Abstract
STX is an agonist for a recently characterized membrane estrogen receptor whose structure has not been identified. We evaluated whether STX suppresses gonadotropin-releasing hormone (GnRH)–induced luteinizing hormone (LH) release from bovine anterior pituitary (AP) cells. We cultured AP cells (n=12) for 3 days in steroid-free conditions, followed by increasing concentrations (0.001, 0.01, 0.1, 1 and 10 nM) of 17β-estradiol or STX for 5 min before GnRH stimulation until the end of the experiment. Estradiol (0.001 to 0.1 nM) significantly suppressed GnRH-stimulated LH secretion, whereas STX did not affect GnRH-stimulated LH secretion at any of the tested concentrations. In conclusion, STX, unlike estradiol, possesses no suppressive effect on GnRH-induced LH release from bovine AP cells.
Keywords: gonadotrope, GPCR, non-genomic effect, ruminant, STX
17β-estradiol, a steroid secreted from ovaries, is a powerful feedback regulator of both hypothalamus and pituitary actions in controlling the secretion of gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) in all domestic animals studied to date. In heifers and cows, blood estradiol concentrations during the luteal phase fluctuate between 0.004 and 0.030 nM [3, 14]. These low concentrations exert a negative feedback effect on GnRH secretion from the arcuate nucleus in the hypothalamus [2, 4, 6]. By contrast, blood estradiol concentrations exceed 0.037 nM [14] prior to ovulation, inducing a pre-ovulatory GnRH surge from the preoptic area [13]. To induce these physiologically important feedback effects from the ovaries to the hypothalamus and pituitary gland, estradiol binds to nuclear-localized estrogen receptors α or β (ERα or ERβ) and alters genomic transcription. In addition to its genomic effect, estradiol mediates rapid, non-genomic suppression of LH, but not FSH secretion, from ovine and bovine anterior pituitaries (AP) [1, 8, 12]. Our previous study suggested that G protein-coupled receptor 30 (GPR30) is expressed in bovine gonadotropes and may partially contribute to the rapid, negative estradiol feedback effect observed in GnRH-induced LH secretion [12]. However, bovine gonadotropes may have another undefined plasma membrane receptor that mediates.
Recently, a novel plasma membrane estradiol receptor involved in mediating non-genomic effects was identified, although its structure, or even its mRNA and amino acid sequences, have yet to be elucidated [11, 12]. Since this receptor can be probed by a non-steroidal diphenylacrylamide compound called STX, it is referred to as the STX receptor. STX is the ligand that specifically binds to the plasma membrane estradiol receptor, but not to ERα, ERβ or GPR30 [9, 11, 15]. STX receptors play important roles in inducing estradiol’s rapid effects in GnRH-producing neurons [9, 16].
To the best of our knowledge, the effects of STX on LH secretion from gonadotropes have not been clarified in any species. Therefore, the present study was planned to investigate the hypothesis that the STX receptor mediates the rapid, negative estradiol feedback effect on the AP by evaluating whether STX suppresses GnRH-induced LH release from cultured bovine AP cells.
All experiments were approved by the Committee on Animal Experiments of the School of Veterinary Medicine at Yamaguchi University. G*Power 3 for windows [5] was used to estimate the required number of anterior pituitaries with an α-error probability of 0.05 and a statistical power of 0.95. Nett et al.[10] reported that the amounts of LH and GnRH receptors in AP were higher during the luteal phase than during the immediate post-estrus period in heifers. Therefore, postpubertal Japanese Black heifers in the middle of the luteal phase (n=12, 26 months old) were stunned using a captive bolt pistol and then exsanguinated by cutting of the throat in a local slaughterhouse in Yamaguchi prefecture. The heads were placed on ice within 5 min of slaughter. Then, APs were obtained within 15 min of slaughter, stored in ice-cold 25 mM HEPES buffer (pH 7.2) containing 10 mM glucose and transported on ice to the laboratory within 4 hr. The experiment was repeated 12 times (i.e., 12 different pituitary glands) using 4 wells per treatment. Enzymatic dispersal of AP cells and culture were performed using a previously described method [7]. Cell viability of greater than 90% was confirmed by Trypan blue exclusion. Cells (2.0 × 105 cells/ml, total 0.5 ml) were plated in 24-well culture plates (MS-80240; Sumitomo Bakelite, Tokyo, Japan) and maintained at 37°C in a humidified atmosphere of 5% CO2 for 82 hr. The wells were washed twice with PBS and then incubated with 490 µl DMEM containing 0.1% BSA for 2 hr. Pretreatment was performed by adding 5 µl of either DMEM containing 0.01% high-quality dimethyl sulfoxide (DMSO; Infinity pure grade, 045–24511, Wako Pure Chemicals, Osaka, Japan) alone or DMEM containing various concentrations (ranging from 0.1 nM to 1,000 nM) of estradiol (052–04041; Wako Pure Chemicals) or STX (donated by Prof. Martin Kelly, Oregon Health and Science University, OR, U.S.A.). For this purpose, DMSO was initially used to dissolve estradiol or STX as 10 mM; then, DMEM containing 0.1% BSA was used to further dilute the solution to provide a final estradiol or STX concentration of 1,000 nM or less. The cells were incubated while gently shaking for 5 min and subsequently treated with 5 µl of DMEM containing 100 nM GnRH (Peptide Institute Inc., Osaka, Japan) for 2 hr to stimulate LH secretion. Thus, the total treatment time with estradiol or STX was 2 hr and 5 min. The pretreatment plus the GnRH treatment yielded a final concentration of 0.001, 0.01, 0.1, 1 or 10 nM estradiol or STX in the wells that had received 0.1, 1, 10, 100 or 1,000 nM of estradiol or STX, respectively, and a final concentration of 1 nM of GnRH. In the preliminary study, LH secretion was stimulated by increasing amounts of GnRH, with a peak at 1 nM GnRH, and reducing secretion at GnRH concentrations higher than 1 nM. Therefore, we used 1 nM of GnRH in this study. The “control” wells contained 5 µl of DMEM containing 0.01% DMSO that had not undergone pretreatment with estradiol, STX or GnRH. The “GnRH” wells contained 5 µl of DMEM containing 0.01% DMSO that had not undergone pretreatment with estradiol or STX, but had been incubated with GnRH for 2 hr. After incubation with GnRH, the medium was collected for radioimmunoassay (RIA) of LH.
The LH concentrations in the culture media were assayed in duplicate by double-antibody RIA using 125I-labeled bLH and anti-oLH antiserum (AFP11743B and AFP192279; National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, CA, U.S.A.). The limit of detection was 0.40 ng/ml, and the intra- and inter-assay coefficients of variation were 3.9 and 6.9%, respectively.
The LH concentrations in the control samples for each pituitary were averaged, and the mean value was set to 100%. The LH concentrations from the 4 replicate wells for each pituitary were averaged, and the mean LH value was expressed as a percentage of the control value. The statistical significance of differences of the LH concentration was analyzed by one-factor analysis of variance (ANOVA) followed by post hoc comparisons using Fisher’s protected least significant difference test. The level of significance was set at P<0.05. Data are expressed as mean ± standard error of the mean (SEM).
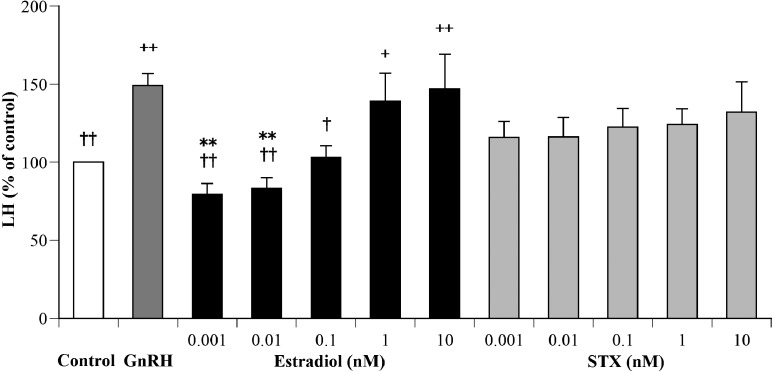
Figure 1 shows the effects of various concentrations of estradiol and STX on GnRH-stimulated LH secretion from the cultured AP cells. The ANOVA had sufficient statistical power (0.96) to show a significant effect of different additives on GnRH-stimulated LH secretion (P<0.05). The mean LH concentration in the medium of GnRH wells (15.3 ± 0.8 ng/ml) was significantly greater (P<0.01) than that of the control wells (10.3 ± 0.7 ng/ml). The wells with 1 nM and 10 nM estradiol displayed significantly greater (P<0.05 and P<0.01, respectively) mean LH concentration than the control wells. The wells with 0.001 to 0.01 nM (P<0.01) and 0.1 nM (P<0.05) of estradiol displayed lower mean LH concentrations than the GnRH wells. The wells with 0.001 and 0.01 nM of estradiol displayed significantly lower (P<0.01) mean LH concentration compared to wells with 10 nM estradiol. None of the wells containing STX displayed significant different mean LH concentrations compared to control or GnRH wells.
Fig. 1.
Effects of various concentrations of estradiol (black bars) or STX (gray bars) in DMEM containing 1 nM GnRH on luteinizing hormone (LH) secretion from cultured bovine AP cells. The LH concentrations in control cells (cultured medium only) were averaged with the mean value set at 100%. The LH concentrations of the treated groups are expressed as a percentage of the control. Each value represents the mean concentration ± SEM (n=12). +P<0.05, ++P<0.01: significant differences compared with the control, †P<0.05, ††P<0.01: significant difference compared with GnRH, **P<0.01: significant differences compared with 10 nM estradiol.
This is the first report evaluating the non-genomic effects of STX on LH secretion from the pituitary, although previous studies have reported on the role of the STX receptor in mediating the rapid, negative estradiol feedback effect on various hypothalamic neurons [9, 11, 16]. We have previously reported that low concentrations of estradiol or the GPR30 agonist, G1, suppressed GnRH-induced LH secretion in bovine AP cells within a 5-min period [12]. Thus, the present results revealed that STX, unlike estradiol or G1, possesses no suppressive effect on GnRH-stimulated LH secretion in the bovine pituitary gland, suggesting that GPR30 may play a more important role in LH secretion from bovine gonadotropes than the STX receptor. Because the STX receptor mediates GnRH secretion from primate neurons by non-genomic mechanism [9], differences in the non-genomic mechanism may exist between gonadotropes and GnRH neurons. Therefore, further studies on the STX receptor in ruminants are required.
The mRNA and amino acid sequences of STX-receptor have not yet been published, and thus far, no specific antibody against the STX receptor has been developed. Therefore, it was impossible to measure expression levels or the cellular localization of the STX receptor in AP cells in any species. Additional tools, including antibodies and antagonists, need to be developed before further characterization of this receptor.
In this study, 10 nM estradiol was found to be less efficient in suppressing GnRH-stimulated LH secretion than the physiological concentrations, 0.01 and 0.1 nM, of estradiol. Further studies are required to clarify the molecular mechanism by which estradiol suppresses LH secretion.
The total treatment time with estradiol or STX was 2 hr and 5 min in this experiment, because estradiol and STX were not removed from the culture medium after the 5 min of pretreatment; they were also able to affect LH secretion from the cultured AP cells during the 2 hr of GnRH stimulation. During the 2 hr and 5 min of treatment, the cultured AP cells continued to secrete LH. Therefore, it should be noted that the differences relative to the control resulted from the effects of estradiol or STX not only during the 5-min pretreatment but also during the 2-hr experiment.
In conclusion, the present results revealed that STX, unlike estradiol, possesses no suppressive effect on GnRH-stimulated LH secretion in the bovine pituitary gland.
Acknowledgments
We greatly appreciate Prof. Martin J. Kelly (Oregon Health and Science University, Oregon, U.S.A.) for providing STX. Faidiban O. Rudolf was supported by Ministry of National Education, Republic of Indonesia by providing scholarship. We thank Urara Nakamura and Kiran Pandey for their help in laboratory works. We also thank Dr. Mitsuhiro Takagi for various important scientific advices. This research was partially supported by the Ministry of Education, Science, Sports and Culture, Grant-in-Aid for Scientific Research (C), 2011–2013 (23580440, Hiroya Kadokawa).
REFERENCES
- 1.Arreguin-Arevalo J. A., Nett T. M.2005. A nongenomic action of 17β-estradiol as the mechanism underlying the acute suppression of secretion of luteinizing hormone. Biol. Reprod. 73: 115–122. doi: 10.1095/biolreprod.105.040329 [DOI] [PubMed] [Google Scholar]
- 2.Clarke I. J.1995. Evidence that the switch from negative to positive feedback at the level of the pituitary gland is an important timing event for the onset of the preovulatory surge in LH in the ewe. J. Endocrinol. 145: 271–282. doi: 10.1677/joe.0.1450271 [DOI] [PubMed] [Google Scholar]
- 3.Endo N., Nagai K., Tanaka T., Kamomae H.2012. Comparison between lactating and non-lactating dairy cows on follicular growth and corpus luteum development, and endocrine patterns of ovarian steroids and luteinizing hormone in the estrous cycles. Anim. Reprod. Sci. 134: 112–118. doi: 10.1016/j.anireprosci.2012.08.018 [DOI] [PubMed] [Google Scholar]
- 4.Evans N. P., Dahl G. E., Glover B. H., Karsch F. J.1994. Central regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by estradiol during the period leading up to the preovulatory GnRH surge in the ewe. Endocrinology 134: 1806–1811. [DOI] [PubMed] [Google Scholar]
- 5.Faul F., Erdfelder E., Lang A. G., Buchner A.2007. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39: 175–191. doi: 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 6.García-Galiano D., Pinilla L., Tena-Sempere M.2012. Sex steroids and the control of the Kiss1 system: developmental roles and major regulatory actions. J. Neuroendocrinol. 24: 22–33. doi: 10.1111/j.1365-2826.2011.02230.x [DOI] [PubMed] [Google Scholar]
- 7.Hashizume T., Soliman E. B., Kanematsu S.1994. Effects of pituitary adenylate cyclase-activating polypeptide (PACAP), prostaglandin E2 (PGE2) and growth hormone releasing factor (GRF) on the release of growth hormone from cultured bovine anterior pituitary cells in vitro. Domest. Anim. Endocrinol. 11: 331–337. doi: 10.1016/0739-7240(94)90004-3 [DOI] [PubMed] [Google Scholar]
- 8.Iqbal J., Latchoumanin O., Clarke I. J.2007. Rapid in vivo effects of estradiol-17β in ovine pituitary gonadotropes are displayed by phosphorylation of extracellularly regulated kinase, serine/threonine kinase, and 3′,5′-cyclic adenosine 5′-monophosphate-responsive element-binding protein. Endocrinology 148: 5794–5802. doi: 10.1210/en.2007-0986 [DOI] [PubMed] [Google Scholar]
- 9.Kenealy B. P., Keen K. L., Rønnekleiv O. K., Terasawa E.2011. STX, a novel nonsteroidal estrogenic compound, induces rapid action in primate GnRH neuronal calcium dynamics and peptide release. Endocrinology 152: 3182–3191. doi: 10.1210/en.2011-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nett T. M., Cermak D., Braden T., Manns J., Niswender G.1987. Pituitary receptors for GnRH and estradiol, and pituitary content of gonadotropins in beef cows. I. Changes during the estrous cycle. Domest. Anim. Endocrinol. 4: 123–132. doi: 10.1016/0739-7240(87)90006-3 [DOI] [PubMed] [Google Scholar]
- 11.Qiu J., Bosch M. A., Tobias S. C., Grandy D. K., Scanlan T. S., Rønnekleiv O. K., Kelly M. J.2003. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J. Neurosci. 23: 9529–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudolf F. O., Kadokawa H.2013. Expression of estradiol receptor, GPR30, in bovine anterior pituitary and effects of GPR30 agonist on GnRH-induced LH secretion. Anim. Reprod. Sci. 139: 9–17. doi: 10.1016/j.anireprosci.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 13.Smith J. T., Li Q., Pereira A., Clarke I. J.2009. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology 150: 5530–5538. doi: 10.1210/en.2009-0712 [DOI] [PubMed] [Google Scholar]
- 14.Spicer L. J., Echternkamp S. E.1986. Ovarian follicular growth, function and turnover in cattle: a review. J. Anim. Sci. 62: 428–451. [DOI] [PubMed] [Google Scholar]
- 15.Tobias S. C., Qiu J., Kelly M. J., Scanlan T. S.2006. Synthesis and biological evaluation of SERMs with potent nongenomic estrogenic activity. ChemMedChem 1: 565–571. doi: 10.1002/cmdc.200500098 [DOI] [PubMed] [Google Scholar]
- 16.Zhang C., Kelly M. J., Rønnekleiv O. K.2010. 17β-Estradiol rapidly increases KATP activity in GnRH via a protein kinase signaling pathway. Endocrinology 151: 4477–4484. doi: 10.1210/en.2010-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]