Abstract
Tumor necrosis factor (TNF)-α induces matrix metalloproteinases (MMPs) that may disrupt skin integrity. We have investigated the effects and mechanisms of exogenous TNF-α on collagen degradation by incubating human skin explants in defined serum-free media with or without TNF-α (10 ng/ml) in the absence or presence of the nonselective MMP inhibitor GM6001 for 8 days. The basal culture conditions promoted type I collagen catabolism that was accelerated by TNF-α (p < 0.005) and accomplished by MMPs (p < 0.005). Levels of the collagenases MMP-8 and MMP-13 were insignificant and neither MMP-2 nor MMP-14 were associated with increased collagen degradation. TNF-α increased secretion of MMP-1 (p < 0.01) but had no impact on MMP-1 quantities in the tissue. Immunohistochemical analysis confirmed similar tissue MMP-1 expression with or without TNF-α with epidermis being the major source of MMP-1. Increased tissue-derived collagenolytic activity with TNF-α exposure was blocked by neutralizing MMP-1 monoclonal antibody and was not due to down-regulation of tissue inhibitor of metalloproteinase-1. TNF-α increased production (p < 0.01), tissue levels (p < 0.005) and catalytic activity of the endogenous MMP-1 activator MMP-3. Type I collagen degradation correlated with MMP-3 tissue levels (rs = 0.68, p < 0.05) and was attenuated with selective MMP-3 inhibitor. Type I collagen formation was down-regulated in cultured compared with native skin explants but was not reduced further by TNF-α. TNF-α had no significant effect on epidermal apoptosis. Our data indicate that TNF-α augments collagenolytic activity of MMP-1, possibly through up-regulation of MMP-3 leading to gradual loss of type I collagen in human skin.
Keywords: Aging, Cytokine, Extracellular matrix proteins, Protease inhibitors, UK370106, C-terminal telopeptide of type I collagen, Type I C-terminal collagen propeptide
Introduction
Collagen is the major extracellular matrix (ECM) component of skin and responsible for its structural integrity. Type I collagen accounts for about 75–80% of the collagen in normal human skin (Lovell et al., 1987). Collagen homeostasis is maintained by degradative and synthetic pathways. This balance can be disturbed by intrinsic and extrinsic factors.
The pleiotropic cytokine tumor necrosis factor (TNF)-α is involved in many physiologic and pathologic processes in the skin (Bashir et al., 2009). For example, ultraviolet radiation up-regulates TNF-α in keratinocytes and fibroblasts that may contribute to the photoaging processes of the dermal ECM (Bashir et al., 2009). The mechanisms behind the direct effects on skin integrity of elevated TNF-α independent of its pro-inflammatory role are poorly delineated.
Hypothetically, TNF-α acts via induction of the matrix metalloproteinases (MMPs), a family of 23 human members that have tissue-destructive potential (Nagase et al., 2006). The collagenases MMP-1, MMP-8 and MMP-13 preferably cleave the native triple helical region of collagen types I, II, III and V. The gelatinases MMP-9 and MMP-2 preferentially degrade type IV collagen although MMP-2 and its endogenous activator MMP-14 exhibit collagenolytic activity in vitro (Fields, 2013). Stromelysins (MMP-3 and MMP-10) have broad substrate specificity but do not cleave native interstitial collagens.
The action of MMPs in tissues is tightly regulated (Ra and Parks, 2007). The activation of secreted latent MMPs involves proteolytic cleavage of the propeptide domain by tissue or plasma proteinases in a stepwise fashion (Suzuki et al., 1990). The organomercurial 4-aminophenylmercuric acetate (APMA) is used to activate MMPs in vitro. Once activated, another line of control of degradation is exerted by tissue inhibitor of metalloproteinases (TIMPs), which bind MMPs with high affinity (Brew and Nagase, 2010).
In monocellular systems, TNF-α up-regulated MMP-1, MMP-3 and MMP-9 (Han et al., 2002; Ravanti et al., 1999; Wong et al., 2001) that was accompanied by increased type I collagenolysis (Meikle et al., 1989). In more complex skin organ cultures that account for important ECM and mesenchymal-epithelial interactions (Tandara and Mustoe, 2011), TNF-α was found to promote activation of MMP-2 via up-regulation of MMP-14 (Han et al., 2001). The same group reported up-regulation of MMP-3 and down-regulation of TIMP-1 with TNF-α exposure (Han et al., 2002). No functional data were coupled to the MMP and TIMP expression profiles or activation mechanisms.
Our primary aim was to study the effect of exogenous TNF-α on overall collagen degradation and specifically on type I collagen turnover in organ-cultured human skin explants. Second, the origin of collagen degrading proteinases was studied using the nonselective MMP inhibitor GM6001. Further elucidation of the collagenolytic processes was performed with TIMP-1, MMP-1 neutralizing antibody and selective MMP-3 inhibitor in a type I collagenase assay. The expression of selected MMPs and TIMPs was also assessed. Finally, viability and apoptosis in cultured skin explants were examined using lactate dehydrogenase (LDH) and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assays.
Materials and methods
Reagents
Reagents as well as MMP-1 (M4696), MMP-3 (HPA007875) and β-actin (A5441) antibodies were purchased from Sigma-Aldrich (St. Louis, MO, USA). Monoclonal MMP-1 antibody (MAB901), IgG1 (MAB002) and rhTIMP-1 were purchased from R&D Systems (Abingdon, UK). Active rhMMP-1 and rhMMP-3 were purchased from PeproTech (Rocky Hill, NJ, USA). MMP-2 was from Millipore (Billerica, MA, USA). Type I collagen antibody (ab138492) was purchased from Abcam (Cambridge, UK).
The IC50 for GM6001 is below 10 nM for the tested MMPs (Ågren et al., 2011). The IC50 for the selective MMP-3 inhibitor UK370106 (Tocris Bioscience, Bristol, UK) has been determined to 23 nM for MMP-3, 34 μM for MMP-2, 1.75 μM for MMP-8, 30 μM for MMP-9, 2.3 μM for MMP-13 and 67 μM for MMP-14. MMP-1 activity was reduced by 20% with 100 μM UK370106 (Fray et al., 2003).
Preparation, allocation to treatment groups, culture conditions and processing of skin explants
The study was approved by the local ethics committee. Healthy female Caucasian patients undergoing elective mammary or abdominal reduction surgery under general anesthesia donated skin after giving their written consent.
The fat-free skin in sterile saline at 4 °C was used within 4 h after removal. From each donor, 115 skin explants were aseptically excised using an 8-mm trephine. Fifteen explants were procured directly (native skin); 5 were fixed in 4% phosphate-buffered paraformaldehyde (PFA) for 24 h at 4 °C (from donors 1–4), 5 were snap frozen and stored at −80 °C, and 5 were subjected to total hydroxyproline determination. The remaining 100 explants were allocated randomly to 10 groups (10 explants per group) for culture.
Explants were incubated without (control) or with rhTNF-α at 10 ng/ml (Han et al., 2002, 2001) in the absence (0 μM GM6001) or presence of 0.01, 0.1, 1.0 and 10 μM GM6001 submerged in 1.0 ml keratinocyte growth medium (KGM)-2 (PromoCell, Heidelberg, Germany) in 24-well tissue culture plates (Nunc, Roskilde, Denmark) at 37 °C in a humidified atmosphere of 5% CO2/air. KGM-2, which contains 0.125 ng/ml epidermal growth factor, 5 μg/ml insulin, 0.33 μg/ml hydrocortisone, 10 μg/ml transferrin, 0.39 μg/ml epinephrine, 4 μl/ml bovine pituitary extract, 6 mmol/l glucose, 50 ng/ml amphotericin-B, 100 μg/ml penicillin, 100 U/ml streptomycin, was supplemented with 1.4 mM CaCl2. The addition of Ca2+ is necessary to maintain epidermal and dermal integrity under serum-free culture conditions (Tavakkol et al., 1999). Cell-culture tested dimethyl sulfoxide was present in all cultures at 0.1% (v/v). Spent media were replaced after 2, 4 and 6 days of incubation with fresh medium containing identical reagents to day 0. The conditioned media from days 4 and 8 were centrifuged at 12,000 × g for 30 min and stored at −80 °C until analyzed. Five of the cultured skin explants were fixed in PFA (from donors 1–4) and 5 were stored at −80 °C until analyzed.
Normal human epidermal keratinocytes
Keratinocytes were isolated from 37-y-old male and maintained according to Furukawa et al. (1987). The cells were incubated without (control) or with TNF-α in 3.0 ml KGM-2 with 1.4 mM Ca2+ in a 6-well tissue culture plate (Nunc). After 2 days of incubation, conditioned media were aspirated and cells detached by 0.25% trypsin/0.02% ethylenediaminetetraacetic acid (EDTA). The total number of cells and viability were determined by automated cell counter (Countess™; Life Technologies, Carlsbad, CA, USA). Cells were lysed in CNTZ buffer (10 mM cacodylate-HCl, pH 6.0, 1.0 M NaCl, 0.01% (v/v) Triton X-100, 1 μM ZnCl2 and 0.2 mg/ml NaN3).
LDH activity
This assay (Roche Diagnostics) was performed in 96-well plates (Nunc). Conditioned media (100 μl) and reaction mixture (100 μl) was incubated for 30 min at ambient temperature protected from light. Fifty microliters stop solution were added and the OD read at 492 nm and 690 nm in a microplate reader (Multiskan MCC/340; Labsystems, Helsinki, Finland).
Collagen degradation
Fragmented collagen in the tissue and released into the media was measured as hydroxyproline colorimetrically (Ågren et al., 2006). The amount of degraded collagen was expressed as μg of hydroxyproline per explant.
Type I collagen degradation and biosynthesis
As an indicator of type I collagen degradation, C-terminal telopeptide of type I collagen (ICTP) was measured by an enzyme immunoassay kit (Orion Diagnostica, Espoo, Finland). De novo synthesis of type I collagen was measured by type I C-terminal collagen propeptide (CICP) released into the conditioned medium (MicroVue; Quidel Corporation, San Diego, CA, USA). Also, day-4 media were centrifuged at 15,000 × g for 15 min using 300 kDa cut-off devices (Vivaspin 500; Sartorius, Epsom, UK) to isolate CICP from type I procollagen (Kopanska et al., 2013).
Histology, MMP-1 immunohistochemistry and TUNEL immunohistofluorescence
The fixed tissues were embedded in paraffin. Serial 5-μm sections were cut from each block. Morphology was assessed in hematoxylin-eosin-stained sections.
MMP-1 immunohistochemistry was performed with the EnVision Flex+ (K8000; Dako, Glostrup, Denmark) polymer peroxidase diaminobenzidine system (Skaland et al., 2010). Tissues were first subjected to heat-induced epitope retrieval for 20 min at 97 °C using Tris-EDTA solution pH 9.0 in the pre-treatment module (Dako). Subsequently, sections were incubated with the MMP-1 monoclonal antibody at 1:10 dilution (50 μg/ml) for 2 h at ambient temperature in the Dako Autostainer Link 48 and treated according to the manufacturer's protocol. Selected sections that were not pre-treated were incubated with the MMP-1 antibody for 18 h at 4 °C. Adjacent sections were incubated with negative isotype control at the same concentration. Sections were counterstained with hematoxylin and cover-slipped. Epidermal and stromal staining were scored separately by blinded senior pathologist (L. H. C.) on a 4-tiered scale: 0, no; +, weak; ++, moderate; +++, intense staining.
TUNEL staining was carried out following pre-treatment with proteinase-K (20 μg/ml) using the ApopTag® fluorescein in situ kit (Millipore). Images were captured using a fluorescence microscope (Eclipse Ti-U, Nikon, Amsterdam, Netherlands) equipped with a digital camera (DS-Qi1Mc, Nikon).
Tissue extraction
Tissues extracts were prepared for 18 h at 4 °C using CNTZ buffer (20 μl/mg tissue) optimized for collagenase extraction (Mirastschijski et al., 2002) and supplemented with EDTA-free proteinase inhibitor cocktail/1 μM pepstatin (Roche Diagnostics, Mannheim, Germany). Tissue extracts were kept at −80 °C until analyzed.
Type I collagenolytic activity assay
Enzymes were incubated with 0.25 μg/ml type I collagen from bovine skin (Millipore) with or without inhibitors/APMA as indicated in a total volume of 40 μl with 25 μM ZnCl2, and 1 mM CaCl2 in the presence of the proteinase inhibitor cocktail and pepstatin at 24 °C for 240 h unless stated otherwise. Samples were electrophoresed on NuPAGE® 4–12% Bis-Tris gels (Life Technologies) under reducing conditions and gels stained with Colloidal Blue (Salsas-Escat et al., 2010). Gels were scanned and the extent of collagen digestion was calculated from the density of the α1, α2, 3/4α1 and 3/4α2 bands (Welgus et al., 1981) determined by ImageJ (National Institutes of Health, Bethesda, MD, USA) and expressed in percentage (%).
MMP and TIMP analyses
MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, MMP-10, MMP-13, TIMP-1, TIMP-2 and TIMP-4 were measured using Quantibody® human MMP/TIMP array (RayBiotech, Norcross, GA, USA). MMP-1 and MMP-3 were quantified by ELISA kits (Boster Biological Technology, Fremont, CA, USA). MMP-2 contents were estimated by gelatin zymography (Mirastschijski et al., 2002) relative to MMP-2 standard run in parallel using densitometry (ImageJ). TIMP-1 was assayed by ELISA kit from PeproTech (Henriksen et al., 2013).
Western blot analyses
Samples were electrophoresed using 10% sodium dodecyl sulfate-polyacrylamide gels under reducing conditions and electrotransferred onto polyvinylidene fluoride (PVDF) membrane (Immobilon® FL, Millipore) or nitrocellulose (Bio-Rad). The membranes were blocked with Odyssey buffer (Li-Cor, Lincoln, NE, USA) and incubated for 18 h at 4 °C with primary antibodies against MMP-1 diluted 1:500, MMP-3 diluted 1:250, type I collagen diluted 1:1,000 (nitrocellulose) and β-actin diluted 1:70,000 (nitrocellulose). The membranes were incubated with matching IRDye®-conjugated secondary antibodies for 1 h at ambient temperature and immunoreactions visualized by infrared imaging (Li-Cor).
β-Casein zymography
Samples were separated by 12% sodium dodecyl sulfate-polyacrylamide gels copolymerized with 0.5 mg/ml bovine β-casein at constant 125 V under nonreducing conditions at 4 °C for 3 h. Gels were renatured in 2.5% Triton X-100 for 30 min and incubated for 140 h at 37 °C in 50 mM Tris–HCl (pH 7.5) containing 10 mM CaCl2, 1 μM ZnCl2 and 0.1% Triton X-100 with or without GM6001 at 10 μM. Gels were stained with Colloidal Blue (Life Technologies).
Statistical analyses
Wilcoxon matched pairs and Spearman rank correlation tests were applied (FigSys, version 2.4.3; Biosoft, Cambridge, UK). p < 0.05 was considered statistically significant. Numerical data are presented as mean ± SEM. n = number of skin donors.
Results
To elucidate the influence and mechanisms of TNF-α on collagen degradation in skin we cultured skin explants ex vivo from six healthy females for 8 days (Table 1).
Table 1.
Demographics of the female donors and hydroxyproline content of skin.
| Donor | Age (years) | Skin typea | Location | Hydroxyproline (μg)b |
|---|---|---|---|---|
| 1 | 38 | II | Breast | 884 ± 80 |
| 2 | 49 | I | Breast | 921 ± 55 |
| 3 | 19 | IV | Breast | 787 ± 55 |
| 4 | 45 | III | Abdomen | 1009 ± 81 |
| 5 | 66 | II | Abdomen | 1171 ± 88 |
| 6 | 34 | II | Abdomen | 1264 ± 114 |
Determined according to Fitzpatrick (1988).
Mean ± SEM of 5 separate 8-mm skin explants before culturing.
Collagen degradation
We first established the time-dependence on collagen degradation in the skin explants by measuring the release of hydroxyproline into the culture medium. From the initiation of culture to day 4 a total of 1.4 ± 0.4 (n = 6) μg hydroxyproline was released. Then a profound increase (p < 0.005) in hydroxyproline liberation occurred to day 8 amounting to 19.8 ± 10.6 (n = 6) μg. Addition of TNF-α to the medium resulted in 1.8 ± 0.5 (n = 6) μg hydroxyproline release over days 0–4 and 38.7 ± 11.3 (n = 6) μg over days 4–8. Overall, TNF-α treatment increased the cumulative hydroxyproline in the medium over 8 days (40.5 ± 11.7 μg) in all six donors (p < 0.005) compared with control (21.2 ± 11.0 μg) treatment (Fig. 1).
Fig. 1.
Effect of TNF-α on collagen degradation of cultured human skin explants monitored by hydroxyproline-containing peptides released into the media after 8 days in culture. The accumulated amount of hydroxyproline from 10 separate organ-cultured 8-mm explants of each of the 6 donors and group (control and TNF-α) was used for the global calculations. Mean ± SEM (n = 6). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The nonselective MMP inhibitor GM6001 was added at 0.01–10 μM to the skin cultures. GM6001 reduced total hydroxyproline release from control skin explants at 1 μM to 3.6 ± 0.6 μg (p < 0.01) and at 10 μM to 1.7 ± 0.2 μg (p < 0.005), and from TNF-α-treated explants at 0.1 μM to 18.4 ± 4.5 μg (p < 0.005), at 1 μM to 4.7 ± 0.8 μg (p < 0.005) and at 10 μM to 1.8 ± 0.3 μg (p < 0.005).
Type I collagen turnover
To specifically address type I collagen turnover, biomarkers for degradation (ICTP) and neosynthesis (CICP) were analyzed in conditioned media.
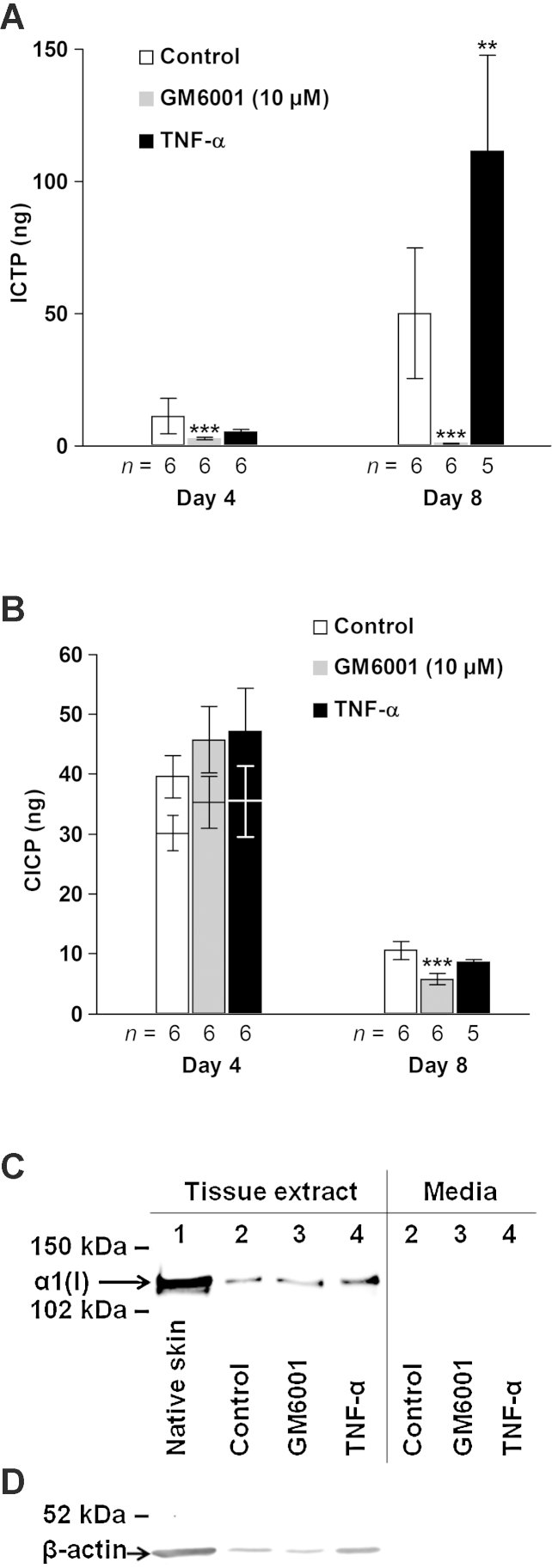
ICTP levels correlated strongly with those of hydroxyproline in control-treated skin explants (rs = 0.82, p < 0.005, n = 12). TNF-α treatment increased (p < 0.01) the ICTP levels compared with control while ICTP levels were reduced (p < 0.005) with 10 μM GM6001 on day 8 (Fig. 2A).
Fig. 2.
Effect of TNF-α and GM6001 on type I collagen turnover assessed by the biochemical markers of degradation ICTP (A), neosynthesis CICP (B) and type I collagen formation (C and D). (A and B) Media from five explants per donor were pooled and then analyzed. CICP levels after ultrafiltration of day-4 samples are indicated by lower bars (B). Mean ± SEM. **p < 0.01, ***p < 0.005 versus control at respective time point. (C and D) Western blot analysis for the α1 chain of type I collagen and β-actin of pooled concentrated (Amicon® Ultra; Millipore) CNTZ tissue extracts (C and D) and conditioned media (C) from 30 individual 8-mm skin explants (5 explants from each of the 6 donors). (C) Loading of tissue extracts was normalized to the β-actin content determined separately (D) and media were adjusted to the corresponding volume to biopsy weight ratio. Lane 1, native skin; 2, control; 3, GM6001 (10 μM); 4, TNF-α. (D) Equal volume (12.5 μl) of the tissue extracts was applied to each well.
CICP levels showed a reverse time course compared with ICTP and decreased (p < 0.005) from day 4 to day 8. In the day-8 samples, CICP levels were statistically unchanged with TNF-α treatment but were reduced (p < 0.005) with 10 μM GM6001. Elimination of type I procollagen by ultrafiltration reduced the CICP levels similarly in the three groups (∼30%) on day 4 (Fig. 2B). Because CICP levels in medium are indirect markers of type I collagen deposition we carried out Western blotting analysis for type I collagen (α1 chain) monomers in tissue extracts of skin explants (Fig. 2C). The tissue-derived type I collagen was reduced appreciably in cultured compared with noncultured skin. TNF-α increased while GM6001 treatment slightly reduced type I collagen levels when normalized to the amount of β-actin (Fig. 2C and D). Type I collagen was not detected in conditioned media (Fig. 2C).
LDH, morphology and apoptosis
The LDH activity in media of control skin cultures decreased (p < 0.005) from day 4 to day 8. LDH was higher with GM6001 (10 μM) treatment on day 8 and with TNF-α days 4 and 8 compared with control (Fig. 3A). In normal human epidermal keratinocytes, TNF-α treatment increased cellular LDH (Fig. 3B). The total number of cells after 2 days treatment was 4.9 ± 0.4 × 105 in control wells and 5.0 ± 0.8 × 105 in TNF-α wells. The corresponding viabilities were 97.0 ± 0.6% and 96.0 ± 0.6%.
Fig. 3.
LDH activity in media from cultured skin explants (A), and in cell lysates of and media from normal human epidermal keratinocytes (B). (A) Individual samples, each comprising the combined media from 5 separate 8-mm skin explants, were analyzed. n = number of skin donors. (B) Confluent keratinocytes of the second passage were treated with or without TNF-α for 2 days. Mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.005 versus control.
Morphologically, no discernible dermal changes occurred with culturing of the skin explants for 8 days possibly with the exception of slightly decreased number of cells compared with native skin. Epidermis had partially separated from dermis with ensuing pyknosis in cultured as opposed to noncultured skin. The degree of epidermal detachment was extensive in the control (40–50% of the length) and TNF-α (30–40%) groups but suppressed with GM6001 (0–20%) treatment. Apoptosis, assessed by TUNEL immunofluorescence, was prominent in epidermis and sparse in dermis of cultured skin explants compared with native skin (Fig. 4A). There was no significant difference in epidermal apoptosis between the control and TNF-α groups (Fig. 4B).
Fig. 4.
Apoptosis assessed by TUNEL immunohistofluorescence. (A) Digoxigenin-labeled 3′-OH DNA termini were detected by sheep polyclonal anti-digoxigenin antibody conjugated with fluorescein (red). Slides were mounted using medium containing 4′,6-diamidino-2-phenylindole (green). Representative sections of native (left), control-treated (middle) and TNF-α-treated (right) skin explants are shown. Epidermis is indicated by dashed line. Scale bars: 500 μm. (B) Total number of TUNEL-positive cells per epidermal area in mm2, determined in two sections from two explants from each donor by two blinded investigators by image analysis (NIS-Elements AR, Nikon), were used for the global calculations. Mean ± SEM (n = 4). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Type I collagenolysis
We next assessed the collagenolytic activity using exogenous native type I skin collagen. Pooled tissue extracts of TNF-α-treated skin explants possessed increased collagenolytic activity (34%) compared with control-treated (19%) skin explants. The difference in collagenolytic activity between TNF-α and control-treated skin was more pronounced with the MMP-activator APMA during the assay. The collagenolytic activity of tissue extracts did not decline appreciably with length of incubation. No collagenolytic activity was detected in native skin (Fig. 5A). The MMP inhibitors GM6001 and TIMP-1 blocked collagenolysis. Moreover, a monoclonal neutralizing antibody against hMMP-1 abolished the collagenolytic activity of tissue extracts of incubated skin explants from the two groups (Fig. 5B). The MMP-1 antibody (10 μg/ml) also completely abrogated the collagenolytic activity (85%) of control day-8 pooled media from donor 1. Selective MMP-3 inhibition with UK370106 reduced type I collagenolysis by about 60% in the presence of APMA but less in the absence of APMA. UK370106 at 1 μM did not decrease the collagenolytic activity of rhMMP-1 (Fig. 5B–D). The assay was linear to about 30% collagenolysis (Fig. 5E). The type I collagen substrate was resistant to trypsin while denatured substrate was completely digested. rhMMP-1 generated the anticipated 3/4 and 1/4 fragments of the α1(I) and α2(I) chains (Fig. 5F).
Fig. 5.
Digestion of native type I collagen by tissue-derived proteinases of native human skin (A), control (A and B) and TNF-α-treated human skin (A–C), active rhMMP-1 (D–F) or trypsin (F). (A–C) Tissue extract pools from 30 individual 8-mm skin explants (5 explants from each of the 6 donors) per group were concentrated 3× (Amicon® Ultra; Millipore). S, substrate. (B–D) Enzymes were incubated for 2 h with inhibitors before the substrate was added. (D) rhMMP-1 was incubated with substrate in the absence or presence of UK370106. (A–D, F) Collagenolytic activity in percentage of type I collagen degradation is shown below each lane. (E) Effect of rhMMP-1 on collagenolysis as a function of concentration and time of incubation (inset, 1 ng/ml). (F) Trypsin (Worthington, Lakewood, NJ, USA) treatment of native or denatured (56 °C, 30 min) substrate was carried out at identical assay conditions. rhMMP-1, 2.5 ng/ml. *, position of trypsin. D, denatured.
MMP profiling of native and cultured skin explants
Collectively, MMPs were responsible for the degradation of endogenous and exogenous type I skin collagen. Our initial approach for identifying the operative MMPs was to analyze pooled tissue extracts using a quantitative MMP/TIMP multiarray (Fig. 6). Clearly, MMP-1 was the dominant MMP in cultured skin explants. MMP-3 was barely detectable in quiescent skin but increased after 8 days of culture. MMP-2 content of native skin was ∼30 ng and increased further in culture. The amount of MMP-8 in native skin was ∼0.1 ng but was reduced after culture. The MMP-9 content was likewise lowered after incubation. Although MMP-10 was detectable in native and incubated skin the levels were insignificant (<0.05 ng/explant). Tissue MMP-13 was nondetectable. The TIMP-1 content of the explants increased after culture whereas TIMP-2 decreased. TIMP-4 was nondetectable in the tissue. From this screening we conclude that MMP-1, MMP-3, MMP-2 and TIMP-1 were the most prominent MMPs/TIMPs in our system and were analyzed on individual samples.
Fig. 6.
MMP and TIMP contents of native skin and cultured skin explants treated without (control) or with TNF-α (10 ng/ml) were measured in pooled tissue extracts by the Quantibody® array and expressed in total amount (ng) per explant. Each pool comprised extracts made from 30 individual 8-mm skin explants (5 explants from each of the 6 donors per group). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Effect of TNF-α on MMP-1, MMP-3, MMP-2 and TIMP-1
There was no difference in total tissue MMP-1 contents between the TNF-α and control groups determined by the ELISA assay. On the other hand, MMP-1 content in media was increased with TNF-α present during incubation (Fig. 7A). Western blot analysis indicated that MMP-1 was in catalytically active glycosylated and nonglycosylated forms in the tissue while both latent and active MMP-1 glycosylated and nonglycosylated forms were found in about equal proportions in the media (Fig. 7B). Immunohistological staining of cultured skin explants clearly showed that MMP-1 was increased throughout the epidermis, being almost exclusively cytoplasmic (++/+++), and slightly reduced (+) extracellularly in the stroma compared with native skin. There were no apparent differences in localization or intensity of the MMP-1 immunoreactivity between control and TNF-α-treated skin explants. Native skin showed predominance of MMP-1 extracellularly in the stroma (++), most intensely in the papillary dermis, and in the nucleus (++) of scattered keratinocytes in epidermis (Fig. 7C). Nuclear MMP-1 was also observed in sections without pre-treatment for antigen retrieval although the signal was weaker than with pre-treatment. In contrast, MMP-1 was entirely cytoplasmic in human oral epithelium (Supplementary Fig. S1).
Fig. 7.
MMP-1 and MMP-3 expression in control and TNF-α-treated skin analyzed by ELISA (A and D), Western blot (B and E) and β-casein zymography (F), and MMP-1 immunohistochemistry (C) and MMP-3 correlation to ICTP (G) after 8 days of incubation. (A and D) Pooled tissue extracts and media from five explants per donor and group were assayed. Mean ± SEM. **p < 0.01, ***p < 0.005 versus control. n = number of skin donors. (B and E) The PVDF membrane was first probed with the polyclonal MMP-1 antibody (B), then stripped and reprobed with the polyclonal MMP-3 antibody (E). Lanes 1 and 3, control; 2 and 4, TNF-α; 1 and 2, pooled tissue extract (18 μl/lane) from 30 individual 8-mm skin explants from the 6 donors (1–6) per group; lanes 3 and 4, pooled media (18 μl/lane) from 24 individual 8-mm skin explants from 5 donors (media from donor 1 was lost) per group. Std.: 42.7 kDa rhMMP-1 (14 ng). Glycosylated and nonglycosylated latent and active MMP-1/MMP-3 doublets are indicated. (C) Representative sections of native (a, d and g), or cultured skin explants from control (b, e and h) and TNF-α groups (c, f and i) treated with primary monoclonal MMP-1 antibody that detects latent and active forms (a–f) or with isotype (negative) control (g–i). (a–c, 40×; d–i, 900×). (F) Casein gels were incubated in the absence (Buffer) or presence of 10 μM GM6001. Lane 1, rhMMP-3 (5 ng); 2, pooled media from control-treated explants (15 μl); 3, pooled media from TNF-α-treated explants (15 μl); 4, rhMMP-1 (2 ng). Mark12™ (Life Technologies) molecular weight marker was run in parallel lane. Upper doublets represent latent forms of MMP-3 (upper bands) and MMP-1 (lower bands) and lower doublets active forms of MMP-3 (upper bands) and MMP-1 (lower bands). (G) Tissue MMP-3 contents and corresponding ICTP in media. Each symbol as indicated in Fig. 1 represents pooled tissue extracts and media from five explants per donor. The TNF-α-treated skin explants of donor 1 is missing due to lost pooled media sample. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
MMP-3 contents in tissue as well as in media were raised in the TNF-α group compared with the control group measured by ELISA (Fig. 7D). Most of the increased MMP-3 with TNF-α was attributed to increased active MMP-3 species. The faint bands in lanes 3 and 4 below latent and active MMP-3 bands are signal from residual bound MMP-1 antibody due to incomplete stripping (Fig. 7E). Increased catalytic activity of MMP-3 in the TNF-α group was demonstrated by β-casein zymographic analysis. GM6001 at 10 μM completely abrogated the appearance of all caseinolytic bands (Fig. 7F). MMP-3 tissue contents correlated with type I collagen degradation measured as ICTP released into the media (Fig. 7G).
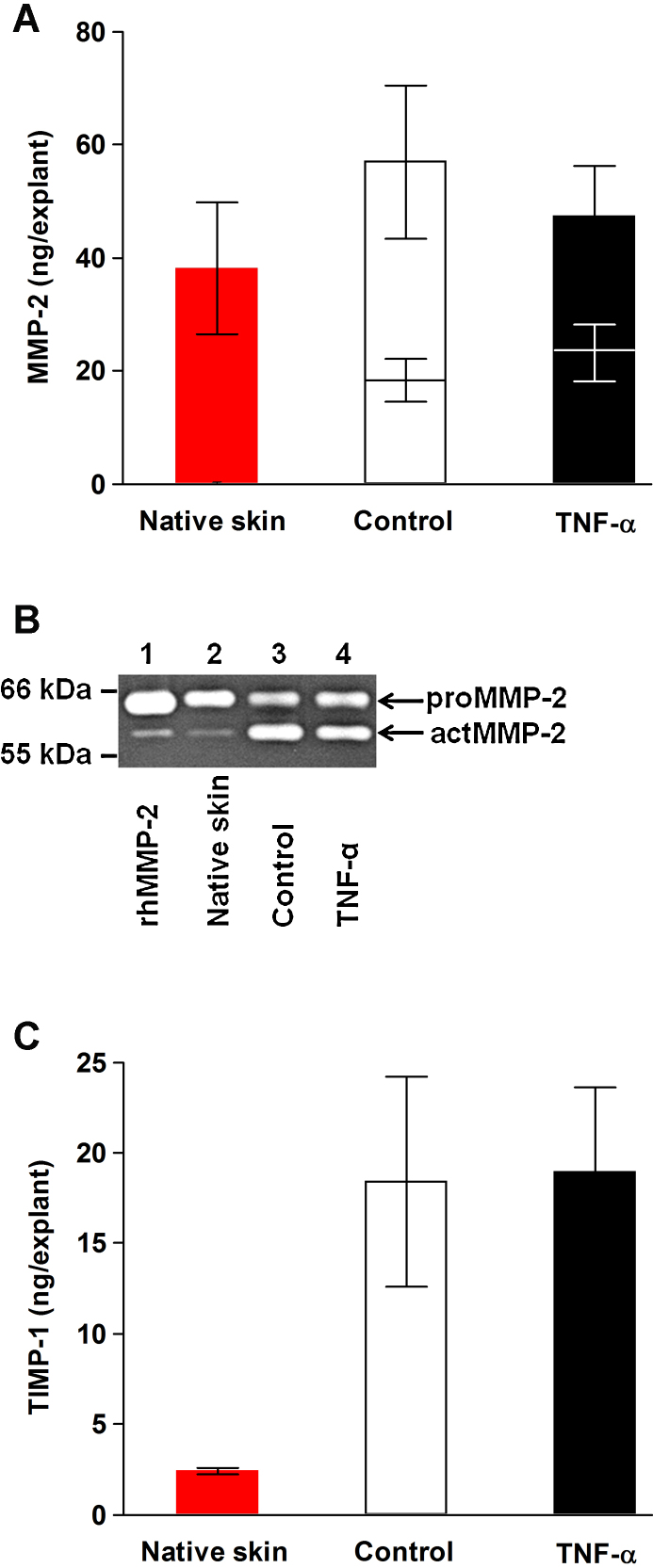
MMP-2 tissue levels were semiquantified by gelatin zymography (Fig. 8A and B). No significant effects of TNF-α treatment on either total or active MMP-2 were observed.
Fig. 8.
MMP-2 (A and B) and TIMP-1 (C) tissue levels. (A) Total and active (lower bars) MMP-2 contents estimated by gelatin zymography. (B) In the zymogram, MMP-2 standard and pooled tissue extracts (1.0 μl) from 30 individual 8-mm skin explants per group were loaded into each lane. Lane 1, rhMMP-2 (50 pg; PF037); 2, native skin; 3, control; 4, TNF-α. (C) TIMP-1 levels determined by ELISA. (A and C) Pooled tissue extracts from five separate native or organ-cultured 8-mm skin explants from each of the donors and group were used for the analyses. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) Mean ± SEM (n = 6).
Tissue TIMP-1 quantities did not differ between TNF-α-treated and control-treated skin although they were increased compared with native skin (Fig. 8C).
Discussion
TNF-α is a multifunctional cytokine with profound effects on the skin (Bashir et al., 2009). In the present study, TNF-α accelerated collagen degradation in cultured human skin explants. This was achieved independently of the secondary effects of TNF-α on inflammation observed in vivo.
Collagen breakdown was measured indirectly by released hydroxproline-containing peptides into the culture medium. In a similar experimental set-up to ours, Koob et al. (1980) found that collagen degradation paralleled de novo protein synthesis. Because insoluble type I collagen is the major dermal component (Lovell et al., 1987), its degradation was specifically measured by soluble ICTP fragments. ICTP correlated with hydroxyproline suggesting that type I collagen was the main endogenous substrate in our organ-culture system. Furthermore, we could confirm that ICTP is a robust biomarker of tissue MMP activity (Garnero et al., 2003).
Although hydroxyproline and ICTP release was MMP driven we can only speculate on the specific MMPs influenced by TNF-α. Native type I collagen is cleaved into 3/4 and 1/4 fragments by the classical collagenases MMP-1, MMP-8 and MMP-13. Expectedly, MMP-8 and MMP-13 tissue levels were negligible compared with MMP-1 (Tandara and Mustoe, 2011). Moreover, tissue-derived proteolytic enzymes from cultured skin explants cleaved native type I collagen molecules into 3/4 and 1/4 fragments. This collagenolytic activity was blocked by a neutralizing MMP-1 antibody. TNF-α treatment increased the amount of released MMP-1 but not the MMP-1 bound to the tissue due to lack of binding of the proform of MMP-1 to collagen (Murphy et al., 1992). Considerably less of active rhMMP-1 than the MMP-1 quantity present in the tissue was required to degrade the corresponding amount of type I collagen. This discrepancy was most likely due to the presence of the endogenous TIMP inhibitors in tissue extracts. Importantly, TNF-α did not significantly influence TIMP-1 tissue content. Taken together, these findings strongly suggest that MMP-1 was activated indirectly by TNF-α causing the increased type I collagenolysis.
MMP-1 activation processes are multiple and complex (Ra and Parks, 2007). MMP-3 is coordinately synthesized with MMP-1 and is induced by TNF-α in whole human skin (Han et al., 2002; Wong et al., 2001). MMP-3 has the capacity to render full collagenolytic activity to MMP-1 (Suzuki et al., 1990). Here, the involvement of MMP-3 in type I collagenolysis was indicated by the significant correlation with ICTP levels. Up-regulation of MMP-3 by TNF-α may thus account for excessive collagen degradation through its unique capacity to fully activate MMP-1 by cleavage of the Gln80-Phe81 bond (Suzuki et al., 1990). These subtle conformational changes of MMP-1 could not be detected by Western blot analysis but we were able to attenuate tissue-associated MMP-1 activation using a selective MMP-3 inhibitor.
In periosteal rabbit connective tissue, MMP-2 and not MMP-1 accounted for collagen breakdown (Kerkvliet et al., 1999). This deviation from our results may be explained by the lack of epithelium in their tissue. In our culture system, epidermis was the major source of MMP-1. Differences in collagenolytic activity was not attributed to MMP-2 (Crabbe et al., 1994) because the levels of active MMP-2 did not differ significantly between the two groups. For the same reason, the MMP-2 activator MMP-14 was unlikely regulated by TNF-α in our experiments. Furthermore, type I collagenolysis was efficiently blocked by TIMP-1 which is an extremely poor inhibitor of MMP-14 (Sabeh et al., 2009). Collectively, neither MMP-2 nor MMP-14 seemed to contribute significantly to the TNF-α-mediated degradation of type I collagen in human skin.
Formation of type I collagen requires processing of C-propeptides and N-propeptides of procollagen by zinc-dependent endopeptidases, e.g. BMP-1 and ADAMTS-2 in skin (Canty and Kadler, 2005). Surprisingly, similar hydroxamate-based compounds to GM6001 are poor inhibitors of procollagen C-proteinase (Ovens et al., 2000). Thus, the observed inhibited release of the CICP could be attributed to slight cytotoxicity of GM6001 that was manifested by increased LDH release. Although there was a trend of increased type I collagen deposition with TNF-α this was not reflected in elevated CICP levels possibly due to increased N-proteinase activity by TNF-α (Harrison et al., 2006).
An incidental finding was the distinct and specific nuclear preference of MMP-1 in keratinocytes in quiescent skin. We could find only one publication reporting nuclear MMP-1 and this was in breast carcinoma cells (Boström et al., 2011). MMP-1 has also been ascribed an anti-apoptotic effect (Limb et al., 2005). Notably, in human oral epithelium MMP-1 was located in the cytosol.
The increased LDH in media with TNF-α treatment without concomitant increased epidermal apoptosis is a conundrum and difficult to explain. Our studies on primary human epidermal keratinocytes indicate that TNF-α increased LDH activity per se rather than causing membrane damage. This could explain the increased LDH release from the cultured skin explants. TNF-α did not induce epidermal apoptosis in agreement with earlier findings for primary keratinocytes (Zimmermann et al., 2011). Moreover, we observed a general decline in viability and gradual separation of epidermis from dermis over the 8-day culture period, which are known phenomena of organ-cultured skin (Kleszczyski and Fischer, 2012). These processes may have increased the release of intracellular cathepsins which possess collagenolytic activity (Wagenaar-Miller et al., 2007). Because the collagenolytic activity of the conditioned media could be blocked by the neutralizing MMP-1 antibody cathepsins did not appear to play a significant role in collagen degradation. Epidermolysis was reduced with GM6001 indicating some involvement of MMPs in this process as indicated in another study (Mol et al., 2009).
In summary, TNF-α promoted collagen degradation in cultured human skin via the collagenolytic MMP-1 and MMP-3 axis. This model of dermal degeneration due to MMP interactions with interstitial collagens may be implicated for down-stream effects of other external stimuli such as ultraviolet radiation.
Acknowledgements
Omid Niazi and Vibeke Pless assisted with laboratory work. Andreas Nordholm-Carstensen was helpful with the statistical analyses and Peter-Martin Krarup with images. Sofia Tedelind and Antonia J. Caliani performed the TUNEL analyses. The research leading to these results has received funding from the European Research Council under the European Community's Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 243195 to Ursula Mirastschijski. This work was also supported by The Pharmacy Foundation of 1991, Denmark.
Appendix A. Supplementary data
The following are the supplementary data to this article:

References
- Ågren M.S., Andersen T.L., Andersen L., Schiødt C.B., Surve V., Andreassen T.T. Nonselective matrix metalloproteinase but not tumor necrosis factor-alpha inhibition effectively preserves the early critical colon anastomotic integrity. Int. J. Colorectal Dis. 2011;26:329–337. doi: 10.1007/s00384-010-1106-3. [DOI] [PubMed] [Google Scholar]
- Ågren M.S., Andersen T.L., Mirastschijski U., Syk I., Schiødt C.B., Surve V. Action of matrix metalloproteinases at restricted sites in colon anastomosis repair: an immunohistochemical and biochemical study. Surgery. 2006;140:72–82. doi: 10.1016/j.surg.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Bashir M.M., Sharma M.R., Werth V.P. TNF-alpha production in the skin. Arch. Dermatol. Res. 2009;301:87–91. doi: 10.1007/s00403-008-0893-7. [DOI] [PubMed] [Google Scholar]
- Boström P., Söderström M., Vahlberg T., Söderström K.O., Roberts P.J., Carpén O. MMP-1 expression has an independent prognostic value in breast cancer. BMC Cancer. 2011;11:348. doi: 10.1186/1471-2407-11-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim. Biophys. Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty E.G., Kadler K.E. Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 2005;118:1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- Crabbe T., O’Connell J.P., Smith B.J., Docherty A.J. Reciprocated matrix metalloproteinase activation: a process performed by interstitial collagenase and progelatinase A. Biochemistry. 1994;33:14419–14425. doi: 10.1021/bi00252a007. [DOI] [PubMed] [Google Scholar]
- Fields G.B. Interstitial collagen catabolism. J. Biol. Chem. 2013;288:8785–8793. doi: 10.1074/jbc.R113.451211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- Fray M.J., Dickinson R.P., Huggins J.P., Occleston N.L. A potent, selective inhibitor of matrix metalloproteinase-3 for the topical treatment of chronic dermal ulcers. J. Med. Chem. 2003;46:3514–3525. doi: 10.1021/jm0308038. [DOI] [PubMed] [Google Scholar]
- Furukawa F., Huff J.C., Weston W.L., Norris D.A. Serum-free serial culture of adult human keratinocytes from suction-blister roof epidermis. J. Invest. Dermatol. 1987;89:460–463. doi: 10.1111/1523-1747.ep12460904. [DOI] [PubMed] [Google Scholar]
- Garnero P., Ferreras M., Karsdal M.A., Nicamhlaoibh R., Risteli J., Borel O. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J. Bone Miner. Res. 2003;18:859–867. doi: 10.1359/jbmr.2003.18.5.859. [DOI] [PubMed] [Google Scholar]
- Han Y.P., Nien Y.D., Garner W.L. Tumor necrosis factor-alpha-induced proteolytic activation of pro-matrix metalloproteinase-9 by human skin is controlled by down-regulating tissue inhibitor of metalloproteinase-1 and mediated by tissue-associated chymotrypsin-like proteinase. J. Biol. Chem. 2002;277:27319–27327. doi: 10.1074/jbc.M202842200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.P., Tuan T.L., Wu H., Hughes M., Garner W.L. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J. Cell Sci. 2001;114:131–139. doi: 10.1242/jcs.114.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C.A., Gossiel F., Bullock A.J., Sun T., Blumsohn A., Mac Neil S. Investigation of keratinocyte regulation of collagen I synthesis by dermal fibroblasts in a simple in vitro model. Br. J. Dermatol. 2006;154:401–410. doi: 10.1111/j.1365-2133.2005.07022.x. [DOI] [PubMed] [Google Scholar]
- Henriksen N.A., Sørensen L.T., Jorgensen L.N., Ågren M.S. Circulating levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with incisional hernia. Wound Repair Regen. 2013;21:661–666. doi: 10.1111/wrr.12071. [DOI] [PubMed] [Google Scholar]
- Kerkvliet E.H., Docherty A.J., Beertsen W., Everts V. Collagen breakdown in soft connective tissue explants is associated with the level of active gelatinase A (MMP-2) but not with collagenase. Matrix Biol. 1999;18:373–380. doi: 10.1016/s0945-053x(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Kleszczyski K., Fischer T.W. Development of a short-term human full-thickness skin organ culture model in vitro under serum-free conditions. Arch. Dermatol. Res. 2012;304:579–587. doi: 10.1007/s00403-012-1239-z. [DOI] [PubMed] [Google Scholar]
- Koob T.J., Jeffrey J.J., Eisen A.Z., Bauer E.A. Hormonal interactions in mammalian collagenase regulation. Comparative studies in human skin and rat uterus. Biochim. Biophys. Acta. 1980;629:13–23. doi: 10.1016/0304-4165(80)90260-3. [DOI] [PubMed] [Google Scholar]
- Kopanska K.S., Powell J.J., Jugdaohsingh R., Bruggraber S.F. Filtration of dermal fibroblast-conditioned culture media is required for the reliable quantitation of cleaved carboxy-terminal peptide of collagen type I (CICP) by ELISA. Arch. Dermatol. Res. 2013;305:741–745. doi: 10.1007/s00403-013-1370-5. [DOI] [PubMed] [Google Scholar]
- Limb G.A., Matter K., Murphy G., Cambrey A.D., Bishop P.N., Morris G.E. Matrix metalloproteinase-1 associates with intracellular organelles and confers resistance to lamin A/C degradation during apoptosis. Am. J. Pathol. 2005;166:1555–1563. doi: 10.1016/S0002-9440(10)62371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell C.R., Smolenski K.A., Duance V.C., Light N.D., Young S., Dyson M. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br. J. Dermatol. 1987;117:419–428. doi: 10.1111/j.1365-2133.1987.tb04921.x. [DOI] [PubMed] [Google Scholar]
- Meikle M.C., Atkinson S.J., Ward R.V., Murphy G., Reynolds J.J. Gingival fibroblasts degrade type I collagen films when stimulated with tumor necrosis factor and interleukin 1: evidence that breakdown is mediated by metalloproteinases. J. Periodont. Res. 1989;24:207–213. doi: 10.1111/j.1600-0765.1989.tb02007.x. [DOI] [PubMed] [Google Scholar]
- Mirastschijski U., Impola U., Karsdal M.A., Saarialho-Kere U., Ågren M.S. Matrix metalloproteinase inhibitor BB-3103 unlike the serine proteinase inhibitor aprotinin abrogates epidermal healing of human skin wounds ex vivo. J. Invest. Dermatol. 2002;118:55–64. doi: 10.1046/j.0022-202x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- Mol M.A., van den Berg R.M., Benschop H.P. Involvement of caspases and transmembrane metalloproteases in sulphur mustard-induced microvesication in adult human skin in organ culture: directions for therapy. Toxicology. 2009;258:39–46. doi: 10.1016/j.tox.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Murphy G., Allan J.A., Willenbrock F., Cockett M.I., O’Connell J.P., Docherty A.J. The role of the C-terminal domain in collagenase and stromelysin specificity. J. Biol. Chem. 1992;267:9612–9618. [PubMed] [Google Scholar]
- Nagase H., Visse R., Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ovens A., Joule J.A., Kadler K.E. Design and synthesis of acidic dipeptide hydroxamate inhibitors of procollagen C-proteinase. J. Pept. Sci. 2000;6:489–495. doi: 10.1002/1099-1387(200009)6:9<489::AID-PSC282>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Ra H.J., Parks W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanti L., Heino J., López-Otin C., Kähäri V.M. Induction of collagenase-3 (MMP-13) expression in human skin fibroblasts by three-dimensional collagen is mediated by p38 mitogen-activated protein kinase. J. Biol. Chem. 1999;274:2446–2455. doi: 10.1074/jbc.274.4.2446. [DOI] [PubMed] [Google Scholar]
- Sabeh F., Li X.Y., Saunders T.L., Rowe R.G., Weiss S.J. Secreted versus membrane-anchored collagenases: relative roles in fibroblast-dependent collagenolysis and invasion. J. Biol. Chem. 2009;284:23001–23011. doi: 10.1074/jbc.M109.002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salsas-Escat R., Nerenberg P.S., Stultz C.M. Cleavage site specificity and conformational selection in type I collagen degradation. Biochemistry. 2010;49:4147–4158. doi: 10.1021/bi9021473. [DOI] [PubMed] [Google Scholar]
- Skaland I., Nordhus M., Gudlaugsson E., Klos J., Kjellevold K.H., Janssen E.A. Evaluation of 5 different labeled polymer immunohistochemical detection systems. Appl. Immunohistochem. Mol. Morphol. 2010;18:90–96. doi: 10.1097/PAI.0b013e3181b0eaad. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Enghild J.J., Morodomi T., Salvesen G., Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin) Biochemistry. 1990;29:10261–10270. doi: 10.1021/bi00496a016. [DOI] [PubMed] [Google Scholar]
- Tandara A.A., Mustoe T.A. MMP- and TIMP-secretion by human cutaneous keratinocytes and fibroblasts – impact of coculture and hydration. J. Plast. Reconstr. Aesthetic Surg. 2011;64:108–116. doi: 10.1016/j.bjps.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakkol A., Varani J., Elder J.T., Zouboulis C.C. Maintenance of human skin in organ culture: role for insulin-like growth factor-1 receptor and epidermal growth factor receptor. Arch. Dermatol. Res. 1999;291:643–651. doi: 10.1007/s004030050469. [DOI] [PubMed] [Google Scholar]
- Wagenaar-Miller R.A., Engelholm L.H., Gavard J., Yamada S.S., Gutkind J.S., Behrendt N. Complementary roles of intracellular and pericellular collagen degradation pathways in vivo. Mol. Cell. Biol. 2007;27:6309–6322. doi: 10.1128/MCB.00291-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H.G., Jeffrey J.J., Eisen A.Z. The collagen substrate specificity of human skin fibroblast collagenase. J. Biol. Chem. 1981;256:9511–9515. [PubMed] [Google Scholar]
- Wong W.R., Kossodo S., Kochevar I.E. Influence of cytokines on matrix metalloproteinases produced by fibroblasts cultured in monolayer and collagen gels. J. Formos. Med. Assoc. 2001;100:377–382. [PubMed] [Google Scholar]
- Zimmermann M., Koreck A., Meyer N., Basinski T., Meiler F., Simone B. TNF-like weak inducer of apoptosis (TWEAK) and TNF-alpha cooperate in the induction of keratinocyte apoptosis. J. Allergy Clin. Immunol. 2011;127:200–207. doi: 10.1016/j.jaci.2010.11.005. [DOI] [PubMed] [Google Scholar]