Abstract
Stone formation in the urinary tract is a common phenomenon with associated morbidity. The exact physicochemical factors responsible for stone formation are not clearly known. Over the past decade considerable interest has been generated in defining the role of nanobacteria in urinary stone formation. A review of the available literature has been carried out to give insights into their nature and outline their role in stone formation. The two aspects of nanobacteria that need to be considered include its biological nature and the other merely as mineralo-protein complexes. Though the current literature favors the concept of mineralo-protein particles, further research is needed to clearly define their nature. Whether living or nonliving, these apatite forming nanoparticles appear to play role in kidney stone formation.
Keywords: Calcifying nanoparticles, fetuin, kidney stones, mineralo-protein complexes, nanobacteria
INTRODUCTION
Stones in the urinary system are known since antiquity with the earliest report in an Egyptian mummy and a reference to stone disease in the Hippocratic oath.[1] The exact pathogenesis or physicochemical mechanism for urolithiasis is still not firmly established. Randall first described interstitial crystal plaques of calcium phosphate (CaP) in the papillary tips of stone formers.[2] The presence of CaP plaques even in calcium oxalate (CaOx) stone formers gave the nucleation concept where the former served as the nucleation surface for initiation and propagation of CaOx crystallization. Over the past decade, there have been several reports on the role of nanobacteria (NB) in urolithiasis but there exists a controversy over their nature.[3,4,5,6,7,8,9] In this article, we review the changing concepts about the nature of nanobacteria along with the available evidence for their role in urolithiasis.
A Pubmed search was made in August 2013 using key words nanobacteria (145 citations) and calcifying nanoparticles (34 citations). After excluding duplicates, all English language articles were reviewed by title and abstract. Studies describing methods of cultivation, isolation, in vitro or in vivo properties of nanobacteria, and articles investigating their role in nephrolithiasis were selected for the review. Articles on association of nanobacteria with other pathologic conditions were excluded. Full text of selected articles was obtained for detailed evaluation. These articles were cross-referenced to find any relevant article that was undetected during initial search. All articles fulfilling above-mentioned criteria were reviewed.
NANOBACTERIA ONTOLOGY
Origin and nomenclature
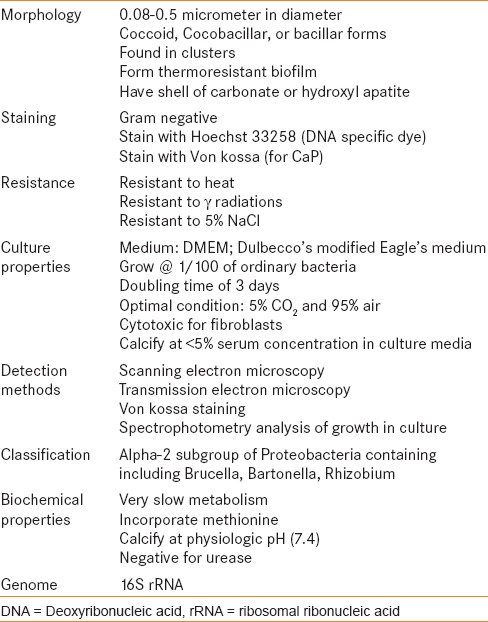
In mathematics “nano” is defined as 10-9. Folk first reported “nannobacteria”, (0.1 mμ m dwarf forms) in the sediments of hot springs in Italy and felt that they played a prominent role in the precipitation of carbonate minerals.[10] Similar forms were discovered on the Martian meteorite ALH84001.[11] A Finnish group claimed isolating such forms from blood and blood products and described their properties in detail [Table 1].[7] The same authors had worked for years on purported nanobacteria, but their reports were turned down by the microbiology world due to the controversies surrounding their size and form.[12,13] Over the years, attempts have been made to isolate nanobacteria and study their behavior in detail.[8,9,14,15,16,17,18,19]
Table 1.
Characteristics of Nanobacteria as described by Kajander et al.[7]
Changing concepts
After the initial report on the biologic nature of nanobacteria by Kajander et al., Cisar et al., attempted isolation of NB using the conventional method described earlier and reached a completely different conclusion about their nature.[8] Cisar et al., through their experiment, provided alternative interpretation of nanobacteria isolates from human kidney stones. In their experiment, the coccoid bodies, similar in morphology to those described by Kajander, were seen under scanning electron microscope (SEM), Calcium phosphate (CaP) was detected by energy-dispersive x-ray microanalysis (EDX), and apatite presence in them was established by X-ray diffraction. These coccoid bodies were also stained with Hoechst 33258 (dye used to stain DNA) as earlier. However, the nucleic acid could not be isolated after decalcification. Polymerase chain reaction (PCR) performed with 16S rDNA primers yielded nucleotide sequence that was found to be identical to 16S rDNA of Phyllobacterium myrsinacearum, a contaminant in PCR studies.[8] The authors concluded that the 16S rDNA reported earlier in nanobacteria may actually be due to environmental contamination. Presence of apatite forming coccoid bodies in the absence of any credible evidence of biologic material (DNA or RNA) was explained by the nucleating property of apatite. Dulbecco's modified Eagle's medium (DMEM) is rich in calcium (Ca) and phosphorous (P). In the presence of high concentration of Ca and P in DMEM, apatite crystals can account for initiation, sustenance, and transferability of biomineralization.[8,20] Following this report, many successful and a few unsuccessful attempts were made to cultivate these nanoparticles but their exact nature remained undefined.[4,9,21,22] Presence of immune response against these particles supported their biologic nature despite unsuccessful attempts to isolate DNA or RNA from them.[3]
In order to better define the nature of these propagating calcifying agents, a comprehensive analysis was undertaken with Nanobacterium sp. the original strain provided by Kajander.[23] After 10 days, biomineralization was clearly visible in DMEM culture. PCR products were similar to contaminants, attempts to extract RNA failed, DNAse and RNAse did not affect culture, propagation was suppressed following treatment with UV radiations, acidic pH, and trypsin (protein denaturation), and antibiotics failed to significantly alter growth. After demineralization with Ethylenediaminetetraacetic acid (EDTA) the core was analyzed with Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were identified in the core, including a high concentration of bovine fetuin that can incite immune response.[23] In fact, soluble extract from kidney stones positive for anti nanon antibodies showed the presence of fetuin on SDS-PAGE and western blot analysis.[23] This report refuted the whole literature on nanobacteria. Kajander, who coined the term nanobacteria, used the term calcifying nanoparticles in a later report.[24]
Subsequently, protein-apatite interactions were studied in vitro and in vivo in human and fetal bovine serum.[25] Serum proteins progressively bind with Ca and P in serum and upon reaching saturation form mineralo-protein complexes resembling NB, both chemically and morphologically.[25] Addition of precipitating ions to cell culture medium also generated mineral nanoparticles resembling NB.[26] Albumin, fetuin, and apolipoprotein A1 were the main constituent proteins and antibodies previously deemed specific for NB in fact reacted with albumin and/or fetuin.[26] It is possible that addition of proteins as serum to DMEM culture medium that is rich in Ca and P might have resulted in precipitation of apatite-protein complexes. Earlier literature on CNP was based on the assumption that gamma treatment of serum sterilizes the fluid entirely. But critical evaluation of gamma irradiated serum showed that it retains capability to form complexes in the presence of high ion concentration.[27]
Proteomic evaluation of these CNP revealed many other proteins as constituents: albumin, fetuin A, fetuin B, fetal hemoglobin, and EF-Tu, EF-G.[28] Demonstration of EF-Tu in these CNP added heat to the controversy. The source of other proteins can be traced to human or bovine serum, unlike the prokaryotic protein EF-Tu. The presence of elongation factors (EF-Tu, EF-G) in an apparently lifeless particle was an enigma. This revived the concept of biologic nature of nano particles.[19,28] There have been claims of successful treatment of NB associated diseases with anti NB therapy, comET® (Nanobac Life Sciences, Tampa, Florida) and antibiotics.[21,29,30] Unfortunately, these studies were not randomized and were poorly blinded. Tetracyclin and ampicillin that claimed to possess antiNB activity are calcium chelators.[29] Even patented comET consists of 500 mg tetracycline, a proprietary nutraceutical, and EDTA.
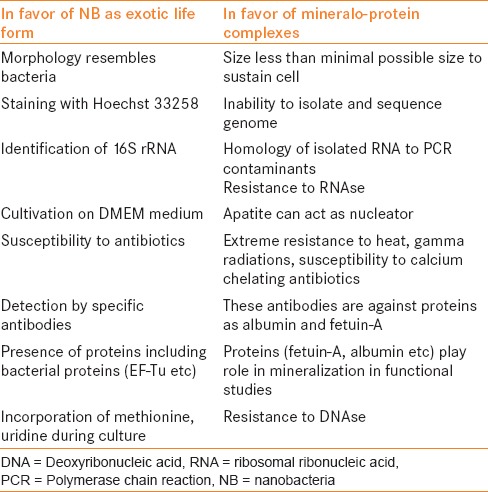
There are arguments in favor and against the biologic nature of these CNP [Table 2] and current understanding is that these are actually mineralo-protein complexes. Whether they are biologic or physicochemical phenomenon, do they have any role in renal stone formation? This review will elaborate on their association with stones with the available evidence.
Table 2.
Arguments on the nature of nanobacteria
CALCIFYING NANOPARTICLES AND STONE DISEASE: WHAT IS THE EVIDENCE
Role of apatite in stone disease
Renal stone formation is a form of biomineralization. Biomineralization can be conceptualized as incorporation of inorganic minerals into organic structural macromolecules (carbohydrates, lipids, and proteins) to form hard structures like bone, teeth, shells, spicules etc., The mineral phase of these tissues is hydroxylapatite (HA).[31] CaOx stones are the most common renal stones. Majority of idiopathic CaOx stones arise attached to Randall's plaque that itself is composed of apatite.[2] It has been observed that the majority of CaOx stones also contain CaP in the form of apatite at its core.[32,33] Experimental studies demonstrated that Randall's plaques begin in the basement membrane of the thin loop of Henle and extend into the interstitium.[34,35] Apatite has crucial role in pathogenesis of renal stones.
Direct evidence
Early experiments made it clear that these new nano particles form an apatite shell around them. In experimental studies they exerted cytotoxic effect on 3T6 fibroblasts and were localized in intracellular space.[36] Akerman et al., radio labeled CNP with 99mTc and injected it intravenously into rabbits to study their in vivo distribution with single photon emission computed tomography (SPECT) imaging.[37] There was tissue specific distribution and high accumulation was achieved in kidneys and urine 10 min after injection.[37] These CNP accessed urine through tubular cells using endocytic transport mechanism. Tubular cells take these cytotoxic CNP and we know from the previous studies that apatite plaque originates in the basement membrane of the thin loop of Henle.[34]
Investigators have isolated these nanoparticles from the majority of kidney stones.[3,4,9,38,39,40] Each one has differently interpreted the nature of these particles but isolation of coccoid nanoparticles with apatite shell has been reported unanimously. Taking a step nearer to prove their role in nephrolithiasis these particles have been isolated specifically from Randall's plaques.[41] CNP multiply 4.6 times faster in conditions of zero gravity. The increased incidence of renal stones in astronauts has been linked with CNP after their isolation from a stone recovered from the urine of an astronaut.[5,39] It is likely that CNP play crucial role in nephrolithiasis.
Indirect evidence
There is indirect evidence that CNPs play an important role in nephrolithiasis. An association between athelosclerosis, coronary artery disease, carotid artery stenosis and kidney stones has recently been found.[42,43] CNPs and fetuin-A are present in atherosclerotic plaques in human arteries and cardiac valves.[14] The central role of CNP in stone formation and atherosclerosis explains the association between kidney stones and myocardial infarction, hypertension, and cerebral stroke.
Probable mechanism of promoting nephrolithiasis
Fetuin: Inhibitor of mineralization
Fetuin (fetuin-A, apha-2-HS-glycoprotein, or alpha-2-Heremans-Schmid glycoprotein) was discovered from fetal bovine serum nearly 70 years ago in 1944, but its physiologic importance has only recently been recognized.[44] Fetuin-A is a 45-kDa plasma protein secreted by the liver.[45] It acts as an inhibitor of calcium phosphate mineral (apatite) precipitation by formation of complexes with Ca and P.[46] It is the key protein in the core of CNP. Low urinary levels of fetuin have been found in patients with documented urolithiasis[47] and those with fetuin-A gene polymorphism are at a higher risk of CaOx nephrolithiasis.[45] In addition to nephrolithiasis fetuin has been linked to other calcification related pathologies in the body. The patients on dialysis have low serum fetuin-A levels and they are more prone to coronary or other calcifications.[48] Fetuin-A has been isolated from kidney stones and calcified vascular foci.[23]
Dual inhibition-seeding concept
This is a phenomenon where the presence of inhibitory proteins suppresses apatite nucleation, until inhibitory influences are overcome with time in the presence of excess calcium or phosphate present in culture medium or in body fluids, when mineral–protein complexes precipitate and seed apatite propagation.[49] It is known that serum derived proteins; fetuin, albumin, are the main constituents of CNB. The role of fetuin and to a lesser extent albumin is to inhibit mineralization by binding with ions and making them more soluble.[46] [Figure 1] However, at saturation or at near saturation concentration this inhibition is overcome and fetuin gets precipitated as mineralo-protein complexes.[25,26,49] These apatite nuclei can grow in size and form crystals resembling those seen in Randall's plaques. [Figure 1] Dual inhibitory-seeding concept explains that CNP are formed predominantly by deployment of calcium inhibitory pathways and how inhibitors can act as seeding nuclei in the presence of favorable mineral concentrations.
Figure 1.
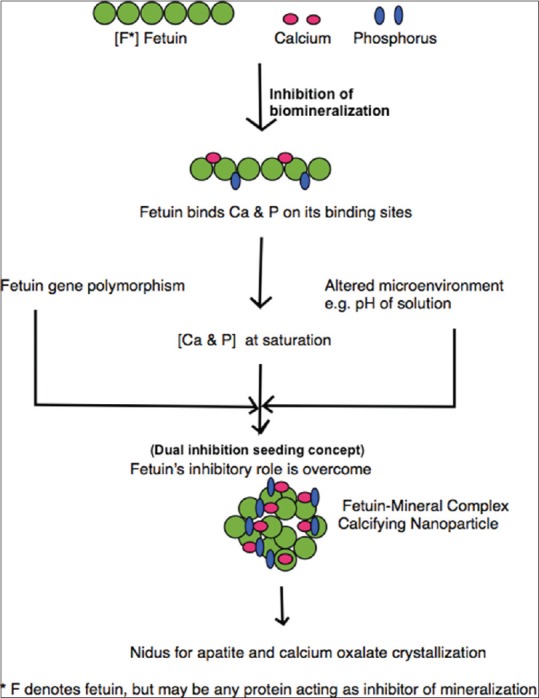
Flow chart showing proposed pathway of formation of calcifying nanoparticles and their role in nephrolithiasis
CONCLUSION
Nanoparticles have come a long way from fossils of Martian life, to exotic life forms on earth and finally mineralo-protein complexes. Though the current understanding is that they are mineralo–protein complexes, the finding of prokaryotic proteins on proteomics puts a query on their exact nature.
Whether these calcifying nanoparticles are exotic life forms or simply mineralo-protein complexes, there is enough evidence to show that CNP plays a pivotal role in inducing calcification and stone formation. They are cytopathic, localized in high concentration in kidneys, excreted in urine, isolated from kidney stones, found in Randall's plaques, associated with atherosclerotic diseases, and form apatite that is the component of Randall's plaques and majority of renal stones. They also cause stone formation in animal models. Continued research is needed to solve the controversy of whether they are living or nonliving.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Modlin M. A history of urinary stone. South Afr Med J. 1980;58:652–5. [PubMed] [Google Scholar]
- 2.Randall A. The origin and growth of renal calculi. Ann Surg. 1937;105:1009–27. doi: 10.1097/00000658-193706000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciftçioglu N, Björklund M, Kuorikoski K, Bergström K, Kajander EO. Nanobacteria: An infectious cause for kidney stone formation. Kidney Int. 1999;56:1893–8. doi: 10.1046/j.1523-1755.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- 4.Khullar M, Sharma SK, Singh SK, Bajwa P, Shiekh FA, Sheikh FA, et al. Morphological and immunological characteristics of nanobacteria from human renal stones of a north Indian population. Urol Res. 2004;32:190–5. doi: 10.1007/s00240-004-0400-3. [DOI] [PubMed] [Google Scholar]
- 5.Ciftçioglu N, Haddad RS, Golden DC, Morrison DR, McKay DS. A potential cause for kidney stone formation during space flights: Enhanced growth of nanobacteria in microgravity. Kidney Int. 2005;67:483–91. doi: 10.1111/j.1523-1755.2005.67105.x. [DOI] [PubMed] [Google Scholar]
- 6.Shiekh FA, Khullar M, Singh SK. Lithogenesis: Induction of renal calcifications by nanobacteria. Urol Res. 2006;34:53–7. doi: 10.1007/s00240-005-0034-0. [DOI] [PubMed] [Google Scholar]
- 7.Kajander EO, Kuronen I, Akerman KK, Pelttari A, Ciftcioglu N. Nanobacteria from blood: The smallest culturable autonomously replicating agent on Earth. ProcSPIE. 1997;3111:420–8. [Google Scholar]
- 8.Cisar JO, Xu DQ, Thompson J, Swaim W, Hu L, Kopecko DJ. An alternative interpretation of nanobacteria-induced biomineralization. Proc Natl Acad Sci U S A. 2000;97:11511–5. doi: 10.1073/pnas.97.21.11511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drancourt M, Jacomo V, Lépidi H, Lechevallier E, Grisoni V, Coulange C, et al. Attempted isolation of Nanobacterium sp. microorganisms from upper urinary tract stones. J Clin Microbiol. 2003;41:368–72. doi: 10.1128/JCM.41.1.368-372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folk RL. SEM imaging of bacteria and nannobacteria in carbonate sediments and rocks. J Sediment Res. 1993;63:990–9. [Google Scholar]
- 11.McKay DS, Gibson EK, Jr, Thomas-Keprta KL, Vali H, Romanek CS, Clemett SJ, et al. Search for past life on Mars: Possible relic biogenic activity in martian meteorite ALH84001. Science. 1996;273:924–30. doi: 10.1126/science.273.5277.924. [DOI] [PubMed] [Google Scholar]
- 12.Maniloff J. Nannobacteria: Size limits and evidence. Science. 1997;276:1776–7. doi: 10.1126/science.276.5320.1773e. [DOI] [PubMed] [Google Scholar]
- 13.Glass JI, Assad-Garcia N, Alperovich N, Yooseph S, Lewis MR, Maruf M, et al. Essential genes of a minimal bacterium. Proc Natl Acad Sci U S A. 2006;103:425–30. doi: 10.1073/pnas.0510013103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller VM, Rodgers G, Charlesworth JA, Kirkland B, Severson SR, Rasmussen TE, et al. Evidence of nanobacterial-like structures in calcified human arteries and cardiac valves. Am J Physiol Heart Circ Physiol. 2004;287:1115–24. doi: 10.1152/ajpheart.00075.2004. [DOI] [PubMed] [Google Scholar]
- 15.Puskás LG, Tiszlavicz L, Rázga Z, Torday LL, Krenács T, Papp JG. Detection of nanobacteria-like particles in human atherosclerotic plaques. Acta Biol Hung. 2005;56:233–45. doi: 10.1556/ABiol.56.2005.3-4.7. [DOI] [PubMed] [Google Scholar]
- 16.Ciftcioglu N, McKay DS, Mathew G, Kajander EO. Nanobacteria: Fact or fiction? Characteristics, detection, and medical importance of novel self-replicating, calcifying nanoparticles. J Investig Med. 2006;54:385–94. doi: 10.2310/6650.2006.06018. [DOI] [PubMed] [Google Scholar]
- 17.Mathew G, Mckay DS, Ciftçioglu N. Do blood-borne calcifying nanoparticles self-propagate? Int J Nanomedicine. 2008;3:265–75. [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Y, Zhang D, Lu H, Luo S, Shen X. Association between calcifying nanoparticles and placental calcification. Int J Nanomedicine. 2012;7:1679–86. doi: 10.2147/IJN.S29786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutikhin AG, Brusina EB, Yuzhalin AE. The role of calcifying nanoparticles in biology and medicine. Int J Nanomedicine. 2012;7:339–50. doi: 10.2147/IJN.S28069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boskey AL. Biomineralization: Conflicts, challenges, and opportunities. (83-91).J Cell Biochem Suppl. 1998:30–31. [PubMed] [Google Scholar]
- 21.Shoskes DA, Thomas KD, Gomez E. Anti-nanobacterial therapy for men with chronic prostatitis/chronic pelvic pain syndrome and prostatic stones: Preliminary experience. J Urol. 2005;173:474–7. doi: 10.1097/01.ju.0000150062.60633.b2. [DOI] [PubMed] [Google Scholar]
- 22.Wen Y, Li Y, Yang Z, Wang X, Wei H, Liu W, et al. Detection of nanobacteria in serum, bile and gallbladder mucosa of patients with cholecystolithiasis. Chin Med J (Engl) 2005;118:421–4. [PubMed] [Google Scholar]
- 23.Raoult D, Drancourt M, Azza S, Nappez C, Guieu R, Rolain JM, et al. Nanobacteria are mineralo fetuin complexes. PLoS Pathog. 2008;4:e41. doi: 10.1371/journal.ppat.0040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kajander EO. Nanobacteria--propagating calcifying nanoparticles. Lett Appl Microbiol. 2006;42:549–52. doi: 10.1111/j.1472-765X.2006.01945.x. [DOI] [PubMed] [Google Scholar]
- 25.Young JD, Martel J, Young D, Young A, Hung CM, Young L, et al. Characterization of granulations of calcium and apatite in serum as pleomorphic mineralo-protein complexes and as precursors of putative nanobacteria. PloS One. 2009;4:e5421. doi: 10.1371/journal.pone.0005421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young JD, Martel J, Young L, Wu CY, Young A, Young D. Putative nanobacteria represent physiological remnants and culture by-products of normal calcium homeostasis. PloS One. 2009;4:e4417. doi: 10.1371/journal.pone.0004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martel J, Wu CY, Young JD. Critical evaluation of gamma-irradiated serum used as feeder in the culture and demonstration of putative nanobacteria and calcifying nanoparticles. PloS One. 2010;5:e10343. doi: 10.1371/journal.pone.0010343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiekh FA, Charlesworth JE, Kim SH, Hunter LW, Jayachandran M, Miller VM, et al. Proteomic evaluation of biological nanoparticles isolated from human kidney stones and calcified arteries. Acta Biomater. 2010;6:4065–72. doi: 10.1016/j.actbio.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cíftçíoglu N, Miller-Hjelle MA, Hjelle JT, Kajander EO. Inhibition of nanobacteria by antimicrobial drugs as measured by a modified microdilution method. Antimicrob Agents Chemother. 2002;46:2077–86. doi: 10.1128/AAC.46.7.2077-2086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silay MS, Miroglu C. The risk of urolithiasis recurrence may be reduced with anti-nanobacterial therapy. Med Hypotheses. 2007;68:1348–50. doi: 10.1016/j.mehy.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Tadic D, Peters F, Epple M. Continuous synthesis of amorphous carbonated apatites. Biomaterials. 2002;23:2553–9. doi: 10.1016/s0142-9612(01)00390-8. [DOI] [PubMed] [Google Scholar]
- 32.Grases F, March JG, Conte A, Costa-Bauzá A. New aspects on the composition, structure and origin of calcium oxalate monohydrate calculi. Eur Urol. 1993;24:381–6. doi: 10.1159/000474333. [DOI] [PubMed] [Google Scholar]
- 33.Abraham PA, Smith CL. Evaluation of factors involved in calcium stone formation. Miner Electrolyte Metab. 1987;13:201–8. [PubMed] [Google Scholar]
- 34.Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, et al. Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607–16. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evan AP, Coe FL, Rittling SR, Bledsoe SM, Shao Y, Lingeman JE, et al. Apatite plaque particles in inner medulla of kidneys of calcium oxalate stone formers: Osteopontin localization. Kidney Int. 2005;68:145–54. doi: 10.1111/j.1523-1755.2005.00388.x. [DOI] [PubMed] [Google Scholar]
- 36.Çiftçioglu N, Kajander EO. Interaction of nanobacteria with cultured mammalian cells. Pathophysiology. 1998;4:259–70. [Google Scholar]
- 37.Akerman KK, Kuikka JT, Ciftcioglu N, Parkkinen J, Bergstroem KA, Kuronen I, et al. Radiolabeling and in-vivo distribution of nanobacteria in rabbits. Proc SPIE. 1997;3111:436–42. [Google Scholar]
- 38.Kajander EO, Ciftçioglu N. Nanobacteria: An alternative mechanism for pathogenic intra- and extracellular calcification and stone formation. Proc Natl Acad Sci U S A. 1998;95:8274–9. doi: 10.1073/pnas.95.14.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones JA, Ciftcioglu N, Schmid JF, Barr YR, Griffith D. Calcifying nanoparticles (nanobacteria): An additional potential factor for urolithiasis in space flight crews. (e11-3).Urology. 2009;73:210. doi: 10.1016/j.urology.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 40.Kumon H, Matsumoto A, Uehara S, Abarzua F, Araki M, Tsutsui K, et al. Detection and isolation of nanobacteria-like particles from urinary stones: Long-withheld data. Int J Urol. 2011;18:458–65. doi: 10.1111/j.1442-2042.2011.02763.x. [DOI] [PubMed] [Google Scholar]
- 41.Ciftçioğlu N, Vejdani K, Lee O, Mathew G, Aho KM, Kajander EO, et al. Association between Randall's plaque and calcifying nanoparticles. Int J Nanomedicine. 2008;3:105–15. doi: 10.2147/ijn.s2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rule AD, Roger VL, Melton LJ, 3rd, Bergstralh EJ, Li X, Peyser PA, et al. Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol. 2010;21:1641–4. doi: 10.1681/ASN.2010030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reiner AP, Kahn A, Eisner BH, Pletcher MJ, Sadetsky N, Williams OD, et al. Kidney stones and subclinical atherosclerosis in young adults: The CARDIA study. J Urol. 2011;185:920–5. doi: 10.1016/j.juro.2010.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mori K, Emoto M, Inaba M. Fetuin-A: A multifunctional protein. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:124–46. doi: 10.2174/187221411799015372. [DOI] [PubMed] [Google Scholar]
- 45.Aksoy H, Aksoy Y, Ozturk N, Aydin HR, Yildirim AK, Akçay F. Fetuin-A gene polymorphism in patients with calcium oxalate stone disease. Urology. 2010;75:928–32. doi: 10.1016/j.urology.2009.08.058. [DOI] [PubMed] [Google Scholar]
- 46.Price PA, Lim JE. The inhibition of calcium phosphate precipitation by fetuin is accompanied by the formation of a fetuin-mineral complex. J Biol Chem. 2003;278:22144–52. doi: 10.1074/jbc.M300744200. [DOI] [PubMed] [Google Scholar]
- 47.Stejskal D, Karpisek M, Vrtal R, Student V, Solichova P, Fiala R, et al. Urine fetuin-A values in relation to the presence of urolithiasis. BJU Int. 2008;101:1151–4. doi: 10.1111/j.1464-410X.2007.07432.x. [DOI] [PubMed] [Google Scholar]
- 48.Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Böhm R, et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: A cross-sectional study. Lancet. 2003;361:827–33. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 49.Wu CY, Martel J, Young D, Young JD. Fetuin-A/albumin-mineral complexes resembling serum calcium granules and putative nanobacteria: Demonstration of a dual inhibition-seeding concept. PloS One. 2009;4:e8058. doi: 10.1371/journal.pone.0008058. [DOI] [PMC free article] [PubMed] [Google Scholar]


