Abstract
Hepatocellular carcinoma (HCC) frequently develops in a pro-inflammatory and pro-fibrogenic environment with hepatic stellate cells (HSCs) remodeling the extracellular matrix composition. Molecules secreted by liver tumors contributing to HSC activation and peritumoral stromal transformation remain to be fully identified. Here we show that conditioned medium from HCC cell lines, Hep3B and HepG2, induced primary mouse HSCs transdifferentiation, characterized by profibrotic properties and collagen modification, with similar results seen in the human HSC cell line LX2. Moreover, tumor growth was enhanced by coinjection of HepG2/LX2 cells in a xenograft murine model, supporting a HCC-HSC crosstalk in liver tumor progression. Protein microarray secretome analyses revealed angiogenin as the most robust and selective protein released by HCC compared to LX2 secreted molecules. In fact, recombinant angiogenin induced in vitro HSC activation requiring its nuclear translocation and rRNA transcriptional stimulation. Moreover, angiogenin antagonism by blocking antibodies or angiogenin inhibitor neomycin decreased in vitro HSC activation by conditioned media or recombinant angiogenin. Finally, neomycin administration reduced tumor growth of HepG2-LX2 cells coinjected in mice. In conclusion, angiogenin secretion by HCCs favors tumor development by inducing HSC activation and ECM remodeling. These findings indicate that targeting angiogenin signaling may be of potential relevance in HCC management.
Tumor microenvironment is known to modulate the progression of human cancers1. In particular, hepatocellular carcinoma (HCC), the most common type of liver tumor, develops in a multicellular milieu in which parenchymal and non-parenchymal hepatic cells coexist with non-hepatic infiltrating cells, mostly inflammatory, providing an adequate cellular scenario that facilitates HCC progression2,3.
The communication of tumor cells with stromal cells within the extracellular matrix (ECM) paves the way for HCC development. Therefore, targeting stromal cells or interfering with the reciprocal cross-talk between stromal and tumor cells may stand as a critical strategy for cancer therapy1,2. In this regard, hepatic stellate cells (HSCs) transform during chronic liver injury from a quiescent state into a myofibroblast-like phenotype, which proliferate and migrate towards areas of necrosis and regeneration, as described in several pathological conditions4,5. Besides their participation in ECM production and degradation, activated HSCs are an important source of hepatic cytokines such as TGF-β, PDGF, HGF, CTGF, FGF, and VEGF, and recruit inflammatory cells, mono- and polymorphonuclear leukocytes that in turn produce chemokines, including MCP-1, CCL21, RANTES, CCR5.
Recent data point out that HSC transformation represents a crucial cell reprogramming event that shifts HSC from a normal vitamin A-storing to an ECM-remodeling phenotype5, favoring a tumorigenic milieu for HCC. For instance, the amount of peritumoral activated HSCs after curative resection predict early recurrence and poor clinical outcome in patients with HCC6. Moreover, HCC-HSC cross-talk generates a permissive proangiogenic microenvironment, particularly by inducing VEGF-A and MMP9 expression in HSCs and increasing motility in hepatocytes7. However, the identification of HCC-secreted mediators that activate surrounding HSCs and consequently facilitate cancer progression remains to be fully explored.
Angiogenin was the first isolated tumor-derived protein with in vivo angiogenic activity8 featuring a ribonuclease activity that stimulates ribosomal RNA (rRNA) transcription and cell proliferation9. Increased angiogenin serum levels have been associated with the incidence and severity of several human tumors10,11,12, including HCC13,14. Hepatocytes release angiogenin extracellularly15, which is first taken up by a specific transporter in endothelial and cancer cells, and then undergoes translocation to the nucleus through a phospholipase C dependent mechanism16. Angiogenin direct binding to the promoter region of ribosomal DNA induces rRNA transcription required for ribosomal biogenesis and the action of angiogenic factors, being essential for cell growth and proliferation. Neomycin, an aminoglycoside antibiotic, interferes with angiogenin nuclear targeting resulting in its perinuclear sequestration17, thus blocking angiogenin-induced cell proliferation and angiogenesis12,17,18. Interestingly, angiogenin is upregulated by hypoxic conditions in melanoma19 and other tumor cells20, and by inducers of acute-phase response in human HepG2 cells21. Angiogenin has been proposed as a putative noninvasive marker for monitoring HCC13, and increased angiogenin expression in patients with HCC correlates with major tumor vascularity and mortality14. However, the role that angiogenin plays in HSC activation has not been previously addressed. Thus, our aim was to analyze if angiogenin is secreted by HCC and to examine the role of angiogenin in HSC activation and HCC-HSC cross-talk in liver cancer.
Materials and Methods
Reagents
DMEM, Trypsin-EDTA, Penicillin-streptomycin, TRIzol, FBS, were from Invitrogen (Paisley, United Kingdom). All tissue culture ware was from Nunc (Roskilde, Denmark). Biotin Blocking System, peroxidase substrate (DAB), peroxidase buffer, and hematoxylin were from DAKO (Glostrup, Denmark). Aquatex was from Merck (Darmstadt, Germany). The ABC kit was from Vecstain (Burlingame, CA). Proteinase inhibitors were from Roche (Madrid, Spain). ECL western blotting substrate was from Pierce (Thermo Fisher Scientific, Rockford, IL). Neomycin and recombinant Angiogenin was from Sigma-Aldrich, and unless otherwise stated, all other reagents were also from Sigma-Aldrich (St. Louis, MO).
Cell culture and conditioned medium preparation
Human liver tumor cell lines Hep3B and HepG2 (European Collection of Animal Cell Cultures (ECACC)), and the human immortalized HSCs (LX2)22,23 were routinely grown in DMEM culture medium supplemented with 10% fetal bovine serum (FBS), and antibiotics at 37°C and 5% CO2. For conditioned medium (CM) preparation, HepG2, Hep3B, or LX2 cells were grown until optimal confluence (80–100%). Cell monolayers were washed three times in sterile 1x PBS and replenished with 15 mL of DMEM (serum free for microarray assays). After 24 hours, media were removed, filtered (0.22 micron) and stored at −20°C.
HSCs isolation and culture
Livers from wild-type male C57BL/6 mice (12−15 weeks old) were perfused with collagenase and HSCs cultured as previously described22,23. Culture purity, assessed routinely by retinoid autofluorescence at 350 nm, was >95%. Cells were cultured in DMEM supplemented with 10% FBS, and antibiotics at 37°C and 5% CO2. Since HSCs become activated after its culture in plastic in a time-dependent fashion, experiments to observe protein or mRNA content were always performed with cells at the same time of culture, with previous administration of CM, recombinant angiogenin or inhibitors for the indicated periods of time.
Immunofluorescence
LX2 cells were grown in coverslips, rinsed with PBS, fixed in formalin and permeabilized in saponin for 10 min. After incubation with PBS containing 5% BSA for 1 hour, monoclonal anti-angiogenin (C-1) (sc-74528 Santa Cruz Biotechnologies, 1:50 in PBS-BSA, 2 h, RT) was added. Later, coverslips were incubated with Alexa Fluor 488 anti-mouse (Molecular Probes, 1:500, 1 h, RT) and Hoechst 33258 (Sigma, 0.2 μg/ml) mounted. Confocal images and single optical sections were collected using a Leica SP2 laser scanning confocal microscope.
RNA silencing
HepsG2 and Hep3B cells were transfected with lentiviral particles containing expression constructs, each encoding target-specific 19–25 nt (plus hairpin) shRNA, designed to knockdown gene expression of angiogenin (shANG, sc-39291-V, Santa Cruz Biotechnologies) or control particles (shCTRL, sc-108080). Hepatoma cells stably expressing shRNAs were isolated by puromycin selection.
Antibody Array
RayBio® Human Angiogenesis Antibody Array 1 and 2 (G-Series, from RayBiotech), which allows simultaneous detection of 43 angiogenic-related proteins (Array components in Suppl. Fig. 1), were used to assess secretion profiles in conditioned media (CM) of HepG2, Hep3B and LX2 cells, according to manufacturer's instructions. Representative medium (DMEM, 10% FBS), not exposed to cells, was used as negative control. Arrays were visualized using ImageQuant LAS4000 software (GE Healthcare) and analyzed with ImageJ (National Institutes of Health).
Tumor animal model
All animal procedures were performed according to protocols approved by the Animal Experimentation Ethics Committee from the University of Barcelona. For subcutaneous tumor model, Male Swiss nude mice, 5–6 week old, were kept under pathogen-free conditions with free access to standard food and water. HepG2 cells (2 × 106), Hep3B cells (2 × 106), LX2 cells (2 × 106) alone or in combination HepG2/LX2 cells (2 × 106/0.4 × 106), Hep3B/LX2 cells (2 × 106/0.4 × 106), were injected subcutaneously into the flanks of mice in 200 μL DMEM media without FBS24,25. To analyze neomycin effect on tumor growth, three days later mice were randomly divided into 2 experimental groups: saline (control) or Neomycin (60 mg/Kg) were subcutaneously injected daily, as previously reported18. Tumors were measured periodically with a vernier caliper, and the volume was calculated as length × width2 ×0.5.
H&E and immunohistochemical staining
Livers were fixed and 7-μm sections were routinely stained with H&E or paraffin sections (5-μm) were prepared following standard procedures22,23,26. The antibodies used were rat mAb anti-CD34 (1:50 dilution, ab8158, Abcam), goat polyclonal anti-antiogenin (C1) (1:200, sc-74528, Santa Cruz Biotechnology), mAb anti-PCNA antibody (PC10) (1:200 dilution, sc-56, Santa Cruz Biotechnology) and anti-CD34 (1:100 dilution, sc-18917, Santa Cruz Biotechnology). The slices were examined with a Zeiss Axioplan microscope equipped with a Nikon DXM1200F digital camera. PCNA index was quantified in four randomly selected fields from each animal, and CD34 positive areas analyzed using ImageJ software.
SDS-PAGE and immunoblot analysis
Cell lysates were prepared in RIPA buffer plus proteinase inhibitors. Samples containing 10 to 30 μg were separated by 8–10% SDS-PAGE. Proteins were transferred to nitrocellulose membranes, blocked in 8% nonfat milk for 1 h at room temperature, and incubated overnight at 4°C with primary antibodies mAb anti-α-SMA (A2547, Sigma-Aldrich), mAb anti-Angiogenin (sc-74528, Santa Cruz Biotechnologies), mAb anti-PCNA (sc-56, Santa Cruz Biotechnologies), rabbit polyclonal anti-cathepsin B (06-480, Millipore), rabbit polyclonal anti-MMP9 (sc-6841-R, Santa Cruz Biotechnologies), mAb anti-β-actin (A1978, Sigma-Aldrich) and developed with ECL-peroxidase system.
RNA isolation and real time RT-PCR
Total RNA was isolated with TRIzol reagent. Real-time RT-PCR was performed with SensiFAST SYBR One-Step Kit (Bioline. Ecogen, Barcelona, Spain) following the manufacturer's instructions22,23,26. The primers sequences used were: human α-SMA, Fw 5′-TTCAGCTTCCCTGAACACCAC-3′, Rv 5′-CAGAGCCATTGTCACACACC -3′ (GenBank# NM_001613); human TGF-β, Fw 5′-GAGCCTGAGGCCGACTACTA-3′ and Rv 5′-CGGAGCTCTGATGTGTTGAA-3′ (GenBank# NM_000660); human β-actin, Fw: 5′-GGACTTCGAGCAAGAGATGG-3′and Rv: 5′-AGGAAGGAAGGCTGGAAGAG -3′ (GenBank# NM_001101), human RPII, Fw: 5′-GACACAGGACCACTCATGAAGT-3′ and Rv: 5′-GTGCGGCTGCTTCCATAAG-3′ (GenBank # NM_000937); human 45S, Fw: 5′-CTGACACGCTGTCCTCTGG-3′ and Rv: 5′- GGTCACCGGTAGGCCAGA-3′ (GenBank# NM_007742); mouse α-SMA, Fw 5′-ACTACTGCCGAGCGTGAGAT-3′ and Rv 5′-AAGGTAGACAGCGAAGCCAA -3′ (GenBank# NM_007392); mouse TGF-β, Fw 5′-GTCAGACATTCGGGAAGCAG-3′ and Rv 5′- GCGTATCAGTGGGGGTCA-3′ (GenBank# NM_011577); mouse Col1A1, Fw: 5′-ACTTCACTTCCTGCCTCAG-3′ and Rv: 5′-TGACTCAGGCTCTTGAGGGT-3′ (GenBank# NM_007742); mouse β-actin, Fw: 5′-GACGGCCAGGTCATCACTAT-3′and Rv: 5′-CGGATGTCAACGTCACACTT -3′ (GenBank# NM_007393).
Human serum samples
Patients (adult males) gave written consent in accordance with the Declaration of Helsinki, and the protocol, approved by ethical committees from the Hospital Clinic, followed ethical guidelines on handling of human samples. Additional data of patients is provided in Suppl. Fig. 2.
Statistical analyses
All images display representative data from at least 3 independent observations, unless indicated. Results are expressed as mean ± SEM and the statistical significance of differences (p value less than 0.05) was performed using the unpaired, non-parametric Student's t test, unless indicated.
Results
Conditioned medium from hepatoma cells transforms primary murine HSCs in vitro
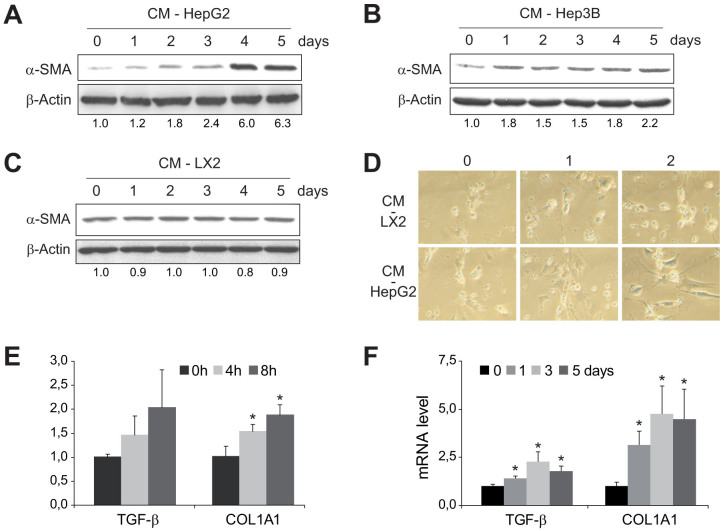
7-day old primary mouse HSCs were collected after being exposed for up to 5 days to conditioned medium from human cell lines HepG2 (CM-HepG2) or Hep3B (CM-Hep3B) to verify the capacity of hepatoma-derived CM to induce HSC activation27. Compared to 7-day old non-treated HSCs, exposure to CM-HepG2 induced significant changes in α-SMA expression (Fig. 1A), while exposure to CM-Hep3B augmented also α-SMA expression but to a lesser extent (Fig. 1B). In fact, changes in HSC morphology, indicative of HSC transformation, were observed in HSCs as soon as 2 days after exposure to CM-HepG2 (Fig. 1D). However, addition of medium from LX2 cells (CM-LX2), a cell line of human activated stellate cells, induced no visible changes in HSC appearance (Fig. 1D) or α-SMA expression (Fig. 1C). Moreover, COL1A1 mRNA levels displayed increased expression in HSCs within hours of CM-HepG2 exposure (Fig. 1E), showing significant increases as soon as 4 h after CM addition, before the overexpression of other profibrogenic genes such as TGF-β. In addition, enhanced mRNA expression of HSC-activated genes, such as TGF-β and COL1A1, was exhibited for several days (Fig. 1F).
Figure 1. HSCs are activated by conditioned medium (CM) from HepG2 and Hep3B cells.
Representative western blot showing α-SMA activation primary murine HSCs (day 7), previously exposed to conditioned medium from HepG2 (A), Hep3B (B) or LX2 cells (C) for 0–5 days, using β-actin levels as a control. D, Microscopic images of morphological changes in HSCs preteated with CM from HepG2 or LX2 during 0, 1 or 2 days. E and F, mRNA quantification of TGF-β and COL1A1 in 7-days-old HSCs after previous CM-HepG2 addition for the indicated periods of time. (n = 3). *, p ≤ 0.05, specific time vs. 0 time point.
HCC-secretome further activates LX2 cells in vitro and HSC-HCC co-implantation promotes tumor growth
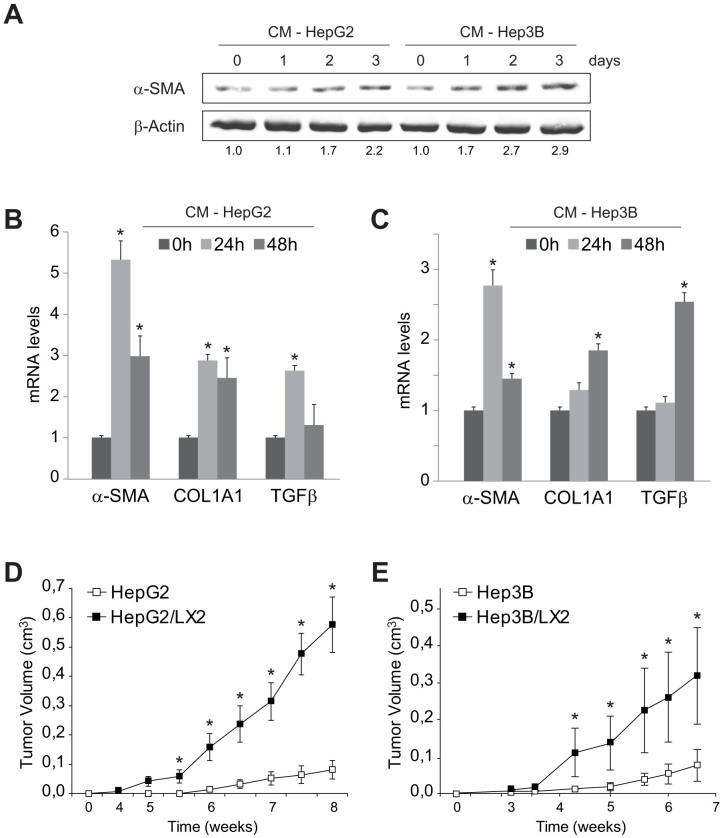
To verify if hepatoma-derived medium could also potentiate human HSC cell activation, LX2 cells were exposed to conditioned medium from HepG2 and Hep3B. Although LX2 is a clonal myofibroblastic HSC cell line, frequently used as a surrogate of human activated HSCs, both cell lines further increased LX2 activation monitored by α-SMA (Fig. 2A), supporting the pro-fibrogenic capacity of HCC secretome. Moreover, either HepG2 (Fig. 2B) or Hep3B (Fig. 2C) enhanced the mRNA expression of genes induced during HSC transformation, such as TGFβ, COL1A1 or α-SMA. Of note, CM from HepG2 cells induced a more potent stimulation, maybe indicating a major content of HSC-activating molecules.
Figure 2. LX2 cells are activated by conditioned medium (CM) from HepG2 and Hep3B cells and promote tumor growth in mice.
A, Representative western blot showing α-SMA activation, using β-actin levels as a control, of human LX2 cells previously exposed to conditioned medium from HepG2 and Hep3B. B and C, mRNA levels of genes associated to HSC activation measured in LX2 cells exposed to conditioned medium from HepG2 and Hep3B. D and E, Evolution of tumor growth in a murine subcutaneous model of Hep3B and HepG2 cells injected alone or combined with LX2 cells. (n = 3). *, p ≤ 0.05, Hep3B/LX2 and HepG2/LX2 vs. Hep3B and HepG2, respectively.
Once observed the ability of liver tumor cells to induce HSC activation, we wanted to verify if HCC-HSC association may promote hepatocarcinoma tumor growth in vivo. To do so, HepG2, LX2 or a combination of both cells (HepG2/LX2, ratio 5:1) were injected in the flanks of nude mice. Injection of LX2 cells alone did not generate any tumor, as previously observed with activated HSCs28. However, LX2 cells injected in combination with HepG2 greatly enhanced subcutaneous tumor growth over time, compared to HepG2 cells alone (Fig 2D). Similarly, Hep3B/LX2-derived tumors also exhibited accelerated cancer progression, compared to Hep3B tumors (Fig. 2E).
Angiogenin is secreted by HCC cells
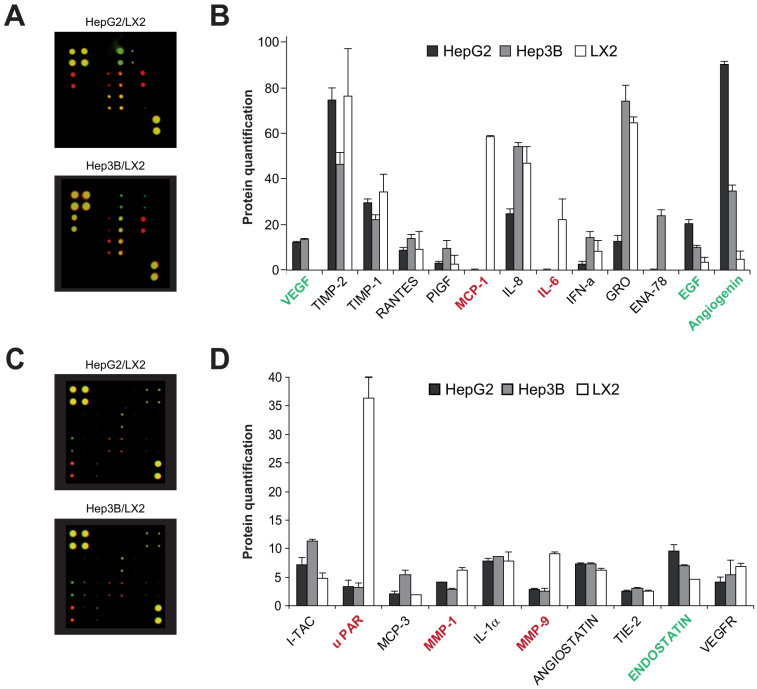
Since HepG2 and Hep3B cell lines were able to activate HSCs, while another human liver cell line such as LX2 did not, we analyzed a panel of cytokines, chemokines, growth factors and angiogenic factors in an attempt to identify activating molecules in the differential protein profile secreted by HCC and LX2 cells using commercial protein microarrays (Suppl. Fig. 1). In comparing the protein expression of HepG2 and Hep3B with respect to LX2 cells (Fig. 3), we observed that out of the 43 target proteins, angiogenin exhibited a marked increased expression (up to 10–20 fold) in HCC cells, being particularly high in HepG2 secretome (Fig 2B). Other proteins, such as vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF), previously described as HSC activators, were clearly enhanced (Fig. 2B), while moderate increases expression of endostatin and I-TAC (CXCL11), in Hep3B secretome, were also detected (Fig. 2D). On the other hand, enhanced extracellular expression of MCP-1, IL-6 (Fig 1B), uPAR, MMP1 and MMP9 (Fig. 2D) were observed in LX2 cells compared to hepatoma cell lines, reflecting the endogenous activation of LX2 cells. Since the role of angiogenin in HSC biology has not been previously addressed, we next focused on the impact and mechanisms underlying the HSC activation by angiogenin.
Figure 3. Differential profile of angiogenesis-related proteins in the secretome of hepatoma (HepG2 and Hep3B) and human HSC activated (LX2) cells.
A and B, Representative merged images of antibody microarrays for protein detection in conditioned medium (CM) from HepG2 or Hep3B (green) and compared to CM-LX2 (red) protein pattern. B and D, protein quantification was measured after calibration with internal standards and background controls. (n = 2). Indicated in red the proteins more expressed in LX2 respect to hepatoma cells, and in green the proteins more expressed in Hep3B/HepG2 compared to LX2.
Recombinant angiogenin induces HSC activation and its elimination from CM-HepG2 reduces HSC transformation
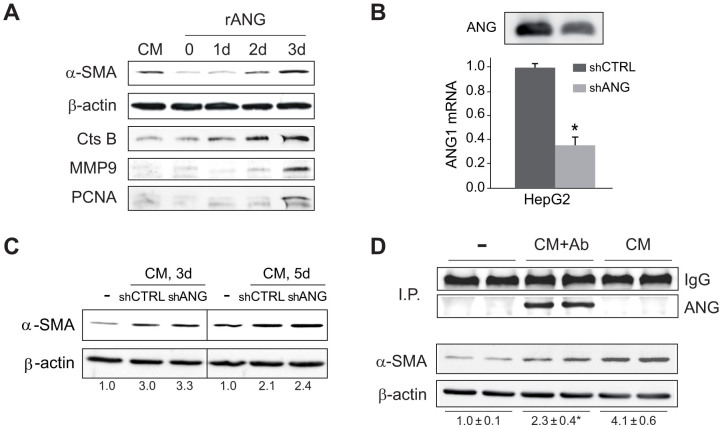
Increased serum angiogenin levels have been detected in patients with different types of cancer, and in particular, poor HCC prognosis correlates with angiogenin expression14. However, no evidence of angiogenin effect on HSC pathophysiology has been previously reported. To address whether angiogenin may reproduce the effect of HCC-derived CM on HSC activation, primary HSCs were exposed to recombinant angiogenin (rANG) to monitor fibrogenic markers. Similar to CM-HepG2-treated HSC cells, angiogenin-treated HSCs displayed higher α-SMA proteins levels than untreated cells (Fig. 4A). Moreover, other proteins that participate in ECM remodeling, such as cathepsin B (CtsB) or MMP9, or indicative of HSC proliferation, such as PCNA, were also elevated by rANG administration (Fig. 4A).
Figure 4. Recombinant angiogenin activates HSCs and angiogenin depletion from conditioned medium reduces HSC activation.
A, Primary murine HSCs were exposure to recombinant angiogenin (rANG, 1 μg/ml) for several days (0 to 3) and phenotypic transformation was detected by changes in α-SMA, PCNA, CtsB or MMP9 expression at day 7. B, Angiogenin protein (up) and mRNA (down) levels of HepG2 cells stably transfected with shRNA control (shCTRL) or against angiogenin (shANG). C, α-SMA protein expression in HSCs after 4 or 5 days of exposure to control medium (-) or conditioned medium from HepG2 cells with shCTRL or shANG transfection. D, α−SMA protein expression after HSC treatment (day 7) with control medium (−), CM-HepG2 (CM) and CM-HepG2 where angiogenin content was previously depleted by immunoprecipitation (CM+Ab), as denoted by angiogenin detection in agarose beads.
To evaluate if reduction of angiogenin expression in HepG2 medium modulates HSC activation by CM-HepG2, we generated HepG2 cells stably expressing control or anti-angiogenin vector coding for shRNA against angiogenin. Although, RNA silencing was effective in decreasing angiogenin mRNA levels and protein expression in HepG2-released medium (Fig. 4B), this reduction was insufficient to decrease HSC activation (Fig. 4C) by CM. Moreover, no changes in tumor incidence or tumor growth were detected between groups when HepG2 cells stably transfected with shANG or shCTRL were coinjected with LX2 cells in nude mice (data not shown).
Since RNA interfering did not completely eliminate angiogenin expression, we examined an alternative fashion to deplete angiogenin content using an anti-angiogenin antibody, that, as seen, quantitatively immunoprecipitated angiogenin from CM-HepG2 medium (Fig. 4D). Angiogenin depletion significantly reduced HSC activation induced by CM-HepG2 medium (Fig. 4D) suggesting that complete elimination of angiogenin is required to abolish its HSC activating capacity.
Neomycin blocks angiogenin nuclear translocation and reduces angiogenin-induced HSC transformation
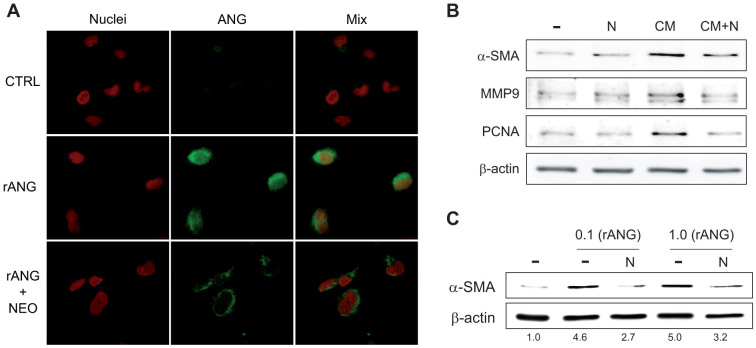
Previous studies have reported that the uptake of angiogenin and its nuclear translocation are required for translational changes and ribosomal biogenesis. Moreover, angiogenin-induced reprogramming may be antagonized by genetic knockdown or by interfering with its nuclear location by the aminoglycoside antibiotic neomycin12,17,18. To verify if angiogenin undergoes a distribution change in activated HSCs, and to validate the potential use of neomycin as an inhibitor of angiogenin, we followed angiogenin distribution in LX2 cells by confocal microscopy (Fig. 5A). After administration of rANG, both nuclear and perinuclear distribution of angiogenin was detected in LX2 cells. Interestingly, neomycin incubation antagonized angiogenin nuclear trafficking, resulting in increased perinuclear location (Fig. 5A), blocked nuclear deposition that precluded interaction of DNA with angiogenin, as indicated by the lack of colocalization with the DNA-binding dye Hoechst 33258. To evaluate if neomycin blockage of angiogenin signaling in HSC is translated in a direct effect on proliferative and profibrotic markers we analyzed neomycin effect on HSC-treated with conditioned medium and with rANG. In accordance with a role of angiogenin in HSC transformation, neomycin reduced the phenotypic changes induced by CM-HepG2 in primary HSCs as indicated by α-SMA, MMP9 and PCNA expression (Fig. 5B). Of note, neomycin decreased HSC activation by rANG, measured by α-SMA protein level (Fig. 5C).
Figure 5. Angiogenin nuclear deposition and HSC activation is reduced by neomycin administration.
A, Angiogenin subcellular location (green) was visualized by confocal immunofluorescence in HSCs treated with rANG (1 μg/ml) and/or neomycin preincubation (100 μM) by nuclear (red) co-staining with Hoechst 33258. Representatives images of α-SMA protein expression: B, analyzed after HSC treatment with different doses of angiogenin (0.1 and 1 μg/ml) and/or neomycin (100 μM) for 3 days; and C, after HSC treatment with control medium (−), or CM-HepG2 (CM) and/or neomycin (N) for 5 days.
Angiogenin levels do not increase in patients with ALD
HSC activation is a hallmark of progressive fibrosis during hepatic deterioration in liver diseases. Our data indicates that angiogenin is an inducer of HSC activation in vitro, however, a relevant role of angiogenin in the HSC activation during liver fibrosis has not been previously proposed. To evaluate the contribution of angiogenin on HSC activation during chronic liver injury, we analyzed the levels of angiogenin in serum samples from individuals with different stages of alcoholic liver disease (ALD). No differences in angiogenin serum content were observed in alcoholic patients with fibrosis or compensated cirrhosis compared to control individuals (Fig. 6). Of note, cirrhotic decompensated patients displayed an important reduction of angiogenin levels, in line with previous results14. Therefore, although angiogenin secreted by hepatoma cells, might modify liver cancer microenvironment via HSC-induced matrix remodeling, and angiogenin serum levels have been found increase in HCC patients as the tumor vascularity increase14, angiogenin concentration is not a reliable pre-neoplasic marker for liver cancer development, at least in a background of ALD. Therefore, angiogenin induced by hepatoma cells may induce further activation of already active HSCs, as observed in LX2 cells, contributing to HCC development, but without playing a role in initial HSC activation during liver fibrosis.
Figure 6. Angiogenin serum levels are not increased in ALD.
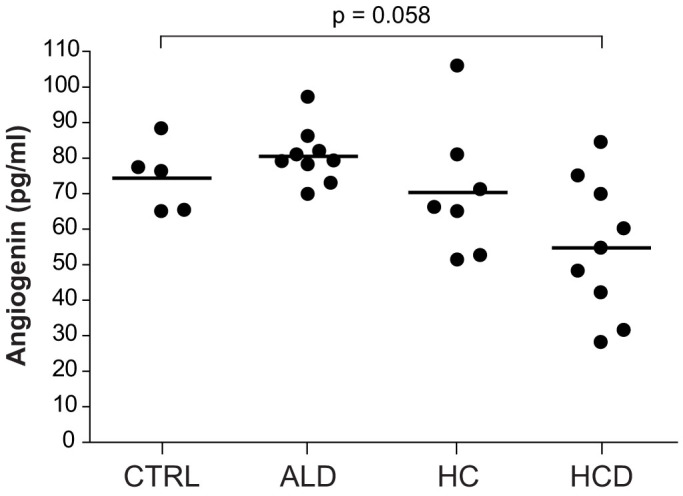
Angiogenin serum levels were measured in control individuals (n = 5) and patients with liver pathologies: ALD, alcoholic liver disease (n = 9, age: 48.1 ± 3.8), HC, hepatic cirrhosis (n = 7, age: 58.1 ± 2.4, MELD: 10.3 ± 1.6), and HCD, hepatic cirrhosis decompensated (n = 9 age: 51.3 ± 2.2, MELD: 14.3 ± 1.8). Additional data of patients is provided in Suppl. Fig. 2.
Neomycin administration markedly reduces HepG2/LX2 in vivo tumor growth
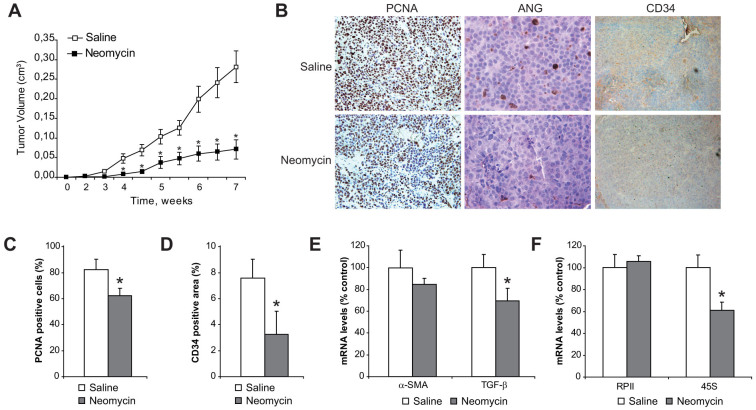
To explore the potential therapeutic relevance of neomycin in HCC, we followed tumor growth in the xenograft model with or without in vivo treatment with neomycin. Nude mice were treated daily with neomycin or saline subcutaneously to follow the growth of tumor growth in xenografts injected with HepG2 cells with or without LX2 cells. While no significant changes were observed in tumor multiplicity between groups (7 out of 10 in saline group, vs. 6 out of 10 in neomycin group), tumor growth determined by tumor volume (Fig. 7A) was clearly lower in neomycin-treated mice. Of note, tumors from neomycin-treated animals emerged slower, and tumor development was significantly diminished in equal size tumors (e.g. estimated doubling time for 50 mm3 tumors was 6.0 ± 1.2 days in saline-treated animals vs. 10.1 ± 2.1 in neomycin treated mice). In fact, HCC proliferation was reduced in neomycin-treated animals (Fig. 7B), as quantified in tumor slides by PCNA index measurement (Fig. 7C), while a reduction in tumor vascularization was determined by CD34 immunohistochemistry (Fig. 7B), and the positive areas quantified (Fig. 7D).
Figure 7. Neomycin administration reduces HCC/HSC tumor growth by blocking angiogenin nuclear translocation and HCC development.
A, measurement of tumors from nude mice subcutaneously injected with HepG2/LX2 cells and treated intraperitonealy with saline or neomycin for eight weeks. B, Representative images of tumor cell proliferation by PCNA detection (40×), angiogenin levels (80×) and CD34 (40×) were visualized in tumor samples from mice treated with neomycin or saline. C, Quantification of PCNA positive cells in tumor slides. D, Quantification of CD34 positive areas in tumor slides. E, mRNA quantification of α-SMA and TGF-β in tumors, using β-actin as control. (n ≥ 6). *, p ≤ 0.05, neomycin vs. saline-injected animals. F, RNA quantification of RNA polymerase II (RPII) and 45S ribosomal RNA in tumors, as above.
Consistent with reduced HSC activation, tumors from neomycin-treated mice displayed significant decreased TGF-β mRNA levels (Fig. 7E). Since nuclear angiogenin participates in rRNA transcription29, a rate-limiting step in ribosome biogenesis, which can be monitored by measurement of the steady-state level of 45S rRNA, we analyzed 45S rRNA expression. Neomycin treatment reduced significantly 45S rRNA levels, without detectable changes in RNA polymerase II (RPII) levels (Fig. 7F), discarding nonspecific effects of neomycin on RNA synthesis. In line with lower ribosomal activity, angiogenin nuclear location was almost absent in slides from neomycin-treated tumor-bearing mice (Fig. 7B) resulting in reduced cell proliferation, as indicated by PCNA (Fig. 7C) and tumor growth (Fig. 7A).
To address whether neomycin antagonizes HCC growth by directly regulating tumor cell proliferation, HepG2 cells were treated with neomycin in vitro. As seen, neomycin treatment with did not cause any decrease in HepG2 cell growth, even at high doses (2 mM) for up to a week (Supplemental Fig. 3), implying that the promoting effect of angiogenin in tumor growth is not due to its direct effect on HepG2 proliferation but most probably by favoring the microenvironment in which HCC develops. Moreover, other liver cancer cell lines such as Hep3B (human), H35 (rat) or Hep1c1c7 (mouse) were also treated with neomycin and no effects on cell proliferation were detected (data not shown). In addition, mice bearing subcutaneously HepG2 cells alone, did not displayed significant changes in tumor growth after neomycin administration (data not shown), suggesting that angiogenin signaling participates in the promotion of HepG2 tumor development induced by LX2 cells.
Discussion
HCC onset and progression is a complex process that requires multifactorial players2,3. Liver injury associated with inflammation and fibrosis precedes the occurrence of HCC, suggesting that HSC activation orchestrates a favorable scenario for HCC growth. Previous observations using a co-culture approach between HCC cells and primary HSC have suggested that activated HSCs play a supportive role in tumor progression by stimulating pro-angiogenic events and promoting tumor survival27,28. The role of disease-specific proteins in the cell secretome is crucial in regulating cell-to-cell and cell-to-extracellular matrix interactions, and the identification of specific molecules present in conditioned media from cell lines is an interesting approach for biomedical research29. Comparing the protein content of two well-studied hepatoma cell lines (HepG2 and Hep3B) to the secretome composition of a human myofibroblastic cell line (LX2) has obvious limitations, but may provide hints of molecules involved in the cellular cross-talk during liver cancer implantation and progression. By doing so, our data identify angiogenin as novel player in a positive loop by which HCC mediates HSC activation, which accelerates and amplifies HCC development (Figure 8). Our findings indicate that HCC-mediated secretion of angiogenin induces, or even aggravate, HSC activation promoting a phenotype with ECM remodeling activity. On the contrary, angiogenin antagonism may affect cancer microenvironment, reducing vessel formation and slowing down tumor growth in in vivo HCC models.
Figure 8. Schematic representation of angiogenin role in HCC/HSC crosstalk.
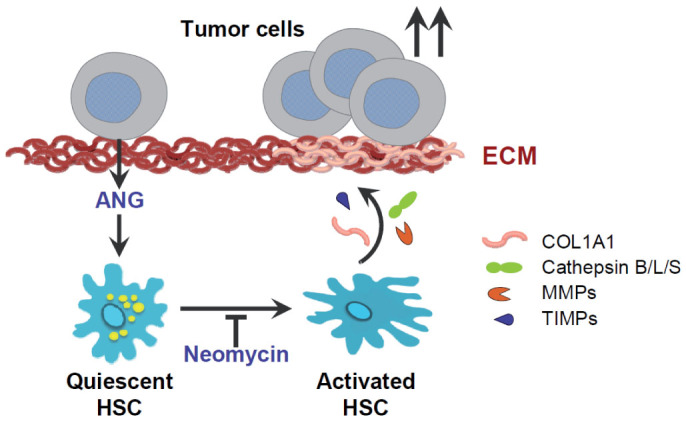
Angiogenin secreted from hepatoma cells is an inducer of HSC transformation by changing numerous proteins involved in ECM remodeling. Among them, specific fibrillar components such as collagen, type I, alpha 1, encoded by the COL1A1 gene, are induced, as well as abnormal expression of enzymes that degrade type IV and V collagens and other extracellular matrix proteins, such us MMP9, tissue inhibitors of metalloproteinases (TIMP) or cysteine proteinases such as cathepsin B. Consequently, physiologic ECM formation is altered due to HSC induction, providing a profibrogenic environment that facilitates tumor growth. In summary, liver tumor promotes its own development via angiogenin-dependent HSC activation, and antagonism of angiogenin signaling, as neomycin does, may be an interesting approach to halter liver cancer progression.
Consistent with previous observations that increased angiogenin levels in cancer stroma contribute to ECM degradation and promote tumor cell invasion10,17, we provide evidence that angiogenin is a critical HCC-derived mediator that stimulates HSC transdifferentiation. However, despite serum angiogenin levels increase in HCC, angiogenin is not expected to be a preneoplasic marker of HCC since patients with developed hepatic fibrosis or cirrhosis show levels similar to control subjects. In fact, a lower angiogenin levels is presented by ALD patients with decompensated cirrhosis, in which most of their HSCs are expected to be activated, suggesting that angiogenin is not playing a relevant role in the initial HSC activation that occurs during liver fibrosis.
Interestingly, increased expression of angiogenin in tumor cells has been observed under hypoxic conditions, as in melanoma cells, and after exposure of HepG2 to inflammatory cytokines including IL-620,21. Since low oxygen tension profile and inflammatory microenvironment are consistent features of HCC development, further enhanced angiogenin levels may be expected in peritumoral areas, inducing HSC phenotypic transformation. In addition, angiogenin plays a prominent role in endothelial cell proliferation induced by other tumor secreted proteins, including VEGF, acidic and basic fibroblast growth factors, and EGF, which requires its nuclear trafficking to exert its ribonuclease activity30. Moreover, angiogenin control of tumor vasculature via hypoxia inducible factor 1 and VEGF has been described in tumor angiogenesis through a NF-κB-dependent PDH2-regulated mechanism31. Therefore, angiogenin directly or via potentiation of other angiogenic factors, stands as a critical player in tumor growth during cancer progression.
Angiogenin is mainly produced by the liver15 and exhibits a high concentration in plasma (250–350 ng/mL) and fast turnover rate with a half-life of 2 h, which is a limitation to the use of anti-angiogenin antibodies or reducing its expression by siRNA approach10,18,32. Consequently, although our findings indicate blockage of angiogenin signaling as feasible strategy to prevent HCC progression and invasiveness, non-advisable large amount of angiogenin antagonists would be needed to neutralize its circulating levels. Fortunately, neomycin, an aminoglycoside antibiotic, has been reported to block nuclear translocation of angiogenin in several tumor models in athymic mice, independently of its antibiotic capacity17. Since angiogenin biological activity, mediated by rRNA transcription, requires its presence in the nucleus, preventing angiogenin nuclear translocation by neomycin does offers an alternative approach to inhibit its action, avoiding potential problems caused by its high blood concentration. Our data indicates that neomycin, that has been used for years in clinical practice as well as its derivatives such as neamine17,18, reduces tumor growth by a direct effect in tumor microvasculature but not directly in HCC proliferation, since we have failed to detect changes after neomycin administration in in vitro growth of different hepatoma cells, in contrast to a previous observation with another cell line33. Furthermore, alterations in angiogenin serum levels has been described among other clinical settings34,35,36,37, in human pathologies such as type 1 diabetes in young patients36 or inflammatory bowel disease37 in which liver fibrosis may emerge as an unwanted complication.
Hopefully, targeting angiogenin with small-molecule inhibitors will be soon a treatment approach, in special if angiogenin role is revealed for different human neoplasias. For instance, N65828, an inhibitor of the ribonucleolytic activity of human angiogenin, has been recently reported to diminish xenograft tumoral growth of T24 bladder carcinoma and HeLa cervical adenocarcinoma cells, through the inhibition of angiogenesis38. Unfortunately, Ki of N65828 toward ANG is 81 μmol/L, a value considered not good enough for drug development12. Before more specific inhibitors are designed and preclinical analysis performed, available medicines with known angiogenin-capability, such as neomycin or neamine, are starting to be tested for specific tumors11,12,18,39,40. In line with this approach, therapies in liver cancer must take into account not only the altered pathways in HCCs but also how the inhibition of tumor-engaged cascades might affect the surrounding stroma, and particularly HSCs41. It is know that HSC activation during fibrogenesis provides a natural landscape where incipient HCC cells are stimulated. Now, our data identify a new role in liver tumors for angiogenin by participating in HCC/HSC crosstalk, and provide compelling evidence pointing to angiogenin targeting as a potential novel strategy for HCC management.
Author Contributions
C.B., M.S., A.T., G.M.N., L.M. and A.d.M. performed the experiments; C.G.R. and J.C. analyzed clinical data and discuss the results; C.B. drafted the manuscript; J.F.C., M.M. and A.M. designed the experiments and revised the manuscript; all authors have read and approved the manuscript.
Supplementary Material
Suppl. Figure 1-3
Acknowledgments
Authors are indebted to Susana Nuñez for her technical support, and Dr. P. Garcia de Frutos for his insightful comments. Most of the work of this study was carried out at the Esther Koplowitz Centre (CEK). This study was funded by grants from the Instituto de Salud Carlos III (FIS PI12/00110, PI09/00056 to A.M., FIS PI10/02114, PI13/00374 to M.M., PI12/01265 to J.C. and PI11/0325 to J.F.C.), Ministerio de Economía y Competitividad (SAF 2012/34831 to J.F.C. and SAF2011-23031 to C.G.R.) and co-funded by FEDER (Fondo Europeo de Desarrollo Regional, Unión Europea. “Una manera de hacer Europa”); center grant P50-AA-11999 from Research Center for Liver and Pancreatic Diseases, US NIAAA to J.F.C.); Fundació la Marató de TV3 to J.F.C., Mutua Madrilea (AP103502012) to C.G.R., and by CIBERehd from the Instituto de Salud Carlos III.
References
- Hanahan D. & Coussens L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012). [DOI] [PubMed] [Google Scholar]
- Hernandez-Gea V., Toffanin S., Friedman S. L. & Llovet J. M. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 144, 512–527 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. & Iredale J. P. The inflammatory microenvironment of HCC - the plot becomes complex. J. Hepatol. 54, 853–855 (2011). [DOI] [PubMed] [Google Scholar]
- Friedman S. L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 88, 125–172 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L., Sheppard D., Duffield J. S. & Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci. Transl. Med. 5, 167sr161, 10.1126/scitranslmed.3004700 (2013). [DOI] [PubMed] [Google Scholar]
- Ju M. J. et al. Peritumoral activated hepatic stellate cells predict poor clinical outcome in hepatocellular carcinoma after curative resection. Am. J. Clin. Pathol. 131, 498–510 (2009). [DOI] [PubMed] [Google Scholar]
- Coulouarn C. et al. Hepatocyte-stellate cell cross-talk in the liver engenders a permissive inflammatory microenvironment that drives progression in hepatocellular carcinoma. Cancer Res. 72, 2533–2542 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett J. W. et al. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry 24, 5480–5486 (1985). [DOI] [PubMed] [Google Scholar]
- Tsuji T. et al. Angiogenin is translocated to the nucleus of HeLa cells and is involved in ribosomal RNA transcription and cell proliferation. Cancer Res. 65, 1352–1360 (2005). [DOI] [PubMed] [Google Scholar]
- Shimoyama S. & Kaminishi M. Angiogenin: its clinical implications in malignancy. Austral-As. J. Cancer. 1, 32–36 (2001). [Google Scholar]
- Yoshioka N., Wang L., Kishimoto K., Tsuji T. & Hu G. F. A therapeutic target for prostate cancer based on angiogenin-stimulated angiogenesis and cancer cell proliferation. Proc. Natl. Acad. Sci. U. S. A. 103, 14519–14524 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibaragi S. et al. Angiogenin-stimulated rRNA transcription is essential for initiation and survival of AKT-induced prostate intraepithelial neoplasia. Mol. Cancer Res. 7, 415–424 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas V. R., Maluf D. G., Archer K. J., Yanek K. C. & Fisher R. A. Angiogenesis soluble factors as hepatocellular carcinoma noninvasive markers for monitoring hepatitis C virus cirrhotic patients awaiting liver transplantation. Transplantation 84, 1262–1271 (2007). [DOI] [PubMed] [Google Scholar]
- Hisai H. et al. Increased expression of angiogenin in hepatocellular carcinoma in correlation with tumor vascularity. Clin. Cancer Res. 9, 4852–4859 (2003). [PubMed] [Google Scholar]
- Weiner H. L., Weiner L. H. & Swain J. L. Tissue distribution and developmental expression of the messenger RNA encoding angiogenin. Science 237, 280–282 (1987). [DOI] [PubMed] [Google Scholar]
- Li S. & Hu G. F. Emerging role of angiogenin in stress response and cell survival under adverse conditions. J. Cell Physiol. 227, 2822–2826 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G. F. Neomycin inhibits angiogenin-induced angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 95, 9791–9795 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibaragi S. et al. Neamine inhibits prostate cancer growth by suppressing angiogenin-mediated rRNA transcription. Clin. Cancer Res. 15, 1981–1988 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A. et al. Hypoxia-induced up-regulation of angiogenin in human malignant melanoma. Cancer Res. 59, 1578–1583 (1999). [PubMed] [Google Scholar]
- Kishimoto K. et al. Hypoxia-induced up-regulation of angiogenin, besides VEGF, is related to progression of oral cancer. Oral Oncol. 48, 1120–1127 (2012). [DOI] [PubMed] [Google Scholar]
- Verselis S. J., Olson K. A. & Fett J. W. Regulation of angiogenin expression in human HepG2 hepatoma cells by mediators of the acute-phase response. Biochem. Biophys. Res. Commun. 259, 178–184 (1999). [DOI] [PubMed] [Google Scholar]
- Moles A., Tarrats N., Fernandez-Checa J. C. & Mari M. Cathepsins B and D drive hepatic stellate cell proliferation and promote their fibrogenic potential. Hepatology 49, 1297–1307 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrats N. et al. Critical role of tumor necrosis factor receptor 1, but not 2, in hepatic stellate cell proliferation, extracellular matrix remodeling, and liver fibrogenesis. Hepatology 54, 319–327 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A. et al. Pharmacological inhibition or small interfering RNA targeting acid ceramidase sensitizes hepatoma cells to chemotherapy and reduces tumor growth in vivo. Oncogene 26, 905–916 (2007). [DOI] [PubMed] [Google Scholar]
- Lluis J. M. et al. GD3 synthase overexpression sensitizes hepatocarcinoma cells to hypoxia and reduces tumor growth by suppressing the cSrc/NF-kappaB survival pathway. PloS One 4, e8059, 10.1371/journal.pone.0008059 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A. et al. Acidic sphingomyelinase controls hepatic stellate cell activation and in vivo liver fibrogenesis. Am. J. Pathol. 177, 1214–1224 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Bru P. et al. Hepatocarcinoma cells stimulate the growth, migration and expression of pro-angiogenic genes in human hepatic stellate cells. Liver Int. 30, 31–41 (2010). [DOI] [PubMed] [Google Scholar]
- Amann T. et al. Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Sci. 100, 646–653 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling P. & Clynes M. Conditioned media from cell lines: a complementary model to clinical specimens for the discovery of disease-specific biomarkers. Proteomics 11, 794–804 (2011). [DOI] [PubMed] [Google Scholar]
- Kishimoto K., Liu S., Tsuji T., Olson K. A. & Hu G. F. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene 24, 445–456 (2005). [DOI] [PubMed] [Google Scholar]
- Chan D. A. et al. Tumor vasculature is regulated by PHD2-mediated angiogenesis and bone marrow-derived cell recruitment. Cancer Cell 15, 527–538 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama S. et al. Increased angiogenin expression in pancreatic cancer is related to cancer aggressiveness. Cancer Res. 56, 2703–2706 (1996). [PubMed] [Google Scholar]
- Zhao J. et al. Neamine inhibits cell proliferation, migration, and invasion in H7402 human hepatoma cells. Saudi Med. J. 31, 1309–1314 (2010). [PubMed] [Google Scholar]
- Ricciardolo F. L. et al. Expression of vascular remodelling markers in relation to bradykinin receptors in asthma and COPD. Thorax 68, 803–811 (2013). [DOI] [PubMed] [Google Scholar]
- Ning R. et al. Neamine induces neuroprotection after acute ischemic stroke in type one diabetic rats. Neuroscience 257, 76–85 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarelli F. et al. Serum angiogenin concentrations in young patients with diabetes mellitus. Eur. J. Clin. Invest. 32, 110–114 (2002). [DOI] [PubMed] [Google Scholar]
- Oikonomou K. A. et al. Angiogenin, angiopoietin-1, angiopoietin-2, and endostatin serum levels in inflammatory bowel disease. Inflamm. Bowel Dis. 17, 963–970 (2011). [DOI] [PubMed] [Google Scholar]
- Miyake M., Goodison S., Lawton A., Gomes-Giacoia E. & Rosser C. J. Angiogenin promotes tumoral growth and angiogenesis by regulating matrix metallopeptidase-2 expression via the ERK1/2 pathway. Oncogene, 10.1038/onc.2014.2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K. et al. Neamine inhibits oral cancer progression by suppressing angiogenin-mediated angiogenesis and cancer cell proliferation. Anticancer Res. 34, 2113–2121 (2014). [PMC free article] [PubMed] [Google Scholar]
- Bottero V. et al. Kaposi's sarcoma-associated herpesvirus-positive primary effusion lymphoma tumor formation in NOD/SCID mice is inhibited by neomycin and neamine blocking angiogenin's nuclear translocation. J. Virol. 87, 11806–11820 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulouarn C. & Clement B. Stellate cells and the development of liver cancer: therapeutic potential of targeting the stroma. J. Hepatol. 60, 1306–1309 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure 1-3