Abstract
The analgesic effect of wound infiltration with bupivacaine was evaluated in cats undergoing bilateral mastectomy. Twenty-one female cats with mammary gland tumors were anesthetized with propofol and oxygen-isoflurane anesthesia following premedication with atropine. In the trial group (Group I; n=11), 30 ml of saline containing 2 mg/kg of bupivacaine was infiltrated topically into the surgical wound right after removal of the mammary glands, whereas only saline solution was infiltrated in the control group (Group II; n=10). At the same time, carprofen (4 mg/kg) was also administered subcutaneously in both groups. Behavioral signs of pain were monitored during the recovery period after general anesthesia. In order to examine the behavioral changes associated with acute pain, a questionnaire was prepared and given to the owners to be completed 4 hr and then 10 hr after the operation. According to the owners’ anwers to the questionnaire, a pain score was specified using a “numerical rating scale” for each cat. Although some cats showed mild to moderate pain, the pain score recorded at 4 hr after the operation was significantly lower in Group I (P<0.001). No significant difference was found at 10 hr after the operation between the groups. The incidence of vocalization, aggression and convulsion within 2 hr after the operation was also lower in Group I. In conclusion, wound infiltration with bupivacaine before incisional closure provided reliable analgesia at least 4 hr after bilateral radical mastectomy in cats.
Keywords: bupivacaine, carprofen, feline, pain, radical mastectomy
Mammary gland tumors are the third most common type of tumor and account for 17% of all tumors in female cats [5, 22]. Feline malignant tumors grow rapidly [12], and most feline mammary tumors are malignant. Therefore, early diagnosis and aggressive therapy have a significant influence on survival rates. In cats, unilateral or bilateral radical mastectomies are recommended as the treatment for mammary tumors [13, 23]. However, radical mastectomies may produce severe postoperative pain in cats [20].
Pain is defined as an unpleasant, complex and multidimensional sensory or emotional experience associated with actual or potential tissue damage [11, 20]. Feeling pain has some advantages, like limiting the activities that could cause further injury, forcing the patient to rest and enabling individuals to learn to avoid dangerous stimuli in the future. Constant pain has many disadvantages, like sensitization of the central nervous system, induction of distress in patients, retardation of recovery, induction of self-injury, decreased cardiovascular function and appetite, increased risk of infection and disseminated intravascular coagulation (DIC), induction of poor anesthetic recovery in the postoperative period and effects on respiration and therefore increases the risk of pneumonia (especially after upper abdominal and thoracic surgery) [20, 32]. In the not too distant past, cats were frequently undertreated for pain, despite the fact that they need analgesia like all other animals, particularly in the first 12 to 24 hr after surgery [4, 18, 26]. The reasons for undertreatment of pain in cats were the limited number of safe analgesics, difficulty in recognizing pain, an insufficient amount of published information and avoidance of the side effects of known analgesics [16, 18]. These undesirable situations have been improving in some countries in which veterinarians are legally allowed to use many analgesic including potent opioids (morphine and fentanyl), ketamine and nonsteroidal anti-inflammatory drugs (NSAIDs) approved for animals [8, 19]. On the other hand, veterinary practitioners are still confused about pain management in many countries with wide restrictions on the use of anesthetics and analgesics.
Local anesthetics and local blocks have been used for a long time in veterinary pain management practise in most countries in the world [16, 27, 32]. Also, NSAIDs have been used for many years for postoperative care in animals [14, 20, 27, 32], and the use of carprofen, ketoprofen and meloxicam is welldocumented in cats. However, preemptive use of carprofen may decrease the blood flow to the kidney during anesthesia and may lead to hemorrhage at the operation site [20]. Local anesthetic agents (lidocaine, ropivacaine, bupivacaine, mepivacaine, etidocaine, etc.) can be injected into or sprayed onto the operation site, and a rapid onset of analgesic action with low toxicity occurs [1, 9, 16, 27]. One of the local anesthetic agents, bupivacaine, requires a longer time for onset of anesthesia (15 min), and its duration (4–6 hr) is more than twice that of lidocaine [26, 30, 32]. Therefore, surgical wound infiltration with bupivacaine is expected to be suitable for postoperative pain management in cats undergoing the radical mastectomy.
For ethical reasons and the medical reasons mentioned above, pain is something that needs medical attention. The aim of the current study is to provide an auxiliary analgesic method to manage a better postanesthetic recovery period until the primary analgesic reaches its effective level in the circulation.
MATERIALS AND METHODS
Animals: Twenty-one 8- to 16-year-old mixed-breed and purebred female cats with body weights of 2.3 to 8 kg that had undergone bilateral radical mastectomy were used in this study. After general and mammary gland examinations, all the cats had a thoracic radiograph and abdominal ultrasound scan, and their mammary gland tumors were staged according to the tumor node metastasis (TNM) classification system [24]. The study was carried out with the consent of the owners.
Anesthesia and Surgery: The cats were fasted for 10–12 hr prior to surgery. In all cases, 0.04 mg/kg intramuscular atropine sulphate (Atropin®; Vetas, Istanbul, Turkey) was administered as a pre-medicant, and 4–6 mg/kg intravenous propofol (Pofol®; Dongkook, Seoul, South Korea) was used for the induction of anesthesia. General anesthesia was maintained with isoflurane (Isoflurane®; Rhodia Organique, Bristol, U.K.) in oxygen delivered via an endotracheal tube. Fluid therapy was administered during anesthesia using Lactated Ringer’s solution (Laktatlı Ringer Sol. 500 ml, I.E. Ulagay, Istanbul, Turkey) at 3 ml/kg/hr as recommended by Davis et al.[3]. The operations were performed by the same surgeon in this study. The cats were randomly divided into two groups. In the trial group (Group I) (n=11), after removal of the mammary glands, a pour-on analgesia was administered to the surgical area with 30 ml of sterile saline (Izotonik Sol. 100 ml, Eczacıbaşı Baxter, Istanbul, Turkey) containing 2 mg/kg bupivacaine (Marcaine® 0.5%; AstraZeneca, Istanbul, Turkey) solution, whereas in the control group (Group II) (n=10), only 30 ml saline was used. The solution was kept on the area for at least 5 min on a sterile gauze (Fig. 1), and the sterile gauze was left on the surgical area until the last few sutures. Carprofen (2 mg/kg, Rimadyl®; Pfizer, Istanbul, Turkey) was injected subcutaneously into all animals right after removal of mammary glands in both groups as the primary analgesic. The same dosage of carprofen was injected again 48 hr after the first injection. Respiratory rates and heart rates were recorded. Systolic and diastolic blood pressures of the cats were also measured from the base of tail with a VET Memo Diagnostic High Definition Oscillometry (HDO) monitor (S+B medVET GmbH, Babenhausen, Germany) at 10-min intervals during the operation.
Fig. 1.
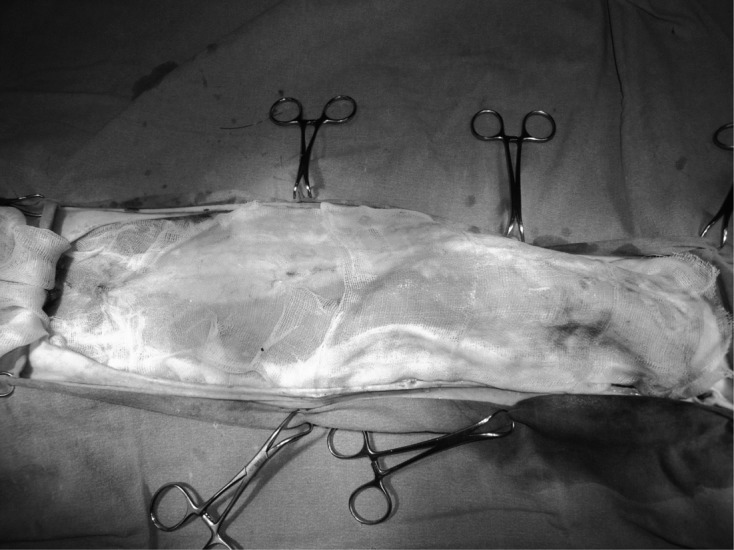
Irrigation solution was administered to the surgical area through an impregnated gauze after the removal of the mammary glands.
Pain assessment in the early period of anesthesia recovery: Respiratory rate, heart rate and blood pressure were measured in the first 2 hr postoperation (at 0, 15, 30, 60, 90 and 120 min; extubation time was defined as 0th min). Behavioral signs of pain (vocalizing, aggression and convulsion) [11, 14, 20, 29, 31] were also monitored for 2 hr in the recovery period of general anesthesia. Sedation scores of the cats were also evaluated by a blinded investigator (B.D.) during the 2 hr after extubation. A simple descriptive scale of 1 to 4 was used to evaluate the degree of sedation. Sedation rate was scored as 1 if the cat was wide awake, 2 if the cat reacted to both vocal and physical stimulations, 3 if the cat responded only to physical stimulation and 4 if there was no response to the stimulations. This scoring system was modified from that of Lascelles et al. [17].
Pain assessment after discharge of patients: The cats were discharged to the owner 2 hr after the operation. The owners were educated about the states of emergency including clinical signs of severe pain and allowed to get in contact with the working team whenever they needed. The owners and cats were invited to the clinic on the 2 days after the operation for treatment of pain, sutures and general condition. The cats were treated again on the 7th and 14th days after operation. On the 14th day, the sutures were removed.
In order to examine the behavioral changes associated with acute pain after discharge of the patients, a questionnaire was prepared meticulously and given to the owners to be completed 4 hr and again 10 hr after the operation. The questionnaire was divided into four sections; 1) the first section asked for patient information (age, breed, reproductive history and mammary mass history), 2) the second section was investigated for the behavioral changes (growling, hissing, depression, restlessness, hiding, biting or scratching the operation area and reluctance to move) and respiratory rate with and without interaction with the cat, 3) the third section investigated behavioral changes and respiratory rate while the owner gently touched the operation site for the purpose of applying firm pressure to the surgical area, and 4) the last section was related to information about the times at which urination, defecation and eating were first observed after the operation. Also, pictures that illustrated facial expressions and body postures were included in the questionnaires. The questionnaries were evaluated by a blinded investigator (B.D.), and pain scores of the cats were specified using the following “numerical rating scale” [32]:
0. No pain, no overt signs of discomfort, no adverse reaction to firm pressure
1. Mild pain, no overt signs of discomfort, adverse reaction to firm pressure
2. Moderate pain, some overt signs of discomfort, made worse by firm pressure
3. Severe pain, overt signs of persistent discomfort, made worse by firm pressure
Data analyses: The effect of surgical wound infiltration with bupivacaine on the pain score was evaluated by using the independent samples t-test. The chi-square test was used for evaluation of behavioral signs during the first two hr post operation. Repeated measures of ANOVA was performed with the SPSS 10.0 statistical package (SPSS, 1999) to analyze data for cardiovascular and respiratory parameters [6]. Differences between the groups in terms of time-dependent changes (group×time interactions) were analyzed by using a General Linear Model (GLM).
RESULTS
The characteristic features, reproductive histories, histopathological results and outcomes of the 21 cats are summarized in Table 1. The mammary gland tumors were staged between T1N0M0 and T3N1M0 in all cats before surgery. No failure of wound healing at the operation site was observed in either group. There was no difference in outcome of the patients between the groups. Five owners from Group I and eight owners from Group II got in contact with our working team within approximately 4 hr after operation. After a detailed anamnesis, it was considered that three of the owners in Group I and two of the owners in Group II worried unnecessarily. However, hissing and dysphoria ere reported for one of the other cat in Group I and the six other cats in Group II.
Table 1. Characteristic features, reproductive histories, histopathological results and outcomes of the patients.
| No. | Group | Age | Breed | Neutered +/− | Mammary glands with a mass | TNM | Histopathology | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 15 | Persian | + | Caudo-thoracal | T2N0M0 | Tubular carcinoma | Still alive at 12 months |
| Abdominal | ||||||||
| 2 | 1 | 15 | Mix | + | Cranio-thoracal | T3N1M0 | Solid adenocarcinoma | Died within 5 months |
| 3 | 1 | 10 | Mix | + | Cranio-thoracal | T3N1M0 | Solid adenocarcinoma | Died within 6 months |
| 4 | 1 | 15 | Siamese | + | Abdominal | T2N1M0 | Tubular adenocarcinoma | Died within 2 months (renal insufficiency) |
| Inguinal | ||||||||
| 5 | 1 | 8 | Turkish Angora Cat | + | All glands | T3N1M0 | Solid adenocarcinoma | Died within 2 months |
| 6 | 1 | 10 | Persian | + | Abdominal | T2N0M0 | Solid adenocarcinoma | Died within 1 year |
| Inguinal | ||||||||
| 7 | 1 | 11 | Mix | + | Abdominal | T2N1M0 | Tubular adenocarcinoma | Died within 6 months (renal insufficiency) |
| Inguinal | ||||||||
| 8 | 1 | 13 | Mix | – | Inguinal | T1N0M0 | Solid adenocarcinoma | Still alive at 12 months |
| 9 | 1 | 14 | Turkish Angora Cat | + | Caudo-thoracal | T2N0M0 | Tubular adenocarcinoma | Still alive at 12 months |
| 10 | 1 | 12 | Mix | – | All glands | T3N1M0 | Tubular adenocarcinoma | Died within 2 months |
| 11 | 1 | 9 | Mix | – | Inguinal | T2N0M0 | Solid adenocarcinoma | Still alive at 12 months |
| 12 | 2 | 15 | Mix | – | All glands | T3N0M0 | Tubulopapillary adenocarcinoma | Died within 11 months |
| 13 | 2 | 16 | Mix | + | All glands | T3N1M0 | Tubular adenocarcinoma | Died within 3 months |
| 14 | 2 | 12 | Mix | – | All glands | T3N1M0 | Tubulopapillary adenocarcinoma | Died within 5 months |
| 15 | 2 | 12 | Siamese | – | Thoracal glands | T1N0M0 | Intraductal adenocarcinoma | Died within 1 year |
| 16 | 2 | 11 | Mix | – | All glands | T3N1M0 | Tubulopapillary adenocarcinoma | Died within 1 month (renal insufficiency) |
| 17 | 2 | 8 | Persian | + | All glands | T2N0M0 | Cystic adenomatous hyperplasia | Still alive at 12 months |
| 18 | 2 | 10 | Mix | ORS* | Inguinal | T3N1M0 | Tubular adenocarcinoma | Died within 5 moths |
| 19 | 2 | 12 | Persian | + | All glands | T2N1M0 | Solid adenocarcinoma | Died within 8 months |
| 20 | 2 | Unknown | Mix | + | Caudo-thoracal | T2N0M0 | Tubular adenocarcinoma | Died within 1 year |
| Abdominal | ||||||||
| 21 | 2 | 14 | Mix | – | Inguinal | T3N1M0 | Tubular adenocarcinoma | Died within 3 months |
*Ovarian remnant syndrome.
No significant difference was detected in respiratory rate, heart rate and systolic and diastolic blood pressure between the groups during surgery. These cardiorespiratory parameters did not change significantly after surgical wound infiltration with bupivacaine or saline in either of the groups.
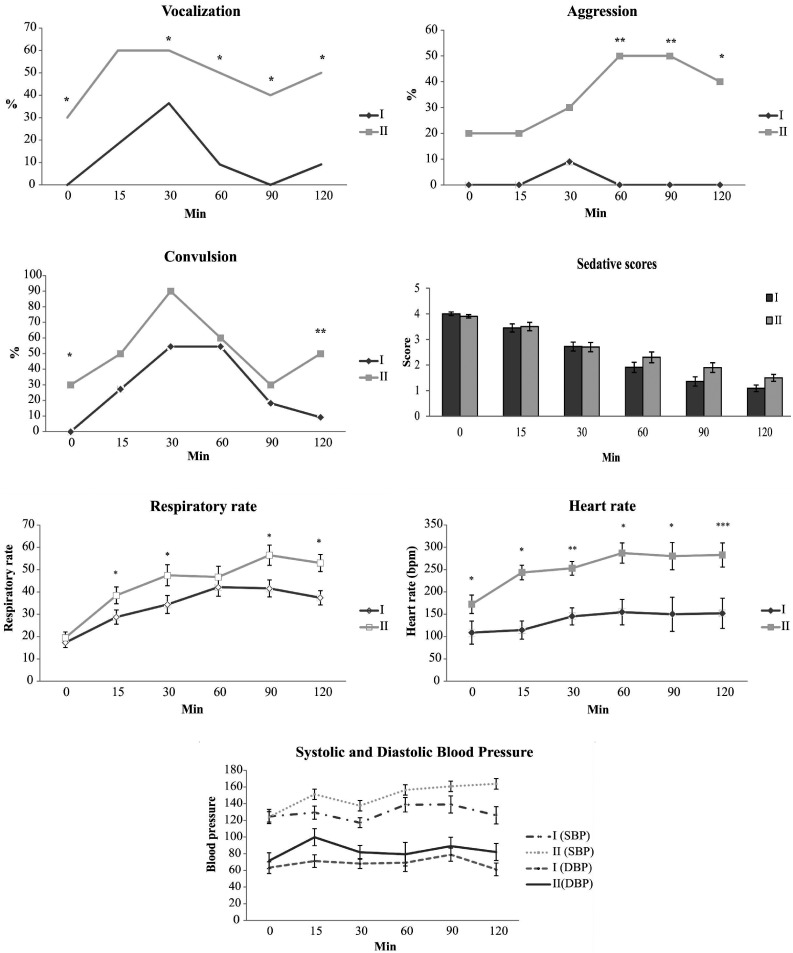
Changes in behavioral signs of pain, sedation score, systolic and diastolic blood pressure, respiratory rate and heart rate of the cats during the 2 hr postoperation are presented in Fig. 2. According to the repeated measures of ANOVA analysis, no significant difference was detected in sedation score between the groups, whereas the difference between the terms was significant (P<0.001). The differences between the groups for systolic and diastolic blood pressure and group×time interactions were insignificant. However, the heart rate and respiratory rate of Group I were significantly lower than those of Group II. Significant reductions in the incidences of behavioral signs of pain were detected during the postanesthetic period in Group I.
Fig. 2.
Changes in behavioral signs of pain (vocalization, aggression and convulsion), respiratory rate, heart rate, and systolic and diastolic blood pressure. Significant difference between the groups: *P<0.05, **P<0.01, ***P<0.001.
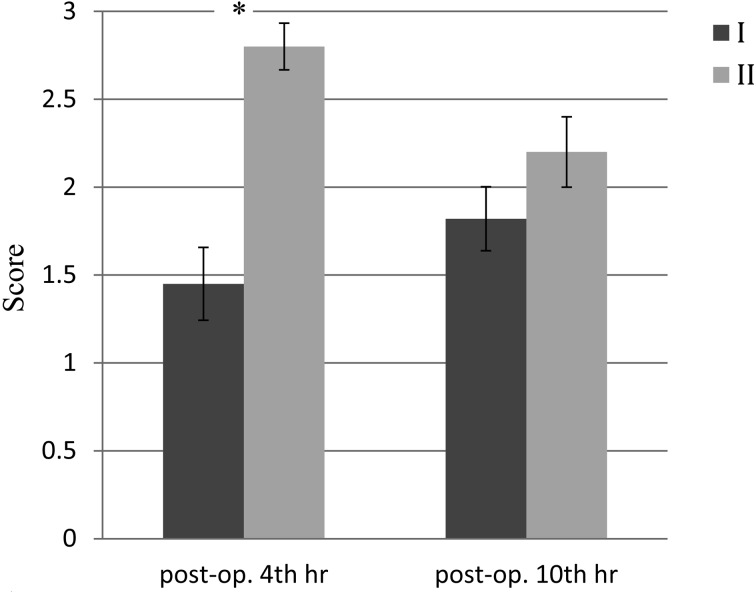
Urination was first observed at 10 ± 1.2 hr and 11 ± 1 hr after surgery in Group I and Group II, respectively. Defecation was first observed at 22 ± 3 hr and 25 ± 2 hr after surgery in Group I and Group II, respectively. Voluntary eating was observed at 12 ± 2 hr and 26 ± 2 hr after surgery in Group I and Group II, respectively. The time at which eating was first observed was statistically earlier in Group I than in Group II (P<0.05). Mean pain scores for the groups are presented in Fig. 3. The pain score for the 4th hr post operation in Group I was significantly lower than that for Group II (P<0.001), whereas no significant difference in the score was found between the groups at 10 hr after the operation. The pain scores between the 4 th and 10 th hr in each group were insignificant.
Fig. 3.
Mean pain scores for the groups at 4 and 10 hr after the operation. Significant difference between the groups: *P<0.001.
DISCUSSION
The amount of postoperative pain varies depending upon the site, duration and nature of the surgical procedure. Surgeries of the mammary glands, ears, eyes and joints are associated with severe postoperative pain [20]. An animal with postoperative pain is more likely to have a poor anesthetic recovery, decreased appetite, slower wound healing and increased anxiety. One of the goals of an anesthetist is to provide adequate analgesia to allow the patient to move, eat and sleep without discomfort, particularly in the first 24 hr after an operation [4, 20]. In the current study, the pain score of Group I was significantly lower than that of Group II at 4 hr after the operation, in accordance with the purpose of the study. Although there was no difference between the pain scores of the groups at 10 hr after the operation, the time at which voluntary eating was first observed after surgery was earlier in Group I. Stress and sympathetic activity caused by pain affect the endocrine system and cause a negative nitrogen balance, disorders in glucose balance and loss of appetite [2]. The results showing an earlier time at which voluntary eating was first observed in Group I may be responsible for the earlier pain control and less negative effect on the endocrine system provided by the surgical wound infiltration with bupivacaine.
According to the telephone conversations with the owners within approximately 4 hr after the operation, it was learned that hissing and dysphoria had been observed in one cat from Group I and five cats from Group II. These findings were associated with a high pain score. However, any additional medical treatment with carprofen would be risky for our geriatric patients, because only 4–5 hr elapsed since the first carprofen injection. Parton et al. [25] reported that the half-life of carprofen after intravenous administration to healthy adult cats ranged 9 to 49 hr. On the other hand, opioids may cause dysphoria in cats [32], and this would increase the complaints. Moreover, opioids are not available or licensed for veterinary use in some countries. No medical treatment was suggested to the owners. However, non-pharmacological methods, such as soft padded beddings in a warm, though not too hot, room in a stress-free, quiet and dim-lit environment [14, 32] were recommended. When our team got in contact with the owners one hr after the first conversation, it was that, at least, a reduction in complaints had been observed in the cats. Brearley [1] reported that local bupivacaine improves postoperative respiratory functions when applied peripherally via a thoracic drain. Tobias et al.[31] compared four different preoperative analgesic methods (intramuscular butorphanol; oral carprofen, subcutaneous ketoprofen; and infiltration block with bupivacaine) in cats who underwent ovariohysterectomy. According to their results, all of the methods induced analgesic effects; however, the bupivacaine infiltration block provided additional analgesia. In our study, the surgical wound infiltration with bupivacaine provided better analgesia in the cats that underwent the bilateral radical mastectomies, although the cats were exposed to much more tissue trauma than the cats in the study of Tobias et al.[31]. Surgical wound infiltration with bupivacaine was able to provide an improvement in analgesia even with such a large amount of tissue trauma.
Despite the beneficial effects, some adverse effects may occur after the administration of local anesthetics, including allergy, sedation, respiratory depression, seizures, hyperexcitability, tissue irrigation and coma [21, 30]. No systemic toxicity occurred in our cats in Group I. In addition, no significant difference was detected between the groups in the sedation rates of the cats after the early postoperative period. These results can be interpreted as indicating that surgical wound infiltration with bupivacaine at a dosage of 2 mg/kg is a safe analgesic method in cats.
Although controversial, it is generally accepted that pain management is performed prevent rather than treat postoperative pain [15]. There are some benefits to administer analgesic agents preoperatively [17]. However, some side effects may occur with preoperative usage of NSAIDs [20]. Parton et al.[25] administered carprofen to healthy cats intravenously and reported that the intravenous administration of carprofen did not cause bleeding or ulceration of the gastric mucosa or change the biochemical and hematological parameters. NSAIDs have not been indicated in renal disease in cats unless impaired renal blood flow occurs [33]. Differently from the study of Parton et al.[25], the cats were anesthetized in our study. As anesthesia causes adecrease in renal blood flow [10], it was thought that carprofen might cause renal disturbance in the cats, and therefore, carprofen was not used preemptively in this study. Regarding opioids, they are not accessible in some countries. Taking into account all of these things, selection of the analgesic agent and its administration time may depend on the practitioner’s preference, the accessibleness of the analgesics in the country and the status of the patient. Carprofen was the best choice among the available analgesic drugs in the country in which the study was carried out. In this study, carprofen was administered during the surgery, that is, right after removal of the mammary glands in both groups. In this way, we reduced the risk of hemorrhage and renal disturbance in our oncologic and mostly geriatric patients.
Cats tend to hide pain as a protection mechanism [14]; therefore, assessing pain in cats is complex [7, 32]. There are considerable variations within a species [27]. Neuroendocrine tests including norepinephrine, epinephrine, cortisol and blood glucose may be run to recognize pain. However, these neurochemicals are not specific for pain in cats. As physiological parameters are influenced by many factors, behavioral changes are accepted as an indicator of pain in cats [11, 14, 20, 28, 29]. In light of this information, a behavioral assessment rating system was used rather than neuroendocrine tests for obtaining the pain scores of the cats in this study. Loss of normal behavior of the patient (decrease in appetite, grooming and/or activity), existence of abnormal behaviors (vocalization, aggression, hiding, decreased interaction with the owner, restlessness, biting/scratching the surgical area, hissing, reluctance to move and failure to use litter box) and reaction to touch are usually associated with acute pain in cats [7, 14, 27, 29, 32]. Hellyer et al. [14] reported that some cats may mask behavioral signs of pain while hospitalized and that pain may only become evident at home. They also indicated that anxiety and fear can amplify pain in cats and that hospitalization is one of the main reasons for anxiety in cats. Therefore, the cats were not hospitalized in this study. But, the owners were educated about identification of pain and when to get in contact with the veterinarian. Epstein [7] suggested the following method for evaluation of pain in cats: inspection of the cat with and without interaction and palpation of the surgical area. This was considered while preparing the questionnarie for the present. In the cats with a high pain score, behavioral changes related to pain occurred with and without interaction and palpation of the painful area. The information provided by Epstein [7] was considered when preparing the questionnaire for this study. In the second section of the questionnarie, the owners were requested to answer the questions with and without interaction with the cat. In the third section, the questions were answered while the owners were gently touching the operation site.
Aggression and vocalization were the most distinct behavioral changes associated with pain in the cats of this study. While almost no aggression was seen in Group I during recovery from anesthesia, Group II, which had a decreased sedation rate, exhibited the opposite results. Significant differences were noted regarding aggression and vocalization with better analgesia in Group I. Convulsions were evaluated as an expression of coming out of anesthesia rather than pain.
The mean pain score of Group I was significantly lower than that of Group II at 4 hr after the operation, whereas no significant difference was found at 10 hr after the operation. The differences noted at 4 hr after the operation can be explained by the analgesic effect of the surgical wound infiltration with bupivacaine in Group I. However, by 10 hr after the operation, it is most likely that the effects of bupivacaine had worn off and that carprofen had reached and effective dosage in both groups. In addition, some cats showed mild to moderate pain (pain score 1–2) at 4 and 10 hr after the operation, even in Group I. The combination of intraoperative carprofen and surgical wound infiltration with bupivacaine improved postoperative analgesia, but was insufficient to control postoperative pain induced by bilateral radical mastectomy in the cats. It is necessary for veterinary practitioners to spare no effort in adopting the maximum treatment possible to reduce postoperative pain in cats undergoing bilateral radical mastectomy in their respective countries.
Under the conditions of our study, we concluded that surgical wound infiltration with bupivacaine (2 mg/kg) before incisional closure provides reliable analgesia in the postanesthetic period at least 4 hr after an operation.
Acknowledgments
An abstract of this paper was presented as a poster presentation at the 16th EVSSAR Congress, Toulouse, France, held on 5–6 July 2013. The authors are grateful to Prof. Dr. Bülent Ekiz for the statistical analysis.
REFERENCES
- 1.Brearley J. C.2011. Local anesthetics and local anesthesia/analgesia. In: 17th FECAVA Eurocongress “Modern Veterinary Practises”, Istanbul, Turkey, 7–10 September. [Google Scholar]
- 2.Çöçelli L. P., Bacaksız B. D., Ovayolu N.2008. The Nurse Factor in Pain Therapy. Gaziantep Med. J. 14: 53–58. [Google Scholar]
- 3.Davis H., Jensen T., Johnson A., Knowles P., Meyer R., Rucinsky R., Shafford H., American Association of Feline Practicioners American Animal Hospital Association 2013. 2013. AAHA/AAFP fluid therapy guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 49: 149–159. doi: 10.5326/JAAHA-MS-5868 [DOI] [PubMed] [Google Scholar]
- 4.Dohoo S. E., Dohoo I. R.1996. Postoperative use of analgesics in dogs and cats by Canadian veterinarians. Can. Vet. J. 37: 546–551. [PMC free article] [PubMed] [Google Scholar]
- 5.Dorn C. R., Taylor D. O. N., Schneider R., Hibbard H. H., Klauber M. R.1968. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J. Natl. Cancer Inst. 40: 307–318. [PubMed] [Google Scholar]
- 6.Ekiz B., Ekiz E. E., Yalçıntan H., Koçak Ö., Yılmaz A., Güneş H.2012. The effects of transport stress on certain welfare parameter and behaviours in Red Karaman, Imroz, Sakız and Karakul rams. J. Fac. Vet. Med. Ist. Uni. 38: 15–28. [Google Scholar]
- 7.Epstein M. E.2010. Postsurgical assessment of pain in dogs and cats. In: The 82nd Annual Westerns Veterinary Conference, Las Vegas, Nevada, U.S.A., 14–18 February.
- 8.Federation of Veterinarians of Europe. 2014. Ketamine use in Veterinaty Medicine. http://www.fve.org/news/position_papers/medicines/fve_00_043_ketamine_use_vet_med.pdf (Last arrival date 10/06/2014).
- 9.Gaynor J., Muir W.2002. Therapy for the alleviation of pain. pp. 113–333. In: Handbook of Veterinary Pain Management, (Gaynor, J. S. and Muir, W. eds.), Mosby Elsevier Publishing, St Louis. [Google Scholar]
- 10.Greene S. A., Graurer G. F.2007. Renal disease. pp. 915–919. In: Lumb&Jones’ Veterinary Anesthesia and Analgesia, (Tranquilli, W. J., Thurmon, J. C., Grimm, K. A. and Ames, I. A. eds.) Blackwell Publishing, London. [Google Scholar]
- 11.Grimm K. A.2002. pp. 327–329. Pain Management for Small Animals. In: Veterinary Anesthesia and Pain Management Secrets, (Greene, S. A. ed.), Hanley& Belfus, Philadelphia. [Google Scholar]
- 12.Hayden D. W., Nielsen S. W.1971. Feline mammary tumours. J. Small Anim. Pract. 12: 687–698. doi: 10.1111/j.1748-5827.1971.tb06197.x [DOI] [PubMed] [Google Scholar]
- 13.Hayes A. A., Mooney S.1985. Feline mammary tumors. Vet. Clin. North Am. Small Anim. Pract. 15: 513–520. [DOI] [PubMed] [Google Scholar]
- 14.Hellyer P., Rodan I., Brunt J., Downing R., Hagedorn J. E., Robertson S. A., AAHA/AAFP Pain Management Guidelines Task Force Members. 2007. AAHA/AAFP pain management guidelines for dogs and cats. J. Feline Med. Surg. 9: 466–480. doi: 10.1016/j.jfms.2007.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamont L. A., Tranquilli W. J., Grimm K. A.2000. Physiology of pain. Vet. Clin. North Am. Small Anim. Pract. 30: 703–728. doi: 10.1016/S0195-5616(08)70003-2 [DOI] [PubMed] [Google Scholar]
- 16.Lamont L. A.2002. Feline perioperative pain management. Vet. Clin. North Am. Small Anim. Pract. 32: 747–763. doi: 10.1016/S0195-5616(02)00028-1 [DOI] [PubMed] [Google Scholar]
- 17.Lascelles B. D. X., Cripps P., Mirchandani S., Waterman A. E.1995. Carprofen as an analgesic for postoperative pain in cats: dose titration and assessment of efficacy in comparison to pethidine hydrochloride. J. Small Anim. Pract. 36: 535–541. doi: 10.1111/j.1748-5827.1995.tb02805.x [DOI] [PubMed] [Google Scholar]
- 18.Lascelles B., Capner C., Waterman-Pearson A. E.1999. A survey of current British Veterinary attitudes to peri-operative analgesia for cats and small mammals. Vet. Rec. 145: 601–604. doi: 10.1136/vr.145.21.601 [DOI] [PubMed] [Google Scholar]
- 19.Lee H. K., Lebkowska-Wieruszewska B., Kim T. W., Kowaski C. J., Giorgi M.2013. Pharmacokinetics of the novel atypical opioid tapentadol after intravenous, intramuscular and subcutaneous administration in cats. Vet. J. 198: 620–624. doi: 10.1016/j.tvjl.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 20.McKelvey D., Hollingshead K. W.2000a. Analgesia. pp. 315–346. In: Veterinary Anesthesia and Analgesia, (McKelvey, D. and Hollingshead, K.W. eds.), Mosby, St Louis. [Google Scholar]
- 21.McKelvey D., Hollingshead K. W.2000b. Special Techniques. pp. 294–299. In: Veterinary Anesthesia and Analgesia, (McKelvey, D. and Hollingshead, K.W. eds), Mosby, St Louis. [Google Scholar]
- 22.Moulton J. E.1990. Mammary Tumors of the Cat. pp. 547–552. In: Tumors in Domestic Animals, (Moulton, J.E. ed.), University of California Press, Berkeley. [Google Scholar]
- 23.Novosad C. A.2003. Principles of treatment for mammary gland tumors. Clin. Tech. Small Anim. Pract. 18: 107–109. doi: 10.1053/svms.2003.36625 [DOI] [PubMed] [Google Scholar]
- 24.Owen L. N.1980. TNM Classification of tumors in domestic animals. WHO, Geneva, apps.who.int/iris/bitstream/10665/68618/1/VPH_CMO_80.20_eng.pdf. Accessed 22 January 2014.
- 25.Parton K., Balmer T. V., Boyle J., Whittem T., MacHon R.2000. The pharmacokinetics and effects of intravenously administered carprofen and salicylate on gastrointestinal mucosa and selected biochemical measurements in healthy cats. J. Vet. Pharmacol. Ther. 23: 73–79. doi: 10.1046/j.1365-2885.2000.00253.x [DOI] [PubMed] [Google Scholar]
- 26.Robertson S. A., Taylor P. M.2004. Pain management in cats—past, present and future. Part 2. Treatment of pain—clinical pharmacology. J. Feline Med. Surg. 6: 321–333. doi: 10.1016/j.jfms.2003.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson S. A.2005. Assessment and management of acute pain in cats. J. Vet. Emerg. Crit. Care 15: 261–272. doi: 10.1111/j.1476-4431.2005.00172.x [DOI] [Google Scholar]
- 28.Smith J. D., Allen S. W., Quandt J. E.1999. Changes in cortisol concentration in response to stress and postoperative pain in client-owned cats and correlation with objective clinical variables. Am. J. Vet. Res. 60: 432–436. [PubMed] [Google Scholar]
- 29.Taylor P. M., Robertson S. A.2004. Pain management in cats—past, present and future. Part 1. The cat is unique. J. Feline Med. Surg. 6: 313–320. doi: 10.1016/j.jfms.2003.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurmon J. C., Tranquilli W. J., Benson G. J.1999. Perioperative Pain and Its Management. pp. 28–59. In: Essentials of Small Animal Anesthesia and Analgesia, (Thurmon, J. C., Tranquilli, W. J. and Benson, G. J., eds.). Lippincott Williams&Wilkins, Philadelphia. [Google Scholar]
- 31.Tobias K. M., Harvey R. C., Byarlay J. M.2006. A comparison of four methods of analgesia in cats following ovariohysterectomy. Vet. Anaesth. Analg. 33: 390–398. doi: 10.1111/j.1467-2995.2005.00282.x [DOI] [PubMed] [Google Scholar]
- 32.Waterman-Pearson A. E.1999. Analgesia. pp. 59–71. In: BSAVA Manual of Small Animal Anesthesia and Analgesia, (Seymour, C. and Gleed, R. eds.), British Small Animal Veterinary Association. [Google Scholar]
- 33.Wolf D. C., Lenz S. D., Carlton W. W.1991. Renal papillary necrosis in two domestic cats and a tiger. Vet. Pathol. 28: 84–87. doi: 10.1177/030098589102800113 [DOI] [PubMed] [Google Scholar]