Abstract
Cytochromes P450 (P450) are important for not only drug metabolism and toxicity, but also biosynthesis and metabolism of cholesterol and bile acids, and steroid synthesis. In cynomolgus macaques, widely used in biomedical research, we have characterized P450 cDNAs, which were isolated as expressed sequence tags of cynomolgus macaque liver. In this study, cynomolgus CYP7A1, CYP17A1, CYP20A1, CYP27A1 and CYP51A1 cDNAs were characterized by sequence analysis, phylogenetic analysis and tissue expression pattern. By sequence analysis, these five cynomolgus P450s had high sequence identities (94–99%) to the human orthologs in amino acids. By phylogenetic analysis, each cynomolgus P450 was more closely related to the human ortholog as compared with the dog or rat ortholog. By quantitative polymerase chain reaction, among the 10 tissue types, CYP7A1 and CYP17A1 mRNAs were preferentially expressed in liver and adrenal gland, respectively. Cynomolgus CYP27A1 and CYP51A1 mRNAs were most abundantly expressed in liver and testis, respectively. Cynomolgus CYP20A1 mRNA was expressed in all the tissues, including brain and liver. Tissue expression patterns of each cynomolgus P450 were generally similar to that of the human ortholog. These results suggest the molecular similarities of CYP7A1, CYP17A1, CYP20A1, CYP27A1 and CYP51A1 between cynomolgus macaques and humans.
Keywords: cynomolgus monkey, cytochrome P450, sequence analysis, tissue expression
Cytochromes P450 (P450) are a gene superfamily consisting of a large number of heme proteins, metabolizing numerous xenobiotics and endogenous substrates. In human P450s, 57 functional genes and 58 pseudogenes have been identified [7]. Although some P450s (especially CYP1–3) are important enzymes for drug metabolism, other P450s play physiological roles by metabolizing endogenous substrates [6]. For example, CYP17A1 participates in synthesis of sex steroids and thus is important for reproduction [8]. CYP7A1, CYP27A1 and CYP51A1 are involved in biosynthesis and metabolism of cholesterol and bile acids [5]. CYP7A1 is a potential therapeutic target for cholesterol lowering because CYP7A1 is a major determinant of plasma cholesterol levels, and drugs, such as bile acid-binding resin cholestyramine, is known to increase CYP7A1 activity [10]. A mutation of CYP7A1 results in no enzyme activity and increased plasma cholesterol level [9]. Because CYP51 has been found not only in mammals, but also in fungi, plants and prokaryotes, some antifungal drugs, such as imidazoles and triazoles, have been developed to target CYP51 [4]. There are also orphan P450s, including CYP20A1, which have not been fully characterized [2].
Cynomolgus macaques are frequently used in biomedical research due to their evolutionary closeness to humans. We previously conducted expressed sequence tags (EST)-sequencing in the liver samples of three cynomolgus macaques [15], from which we subsequently identified and characterized a number of P450s that are mainly important for drug metabolism [12,13,14]. However, CYP7A1, CYP17A1, CYP20A1, CYP27A1 and CYP51A1 cDNAs, which were also isolated by the EST-sequencing, have not been characterized. In this study, therefore, these P450s were analyzed by sequence analysis, phylogenetic analysis and tissue expression patterns.
For analysis of cynomolgus P450, brain, lung, heart, liver, kidney, adrenal gland, small intestine (jejunum), testis, ovary and uterus were collected from six purpose-bred cynomolgus macaques (three males and three females from Indochina, 4–5 years of age and 3–5 kg), and total RNA was extracted from these tissues as previously described [12]. Pooled samples of these six animals were used to measure mRNA expression. The study was reviewed and approved by the local animal ethics committee. Sequencing of the P450 cDNA was performed using ABI Prism BigDye Terminator v3.0 Ready Reaction Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, U.S.A.), and the sequence data were analyzed using DNASIS Pro (Hitachi Software, Tokyo, Japan), as described previously [12]. A homology search was performed using BLAST (National Center for Biotechnology Information). The phylogenetic tree was created using the neighbor joining method as described previously [12]. Human and rhesus macaque genome data were analyzed using BLAT (UCSC Genome Bioinformatics). The cynomolgus cDNAs reported in this paper have been deposited to GenBank under accession numbers DQ074791 (CYP7A1), DQ074802 (CYP17A1), KJ922552 (CYP20A1), DQ074803 (CYP27A1) and DQ074804 (CYP51A1).
Sequencing and sequence analysis revealed that cynomolgus CYP7A1, CYP17A1, CYP20A1, CYP27A1 and CYP51A1 identified by EST-sequencing had higher amino acid sequence identities (94–99%) to the human orthologs as compared with those of dogs and rats which are also used in biomedical research (Table 1). By comparing to human CYP7A1 cDNA, cynomolgus CYP7A1 cDNA lacked the 5′ end of the sequence, so that the first 186 amino acid residues were missing. A full-length CYP7A1 cDNA (NM_001193803) was found in GenBank for rhesus macaques, closely related to cynomolgus macaques, raising the possibility that cynomolgus macaques might also express a full-length CYP7A1 transcript. By comparing to human CYP20A1 gene sequence using BLAT, cynomolgus CYP20A1 cDNA lacked the sequence of exon 10, so that this cDNA did not contain a complete open reading frame (ORF) due to the premature termination codon. BLAT analysis showed that CYP20A1 gene of rhesus macaques (closely related to cynomolgus macaques) contained the sequence highly homologous to exon 10 of human CYP20A1 gene. This exon 10 sequence was flanked by AG and GT at the 5′ and 3′ end, respectively, consistent with the consensus sequences for splice junctions in eukaryotic genes, indicating that this alternative splicing event must be accounted for by the factors other than mutations at splicing donor or acceptor sites. Alternatively, mutation (s) at the splicing sites might be present in the cynomolgus macaque genome, the data of which were not available for BLAT analysis.
Table 1. Sequence identities of cynomolgus P450 cDNAs to the human, dog and rat orthologs in amino acid sequences.
| CYP7A1 (%) | CYP17A1 (%) | CYP20A1 (%) | CYP27A1 (%) | CYP51A1 (%) | |
|---|---|---|---|---|---|
| Human | 95 | 94 | 97 | 98 | 99 |
| Dog | 82 | 80 | 94 | 80 | 97 |
| Rat | 82 | 68 | 80 | 72 | 94 |
Amino acid sequence identities were determined using BLAST.
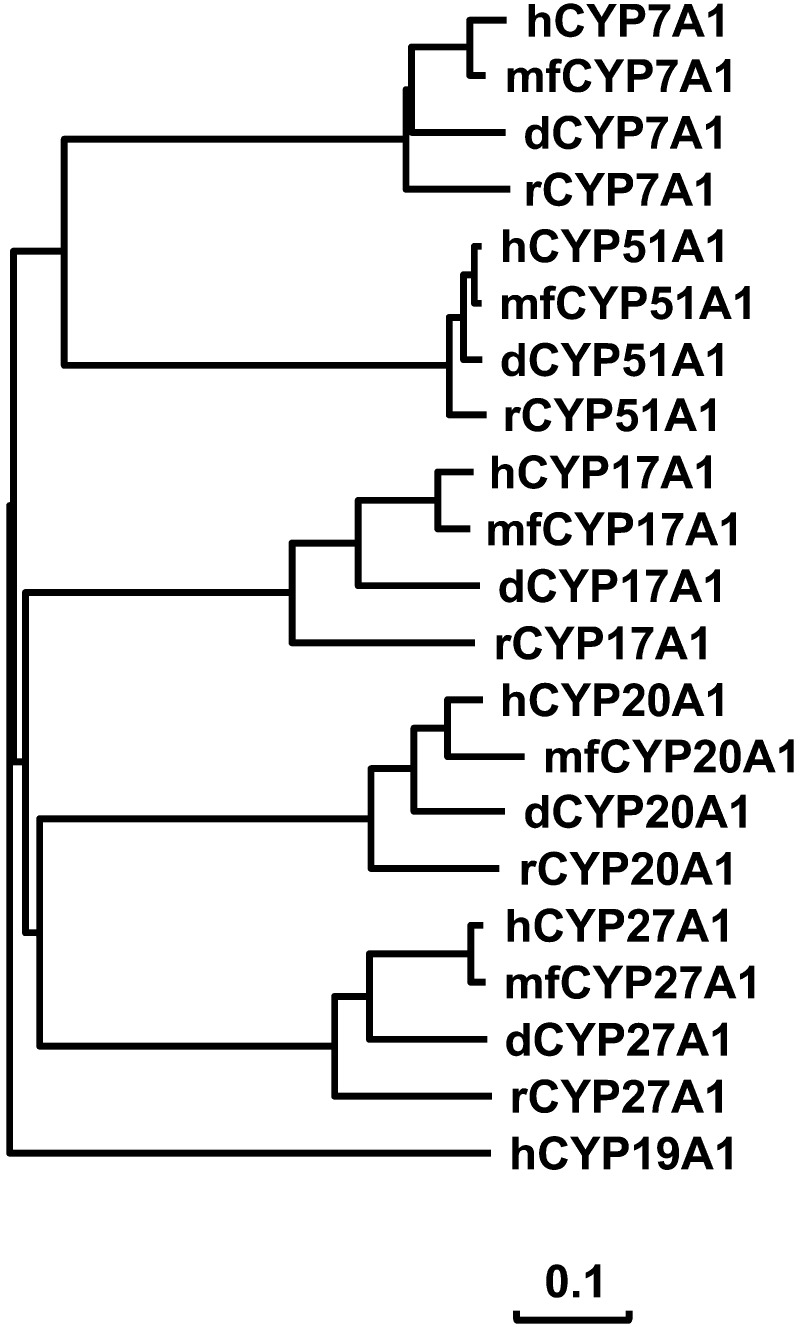
Cynomolgus CYP7A1, CYP17A1, CYP20A1, CYP27A1 and CYP51A1 had the sequence structures characteristic of P450 enzymes, including the heme-binding region. The exception was that the heme-binding region of cynomolgus CYP20A1 could not be determined due to an incomplete ORF as described earlier. Phylogenetic analysis was performed using CYP7A1, CYP17A1, CYP20A1, CYP27A1 and CYP51A1 amino acid sequences of humans, cynomolgus macaques, dogs and rats. The result indicated that cynomolgus CYP7A1, CYP17A1, CYP20A1, CYP27A1 and CYP51A1 were more closely clustered with the human ortholog as compared with the dog or rat ortholog (Fig. 1). These results indicated similarities of these P450s in the primary sequence structures between cynomolgus macaques and humans.
Fig. 1.
Phylogeny of cynomolgus P450s. A phylogenetic tree was created using the neighbor joining method. CYP7A1, CYP17A1, CYP20A1, CYP27A1 and CYP51A1 amino acid sequences were from humans (h), cynomolgus macaques (mf), dogs (d) and rats (r). Human CYP39A1 was used as outgroup. For the distance measurement, the scale bar indicates 0.1 amino acid substitutions per site.
To measure expression of cynomolgus CYP7A1, CYP17A1, CYP20A1, CYP27A1 and CYP51A1 mRNAs, quantitative polymerase chain reaction (qPCR) analysis was performed with brain, lung, heart, liver, kidney, adrenal gland, jejunum, testis, ovary and uterus RNAs as described previously [14] using SYBR Green PCR Master Mix (Applied Biosystems). The primers used were mfCYP7A1 (5qrt1) 5′-AGTCAGCTTGGAAGGCAATC-3′ and mfCYP7A1 (3qrt1) 5′-TTGAGGGAGGCACTGGAA-3′ for CYP7A1, mfCYP17A1 (5qrt1) 5′-ACCATCCGAGAGGTGCTTC-3′ and mfCYP17A1 (3qrt1) 5′-CCTTGTCCACAGCAAACTCA-3′ for CYP17A1, mfCYP20A1 (5qrt1) 5′-CTTTATGCCCTTGGTGTGGT-3′ and mfCYP20A1 (3qrt1) 5′-TGTGCCTGAGAATCCAAGTG-3′ for CYP20A1, mfCYP27A1 (5qrt1) 5′-GGTGTCTGGCTACCTGCACT-3′ and mfCYP27A1 (3qrt1) 5′-CATGTCAGCGTGTTGGATG-3′ for CYP27A1, and mfCYP51A1 (5qrt1) 5′-GCTGCCTTTGCCTAGTTTCA-3′ and mfCYP51A1 (3qrt1) 5′-CGCCCATCCTTGTATGTAGC-3′ for CYP51A1. The primers were used at a final concentration of 200 nM. The raw data were normalized with the 18S ribosomal RNA level to determine the relative expression level.
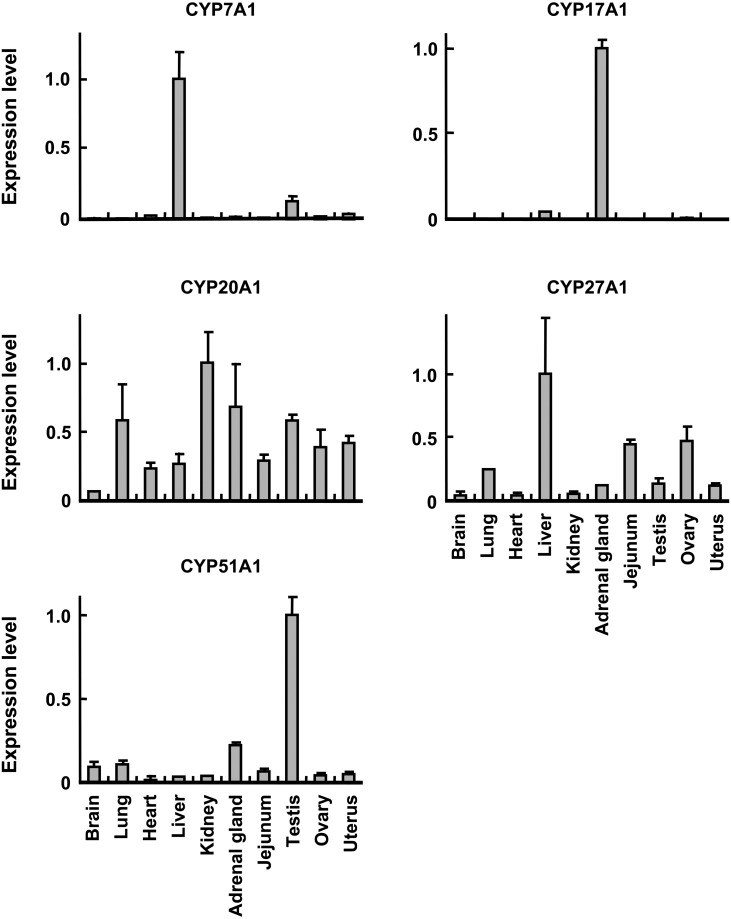
qPCR analysis showed that among the 10 tissue types analyzed, cynomolgus CYP7A1 and CYP17A1 mRNAs were preferentially expressed in liver and adrenal gland, respectively (Fig. 2). Cynomolgus CYP27A1 and CYP51A1 mRNAs were expressed in all the 10 tissue types analyzed, but were most abundantly expressed in liver and testis, respectively (Fig. 2). Cynomolgus CYP20A1 mRNA showed a ubiquitous expression pattern with the most abundant expression in kidney, followed by adrenal gland, testis and lung (Fig. 2). Therefore, cynomolgus CYP7A1, CYP17A1, CYP20A1, CYP27A1 and CYP51A1 mRNAs showed distinct tissue expression patterns.
Fig. 2.
Tissue distribution of cynomolgus P450 mRNA. qPCR was conducted using total RNA of brain, lung, heart, liver, kidney, adrenal gland, small intestine, testis, ovary and uterus as described in text. For each tissue, total RNAs from six animals (three males and three females) were pooled and used for qPCR. Expression level of P450 mRNA was normalized to 18S rRNA level. Values represent the average ± S.D. from three independent amplifications. For graphic representation, the highest value of each P450 mRNA was adjusted to 1.
CYP7A1, CYP27A1 and CYP51A1 are involved in biosynthesis and metabolism of cholesterol and bile acids. CYP7A1, cholesterol 7α-hydroxylase, catalyzes the major rate-limiting step of the classical pathway of bile acids [5]. Human CYP7A1 is considered to be liver-specific [5], coinciding well with a predominant expression of cynomolgus CYP7A1 (Fig. 2). CYP27A1, sterol 27-hydroxylase, catalyzes the oxidation of cholesterol to 27-hydroxycholesterol in the bile acid biosynthesis and participates in other processes of cholesterol homeostasis [5]. CYP27A1 is also involved in metabolism of vitamin D as vitamin D 25-hydroxylase. Human CYP27A1 is expressed in various tissues [9], including liver [1], similar to cynomolgus CYP27A1 (Fig. 2).
CYP51A1, the only P450 involved in cholesterol biosynthesis, catalyzes the oxidative 14α-demethylation of lanosterol [5]. Although human CYP51 is expressed in various tissues [5], the abundant expression is found in testis [3], similar to cynomolgus CYP51A1 (Fig. 2). CYP51 shows stage-specific expression patterns during spermatogenesis, which requires extensive changes of membrane structure [3]. Because cholesterol is involved in modulation of membrane properties [3], CYP51 might play essential roles in spermatogenesis. High sequence identity and similar tissue expression patterns indicate the potential functional similarity of cynomolgus CYP7A1, CYP27A1 and CYP51A1 to the human orthologs in biosynthesis and metabolism of cholesterol and bile acids.
CYP17A1 is involved in steroid synthesis by catalyzing steroid 17α-hydroxylation and 17,20-lyase activity toward production of sex steroids and thus is important for reproduction [8]. Human CYP17A1 is expressed abundantly in adrenal gland [8], similar to cynomolgus CYP17A1 (Fig. 2). In contrast, mouse and rat CYP17 expression appears to be absent in adrenal gland [8]. Moreover, substrate preference of CYP17A1 is somewhat different between rats and humans [8]. Cynomolgus CYP17A1 showed high sequence identity and similar tissue expression pattern to human CYP17A1, suggesting that cynomolgus CYP17A1 might have more similar function to human CYP17A1 as compared with rodent CYP17A1.
CYP20A1 is an orphan isoform in humans, and the function has not been characterized [2]. Human CYP20A1 mRNA is expressed in brain and liver [11], and cynomolgus CYP20A1 was also expressed in these tissues (Fig. 2). Cynomolgus CYP20A1 cDNA identified in this study lacked exon 10 sequence due to alternative splicing. Similarly, other than the full-length CYP20A1 transcript, two alternatively-spliced transcripts are expressed in rats, lacking either exon 2 or exons 2/3 [11]. Such alternatively-spliced transcripts have also been identified in various P450s, including cynomolgus CYP2C93, which lacked exon 2 sequence due to the mutation at the splicing acceptor site of intron 1 [16]. Hence, the full-length CYP2C93 transcript with a complete ORF appears to be expressed in rhesus macaques, but not in cynomolgus macaques, indicating potential differences between the two lineages in a CYP2C93-dependent drug metabolism. It is of great interest to investigate to see if full-length CYP20A1 transcript is expressed in cynomolgus macaques.
In conclusion, cynomolgus CYP7A1, CYP17A1, CYP20A1, CYP27A1 and CYP51A1 were characterized by sequence analysis, phylogenetic analysis and tissue expression pattern. Each of these cynomolgus P450s had a high sequence identity to the human ortholog and was most closely related to the human ortholog as compared with the dog or rat ortholog. Moreover, each of these cynomolgus P450 mRNAs showed a tissue expression pattern generally similar to the human ortholog in the 10 tissue types analyzed. These results suggest the molecular similarities of CYP7A1, CYP17A1, CYP20A1, CYP27A1 and CYP51A1 between cynomolgus macaques and humans. Further investigation of drug-metabolizing capability would help understand the function of these cynomolgus P450s.
Acknowledgments
We sincerely thank Mr. Masahiro Utoh for his support of this work and Mr. Lance Bell for his advice on English writing.
REFERENCES
- 1.Cali J. J., Russell D. W.1991. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J. Biol. Chem. 266: 7774–7778. [PubMed] [Google Scholar]
- 2.Guengerich F. P., Cheng Q.2011. Orphans in the human cytochrome P450 superfamily: approaches to discovering functions and relevance in pharmacology. Pharmacol. Rev. 63: 684–699. doi: 10.1124/pr.110.003525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keber R., Rozman D., Horvat S.2013. Sterols in spermatogenesis and sperm maturation. J. Lipid Res. 54: 20–33. doi: 10.1194/jlr.R032326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lepesheva G. I., Waterman M. R.2007. Sterol 14α-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim. Biophys. Acta 1770: 467–477. doi: 10.1016/j.bbagen.2006.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorbek G., Lewinska M., Rozman D.2012. Cytochrome P450s in the synthesis of cholesterol and bile acids—from mouse models to human diseases. FEBS J. 279: 1516–1533. doi: 10.1111/j.1742-4658.2011.08432.x [DOI] [PubMed] [Google Scholar]
- 6.Nebert D. W., Russell D. W.2002. Clinical importance of the cytochromes P450. Lancet 360: 1155–1162. doi: 10.1016/S0140-6736(02)11203-7 [DOI] [PubMed] [Google Scholar]
- 7.Nelson D. R., Zeldin D. C., Hoffman S. M., Maltais L. J., Wain H. M., Nebert D. W.2004. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14: 1–18. doi: 10.1097/00008571-200401000-00001 [DOI] [PubMed] [Google Scholar]
- 8.Payne A. H., Hales D. B.2004. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 25: 947–970. doi: 10.1210/er.2003-0030 [DOI] [PubMed] [Google Scholar]
- 9.Pikuleva I. A.2006. Cholesterol-metabolizing cytochromes P450. Drug Metab. Dispos. 34: 513–520. doi: 10.1124/dmd.105.008789 [DOI] [PubMed] [Google Scholar]
- 10.Pikuleva I. A.2008. Cholesterol-metabolizing cytochromes P450: implications for cholesterol lowering. Expert Opin. Drug Metab. Toxicol. 4: 1403–1414. doi: 10.1517/17425255.4.11.1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stark K., Wu Z. L., Bartleson C. J., Guengerich F. P.2008. mRNA distribution and heterologous expression of orphan cytochrome P450 20A1. Drug Metab. Dispos. 36: 1930–1937. doi: 10.1124/dmd.108.022020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uno Y., Fujino H., Kito G., Kamataki T., Nagata R.2006. CYP2C76, a novel cytochrome P450 in cynomolgus monkey, is a major CYP2C in liver, metabolizing tolbutamide and testosterone. Mol. Pharmacol. 70: 477–486. doi: 10.1124/mol.106.022673 [DOI] [PubMed] [Google Scholar]
- 13.Uno Y., Hosaka S., Matsuno K., Nakamura C., Kito G., Kamataki T., Nagata R.2007. Characterization of cynomolgus monkey cytochrome P450 (CYP) cDNAs: is CYP2C76 the only monkey-specific CYP gene responsible for species differences in drug metabolism? Arch. Biochem. Biophys. 466: 98–105. doi: 10.1016/j.abb.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 14.Uno Y., Matsuno K., Nakamura C., Utoh M., Yamazaki H.2009. Identification and characterization of CYP2B6 cDNA in cynomolgus macaques (Macaca fascicularis). J. Vet. Med. Sci. 71: 1653–1656. doi: 10.1292/jvms.001653 [DOI] [PubMed] [Google Scholar]
- 15.Uno Y., Suzuki Y., Wakaguri H., Sakamoto Y., Sano H., Osada N., Hashimoto K., Sugano S., Inoue I.2008. Expressed sequence tags from cynomolgus monkey (Macaca fascicularis) liver: a systematic identification of drug-metabolizing enzymes. FEBS Lett. 582: 351–358. doi: 10.1016/j.febslet.2007.12.031 [DOI] [PubMed] [Google Scholar]
- 16.Uno Y., Uehara S., Kohara S., Iwasaki K., Nagata R., Fukuzaki K., Utoh M., Murayama N., Yamazaki H.2011. Newly identified CYP2C93 is a functional enzyme in rhesus monkey, but not in cynomolgus monkey. PLoS ONE 6: e16923. doi: 10.1371/journal.pone.0016923 [DOI] [PMC free article] [PubMed] [Google Scholar]