that of cells incubated without loperamide. The IC50 was determined by nonlinear regression analysis fitting to a dose-response curve using a computer software program (PRISM 4, GraphPad Software, La Jolla, CA, U.S.A.).
To complement cell viability assessment, a second set of cells for each cell line was grown in 6-well tissue culture treated plates. These cells were plated at various concentrations between 75,000–200,000 cells per well depending on growth rate in the flask. After 24 hr, these cells were treated with two different drug concentrations (10 µM and 100 µM), while a control group was treated with media alone. The cells were observed with light microscopy and photographed at 6, 24 and 48 hr after addition of loperamide.
Apoptosis analysis: The proportion of cells undergoing apoptosis was evaluated using 7-AAD (10 µg/ml; Molecular Probes, Invitrogen). Cells were plated in 6-well plates at concentrations ranging from 25,000 to 150,000 cells/well depending on inherent growth rate to ensure that cells did not overgrow by the time of the assay. The cells were incubated for 12–24 hr at 37°C, at which time media were removed, and various concentrations of loperamide (0, 10, and 30 µM) diluted in 10% media were added. Cells were then evaluated with flow cytometry at 6, 24 and 48 hr post addition of drug. Immediately prior to analysis, media were removed, and the attached cells were rinsed with cold PBS, trypsinized and resuspended in media. The cell suspension was centrifuged at 186 × g at 7°C for 7 min, after which the cell recovery was enumerated with an automated cell counter (Cellometer, Nexcelom, Lawrence, MA, U.S.A.). Supernatant was removed, and the cells were resuspended in 100 µl of cold PBS. One hundred µl of 7-AAD, prepared in a supplemented buffer (0.1% bovine serum albumen (BSA), 0.1% NaN3 and 1.0% FBS), was added. The cells were incubated on ice for 15–30 min and evaluated using a LSR-II flow cytometer with FACSDiva 6.0 software (BD Biosciences, San Jose, CA, U.S.A.). A minimum of 10,000 events were collected per sample, and reported values were a percentage of total cells counted. Cells were discriminated into three populations, live cells and early apoptotic or late apoptotic/necrotic cells based on size and 7-AAD uptake as described previously [15, 25]. Briefly, viable cells were of moderate size with minimal 7-AAD uptake, while early apoptotic cells had moderate dye uptake and smaller size, and late apoptotic/necrotic cells were smallest in size with most intense uptake. The excitation wavelength was 488 nm with emission detected with a photomultiplier equipped with a 695/40 band pass filter.
Cell cycle analysis: Cells were grown and treated with loperamide for cell cycle analysis in the same manner as for apoptosis analysis (0, 10 and 30 µM) and allowed to incubate for 6, 24 and 48 hr. Cells were harvested as above for apoptosis analysis and were evaluated with a commercial cell cycle kit (BD CycleTestTM PLUS DNA kit, BD Biosciences) [20]. Briefly, cells were suspended in a sodium citrate, sucrose and dimethyl sulfoxide buffer. The cell suspension was centrifuged for 5 min at 300 × g at room temperature, the supernatant was removed, and the cells were resuspended in the same buffer solution. This wash step was repeated a second time, and the resulting suspension was then centrifuged for 5 min at 300 × g at 23°C ± 5°C, and the supernatant was removed. Fifty µl of trypsin in a spermine tetrahydrochloride buffer was added for the digestion of cell membranes and cytoskeletal elements and allowed to react for 10 min. After incubation, 40 µl of a trypsin inhibitor and RNase buffer was added and incubated for 10 min, after which 40µl of cold (2–8°C) propidium iodide was added and allowed to incubate for another 10 min. The samples were placed on ice and immediately analyzed using flow cytometry to detect propidium iodide fluorescence, using an excitation of 488 nm and emission detected through a 575/26 band pass filterand analyzed with FlowJo software (Tree Star, Ashland, OR, U.S.A.) to determine cell cycle parameters.
Chemosensitization assay: Cells were grown as harvested as for the cellular proliferations assays. After plating, cells were allowed to adhere for 12–24 hr, after which time the cells were treated with doxorubicin alone (Sigma) at concentrations from 0.1 nM–1,000 nM, loperamide alone at 10 µM or a combination of increasing concentrations of doxorubicin (0.1 nM–1,000 nM) with 10 µM of loperamide. The dose of 10 µM was selected as it was near the IC50 value for most cell lines. A separate experiment using 25 µM of loperamide instead of 10 µM was also run for the CMT-12 cell line, since this cell line had a higher IC50 than the other three cell lines. Control cells were treated with media alone at the same volume as used for the drugs. Cells were incubated with the drugs for 72 hr before the Alamar Blue assay was run as described above. The relative viable cell number was expressed as a percentage of control cells.
Statistical analysis: All statistical analyses below were conducted separately for each of the 4 cell lines (CMT-12, CML-1, CTAC and D-17). Cell proliferation or cytotoxicity data (cell counts) were subjected to a 3-way ANOVA. The main effects for this linear model were media (1% vs. 10% FBS), loperamide dose (0, 0.01, 0.1, 1.0, 3.2, 10, 32 and 100 µM) and exposure time (24, 48 and 72 hr). Residuals analysis suggested a Box-Cox power transformation [1] of the cell count response, the results from which are reported. Cell apoptosis data from loperamide exposure were measured as percent viable cells. These data were subjected to a 2-way ANOVA model using dose (0, 10 and 30 µM) and exposure time (6, 24 and 48 hr). The frequency (%) of cells in the G0/G1 phase of the cell cycle was subjected also to a 2-way ANOVA model as the experimental design matched that of the apoptosis data. The synergistic effects of doxorubicin with loperamide (chemosensitization) were measured by a 2-way ANOVA of the cell proliferation response subject to doxorubicin concentration (0, 0.1, 1.0, 10, 100 and 1,000 nM) and loperamide concentration (0 and 10 µM) as main effects. Residuals analysis suggested a weighted least-squares [13] regression method, which was performed and reported as addressing the problem of heteroscedasticity. In addition, the test for a statistically significant (P<0.05) interaction between doxorubicin and loperamide was equivalent to the Bliss test for synergism [21]. Multiple comparisons among levels of the main effects in any of these analyses were conducted using the Tukey-Kramer HSD (honestly significant difference) test [10, 22]. All analyses were performed using JMP® Pro 10.0.
RESULTS
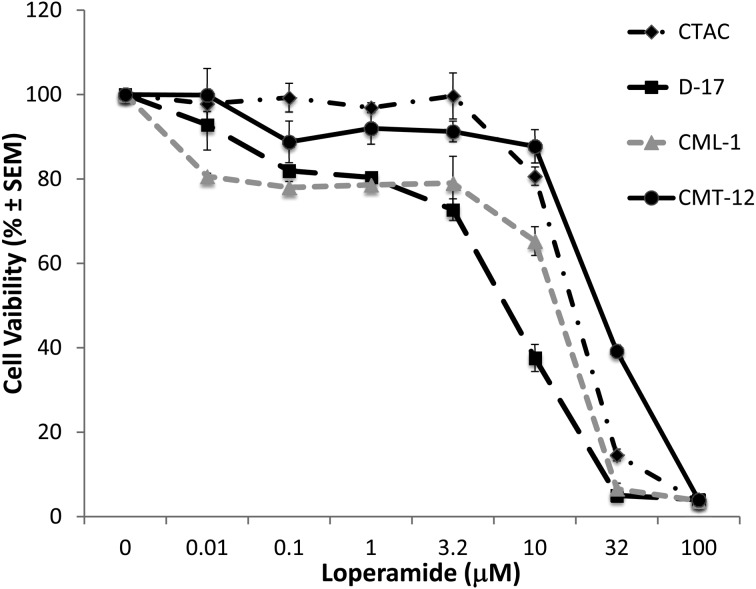
Loperamide induced a dose-dependent cytotoxicity in canine cancer cell lines: Loperamide produced anti-proliferative effects with increasing concentrations in all cell lines tested (Fig. 1). Cell lines had different sensitivities to the cytotoxic effects of loperamide, with the D-17 osteosarcoma cell line most sensitive (Table 1). Specifically, CTAC and CMT-12 cell viability at concentrations ≥32 µM were significantly lower compared to control cells. Cell viability in both the D-17 and CML-1 cell lines was significantly lower at loperamide concentrations ≥10 µM than compared to control cells. Morphological changes supported the cytotoxic effects of loperamide (Fig. 2). There were no significant differences in the IC50 concentrations for any cell line based on timing (24, 48 and 72 hr time points), indicating the loperamide exhibits its maximal effect by 24 hr. In addition, there was no effect of the FBS concentration in the media (1% vs. 10%) on cell survival for either the CTAC or the D-17 cell lines (data not shown). However, there was a positive effect on CML-1 cell line survival with increasing FBS solution strength and negative effect on CMT-12 cell line survival (data not shown).
Fig. 1.
Loperamide impaired cell viability following 72 hr of incubation in a dose dependent fashion in CTAC, D-17, CML-1 and CMT-12 canine cancer cell lines. Viability data were garnered from experiments performed in triplicate and assessed by Alamar Blue assay. Cell viability for both CTAC and CMT-12 was significantly lower than control cells at concentrations ≥32 µM; cell viability for D-17 and CML-1 was significantly lower than control cells at concentrations ≥10 µM.
Table 1. IC50 concentrations of loperamide in canine cancer cells.
| CTAC | D-17 | CML-1 | CMT-12 | |
|---|---|---|---|---|
| IC50 (µM) 24 hr | 20 ± 0.8 | 8.7 ± 0.5 | 19.4 ± 0.6 | 27 ± 0.5 |
| IC50 (µM) 48 hr | 16.2 ± 0.8 | 8.2 ± 0.8 | 16.7 ± 1.1 | 25.5 ± 1.7 |
| IC50 (µM) 72 hr | 19.3 ± 0.5 | 7.2 ± 0.3 | 14.8 ± 1.1 | 25.8 ± 0.3 |
IC50 concentrations for 4 canine cancer cell lines after 24, 48 and 72 hr of incubation with loperamide. There was no difference in IC50 based on time, as analyzed via ANOVA with Tukey post-hoc test. Values are expressed as mean ± SEM from three independent experiments.
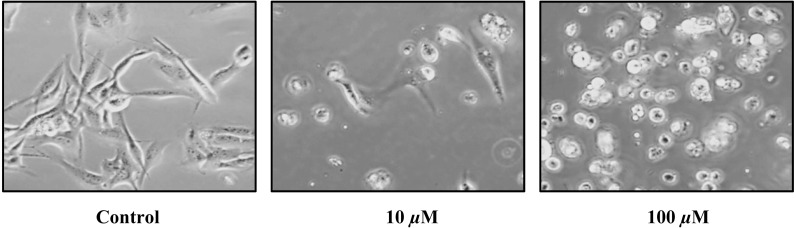
Fig. 2.
Morphological changes of the D-17 cell line after treating with 0, 10 and 100 µM of loperamide for 24 hr. Cell morphology changed from an attached spindle-shaped appearance (control) to detached and rounded with increasing doses of loperamide. This is a representative cell line, as all others showed similar changes.
Loperamide induced a dose and time-dependent apoptosis: Cells were stained with 7-AAD and analyzed via flow cytometry to confirm that loperamide induced cell death. Results in all cell lines showed a dose and time-dependent decrease in viable cells following treatment with loperamide (Table 2). In the absence of loperamide, less than 10% of cells were apoptotic. At 6 hr, there were no significant differences in percentage of apoptotic cells from baseline in any cell line; however, following both 24 and 48 hr of exposure to 30 µM, a significantly higher number of dead cells were detected. There was no difference between the 10 µM group and the control group at any time point for any of the cell lines.
Table 2. Induction of apoptosis following incubation with loperamide.
| CTAC | D-17 | CML-1 | CMT-12 | |
|---|---|---|---|---|
| 6 hr | ||||
| 0 µM | 2.6 ± 0.8 | 6.1 ± 1.7 | 1.9 ± 0.5 | 9.7 ± 1.5 |
| 10 µM | 1 ± 0.2 | 7.6 ± 1.3 | 2.4 ± 0.6 | 8.6 ± 0.6 |
| 30 µM | 2.8 ± 0.5 | 5.8 ± 0.2 | 3.5 ± 1.6 | 10.8 ± 3.2 |
| 24 hr | ||||
| 0 µM | 1.5 ± 0.5 | 2.2 ± 0.6 | 1.5 ± 0.3 | 9.1 ± 2.6 |
| 10 µM | 3.2 ± 0.9 | 2.4 ± 1 | 1.4 ± 0.3 | 6.2 ± 1.7 |
| 30 µM | 39.9 ± 6.1* | 27.6 ± 7.3* | 46.5 ± 13.5* | 25.5 ± 4.4* |
| 48 hr | ||||
| 0 µM | 4.1 ± 1.6 | 1.1 ± 0.2 | 1.8 ± 0.5 | 7.1 ± 0.7 |
| 10 µM | 6.2 ± 2 | 1.4 ± 0.4 | 1.4 ± 0.2 | 9 ± 0.9 |
| 30 µM | 92.7 ± 0.1* | 57.2 ± 4.6* | 86.1 ± 3.5* | 72.8 ± 2.3* |
Percentage of cells undergoing apoptosis at three different time points and three different loperamide concentrations. Values are expressed as mean ± SEM from three independent experiments. An * indicates that there are significantly more apoptotic cells compared to the control (0 µM of drug) as analyzed via ANOVA with Tukey post-hoc test.
Loperamide induced a dose and time-dependent G0/G1 accumulation: A commercially available cell assay for live cells based on standard propidium iodide staining was used to assess cell cycle effects on all cell lines. Loperamide caused a dose and time-dependent G0/G1 accumulation in viable cells (Table 3, Fig. 3). There was no difference between the control and the 10 µM concentrations at any time point.
Table 3. Percentage of cells in G0/G1 following incubation with loperamide.
| CTAC | D-17 | CML-1 | CMT-12 | |
|---|---|---|---|---|
| 6 hr | ||||
| 0 µM | 48.5 ± 3.1 | 45.8 ± 1.2 | 54.6 ± 0.3 | 49.7 ± 1.4 |
| 10 µM | 50 ± 2 | 44.5 ± 0.5 | 55.8 ± 1.2 | 51.5 ± 2 |
| 30 µM | 41.3 ± 1.1 | 44.8 ± 0.6 | 59.7 ± 1.3 | 49 ± 2.3 |
| 24 hr | ||||
| 0 µM | 38.1 ± 3.3 | 52.6 ± 1.5 | 50.6 ± 0.3 | 58.7 ± 1 |
| 10 µM | 53.5 ± 2.1 | 55.5 ± 2.2 | 57.2 ± 1.6 | 59.6 ± 0.2 |
| 30 µM | 56.8 ± 0.8* | 63.4 ± 3.7* | 70 ± 1.9* | 78.2 ± 2.4* |
| 48 hr | ||||
| 0 µM | 52.7 ± 5.1 | 54.4 ± 1.2 | 49.7 ± 3.1 | 80.4 ± 1.1 |
| 10 µM | 51.2 ± 1.6 | 60.7 ± 1.8 | 50.3 ± 1.8 | 77.6 ± 2 |
| 30 µM | 62.8 ± 2.4* | 78.8 ± 1.1* | 71.6 ± 0.8* | 85.7 ± 1.7* |
Percentage of cells in G0/G1 at three different time points and three different loperamide concentrations. Values are expressed as mean ± SEM from three independent experiments. An * indicates that there are significantly more G0/G1 cells compared to the control (0 µM of drug) as analyzed via ANOVA with Tukey post-hoc test.
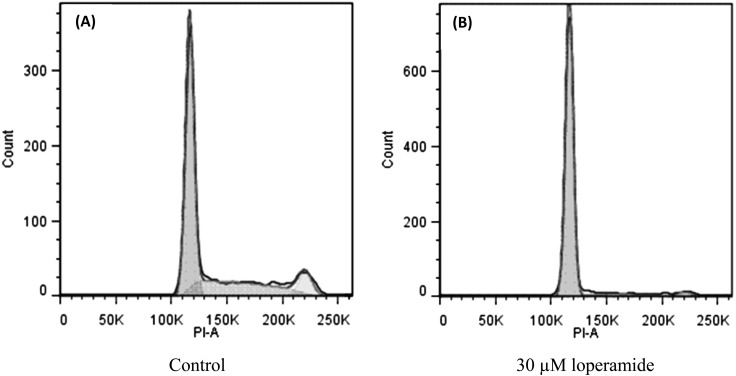
Fig. 3.
Loperamide caused an accumulation of cells in the G0/G1 phase as assessed by flow cytometry. Representative flow cytometry histograms of D-17 cells are displayed for cells treated for 48 hr with loperamide (30 µM) (B) and control (A).
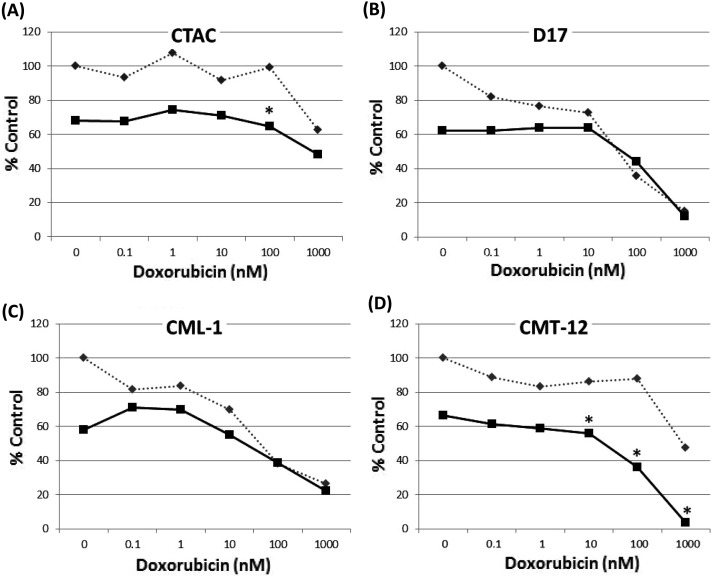
Loperamide sensitized canine cancer cells to doxorubicin: Four cell lines were screened to determine if loperamide increased the sensitivity of cancer cells to the chemotherapeutic drug doxorubicin in vitro. There was no evidence of synergism for the D-17, CML-1 or CMT-12 cell lines with 10 µM loperamide. However, there was synergism between the two drugs at 100 nM of doxorubicin and 10 µM loperamide for the CTAC cell line. Due to the fact that the IC50 of the CMT-12 cell line was higher than the other three cell lines, the experiment was repeated using the same concentrations of doxorubicin, but with 25 µM of loperamide, nearer to the IC50. This combination showed significant synergism at doxorubicin concentrations>10 nM (Fig. 4).
Fig. 4.
Doxorubicin alone is represented by the interrupted line, while the combination of doxorubicin and loperamide is represented by the non-interrupted line. Concentrations that exhibit synergism are represented by the *. Loperamide concentration in A-C is 10 µM, while it is 25 µM in D. The curves are representative samples of experiments performed in triplicate.
DISCUSSION
The development of novel anticancer drugs typically involves significant expense and time. Thus, the discovery of existing drugs with potentially expanded roles beyond their traditional purpose is attractive. Here, loperamide, a commonly used anti-diarrheal in canine and human cancer patients, caused a significant dose-dependent cytotoxicity in 4 different canine cancer cell lines. The IC50 for loperamide ranged from 7.2–27 µM, which correlates well with a study performed on a library of human cancer cell lines, showing IC50 concentrations ranging from 11.8–41.4 µM. As seen in the human study, canine osteosarcoma cells were most sensitive to loperamide, while canine breast carcinoma cells were less sensitive (6). Cell death in the canine cancer cell lines investigated here was mediated in part by apoptosis, as demonstrated through 7-AAD staining and flow cytometry, a technique shown to correlate well with other common apoptosis analyses, such as Annexin-V and propidium iodide [15, 25]. The mechanism behind apoptosis in these cell lines has not been elucidated, although in human cancer cell lines, loperamide has been shown to cause apoptosis via activation of the caspase 3 pathway [5]. Apoptotic canine cancer cells were identified at 24 hr, which was slightly later than with human cancer cells, as apoptosis was evident as early as 6 hr following loperamide exposure [5]. This may be a reflection of different assays used or possibly decreased potency against canine cells; it is possible that with higher doses of loperamide, a significant change in 7-AAD staining would have been noted prior to 24 hr. Interestingly, other opiate agonists, such as etorphine, morphine and buprenorphine, have also been shown to induce apoptosis in some human cell lines [7, 8, 24].
An unexpected finding was that unlike a G2/M arrest seen in human cancer cell lines [5], loperamide caused a dose and time-dependent G0/G1 accumulation in canine cancer cells. Our group’s initial intent was to consider using loperamide as a radiation sensitizer, given that human cancer cell lines are arrested in sensitive phases of the cell cycle [16, 26]. It is still possible that loperamide, or a similar peripheral opiate agonist, might exert synergism with radiotherapy through another mechanism and may be worth exploring. It is unclear why the cells tested here demonstrated G0/G1 arrest rather than G2/M, however, it is possible that canine cancer cells may have altered cell cycle kinetics compared to human cancer cells.
In addition to its negative effects on cell viability and its pro-apoptotic effect, loperamide also demonstrated synergism with doxorubicin in both the CTAC and CMT-12 cell lines at concentrations near the IC50 when combined with doxorubicin. While it is interesting that both of these cell lines are carcinomas, further investigation is indicated to determine if similar effects are seen in other cell types. In a human breast cancer cell line stably transfected with the MDR-1 gene leading to doxorubicin resistance, loperamide was able to reverse this resistance due to high affinity for the p-glycoprotein pump, therefore leading to intracellular accumulation of the chemotherapeutic [27]. While our laboratory has performed preliminary work investigating the immunohistochemical expression of p-glycoprotein in our selected cell lines, further work is needed to determine if there is a relationship between p-glycoprotein expression and loperamide effects. The apparent synergism of doxorubicin and loperamide in some cell lines may also be due to occupation of another shared efflux pump, such as MRP-1, or through alternative mechanisms not related to drug efflux. It may be interesting in future studies to evaluate the effect of loperamide on doxorubicin-resistance canine cell lines compared to naïve cancer cell lines to further elucidate the potential for adjuvant therapy. Low concentrations of loperamide may be capable of improving cell sensitivity to chemotherapy.
A possible limitation of the in vitro work performed here is that the concentrations of loperamide used are above what has safely been used in vivo. One study showed that after a typical dosage of 0.2 mg/kg, blood concentrations of 0.02 µM were achieved [12]. The maximally tolerated dose of loperamide is not known, but toxicities including vomiting, diarrhea and central nervous system depression have been reported at doses as low as 1.25 mg/kg [17]. In dogs with mutations in their p-glycoprotein pumps (ABCB1Δ mutation), toxicity may be seen at doses well tolerated by normal dogs [19]. However, it is certainly possible that the in vitro work done in this study is not a true reflection of the in vivo activity of this drug. It is also important to note that in this work, cells were only exposed to a single dose of loperamide. If loperamide is capable of reversing resistance, doses in the realm of 10 µM may still be necessary, which would be difficult to achieve in dogs unless the target cancer cells are capable of accumulating drug [27]. Loperamide exerted negative effects on the cancer cell lines studied here as early as 24 hr following exposure; given that loperamide may be administered more frequently, chronic dosing may have altered effects. It is also important to note that variations in drug metabolism and patient variability may lead to anti-neoplastic effects in a spontaneous tumor model with lower drug concentrations. Despite these limitations, this study establishes a proof-of-principle in support of loperamide as an anti-cancer agent. Further studies are needed to establish mechanisms of action of its activity as well as in vivo tolerability as part of multi-modality therapy. It is possible that drugs similar to loperamide will exert similar anticancer properties at clinically relevant doses. Loperamide would be an attractive drug in the clinic, as it has minimal side effects, is an over-the-counter drug, and is inexpensive.
In conclusion, results presented here provide novel information regarding a commonly used commercially available drug. Loperamide negatively affected canine cancer cell viability with IC50 values similar to those reported in human cancer cell lines. Loperamide also caused apoptosis in a dose-dependent fashion and induced a G0/G1 cell cycle arrest. In vitro chemosensitivity studies suggest that loperamide may sensitize some canine cancer cells to doxorubicin cytotoxicity. Further work on the efficacy of loperamide or similar peripheral µ-opioid agonist in the management of cancer is warranted.
REFERENCES
- 1.Box G. E. P., Cox D. R.1964. An analysis of transformations. J. R. Stat. Soc., B 3: 39–52. [Google Scholar]
- 2.Cadet P., Rasmussen M., Zhu W., Tonnesen E., Mantione K. J., Stefano G. B.2004. Endogenous morphinergic signaling and tumor growth. Front. Biosci. 9: 3176–3186. doi: 10.2741/1471 [DOI] [PubMed] [Google Scholar]
- 3.Cascinu S., Bichisao E., Amadori D., Silingardi V., Giordani P., Sansoni E., Luppi G., Catalano V., Agostinelli R., Catalano G.2000. High-dose loperamide in the treatment of 5-fluorouracil-induced diarrhea in colorectal cancer patients. Support. Care Cancer 8: 65–67. [DOI] [PubMed] [Google Scholar]
- 4.Choi C. H.2005. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 5: 30. doi: 10.1186/1475-2867-5-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong X. W., Xu Y. H., Chen X. L., Wang Y. X.2012. Loperamide, an antidiarrhea drug, has antitumor activity by inducing cell apoptosis. Pharmacol. Res. 65: 372–378. doi: 10.1016/j.phrs.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 6.Hatzoglou A., Ouafik L., Bakogeorgou E., Thermos K., Castanas E.1995. Morphine cross-reacts with somatostatin receptor SSTR2 in the T47D human breast cancer cell line and decreases cell growth. Cancer Res. 55: 5632–5636. [PubMed] [Google Scholar]
- 7.Hu S., Sheng W. S., Lokensgard J. R., Peterson P. K.2002. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology 42: 829–836. doi: 10.1016/S0028-3908(02)00030-8 [DOI] [PubMed] [Google Scholar]
- 8.Kugawa F., Arae K., Ueno A., Aoki M.1998. Buprenorphine hydrochloride induces apoptosis in NG108-15 nerve cells. Eur. J. Pharmacol. 347: 105–112. doi: 10.1016/S0014-2999(98)00080-6 [DOI] [PubMed] [Google Scholar]
- 9.Krajarng A., Nilwarankoon S., Suksamrarn S., Watanapokasin R.2012. Antiproliferative effect of α-mangostin on canine osteosarcoma cells. Res. Vet. Sci. 93: 788–794. doi: 10.1016/j.rvsc.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 10.Kramer C. Y.1956. Extension of multiple range tests to group means with unequal numbers of replications. Biometr. 12: 307–310. doi: 10.2307/3001469 [DOI] [Google Scholar]
- 11.Lawrence J. A., Huelsmeyer M. K., Thamm D. H., Tumas D. B., Birkus G., Kurzman I., Vail D. M.2013. Noval acyclic nucleotide analogues GS-343074 and GS-424044 demonstrate antiproliferative and pro-apoptotic activity in canine neoplastic cell lines. Vet. Comp. Onc. doi: (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mealey K. L., Waiting D., Raunig D. L., Schmidt K. R., Nelson F. R.2010. Oral bioavailability of P-glycoprotein substrate drugs do not differ between ABCB1-1Δ and ABCB1 wild type dogs. J. Vet. Pharmacol. Ther. 33: 453–460. doi: 10.1111/j.1365-2885.2010.01170.x [DOI] [PubMed] [Google Scholar]
- 13.Neter J., Kutner M. H., Nachtsheim C. J., Wasserman W.1996. In: Applied Linear Statistical Models, 4th ed. McGraw-Hill, Boston. [Google Scholar]
- 14.Nobili S., Landini I., Giglioni B., Mini E.2006. Pharmacological strategies for overcoming multidrug resistance. Curr. Drug Targets 7: 861–879. doi: 10.2174/138945006777709593 [DOI] [PubMed] [Google Scholar]
- 15.Olgun S., Gogal R. M., Jr, Adeshina F., Choudhury H., Misra H. P.2004. Pesticide mixtures potentiate the toxicity in murine thymocytes. Toxicology 196: 181–195. doi: 10.1016/j.tox.2003.09.007 [DOI] [PubMed] [Google Scholar]
- 16.Patel K., Doudican N. A., Schiff P. B., Orlow S. J.2011. Albendazole sensitizes cancer cells to ionizing radiation. Rad. Onc. 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plumb D. C.2011. Loperamide, H. C. L. In: Plumb’s Veterinary Drug Handbook, 7th ed, Wiley, Hoboken. [Google Scholar]
- 18.Rasmussen M., Zhu W., Tonnesen J., Cadet P., Tonnesen E., Stefano G. B.2002. Effects of morphine on tumor growth. Neuroend. Let. 23: 193–198. [PubMed] [Google Scholar]
- 19.Sartor L. L., Bentjen S. A., Trepanier L., Mealey K. L.2004. Loperamide toxicity in a collie with the MDR1 mutation associated with ivermectin sensitivity. J. Vet. Intern. Med. 18: 117–118. doi: 10.1111/j.1939-1676.2004.tb00145.x [DOI] [PubMed] [Google Scholar]
- 20.Shi P., Lai R., Lin Q., Iqbal A. S., Young L. C., Kwak L. W., Ford R. J., Amin H. M.2009. IGF-IR tyrosine kinase interacts with NPM-ALK oncogene to induce survival of T-cell ALK+ anaplastic large-cell lymphoma cells. Blood 114: 360–370. doi: 10.1182/blood-2007-11-125658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slinker B. K.1998. The statistics of synergism. J. Mol. Cell. Cardiol. 30: 723–731. doi: 10.1006/jmcc.1998.0655 [DOI] [PubMed] [Google Scholar]
- 22.Tukey J.1953. A problem of multiple comparisons. Manuscript of 396 pages, Princeton University. [Google Scholar]
- 23.Vail D. M.2009. Supporting the veterinary cancer patient on chemotherapy: neutropenia and gastrointestinal toxicity. Top. Companion Anim. Med. 24: 122–129. doi: 10.1053/j.tcam.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 24.Yin D. L., Ren X. H., Zheng Z. L., Pu L., Jiang L. Z., Ma L., Pei G.1997. Etorphine inhibits cell growth and induces apoptosis in SK-N-SH cells: involvement of pertussis toxin-sensitive G proteins. Neurosci. Res. 29: 121–127. doi: 10.1016/S0168-0102(97)00080-1 [DOI] [PubMed] [Google Scholar]
- 25.Zembruski N. C. L., Stache V., Haefeli W. E., Weiss J.2012. 7-Aminoactinomycin D for apoptosis staining in flow cytometry. Anal. Biochem. 429: 79–81. doi: 10.1016/j.ab.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y., Jiang W., Li B., Yao Q., Dong J., Cen Y., Pan X., Li J., Zheng J., Pang X., Zhou H.2011. Artesunate enhances radiosensitivity of human non-small cell lung cancer A549 cells via increasing NO production to induce cell cycle arrest at G2/M phase. Int. Immunopharmacol. 11: 2039–2046. doi: 10.1016/j.intimp.2011.08.017 [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y., Sridhar R., Shan L., Sha W., Gu X., Sukumar S.2012. Loperamide, an FDA-approved antidiarrhea drug, effectively reverses the resistance of multidrug resistant MCF-7/MDR1 human breast cancer cells to doxorubicin-induced cytotoxicity. Cancer Invest. 30: 119–125. doi: 10.3109/07357907.2011.640653 [DOI] [PMC free article] [PubMed] [Google Scholar]