Abstract
A lack of strategy to counteract hypoxia (pO2< 10–15 mmHg) and technique to repeatedly measure tumor pO2 has restricted therapeutic optimization. We report the results obtained with an innovative anti-angiogenic strategy of recurrent low-dose (metronomic) chemotherapy to modulate hypoxia and growth of the Head and Neck tumor xenografts.
The FaDu tumors were established in the flank of immune deficient mice and EPR oximetry with lithium phthalocyanine crystals was used to follow the temporal changes in tumor pO2 on treatment with gemcitabine including controls for three weeks. The FaDu tumors were hypoxic with a baseline (pre-treatment) pO2 of 2–8 mmHg. A transient increase in the tumor pO2 was evident on day 3 on treatment with a conventional schedule of gemcitabine (150 mg/kg, dl, d8, d15). No significant change in the tumor pO2 on treatment with metronomic gemcitabine (25 mg/kg on dl, d3, d5 for 3 weeks) was observed. However, tumor pO2 increased significantly on d15–dl8 during treatment with a metronomic schedule of 15 mg/kg gemcitabine (dl, d3, d5 for 3 weeks). A modest decrease in the tumor growth was evident on treatment with conventional gemcitabine. Notably, tumor growth was significantly inhibited by metronomic (25 and 15 mg/kg) gemcitabine treatment. The immuno-histochemistry (IHC) analyses of the tumor samples indicate a decrease in HIF-la and TSP-1 on treatment with metronomic gemcitabine.
In conclusion, a significant inhibition of tumor growth on treatment with metronomic gemcitabine was observed; however, the increase in pO2 was dose dependent. EPR oximetry can be used to follow the temporal changes in tumor pO2 to identify a therapeutic window on treatment with metronomic chemotherapy for potential combination with radiotherapy.
Keywords: Chemotherapy, Anti-angiogenesis, Vascular normalization, Head and neck cancer, Partial pressure of oxygen (pO2), Electron paramagnetic resonance (EPR) oximetry, Hypoxia inducible factor (HIF), Thrombospondin (TSP)
1 Introduction
Atypical angiogenesis with leaky vasculature and abnormal blood flow in solid tumors often leads to hypoxic microenvironment, a major cause of resistance to most of the standard therapies, such as radiation and chemotherapy. Furthermore, tumor hypoxia is dynamic i.e. varies with tumor growth and as a consequence of various treatment. In spite of extensive research, a lack of a suitable strategy to effectively reduce tumor hypoxia and technique to measure the dynamic changes in tumor pO2 has restricted the potential optimization of treatment outcome. We are investigating the efficacy of a recently proposed strategy of recurrent low-dose (metronomic) chemotherapy [1] on the pO2 and growth of Head and Neck tumor xenografts. The metronomic schedule is expected to prune the immature neo-vasculature and then remodel the remaining vasculature, leading to the normalization of otherwise abnormal tumor vasculature [2, 3]. The vascular normalization is expected to restore the blood flow and thus oxygenate the tumors, thereby providing a therapeutic window which, if exploited, can improve treatment outcome.
We have successfully used in vivo EPR oximetry to repeatedly measure tissue pO2 of Head and Neck tumor xenografts on treatment with conventional and metronomic schedules of gemcitabine. The results indicate a transient increase in the tumor pO2 on treatment with metronomic gemcitabine; however the extent of increase in pO2 was dose dependent. A significant decrease in the tumor growth was observed on treatment with metronomic gemcitabine as compared to the conventional schedule. Our overall goal is to establish metronomic regimens that can significantly oxygenate and inhibit tumor growth compared to the conventional regimen being tested in clinical trials
2 Methods
2.1 Head and Neck Tumor Xenografts and Experimental Groups
The FaDu tumors were established by injecting 100 μl suspension of 2–2.5 × 106 cells in each flank of 20–22 g NOD scid gamma (NSG) mice (inbred). The ectopic tumors of 6–8 mm in length were obtained in 18–21 days after cell inoculation and then the animals were randomly assigned to four groups: (i) control, (ii) 150 mg/kg gemcitabine in a conventional schedule (dl, d8, d15), (iii) 25 mg/kg gemcitabine in a metronomic schedule (dl, d3, d5 for 3 weeks) and (iv) 15 mg/kg gemcitabine in a metronomic schedule (dl, d3, d5 for 3 weeks). The animals were treated by intraperitoneal injections of gemcitabine (Eli Lilly and Co., IN, USA). A baseline tumor pO2 by EPR oximetry and tumor volume by a standard caliper method (volume = π/6 × length × width2) was measured prior to any treatment on d1 and then the treatment was initiated along with the repeated measurement of tumor pO2 and volume. At the end of the third week, the tumors were excised and IHC analysis was carried out to investigate the levels of HIF-lα and TSP-1 in the control, 150 mg/kg gemcitabine and 15 mg/kg gemcitabine groups.
2.2 In Vivo EPR Oximetry
A 1.2 GHz (L-band) EPR spectrometer was used to repeatedly measure tumor pO2 during treatment with a different dose and schedule of gemcitabine. Two aggregates of LiPc (lithium phthalocyanine, oximetry probe) crystals synthesized in our laboratory were implanted using 25 G needles approximately 4 mm apart and at a depth of 2–3 mm from the surface in each tumor. The pO2 was measured simultaneously from both implants using magnetic field gradients (multi-site EPR oximetry) for 15–20 min and pooled to obtain an average pO2 on each day of the measurement [4, 5].
2.3 Immunohistochemistry Analysis of the Tumors
The HIF-lα (hypoxia marker) and TSP-1 (angiogenic inhibitor) staining was performed on formalin-fixed, paraffin-embedded sections of the FaDu tumors using the standard method [6]. Briefly, the primary antibodies were diluted 1:100 and incubated for 1 h. The sections were subsequently incubated with appropriate secondary antibody and staining was developed using diaminobenzidine solution. The images were acquired on Olympus BX50 microscope at different magnifications for analysis.
2.4 Statistical Analysis
A paired t-test was used to determine the statistical significance of the changes in tumor pO2 compared to dl in each group and an unpaired t-test was used to compare the tumor volume between groups. The tests were two-sided and a change with a p-value<0.05 was considered statistically significant. All data are expressed as mean ± SEM; N is the number of tumors in each group.
3 Results
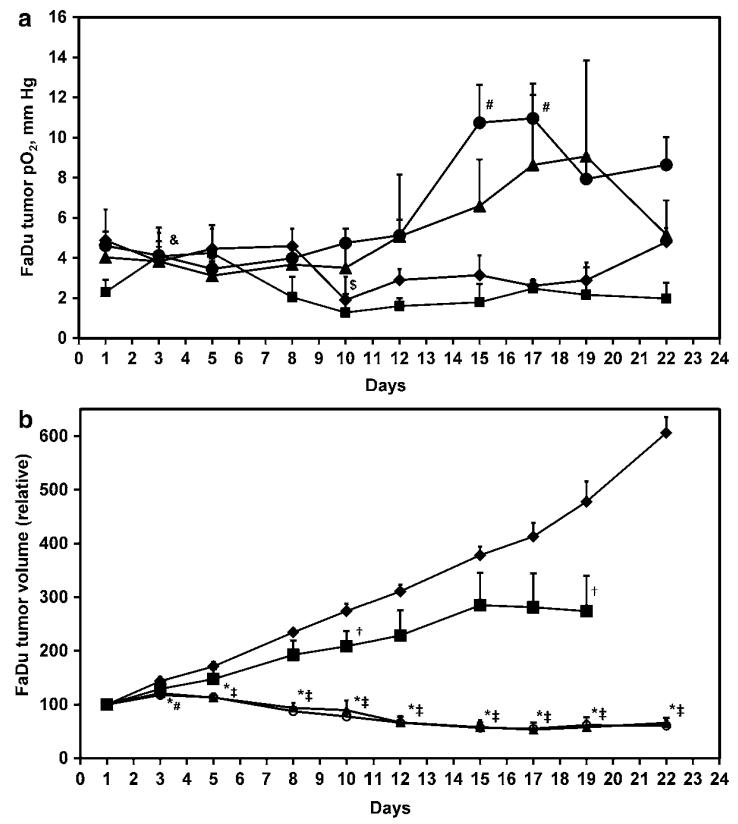
The baseline pO2 in the control, 150 mg/kg gemcitabine, 25 mg/kg gemcitabine and 15 mg/kg gemcitabine were 4.8±1.5, 2.3±0.6, 4.0±0.7 and 4.6±0.7 mmHg respectively, and were not significantly different between the groups (Fig. 14.1a) In these experiments, a significant decline in the tumor pO2 of the control group was evident on d10 but the pO2 returned to baseline on subsequent days. On the othei hand, a significant increase in the tumor pO2 on treatment with 150 mg/kg gemcitabine was observed on d3, which declined to baseline in the subsequent measurements. No significant change in the tumor pO2 was evident on metronomic treatment with 25 mg/kg gemcitabine; however, tumor pO2 significantly increased on d15–dl7 during treatment with 15 mg/kg gemcitabine.
Fig. 14.1.
(a). Temporal changes in the tumor pO2, and (b). Tumor volume in the control (diamond, N = 6–9), conventional chemotherapy (square, 150 mg/kg gemcitabine, d1, d8, d15, N = 7–9) and metronomic chemotherapy (triangle, 25 mg/kg and circle, 15 mg/kg gemcitabine on dl, d3, d5 each week for 3 weeks, N = 12–18). $, p<0.05 day 1 vs. d3–d22, control; &, p<0.05 day 1 vs. d3–d22, 150 mg/kg gemcitabine; #, p<0.05 day 1 vs. d3–d22, 15 mg/kg gemcitabine; †, p<0.05 150 mg/kg gemcitabine vs. control; *, p<0.05 25 mg/kg gemcitabine vs. control; ‡, p<0.05 15 mg/kg gemcitabine vs. control
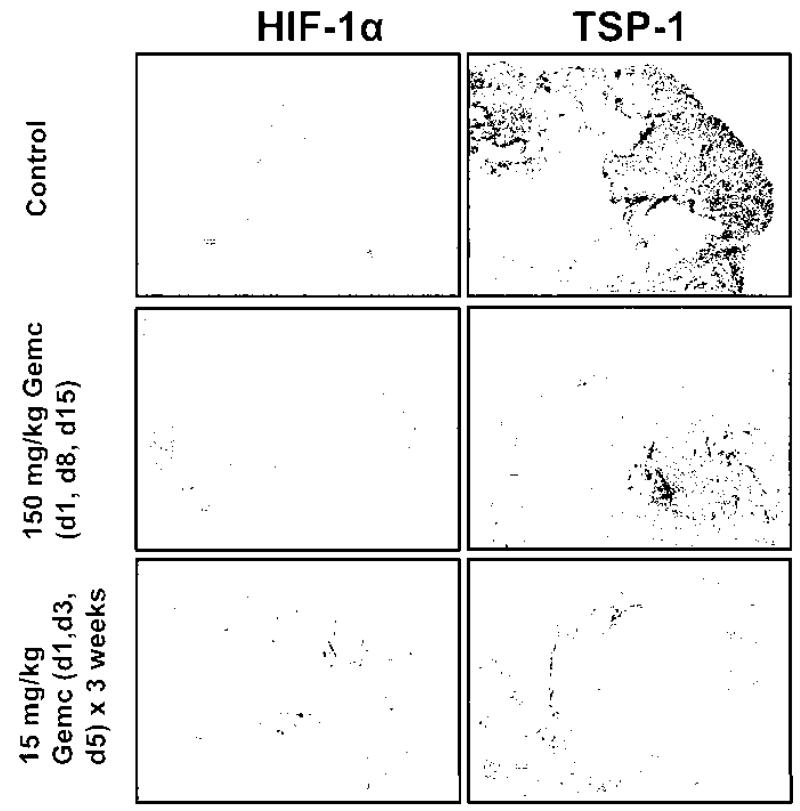
The baseline tumor volume in the control, 150 mg/kg gemcitabine, 25 mg/kg gemcitabine and 15 mg/kg gemcitabine were 342.7 ±23.8,281.8 ± 18.6, 287.6 ± 16.6 and 321.3 ± 19 mm3, respectively, and were not significantly different between the groups (Fig. 14.1b). A modest decrease in the tumor growth was observed on d10 and dl9 on treatment with 150 mg/kg gemcitabine in the conventional schedule. However, a significant and consistent inhibition of tumor growth was observed from d3–d22 on metronomic treatment with 25 and 15 mg/kg gemcitabine. The IHC images indicate a relative decline in HIF-lα and TSP-1 on treatment with metronomic 15 mg/kg gemcitabine compared to the control and conventional 150 mg/kg gemcitabine schedule (Fig. 14.2).
Fig. 14.2.
Immunohistochemistry analysis of the tumor samples in the control, conventional (150 mg/kg gemcitabine, d1, d8, d15) and metronomic (15 mg/kg gemcitabine on d1, d3, d5 for 3 weeks) chemotherapy groups. The scale of magnification is 200 μm in the images
4 Conclusions
The rationale for metronomic chemotherapy was proposed after the observation that treatment with some chemotherapeutics resulted in a mild and reversible anti-angiogenic effect on conventional schedule. Several pre-clinical studies have now confirmed a persistent anti-angiogenic effect using recurrent low-dose schedules, which led to the concept of vascular normalization [2, 3]. We hypothesize that vascular normalization induced by the metronomic approach should restore blood flow into the tumors and thus enhance tissue pO2. We also hypothesize that the techniques that can provide repeated measurement of tumor pO2 will be extremely useful in identifying a therapeutic window during which the tumors are oxygenated during metronomic treatment. Given the role of hypoxia in resistance to radiotherapy, this vital information can be used to schedule irradiations when the tumors are oxygenated (oxygen guided radiotherapy protocols) to improve the treatment outcome.
Our results indicate a significant increase in tumor pO2 on treatment with metronomic gemcitabine compared to the conventional schedule; however, the efficacy was dose dependent. A significant inhibition of tumor growth was observed with both metronomic schedules (25 or 15 mg/kg gemcitabine) investigated in these experiments. The preliminary IHC results suggest a decrease in the levels of HIF-lα on treatment with 15 mg/kg gemcitabine in metronomic schedule, which is in agreement with the increase in tumor pO2 observed in this group. However, a decrease in the levels of TSP-1 was also evident on treatment with metronomic gemcitabine compared to the conventional schedule. This is in contrary to the expected anti-angiogenic effect of metronomic gemcitabine, and warrants further investigation. The ability to repeatedly measure tumor pO2 by EPR oximetry can be used to identify tumors that oxygenate during metronomic treatment for potential combination with radiotherapy. A direct assessment of tumor vasculature by mCT is ongoing to confirm vascular normalization on treatment with metronomic schedules.
Acknowledgments
Pilot Program Project funded by the Norris Cotton Cancer Center, Department of Radiology, and the EPR Center, Geisel School of Medicine, Hanover, NH.
Contributor Information
Nadeem Khan, EPR Center for Viable Systems, Department of Radiology, The Geisel School of Medicine, Hanover, NH 03755, USA; Norris Cotton Cancer Center, Lebanon, NH 03756, USA
Huagang Hou, EPR Center for Viable Systems, Department of Radiology, The Geisel School of Medicine, Hanover, NH 03755, USA; Norris Cotton Cancer Center, Lebanon, NH 03756, USA.
Sassan Hodge, Department of Surgery, Dartmouth-Hitchcock Medical Center, One Medical Center Drive, Lebanon, NH 03756, USA.
Muthulakshmi Kuppusamy, EPR Center for Viable Systems, Department of Radiology, The Geisel School of Medicine, Hanover, NH 03755, USA; Norris Cotton Cancer Center, Lebanon, NH 03756, USA.
Eunice Y. Chen, Department of Surgery, Dartmouth-Hitchcock Medical Center, One Medical Center Drive, Lebanon, NH 03756, USA
Alan Eastman, Department of Pharmacology and Toxicology, The Geisel School of Medicine, Hanover, NH 03755, USA.
Periannan Kuppusamy, EPR Center for Viable Systems, Department of Radiology, The Geisel School of Medicine, Hanover, NH 03755, USA; Norris Cotton Cancer Center, Lebanon, NH 03756, USA.
Harold M. Swartz, EPR Center for the Study of Viable Systems, The Geisel School of Medicine at Dartmouth, Lebanon, NH, USA
References
- 1.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7(8):455–65. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 2.Goel S, Wong AH, Jain RK. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harbor Perspect Med. 2012;2(3):a006486. doi: 10.1101/cshperspect.a006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 4.Mupparaju S, Hou H, Lariviere JP, Swartz H, Jounaidi Y, Khan N. Repeated tumor oximetry to identify therapeutic window during metronomic cyclophosphamide treatment of 9 L gliomas. Oncol Rep. 2011;26(l):281–286. doi: 10.3892/or.2011.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doloff JC, Khan N, Ma J, Demidenko E, Swartz HM, Jounaidi Y. Increased tumor oxygenation and drug uptake during anti-angiogenic weekly low dose cyclophosphamide enhances the anti-tumor effect of weekly tirapazamine. Curr Cancer Drug Target. 2009;9(6):777–788. doi: 10.2174/156800909789271503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi ZR, Itzkowitz SH, Kim YS. A comparison of three immunoperoxidase techniques for antigen detection in colorectal carcinoma tissues. J Histochem Cytochem. 1988;36(3):317–322. doi: 10.1177/36.3.3278057. [DOI] [PubMed] [Google Scholar]