Abstract
Background
Overactive bladder (OAB) is highly prevalent and is associated with considerable morbidity and reduced health-related quality of life. β3-adrenergic receptor (β3-AR) stimulation is a novel alternative to antimuscarinic therapy for OAB.
Objective
The objective of this analysis was to assess the cost effectiveness of the β3-AR agonist mirabegron relative to tolterodine extended release (ER) in patients with OAB from a UK National Health Service (NHS) perspective.
Methods
A Markov model was developed to simulate the management, course of disease, and effect of complications in OAB patients over a period of 5 years. Transition probabilities for symptom severity levels and probabilities of adverse events were estimated from the results of the randomised, double-blind SCORPIO trial in 1,987 patients with OAB. Other model inputs were derived from the literature and on assumptions based on clinical experience.
Results
Total 5-year costs per patient were £1,645.62 for mirabegron 50 mg/day and £1,607.75 for tolterodine ER 4 mg/day. Mirabegron was associated with a gain of 0.009 quality-adjusted life-years (QALYs) with an additional cost of £37.88. The resulting incremental cost-effectiveness ratio (ICER) was £4,386/QALY gained. In deterministic sensitivity analyses in the general OAB population and several subgroups, ICERs remained below the generally accepted willingness-to-pay (WTP) threshold of £20,000/QALY gained. The probability of mirabegron 50 mg being cost effective relative to tolterodine ER 4 mg was 89.4 % at the same WTP threshold.
Conclusions
Mirabegron 50 mg/day is likely to be cost effective compared with tolterodine ER 4 mg/day for adult patients with OAB from a UK NHS perspective.
Electronic supplementary material
The online version of this article (doi:10.1007/s40261-014-0240-z) contains supplementary material, which is available to authorized users.
Key Points
| Overactive bladder (OAB) is a common condition that increases in prevalence with age and has a significant negative impact on quality of life. |
| For the past 40 years, antimuscarinic agents have been the mainstay of therapy for OAB; however, unmet efficacy expectations and side effects, particularly dry mouth, limit persistence with antimuscarinic therapy. |
| Recently, an alternative to antimuscarinic agents has received marketing authorisation; the β3-adrenergic receptor agonist mirabegron is associated with at least similar efficacy to most antimuscarinic therapies and also has a lower incidence of dry mouth. |
| No economic analyses of mirabegron compared to antimuscarinic agents in the treatment of OAB have been performed; therefore, we developed a model to analyse the cost effectiveness of mirabegron 50 mg/day relative to currently available antimuscarinic agents for the treatment of OAB from a UK National Health Service perspective. |
| The model captured the effects of variations in symptom severity over time on quality of life and the influence of treatment discontinuation and switch on costs and health outcomes, as well as direct treatment costs. |
| Compared to tolterodine extended release 4 mg, mirabegron 50 mg was cost effective, with an approximately 90 % probability of cost effectiveness at a willingness-to-pay threshold of £20,000. |
Introduction
Overactive bladder (OAB) is a symptom-defined condition characterised by urinary urgency, usually with urinary frequency and nocturia, and sometimes with urgency incontinence [1]. Urinary urgency is particularly bothersome for persons with OAB [2]. OAB is highly prevalent, with a frequency that increases with age. An estimation model based on sex- and age-stratified prevalence data and International Continence Society definitions [3] suggested that the overall prevalence of OAB was 10.7 % in 2008 and predicted an increase of 20.1 %, from 455 to 546 million, by the year 2018 [4]. This followed earlier data from a population-based survey based on 16,776 interviews in six European countries that showed an overall prevalence of OAB symptoms of 16.6 % in persons aged ≥40 years [5].
Patients with OAB have decreased health-related quality of life (HRQoL), including health-related work impairment, depression and sexual/relationship difficulties [2, 6, 7]. Anxiety and depression are strongly associated with OAB [7], and patients report significant social, psychological, occupational, domestic and physical stigma [8]. The prevalence of OAB and its effects on HRQoL means that effective therapy is needed.
Treatment options for OAB include conservative management (lifestyle advice, bladder training, etc.), followed by drug therapy and surgery where required; the primary pharmacological option has traditionally been muscarinic receptor antagonist therapy (antimuscarinic drugs; e.g. oxybutynin, tolterodine and solifenacin) [9–11]. These inhibit abnormal detrusor contractions in the bladder, but also inhibit muscarinic receptors elsewhere in the body, such as the salivary glands, tear ducts, brain and heart, to varying degrees [12]. This non-specificity results in adverse events, the most common and troublesome of which are dry mouth, blurred vision and constipation [12, 13]. Adverse events, together with unmet efficacy expectations, are the primary reasons for discontinuation of antimuscarinic drugs [13]. Therefore, alternative approaches to OAB therapy have been developed and approved, the most important of which is β3-adrenergic receptor (β3-AR) stimulation to promote storage of urine in the bladder via detrusor relaxation [14].
The β3-AR agonist mirabegron (Betmiga™; Astellas) has been shown in phase III trials to be effective and well tolerated in the management of OAB [15–18]. These trials showed that mirabegron therapy significantly improves outcomes such as the number of incontinence episodes and micturitions per 24 h compared to placebo at 12 weeks [15–17], and is well tolerated with sustained efficacy for up to 12 months [18]. In trials in which patients were randomised to mirabegron, tolterodine or placebo, the incidence of dry mouth with tolterodine was at least threefold that with mirabegron [15, 18]. In these trials, tolterodine produced mean decreases in the number of incontinence episodes and micturitions per 24 h that were not significantly greater than those with placebo at 12 weeks, but were similar to those with mirabegron at 12 months [15, 18].
Economic analyses comparing different treatments are now an important factor in making decisions regarding purchasing, pricing, reimbursement and formulary acceptance. To date, however, no economic analyses of mirabegron in the treatment of OAB have been published. Therefore, a Markov model was developed to analyse the cost effectiveness of mirabegron 50 mg/day relative to currently available antimuscarinic agents for the treatment of OAB. We describe the model in detail and report an analysis of the cost effectiveness of mirabegron 50 mg compared with tolterodine extended release (ER) 4 mg in adult patients with OAB from a UK National Health Service (NHS) payer perspective.
Methods
Model Overview
A Markov model was developed to simulate the therapeutic management, course of disease, and effect of complications in hypothetical cohorts of OAB patients over a 5-year period (Supplementary Figure 1). Several existing models based on a structure developed by Kobelt et al. [19] were already available, but were limited to a time horizon of 1 year and did not model treatment pathways after drop-out. Thus, differences in costs and outcomes related to treatments used after withdrawal could not be accurately estimated. We captured the influence of treatment discontinuation and switch on costs and OAB symptoms. This is particularly important because persistence with antimuscarinic agents affects HRQoL, health status and healthcare resource utilisation [20]. HRQoL was assumed to be dependent on both variations in symptoms over time and the side effects of treatment.
The SCORPIO Trial
SCORPIO was a randomised, double-blind, parallel group, placebo- and active-controlled phase III trial carried out in 27 countries in Europe and Australasia to compare the efficacy and safety of mirabegron 50 and 100 mg daily with those of placebo in patients with OAB treated over 12 weeks [15]. The co-primary endpoints were the change from baseline to final visit in the mean number of incontinence episodes and micturitions per 24 h. Assessment of safety and tolerability was a secondary endpoint. A secondary comparison of the efficacy and safety of treatment with tolterodine ER 4 mg daily and placebo was also performed.
In total, 1,987 patients were randomised. Mirabegron produced statistically significant (p < 0.05) improvements (adjusted mean change from baseline [95 % confidence intervals]) compared with placebo in terms of number of incontinence episodes per 24 h (50 mg: −1.57 [−1.79 to −1.35]; placebo –1.17 [−1.39 to –0.95]) and number of micturitions per 24 h (50 mg: −1.93 [−2.15 to −1.72]; placebo −1.34 [−1.55 to −1.12]). Statistically significant improvements were also noted for other key efficacy endpoints and HRQoL outcomes. Incidences of treatment-emergent adverse events were similar across treatment groups, but the incidence of dry mouth with mirabegron 50 mg was similar to placebo (2.8 and 2.6 %, respectively), whereas it was more than threefold higher in patients receiving tolterodine ER 4 mg (10.1 %) [15].
Model Description
The model was programmed to run in Microsoft Excel 2007 (Microsoft Corp., Redmond, WA, USA). The treatment pathway, based on the model described above, for the analysis is shown in Fig. 1. Transitions are shown in more detail in Supplementary Figure 1. The model simulated changes in symptoms (frequency of micturitions and incontinence episodes, for which the model was run in parallel) at monthly intervals (i.e. 60 cycles over 5 years). Every month, patients could remain on treatment (mirabegron or tolterodine), switch to a treatment with efficacy and price similar to solifenacin or discontinue (i.e. go to ‘no treatment’). A small proportion received botulinum toxin (BTX) after this next line of therapy (this transition was not allowed directly after mirabegron or tolterodine). The probabilities of switch and discontinuation were dependent on adverse events. Patients with adverse events could stay on treatment, but incurred a disutility. Patients who discontinued treatment could naturally improve and thereby transition to a lower severity category after 1 month, or could worsen or stay the same. These patients could also restart their previous treatment, could move to a new treatment, or remain off treatment. In the event of success on BTX, patients were assumed to move to the lowest level of severity and remain there until the end of the simulation.
Fig. 1.
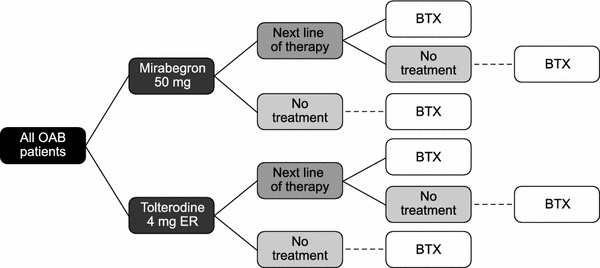
Markov treatment pathway. BTX botulinum toxin, ER extended release, OAB overactive bladder syndrome
The model accounted for the differences in probability of improving or worsening between the short- and long-term. Thus, the probability of improvement was greatest in the first month after treatment initiation, after which it decreased progressively, and was then assumed constant after 3 months.
Several populations were considered. Any patient presenting with OAB formed the base case population. Other populations considered were patients dissatisfied with previous treatment, patients dissatisfied with previous treatment due to lack of efficacy, patients dissatisfied with previous treatment due to intolerance, treatment-naïve patients, elderly patients, female patients, male patients and incontinent patients.
Model Input Parameters
All model input parameters are presented in detail in Supplementary Table 1.
Symptom Severity
Symptoms (micturition frequency and incontinence) had five levels of severity (Table 1). Initial proportions of patients with symptoms of each severity were derived from SCORPIO [15]. Transition probabilities between symptom levels for mirabegron 50 mg and tolterodine ER 4 mg were estimated by applying multinomial logistic regression models to the SCORPIO trial data (see Electronic Supplementary Material and Supplementary Tables 2 and 3).
Table 1.
Symptom severity levels. Definitions and distribution of patients at baseline
| Symptom severity level | Mean episodes/day (n) | Proportion of patients (%) |
|---|---|---|
| Micturition | ||
| 1 | ≤8 | 6.30 |
| 2 | >8–10 | 30.69 |
| 3 | >10–12 | 27.18 |
| 4 | >12–14 | 19.46 |
| 5 | >14 | 16.37 |
| Incontinence | ||
| 1 | 0 | 38.87 |
| 2 | >0–1 | 18.84 |
| 3 | >1–2 | 14.64 |
| 4 | >2–3 | 9.18 |
| 5 | >3 | 18.47 |
Treatment Persistence, Switching and Restarting
Other model inputs are summarised in Table 2. Persistence on mirabegron was extrapolated from the study of Wagg et al. [21], in which data were extracted via medical practice software and anonymised from the records of >1,200,000 registered patients, of whom 4,833 had documented OAB. An additional 12-week observational study of OAB patients in Spain showed that 24 % of patients who changed treatment did so because of adverse events [22]; these results were consistent with those of a large US survey of patients with OAB [13]. As no real-life data are available for persistence with mirabegron, the model assumed that discontinuation rates for patients without adverse events were similar for mirabegron and its comparator. The probability of switching to next-line therapy was obtained from an analysis based on the UK general practice research database [23]. The base-case frequency (Table 2) was derived from 5,424 patients who received first-line tolterodine, of whom 68.92 % discontinued and 26.06 % switched to another medication (most frequently oxybutynin).
Table 2.
Model inputs for the base-case scenario. Also shown are ranges used for the probabilistic sensitivity analysis
| Parameter | Base case value | Sensitivity analysis range | References |
|---|---|---|---|
| Discontinuation and switch | |||
| Treatment discontinuation | |||
| Without AEs | 6.4 % per month | 3.0–14.5 % | Base case and upper limit: Wagg et al. [21]; Sánchez-Ballester et al. [22]; lower limit: assumption |
| With AEs | 90 % per month | 50–100 % | Expert opinion |
| Treatment switcha | 26.1 % | 15.3–50.0 % | Base case: Odeyemi et al. [23]; sensitivity analysis: D’Souza et al. [38]/assumption |
| Treatment restartb | 5.6 % per month | 0–20.0 % | Expert opinion |
| BTX injection | 0.01 % per month | 0–0.05 % | Expert opinion |
| Success with BTX | 79 % | 50–100 % | Wu et al. [24] |
| Adverse events | |||
| Dry mouth | |||
| Mirabegron 50 mg | 2.8 % | 2.1–3.5 % | SCORPIO [15] |
| Tolterodine ER 4 mg | 10.1 % | 8.7–11.5 % | SCORPIO [15] |
| Constipation | |||
| Mirabegron 50 mg | 1.6 % | SCORPIO [15] | |
| Tolterodine ER 4 mg | 2.0 % | SCORPIO [15] | |
AE adverse event, BTX botulinum toxin, ER extended release, OAB overactive bladder syndrome
a Among patients discontinuing OAB treatment
b Split between different medications was assumed to be one-third each for initial, second- and third-line treatments
No data are available in the literature regarding the probability of restarting treatment after a period of no treatment. We assumed an annual probability of 50 % (monthly probability of 5.6 %). The 79 % probability of improvement of symptoms after BTX injection was based on a published cost-effectiveness analysis comparing BTX to antimuscarinic therapy [24].
Adverse Events
Monthly probabilities of adverse events were derived from SCORPIO [15]. Patients were assumed to experience dry mouth or constipation (Table 2). Other adverse events were excluded based on the results of a European cross-sectional survey that showed that these two events are the main drivers of adverse event-related treatment discontinuation with antimuscarinic therapy [25] and the finding that the events reported most frequently with mirabegron occur at a similar incidence with placebo [15].
Utilities
Utility values according to symptom severity and adverse events were derived from EuroQol 5-Dimension (EQ-5D) index scores, based on the UK time trade-off tariff [26], collected in SCORPIO [15]. A linear regression model of EQ-5D utility as a function of symptom severity was developed as described in detail in the Electronic Supplementary Material and Supplementary Table 4. The utility decrement for adverse events was an average, weighted according to the treatment-specific probabilities for the different adverse events. Utilities derived from the overactive bladder questionnaire (OAB-q), using the algorithm developed by Yang et al. [27], were also used.
Resource Utilisation and Costs
Resources and associated costs included in the model are from a UK NHS payer perspective (Table 3). Drug therapy, primary care visits, specialist (urologist) visits, BTX injections and incontinence pads were accounted for. Patients were assumed to use one tablet of mirabegron per day; wastage or partial compliance was not accounted for. Pad utilisation was tiered according to incontinence severity.
Table 4.
Predicted costs (2012) and cost-effectiveness outcomes for the base-case analysis (general OAB population)
| Parameter | Mirabegron strategy | Tolterodine strategy | Cost difference/incremental change |
|---|---|---|---|
| Predicted costs (£) | |||
| Drug acquisition | 451.43 | 343.70 | 107.72 |
| Other OAB treatment | 364.92 | 393.42 | −28.50 |
| GP visit | 101.38 | 105.83 | −4.45 |
| Specialist visit (initial + follow-up) | 405.83 | 423.31 | −17.78 |
| BTX (initial + repeat injections) | 93.66 | 102.78 | −9.11 |
| Incontinence pads | 228.70 | 238.71 | −10.00 |
| Total | 1,645.62 | 1,607.75 | 37.88 |
| Cost effectiveness | |||
| Total costs (£) | 1,645.62 | 1,607.75 | 37.88 |
| QALYs | 3.764 | 3.755 | 0.009 |
| ICER (£/QALY gained per patient) | 4,386 | ||
BTX botulinum toxin, GP general practitioner, ICER incremental cost-effectiveness ratio, OAB overactive bladder syndrome, QALY quality-adjusted life-year
Table 3.
Modelled resource use and costs for the base-case scenario. Also shown are ranges used for the probabilistic sensitivity analysis
| Parameter | Base case value | Sensitivity analysis range | References |
|---|---|---|---|
| Resource use | |||
| Pad utilisation | |||
| Incontinence level 1 | 0.17 | 0.150–0.198 | SCORPIO [15] |
| Incontinence level 2 | 0.75 | 0.687–0.817 | |
| Incontinence level 3 | 1.38 | 1.282–1.486 | |
| Incontinence level 4 | 1.89 | 1.745–2.039 | |
| Incontinence level 5 | 3.34 | 3.167–3.511 | |
| GP consultations | 1 visit at start and at every switch | 0–2 | Expert opinion |
| Specialist consultations | 1.5 visits at start and at every switch | 1–3 | Cardozo et al. [31]/assumption |
| BTX injectionsa | 0.17 per month | 0–0.34 | Expert opinion |
| Costs | |||
| Monthly acquisition cost | |||
| Mirabegron 50 mg | £29.40 | BNF 2011/12 [39] | |
| Tolterodine ER 4 mg | £28.01 | BNF 2011/12 [39] | |
| Solifenacin 5 mg | £28.00 | BNF 2011/12 [39] | |
| GP consultation | £36.00 | PSSRU 2010 [40] | |
| Specialist visit | £96.00 | NHS payment 2010–2011 | |
| BTX injection/reinjection | £1,158/£964 | Nottingham Urology Group [41] | |
| Incontinence pad | £0.16 | AgeUK [42] | |
BNF British National Formulary, BTX botulinum toxin, GP general practitioner, PSSRU Personal Social Services Research Unit
aFollowing successful first injection
Costs are presented in 2012 British pounds (£) and are summarised together with sources in Table 3. An annual discount rate of 3.5 % was applied to costs and health benefits.
Model Outputs
The model was designed to output (i) average annual cumulative costs by treatment strategy; (ii) quality-adjusted life-years (QALYs) gained by using a particular treatment strategy; (iii) and incremental cost-effective ratios (ICERs) expressed as incremental cost per QALY gained.
Sensitivity and Subgroup Analyses
Sensitivity analyses evaluated the impact of assumptions used in the model and variability surrounding model inputs. For deterministic sensitivity analyses, one variable or assumption was changed at a time. One-way sensitivity analyses were conducted on all model parameters associated with uncertainty: proportions of patients by severity level at baseline; transition probabilities between symptom levels; utilities by symptom level; probabilities of treatment-related events; probabilities related to BTX injections; and probabilities associated with adverse events and resource use. Outcomes were computed using confidence limits around each parameter or other fixed values.
A probabilistic sensitivity analysis was also performed. Appropriate statistical distributions were assigned to input parameters (Table 2). Values were drawn at random from statistical distributions for these variables and the process was iterated 5,000 times to provide distributions for ICERs. Cost-effectiveness acceptability curves (CEACs) were generated at various willingness-to-pay (WTP) thresholds. The probabilistic analysis was programmed in visual basic applications for Excel.
Finally, because indirect costs were not included in the model, a sensitivity analysis including indirect costs valued using the human capital approach to determine the effect on the ICER estimate was also performed. Absenteeism was derived from the percentage work time missed, assessed for each patient in the SCORPIO trial using the work productivity and activity impairment (WPAI) questionnaire [28].
Subgroup analyses were also performed.
Results
Base-Case Scenario
On average, patients treated with tolterodine discontinued treatment earlier than those treated with mirabegron (329.79 vs. 391.79 days, respectively). Total costs per patient over 5 years were £1,645.62 for the mirabegron strategy and £1,607.75 for tolterodine from the UK NHS perspective. Medication costs were higher with mirabegron than with tolterodine (£451.43 vs. £343.70), mainly due to the differences in time to discontinuation (Table 4). This was partly offset by the reduced costs of specialist visits and incontinence pad utilisation under the mirabegron strategy.
Based on EQ-5D utilities, the mirabegron strategy was associated with a gain of 0.009 QALYs, and an additional cost of £37.88 over the modelled 5-year period. The resulting ICER was below the WTP threshold of £20,000/QALY gained, at £4,386/QALY gained (Table 4). Based on OAB-q utilities, the number of QALYs gained was 0.01259, leading to an ICER of £3,008/QALY gained.
Subgroup Analyses
Results of cost-effectiveness analyses for different subgroups are summarised in Table 5. Mirabegron was found to be cost effective compared to tolterodine ER 4 mg in all subgroups except the subgroup comprising men (ICER £38,708). The benefit of mirabegron 50 mg was greatest for patients dissatisfied because of adverse events, with an estimated gain of 0.0186 QALYs (EQ-5D) at a cost of £28.37 over 5 years, yielding an ICER of £1,528/QALY gained. ICERs across the other subgroups ranged from £3,091/QALY gained (women only) to £5,736/QALY gained (elderly).
Table 5.
Cost-effectiveness results by subgroup (UK NHS perspective)
| Subgroup | Incremental costs (£) | Incremental QALYs | ICER (£/QALY gained) |
|---|---|---|---|
| General population | 37.88 | 0.0086 | 4,386 |
| Previously treated | 38.07 | 0.0099 | 3,836 |
| Dissatisfied because of lack of efficacy | 40.27 | 0.0091 | 4,446 |
| Dissatisfied because of adverse events | 28.37 | 0.0186 | 1,528 |
| Incontinent | 32.36 | 0.0124 | 2,620 |
| Elderly | 35.19 | 0.0061 | 5,736 |
| Treatment naïve | 40.27 | 0.0076 | 5,315 |
| Women | 37.73 | 0.0122 | 3,091 |
| Men | 43.96 | 0.0011 | 38,708 |
ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-year
Deterministic Sensitivity Analysis
For the sensitivity analyses, health state utilities based on EQ-5D were used. Detailed results are shown in the tornado chart in Fig. 2. The model was most sensitive to the transition probabilities between symptom levels of incontinence and micturition for mirabegron and tolterodine, the distribution of patients by micturition and incontinence severity level at baseline, and the monthly probability of restarting treatment. However, mirabegron was cost effective or dominant compared with tolterodine in all scenarios. The ICER was also found to vary little (from £4,092 to 4,698/QALY gained) when the discount rate was varied from 0 to 6.0 % for costs and outcomes.
Fig. 2.
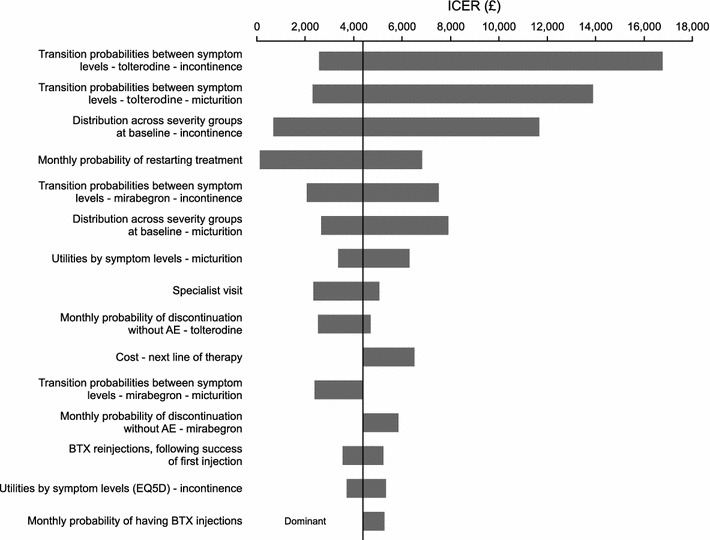
Deterministic sensitivity analysis. AE adverse event, BTX botulinum toxin, EQ5D European Quality of Life questionnaire in five dimensions, ICER incremental cost-effectiveness ratio
Probabilistic Sensitivity Analysis
The probabilistic sensitivity analysis results are represented in the CEAC shown in Fig. 3. At the WTP threshold of £20,000/QALY gained, the probability of mirabegron 50 mg being cost effective relative to tolterodine ER 4 mg was 89.4 %.
Fig. 3.
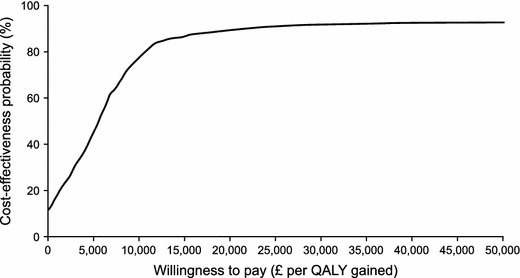
Cost-effectiveness acceptability curve for mirabegron 50 mg vs tolterodine extended release 4 mg; general OAB population. OAB overactive bladder syndrome, QALY quality-adjusted life year
Sensitivity Analysis for Indirect Costs
The inclusion of indirect costs resulted in lower ICERs in all comparisons, with mirabegron being dominant in all populations except the previously treated and men subgroups, in which the ICERS were below the WTP threshold of £20,000/QALY gained (data not shown).
Discussion
The base-case analysis in the present model showed that mirabegron 50 mg/day is cost effective at the WTP thresholds of £20,000/QALY and £30,000/QALY generally applied by the UK National Institute for Health and Clinical Excellence [29]. The one-way deterministic sensitivity analysis demonstrated that the ICER remained below the WTP threshold of £20,000 whenever a parameter was modified. The probabilistic sensitivity analysis, which tested uncertainty around the model parameters through Monte Carlo simulations using appropriate distributions, demonstrated a high probability (89.4 %) that mirabegron 50 mg/day would be cost effective compared with tolterodine ER 4 mg/day at the generally accepted cost-effectiveness threshold of £20,000/QALY gained. Overall, the subgroup analyses also supported the primary result.
To the best of our knowledge, no other economic analyses of mirabegron in the treatment of OAB with which to compare our findings are available in the literature. However, previously published cost-effectiveness models comparing tolterodine ER 4 mg with other antimuscarinic agents suggest that our results are plausible. Speakman et al. [30] developed a 1-year Markov model to evaluate the cost effectiveness of solifenacin 5 mg and 10 mg compared with tolterodine (immediate release 2 mg twice daily or ER 4 mg daily) in OAB from the UK NHS perspective, while a 1-year model developed by Cardozo et al. [31] assessed the cost effectiveness of solifenacin against other antimuscarinic agents commonly used in UK clinical practice, including tolterodine. The predicted total annual costs per patient for tolterodine were £526 and £480, in the Speakman and Cardoza studies respectively [30, 31], compared with an annual cost of £620 in our model when run over a 1-year time horizon. The difference in costs was mainly due to our assumption that all patients initiating a new treatment would consult a specialist. The respective numbers of QALYs for 1 year with tolterodine were 0.705 and 0.710 [30, 31], compared with 0.814 in our model, with the difference being due to our use of a new set of utilities based on EQ-5D data collected in clinical trials of mirabegron. However, the difference between the maximum and minimum utilities in this new model is similar to the range in the earlier model of Speakman et al. [30] (0.145 vs. 0.144). Furthermore, the QALY gain based on our model (0.009) is consistent with these and other previous studies that used EQ-5D (0.004 [30], 0.014 [32] and 0.004 [33]). This suggests that the magnitude of the QALY gain is meaningful in OAB, although it should be noted that as a generic tool EQ-5D is relatively insensitive to changes in OAB symptoms. An analysis mapping EQ-5D scores to those obtained with the OAB-specific OAB-5D instrument has shown that scores are higher with OAB-5D, suggesting that the magnitude of any differences would also be higher [34].
In other analyses carried out from a UK health system perspective, Hakkaart et al. [32] found incremental costs per QALY gained over placebo to be £17,602 and £24,464 with solifenacin 5 mg and 10 mg daily, respectively, in a Markov model run over a 12-month time horizon in patients with OAB, and Getsios et al. [33] showed that a sustained-release formulation of oxybutynin 10 mg dominated immediate-release tolterodine 4 mg. Another analysis has suggested that immediate- and sustained-release oxybutynin and tolterodine ER are all cost-effective options for managing urge incontinence from a UK NHS perspective [35]. These findings suggest strongly that economic comparisons of mirabegron with these and other antimuscarinic agents will be of considerable interest to clinicians and healthcare policy makers. In addition to the analysis reported here, a model based on a mixed treatment comparison (MTC) of mirabegron and antimuscarinic therapy for OAB has also been reported [36, 37]. This demonstrated that the efficacy of mirabegron 50 mg in OAB patients is similar to that of most approved antimuscarinic drugs in the general OAB population and that it has the most favourable tolerability profile, with a significantly lower incidence of dry mouth than any antimuscarinic agent and the same incidence of constipation as placebo [36].
A key strength of our model is that it was designed to capture the effects on HRQoL of variations in symptom severity over time, as well as the impact of treatment discontinuation and switching on costs and health outcomes. In contrast, previous Markov models for this type of analysis have been based on the extensively applied model developed by Kobelt et al. [19], which consists of five health states representing different levels of disease severity with a single absorbing state for drop-outs, who were considered to remain off treatment. We found that the main symptoms of OAB (micturitions and incontinence) had a significant influence on utility independently of each other, with moderate correlations between changes in these symptoms. The progression of these symptoms over time was therefore modelled separately. In addition, we attached increased importance to the modelling of patient pathways after treatment discontinuation, because of the high rates of discontinuation and treatment switching observed in patients with OAB. Finally, the use of a 5-year time horizon, at which point <5 % of patients remained on treatment in the model, and performing sensitivity analyses based on other time-points, at which the conclusions regarding the cost-effectiveness of mirabegron held, ensured that the model reflected the costs and outcomes of managing a chronic condition. Therefore, we believe that our model more accurately represents the clinical management of OAB.
Another strength is that we have analysed the cost effectiveness of therapy in various relevant patient subgroups. These analyses showed that mirabegron was consistently cost effective in most of these subgroups.
In contrast, possible limitations include the assumption that discontinuation rates due to reasons other than adverse events were similar for mirabegron and tolterodine, because no real-life data on persistence with mirabegron are available. We believe that this is a conservative assumption because the MTC demonstrated that the probability of mirabegron being more effective than tolterodine in terms of micturition and incontinence is 87.4 and 88.3 %, respectively [36]. Moreover, no data on the probability of BTX being used to treat OAB following drug therapy and no data on the numbers of visits to specialists were available. Again, we took a conservative approach and assumed that the proportions of patients receiving BTX were small; sensitivity analysis showed that the ICER would increase modestly if no patients received BTX and that mirabegron remained cost effective. The impact of varying the numbers of visits to specialists was also assessed in a sensitivity analysis, which showed that this affected the absolute results, but not sufficiently to change the conclusion that mirabegron is cost effective. Furthermore, number of specialist visits was not one of the main parameters to which the model was sensitive.
The model used in our analysis included direct healthcare costs only, in line with the requirements of UK guidelines for pharmacoeconomic assessments. The failure to include indirect costs in the model represents another potential limitation, but a sensitivity analysis including indirect costs showed that ICERs were lower in all comparisons, with mirabegron being dominant in all populations except the previously treated and men subgroups.
Conclusion
In conclusion, from a UK NHS perspective, mirabegron 50 mg/day appears to be a cost-effective treatment strategy compared with tolterodine ER 4 mg/day for the general population of adults with OAB and for subgroups including those previously treated and those who are treatment naive, patients dissatisfied due to lack of efficacy or adverse events, patients who are incontinent, the elderly, and women.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was sponsored by Astellas Pharma Global Development. Medical writing was provided by Andy Noble of Bioscript Medical, and was funded by Astellas Pharma Global Development. S. Aballéa, K. Desroziers and M. Toumi are employees of Creativ-Ceutical SARL, which was contracted by Astellas Pharma to perform this research. K. Maman, J. Nazir, I. A. O. Odeyemi, A. Garnham and Z. Hakimi are employees of Astellas Pharma.
References
- 1.Abrams P, Artibani W, Cardozo L, Dmochowski R, van Kerrebroeck P, Sand P, et al. Reviewing the ICS 2002 terminology report: the ongoing debate. Neurourol Urodyn. 2009;28:287. doi: 10.1002/nau.20737. [DOI] [PubMed] [Google Scholar]
- 2.Coyne KS, Payne C, Bhattacharyya SK, Revicki DA, Thompson C, Corey R, et al. The impact of urinary urgency and frequency on health-related quality of life in overactive bladder: results from a national community survey. Value Health. 2004;7:455–463. doi: 10.1111/j.1524-4733.2004.74008.x. [DOI] [PubMed] [Google Scholar]
- 3.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 4.Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108:1132–1138. doi: 10.1111/j.1464-410X.2010.09993.x. [DOI] [PubMed] [Google Scholar]
- 5.Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760–766. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- 6.Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int. 2008;101:1388–1395. doi: 10.1111/j.1464-410X.2008.07601.x. [DOI] [PubMed] [Google Scholar]
- 7.Coyne KS, Sexton CC, Kopp ZS, Ebel-Bitoun C, Milsom I, Chapple C. The impact of overactive bladder on mental health, work productivity and health-related quality of life in the UK and Sweden: results from EpiLUTS. BJU Int. 2011;108:1459–1471. doi: 10.1111/j.1464-410X.2010.10013.x. [DOI] [PubMed] [Google Scholar]
- 8.Nicolson P, Kopp Z, Chapple CR, Kelleher C. It’s just the worry about not being able to control it! A qualitative study of living with overactive bladder. Br J Health Psychol. 2008;13:343–359. doi: 10.1348/135910707X187786. [DOI] [PubMed] [Google Scholar]
- 9.Gormley EA, Lightner DJ, Burgio KL, Chai TC, Clemens JQ, Culkin DJ, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188(6 Suppl):2455–2463. doi: 10.1016/j.juro.2012.09.079. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence. Lower urinary tract symptoms. The management of lower urinary tract symptoms in men. London: National Institute for Health and Care Excellence; 2010.
- 11.National Institute for Health and Care Excellence. Urinary incontinence. The management of urinary incontinence in women. London: National Institute for Health and Care Excellence; 2013.
- 12.Madhuvrata P, Cody JD, Ellis G, Herbison GP, Hay-Smith EJ. Which anticholinergic drug for overactive bladder symptoms in adults. Cochrane Database Syst Rev. 2012;1:CD005429. [DOI] [PubMed]
- 13.Benner JS, Nichol MB, Rovner ES, Jumadilova Z, Alvir J, Hussein M, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int. 2010;105:1276–1282. doi: 10.1111/j.1464-410X.2009.09036.x. [DOI] [PubMed] [Google Scholar]
- 14.Kumar V, Templeman L, Chapple CR, Chess-Williams R. Recent developments in the management of detrusor overactivity. Current Opin Urol. 2003;13:285–291. doi: 10.1097/00042307-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Khullar V, Amarenco G, Angulo JC, Cambronero J, Høye K, Milsom I, et al. Efficacy and tolerability of mirabegron, a β(3)-adrenoceptor agonist, in patients with overactive bladder: results from a randomised European–Australian phase 3 trial. Eur Urol. 2013;63:283–295. doi: 10.1016/j.eururo.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Herschorn S, Barkin J, Castro-Diaz D, Frankel JM, Espuna-Pons M, Gousse AE, et al. A phase III, randomized, double-blind, parallel-group, placebo-controlled, multicentre study to assess the efficacy and safety of the β3 adrenoceptor agonist, mirabegron, in patients with symptoms of overactive bladder. Urology. 2013;82:313–320. doi: 10.1016/j.urology.2013.02.077. [DOI] [PubMed] [Google Scholar]
- 17.Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol. 2013;189:1388–1395. doi: 10.1016/j.juro.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Chapple CR, Kaplan SA, Mitcheson D, Klecka J, Cummings J, Drogendijk T, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63:296–305. doi: 10.1016/j.eururo.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 19.Kobelt G, Jönsson L, Mattiasson A. Cost-effectiveness of new treatments for overactive bladder: the example of tolterodine, a new muscarinic agent: a Markov model. Neurourol Urodyn. 1998;17:599–611. doi: 10.1002/(SICI)1520-6777(1998)17:6<599::AID-NAU4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 20.Balkrishnan R, Bhosle MJ, Camacho FT, Anderson RT. Predictors of medication adherence and associated health care costs in an older population with overactive bladder syndrome: a longitudinal cohort study. J Urology. 2006;175:1067–1071. doi: 10.1016/S0022-5347(05)00352-6. [DOI] [PubMed] [Google Scholar]
- 21.Wagg A, Compion G, Fahey A, Siddiqui E. Persistence with prescribed antimuscarinic therapy for overactive bladder: a UK experience. BJU Int. 2012;110:1767–1774. doi: 10.1111/j.1464-410X.2012.11023.x. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-Ballester F, Miranda P, Lizarraga I, Rejas J, Arumi D. Therapeutic benefit in patients switching tolterodine to other novel antimuscarinic agents. Actas Urol Esp. 2014;38:156–163. doi: 10.1016/j.acuro.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Odeyemi IA, Dakin HA, O’Donnell RA, Warner J, Jacobs A, Dasgupta P. Epidemiology, prescribing patterns and resource use associated with overactive bladder in UK primary care. Int J Clin Pract. 2006;60:949–958. doi: 10.1111/j.1742-1241.2006.01057.x. [DOI] [PubMed] [Google Scholar]
- 24.Wu JM, Siddiqui NY, Amundsen CL, Myers ER, Havrilesky LJ, Visco AG. Cost-effectiveness of botulinum toxin a versus anticholinergic medications for idiopathic urge incontinence. J Urol. 2009;181:2181–2186. doi: 10.1016/j.juro.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 25.Compion G, Jackson J, Janes J. Reasons for switching antimuscarinic therapy: results from a European cross-sectional survey of physicians, and patients with OAB. In: 27th Annual Congress of the European Association of Urology: Paris; 2012.
- 26.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Brazier J, Tsuchiya A, Coyne K. Estimating a preference-based single index from the Overactive Bladder Questionnaire. Value Health. 2009;12:159–166. doi: 10.1111/j.1524-4733.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- 28.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 29.Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ. 2004;13:437–452. doi: 10.1002/hec.864. [DOI] [PubMed] [Google Scholar]
- 30.Speakman M, Khullar V, Mundy A, Odeyemi I, Bolodeoku J. A cost-utility analysis of once daily solifenacin compared to tolterodine in the treatment of overactive bladder syndrome. Curr Med Res Opin. 2008;24:2173–2179. doi: 10.1185/03007990802234829. [DOI] [PubMed] [Google Scholar]
- 31.Cardozo L, Thorpe A, Warner J, Sidhu M. The cost-effectiveness of solifenacin vs fesoterodine, oxybutynin immediate-release, propiverine, tolterodine extended-release and tolterodine immediate-release in the treatment of patients with overactive bladder in the UK National Health Service. BJU Int. 2010;106:506–514. doi: 10.1111/j.1464-410X.2009.09160.x. [DOI] [PubMed] [Google Scholar]
- 32.Hakkaart L, Verboom P, Phillips R, Al MJ. The cost utility of solifenacin in the treatment of overactive bladder. Int Urol Nephrol. 2009;41:293–298. doi: 10.1007/s11255-008-9448-2. [DOI] [PubMed] [Google Scholar]
- 33.Getsios D, Caro JJ, Ishak KJ, El-Hadi W, Payne K, O’connel M, et al. Oxybutynin extended release and tolterodine immediate release : a health economic comparison. Clin Drug Investig. 2004;24:81–88. doi: 10.2165/00044011-200424020-00003. [DOI] [PubMed] [Google Scholar]
- 34.Desroziers K, Aballea S, Maman K, Nazir J, Odeyemi I, Hakimi Z. Estimating EQ-5D and OAB-5D health state utilities for patients with overactive bladder. Health Qual Life Outcomes. 2013;11:200. doi: 10.1186/1477-7525-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes DA, Dubois D. Cost-effectiveness analysis of extended-release formulations of oxybutynin and tolterodine for the management of urge incontinence. Pharmacoeconomics. 2004;22:1047–1059. doi: 10.2165/00019053-200422160-00002. [DOI] [PubMed] [Google Scholar]
- 36.Maman K, Aballea S, Nazir J, Desroziers K, Neine ME, Siddiqui E, et al. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol. 2013;65:755–765. doi: 10.1016/j.eururo.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Maman K, Neine M, Briquet B, Nazir J, Odeyemi IAO, Hakimi Z, et al. Cost-effectiveness of mirabegron compared with antimuscarinics for the treatment of patients with overactive bladder in the UK. In: ISPOR 16th Annual European Congress. Dublin; 2013: PUK22.
- 38.D’Souza AO, Smith MJ, Miller LA, Doyle J, Ariely R. Persistence, adherence, and switch rates among extended-release and immediate-release overactive bladder medications in a regional managed care plan. J Manag Care Pharm. 2008;14:291–301. doi: 10.18553/jmcp.2008.14.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.BNF.org. British National Formulary. http://www.bnf.org/bnf/index.htm. Accessed 17 Dec 2013.
- 40.Personal Social Services Research Unit. Unit Costs of Health and Social Care 2010. http://www.pssru.ac.uk/pdf/uc/uc2010/uc2010_s10.pdf. Accessed 27 June 2014.
- 41.Nottingham Urology Group. Bladder BOTOX Injections. http://www.nottinghamurologygroup.co.uk/treatments/bladder-botox-injections. Accessed 17 Dec 2013.
- 42.Age UK. The latest incontinence products & incontinence advice. http://www.ageukincontinence.co.uk/. Accessed 17 Dec 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


