Abstract
Introduction:
We aimed to investigate the long-term outcome of trimodality therapy consisting of transurethral resection of bladder tumor, external beam radiation therapy, and concurrent intra-arterial low dose cisplatin for patients with muscle invasive bladder cancer.
Materials and Methods:
We retrospectively reviewed the medical records of 37 consecutive patients (28 men and 9 women) who underwent trimodality therapy for T2-3N0M0 bladder cancer at our hospital between 1996 and 2011. A total of 60Gy of external beam radiation therapy was administered. A daily low dose of cisplatin was administered intra-arterially through a subcutaneously placed reservoir on the days of radiation therapy. Complete response was defined as no residual cancer in transurethral resection specimens and negative cytology. When a complete response could not be achieved, patients underwent additional intra-arterial chemotherapy.
Results:
Five-year cause specific, disease free, and overall survival rates were 86.4%, 69.7%, and 69.6%, respectively, with a mean follow-up period of 56.5 ± 6.1 months. Five-year cause specific survivals of the complete response group after the trimodality therapy, the complete response group after additional intra-arterial chemotherapy and the non-complete response group were 100% (n = 21), 85.9% (n = 9) and 0% (n = 7), respectively. Five-year overall survivals of the complete response group after the trimodality therapy, the complete response group after additional intra-arterial chemotherapy and the non-complete response group were 82.8%, 85.3% and 0%, respectively.
Conclusions:
This trimodality therapy for muscle invasive bladder cancer could achieve favorable survival rates with bladder preservation and minimal adverse events. This trimodality therapy can be one of the useful treatment options.
Keywords: Bladder preservation, intra-arterial chemotherapy, muscle invasive bladder cancer, radiotherapy
INTRODUCTION
The standard treatment for patients with muscle invasive bladder cancer (MIBC) is radical cystectomy. The 5-year survival rate after radical cystectomy is reported to be less than 60%,[1] and approximately 50% of patients who undergo radical cystectomy eventually die of distant metastases. In addition, quality of life is considerably lowered due to urinary diversion-related problems.
Bladder preservation therapy is considered an alternative to radical cystectomy.[2] A number of studies[3,4,5] report the results of multimodality therapy for MIBC consisting of transurethral resection of the bladder tumor (TUR-BT), external beam radiation therapy (EBRT), and systemic chemotherapy, with 5-year survival rates similar to those in patients who underwent radical cystectomy. These studies suggest that bladder preservation could be achieved using multimodality therapy as a substitute for radical cystectomy. However, systemic chemotherapy may cause unfavorable adverse events, especially in the elderly.
Intra-arterial chemotherapy (IACT) is an effective treatment method for delivering highly concentrated anticancer drugs to the tumor site with less systemic toxicity. It has been reported that multimodality therapy combined with IACT produces survival rates similar to that of radical cystectomy, with bladder preservation and minimal adverse events.[6,7] In the present study, we report our results of trimodality therapy consisting of TUR-BT, EBRT, and IACT in patients with MIBC.
MATERIALS AND METHODS
Patients
This study was approved by the ethical committee of Nara Prefectural Nara Hospital. We reviewed the medical records of 37 consecutive patients (28 men and 9 women) who underwent trimodality therapy (TUR-BT, followed by EBRT and concurrent IACT for MIBC (T2-3N0M0)) at our hospital between 1996 and 2011. This treatment was performed in patients who were unable to undergo radical cystectomy and urinary diversion due to such conditions as advanced age, poor performance status, severe co-morbidity, and refusal to undergo surgery. Consent was obtained after histological confirmation of MIBC following initial TUR-BT. All patients were informed that the standard treatment for MIBC was radical cystectomy, and that this trimodality therapy was currently under investigation. TNM classification was staged according to the International Union Against Cancer Staging System (6th, 2002). Staging information in all cases was obtained through cystoscopy, computed tomography, magnetic resonance imaging, bone scintigraphy, and histological findings obtained by TUR-BT. All adverse events were graded using version 4.0 of the National Cancer Institute Common Terminology Criteria for Adverse Events.
Treatment schedule
Our treatment schedule is shown in Figure 1. Initially, all patients underwent TUR-BT, in which all visible tumors including the muscle layer were resected as deep as possible to confirm the pathological tumor stage and reduce the volume of the tumor. Prior to chemoradiotherapy, a port (P-U CELSITE PORT®, TORAY, Japan) was placed in the femoral subcutaneous tissue and a catheter (ANTHRON P-U CATHETER®, TORAY, Japan) was placed in the internal iliac artery. Coil embolization of superior and inferior gluteal arteries and obturator arteries were performed to modify the blood flow. In 19 patients, ports and catheters were placed bilaterally in the right and left internal iliac arteries, and in the remaining 18 patients in either the right or left iliac artery, according to the location of the main tumor.
Figure 1.
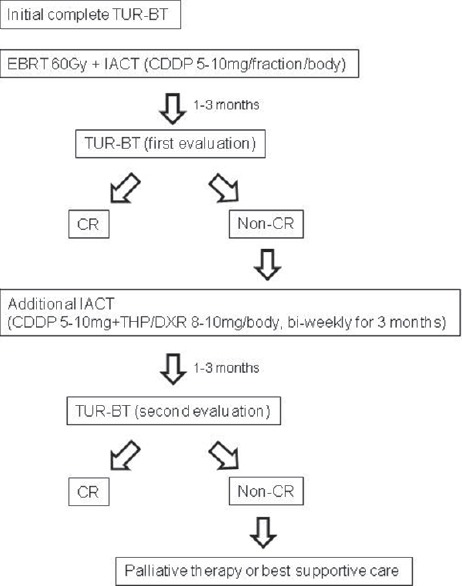
Treatment schedule
Five fractions weekly of 2.0 Gy per fraction, for a total of 40-60 Gy, were delivered to the whole pelvis using the four-field box technique, and an additional 10-20 Gy was administered to the urinary bladder using an opposing two-fold technique with 6 MV photons from a linear accelerator. EBRT was withheld if grade 3 or greater EBRT-related adverse events were observed.
Low dose cisplatin (CDDP, 5-10 mg/body) was administered intra-arterially through the port immediately before every fraction of EBRT, and this IACT was continued until the full dose of EBRT was completed. Two patients were administered carboplatin (a total of 450 and 520 mg, respectively) instead of CDDP because of renal dysfunction. IACT was withheld if grade 3 or greater adverse events appeared. Granulocyte colony-stimulating factor was given if neutrophil counts were below 500/mm3 or total white blood cell counts were below 1000/mm3.
Evaluation/Additional IACT
First evaluation of response: TUR-BT was performed in all patients 1-3 months after chemoradiotherapy for histological evaluation. Complete response (CR) was defined as no residual cancer in TUR specimens and negative cytology after treatment. Non-CR was defined as viable cancer in TUR specimens and/or positive cytology.
Additional IACT/Second evaluation: If a patient did not achieve CR at the first evaluation, additional IACT consisting of CDDP 5-10 mg/body and pirarubicin/doxorubicin (THP/DXR) 8-10 mg/body was administered bi-weekly on an out-patient basis for 3 months. One to 3 months after the completion of additional IACT, patients underwent TUR-BT for a second histological evaluation. The definition of second CR and non-CR was the same as that of the first evaluation.
Follow-up procedures
Follow-up studies of patients who achieved CR included computed tomography, cystoscopy, and urine cytology every 3 months for 2 years, every 6 months for the next 3 years, and every 12 months thereafter.
Statistical analysis
The cause-specific survival, disease-free survival, and overall survival rates were calculated by the Kaplan–Meier method. A P < 0.05 was considered to be significant. All statistical analyses were conducted using Prism software (GraphPad Software, San Diego, CA).
RESULTS
Patient characteristics are presented in Table 1. The mean age was 75.3 ± 1.1 (61-87) years. The histologic type identified in TUR specimens was urothelial carcinoma only. There were 17 patients in T2 and 20 patients in T3. The mean dosage of radiotherapy was 60 ± 0.6 (50-70) Gy. The mean of the total amount of CDDP in IACT concomitant with EBRT was 169.5 ± 9.5 (42-300) mg, and the means of the amount of CDDP and THP/DXR in the additional IACT were 51.3 ± 2.1 (36-60) mg and 54.7 ± 1.8 (40-60) mg, respectively. The total amount of CDDP administered to each patient in the full schedule of treatment was 42 to 300 (mean 190 ± 10.7) mg. The mean follow-up period was 56.5 ± 6.1 (6-137) months.
Table 1.
Patient characteristics (n=37)
The 5-year cause-specific, disease-free, and overall survival rates were 86.4%, 69.7%, and 69.6%, respectively [Figure 2].
Figure 2.
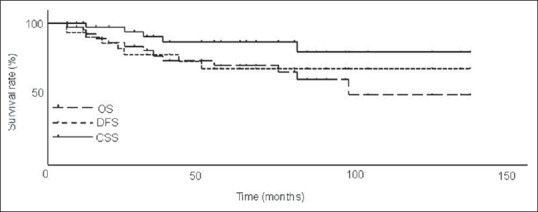
Cause-specific (CSS), disease-free (DFS), and overall survival rates (OS) in all cases (n=37). Five-year CSS, DFS, and OS were 86.4%, 69.7%, and 69.6%, respectively
First evaluation
Twenty-one patients (56.8%) achieved CR at the first evaluation. Among these patients, 2 subsequently developed recurrence of non-muscle invasive bladder tumors. None of the CR patients developed recurrence of muscle invasive tumors or distant metastasis. Seventeen were alive (16 without disease and 1 with non-invasive recurrence), and 4 died of other causes. Of the 16 non-CR patients (43.2%) at the first evaluation, 5 developed local recurrences (3 with non-invasive tumors and 2 with invasive tumors), and 3 had distant metastases. Seven were alive at the latest follow-up (5 without disease and 2 with distant metastases) and 9 died (5 of bladder cancer and 4 of other causes).
Second evaluation
Of the 16 patients who did not achieve CR at the first evaluation, 14 underwent additional IACT with CDDP and THP/DXR, and 2 received no additional treatment due to poor general condition. After completing additional IACT, 9 of 14 patients achieved CR at the second evaluation. Among these patients, 4 developed local recurrences in the bladder (3 non-invasive and 1 invasive tumors), and 2 had distant metastases. Six were alive at the latest follow-up (5 without disease and 1 with distant metastasis) and 3 died (2 of bladder cancer and 1 of other cause). Of the 7 non-CR patients, 1 was alive with distant metastasis and 6 died (3 of bladder cancer and 3 of other causes). The 5-year cause specific survivals of the CR group at the first evaluation, the CR group at the second evaluation and the non-CR group were 100% (n = 21), 85.9% (n = 9) and 0% (n = 7), respectively [Figure 3]. The 5-year cause specific survivals of the CR groups were significantly higher than that of the non-CR group (P < 0.0001, P = 0.0163, respectively). The 5-year overall survivals of the CR group at the first evaluation, the CR group at the second evaluation and the non-CR group were 82.8%, 85.3% and 0%, respectively [Figure 4]. The 5-year overall survivals of the CR groups were significantly higher than that of the non-CR group (P < 0.0001, P = 0.0014, respectively).
Figure 3.
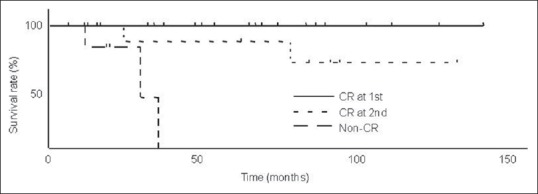
Cause-specific survival rates (CSS) of complete response (CR) at the first evaluation, CR at the second evaluation and the non-CR. Five-year CSS of these three groups were 100% (n=21), 85.9% (n=9) and 0% (n=7), respectively. Five-year CSS of CR groups were significantly higher than that of non-CR group (P<0.0001, P=0.0163, respectively)
Figure 4.
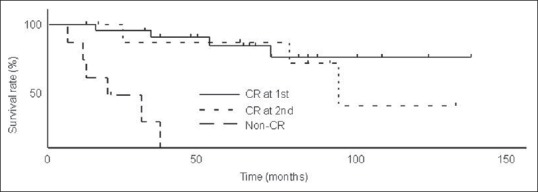
Overall survival rates (OS) of complete response (CR) at the first evaluation, CR at the second evaluation and the non-CR. Five-year OS of these three groups were 82.8% (n=21), 85.3% (n=9) and 0% (n=7), respectively. Five-year OS of CR groups were significantly higher than that of non-CR group (P<0.0001, P=0.0014, respectively)
Adverse events
None of the patients developed grade 4 toxicity. Grade 3 myelosuppression occurred in 2 patients (1 patient was treated with granulocyte colony-stimulating factor due to neutropenia and the other required a blood transfusion). Four patients had infections in their port sites and were forced to remove them. Two patients required conservative treatment in the hospital due to grade 3 gross hematuria. One patient developed severe lower gastrointestinal hemorrhage 2 months after chemoradiotherapy and needed a blood transfusion. One patient had vesicovaginal fistula 84 months after treatment. Other grade 1-2 toxicities were renal dysfunction in 9 patients, gastrointestinal toxicities (anorexia, nausea, and diarrhea) in 9, small bladder capacity in 8, myelosuppression in 5, and acute prostatitis in 1. Toxicities related to port-catheter placement and coil embolization (gluteal pain, imperceptions of the legs, and intermittent claudication) were observed in 8 patients. There was no vascular access related problem.
DISCUSSION
Standard treatment for MIBC is radical cystectomy. However, the morbidity rate reported with radical cystectomy is over 30%.[8] In addition, the 5-year overall survival rate after radical cystectomy is less than 60%.[1]
Recently, considerable interest has been focused on trimodality treatment, consisting of TUR-BT, chemotherapy, and radiotherapy for MIBC as bladder preservation therapy.[2] Shipley, et al.,[9] reported the outcome of patients with MIBC (stage T2-4) treated with trimodality therapy: The CR rate was 64% and the 5-year overall survival rate was 54% with a median follow-up of 7 years. Other groups have reported similar results of trimodality therapy for MIBC.[3,4,5] However, both severe acute and late toxicities associated with systemic chemotherapy and radiotherapy have negative impacts on the advantages of trimodality therapy, especially for the elderly or those who have severe co-morbidity. In addition, because of differences in patients and protocols among studies, it is difficult to compare their results with those of surgery alone and the best regimen for trimodality therapy remains to be elucidated.
IACT has been employed instead of systemic chemotherapy to improve local tumor control and minimize chemotherapy-related toxicities.[10] The concentration of CDDP in bladder tumor tissue was higher with administration via the internal iliac artery than that with intravenous infusion, and CDDP levels in peripheral tissues were almost the same as those with intravenous infusion.[11] Okada, et al.,[12] also reported that platinum levels in internal iliac venous blood after administration of CDDP through the internal iliac artery were higher than those after systemic intravenous administration. No significant difference was found in platinum levels in the systemic blood between intra-arterial and intravenous infusions. Thus, IACT can exert the same treatment effects as intravenous administration even with a lower dose of chemotherapeutic agents, resulting in reducing adverse events.
Recently, Hashine, et al.,[6] reported their favorable results of trimodality therapy using IACT. Ninety-four patients with MIBC were treated with radiotherapy at a total of 44-60 Gy concomitant with IACT using CDDP (20-25 mg/m2, day 1-3) and THP/DXR (15 mg/m2, day 8-10). With a median follow-up period of 72.9 months, 5 and 10-year overall survival rates were 66.6% and 47.4%, and cause-specific survival rates were 76.2% and 67.5%, respectively. Similar results were reported in other studies of radiotherapy and concurrent IACT for MIBC.[7,10] In the present study, the 5-year overall survival rate was 69.6% and cause-specific survival rate was 86.4%, with a mean follow-up period of 56.5 ± 6.1 months, which are comparable to those of previous reports.
In our IACT protocol, low dose CDDP was administered before every fraction of EBRT, and CDDP and THP/DXR were administered every 2 weeks to patients who did not achieve CR at the first evaluation. Grade 3 chemotherapy-related toxicities occurred in only 2 patients (myelosuppression), and grade 1-2 toxicities in 15 patients (myelosuppression, renal dysfunction, and gastrointestinal toxicities). The adverse events observed in this study were obviously fewer than those reported in previous studies of trimodality therapy using systemic chemotherapy[3,4,5,9] and even those using IACT.[6,7,10]
A total of 60 Gy was administered to the majority of our patients (40 Gy to the whole pelvis, 20 Gy to the urinary bladder). Grade 3 EBRT-related toxicities occurred in 4 patients (hematuria, lower gastrointestinal hemorrhage, and vesicovaginal fistula). Some studies showed the potential of reducing the radiation dose with IACT. Ikushima, et al.,[10] reported that the 3-year overall survival rate was 81%, when patients with MIBC were treated with EBRT of 40 Gy and concurrent IACT. Similarly, Sumiyoshi, et al.,[13] reported the 5-year overall survival rate was 74% by treatment with EBRT of 36-50 Gy and IACT. IACT-specific adverse events such as port site infection, gluteal pain, imperceptions of the legs, and intermittent claudication, which were partly due to the embolization of gluteal and obturator arteries, were observed in 12 patients (32%), although these were not severe.
Certain chemotherapeutic agents were reported to act as a radiation sensitizer against tumor cells in addition to their direct cytotoxic effects.[14,15] CDDP has also been shown to sensitize tumor tissues to radiation.[16] The efficacy of EBRT and concurrent low dose CDDP was reported in patients with non-metastatic non-small cell lung cancer: Schaake-Koning et al.,[17] demonstrated in their large scale randomized study that a combination of EBRT and low dose CDDP produced a significantly higher overall survival rate than that with EBRT alone.
In the present study, patients who did not achieve CR at the first evaluation were treated with additional IACT consisting of CDDP and THP/DXR every 2 weeks for 12 weeks. Of the 14 patients who underwent additional IACT, 9 achieved CR at the second evaluation. While it is possible that the residual tumor after initial trimodality therapy was completely resected by TUR performed as the first evaluation, second CR could partly be due to this additional IACT. If the initial trimodality therapy failed, salvage cystectomy should be considered for patients with non-metastatic MIBC. Our patients were considered to be unable to undergo invasive surgical treatment due to advanced age, poor performance status, severe co-morbidity, or refusal to undergo surgery. Traditionally, doxorubicin has been used as part of the MVAC regimen for urothelial carcinomas. Some studies used doxorubicin with CDDP as IACT and reported favorable results.[6,7,10]
We showed that 5-year cause-specific and overall survival rates of patients who achieved CR at the first or second evaluation were significantly higher than those of non-CR patients. Hashine, et al.,[6] reported that 89.4% of patients who were treated with trimodality therapy using IACT obtained CR, and that clinical stage T2 patients without hydronephrosis were good candidates for bladder preservation therapy. We were unable to find any significant factors associated with CR possibly because of the small sample size.
This study has some limitations. The patients were unable to undergo radical cystectomy, thus there is a selection bias. Definitive pathological staging cannot be assessed because radical cystectomy was not performed. Although the tumor stage was confirmed to be at least T2 with transurethral resection, clinical mis-staging may be included. We showed the potential of this trimodality therapy without severe adverse events. Further studies are needed to compare results with radical cystectomy or other bladder preservation therapies for MIBC.
CONCLUSIONS
Patients with MIBC can obtain benefits with trimodality therapy consisting of complete TUR-BT and EBRT with concomitant IACT. However, the standard indication and treatment regimen for trimodality therapy remains to be elucidated.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shariat SF, Karakiewicz PI, Palapattu GS, Lotan Y, Rogers CG, Amiel GE, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: A contemporary series from the Bladder Cancer Research Consortium. J Urol. 2006;176:2414–22. doi: 10.1016/j.juro.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Koga F, Kihara K. Selective bladder preservation with curative intent for muscle-invasive bladder cancer: A contemporary review. Int J Urol. 2012;19:388–401. doi: 10.1111/j.1442-2042.2012.02974.x. [DOI] [PubMed] [Google Scholar]
- 3.Krause FS, Walter B, Ott OJ, Haberle L, Weiss C, Rodel C, et al. 15-year survival rates after transurethral resection and radiochemotherapy or radiation in bladder cancer treatment. Anticancer Res. 2011;31:985–90. [PubMed] [Google Scholar]
- 4.Maarouf AM, Khalil S, Salem EA, ElAdl M, Nawar N, Zaiton F. Bladder preservation multimodality therapy as an alternative to radical cystectomy for treatment of muscle invasive bladder cancer. BJU Int. 2009;107:1605–10. doi: 10.1111/j.1464-410X.2010.09564.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman DS, Winter KA, Shipley WU, Heney NM, Wallace HJ, 3rd, Toonkel LM, et al. Phase I-II RTOG study (99-06) of patients with muscle-invasive bladder cancer undergoing transurethral surgery, paclitaxel, cisplatin, and twice-daily radiotherapy followed by selective bladder preservation or radical cystectomy and adjuvant chemotherapy. Urology. 2009;73:833–7. doi: 10.1016/j.urology.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 6.Hashine K, Kusuhara Y, Miura N, Shirato A, Sumiyoshi Y, Kataoka M. Bladder preservation therapy conducted by intra-arterial chemotherapy and radiotherapy for muscle invasive bladder cancer. Jpn J Clin Oncol. 2009;39:381–6. doi: 10.1093/jjco/hyp023. [DOI] [PubMed] [Google Scholar]
- 7.Mori K, Nomata K, Noguchi M, Eguchi J, Hayashi N, Kanetake H. Long-term follow up of patients with invasive bladder carcinoma receiving combined cisplatin-based intra-arterial chemotherapy and radiotherapy. Int J Urol. 2007;14:591–4. doi: 10.1111/j.1442-2042.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 8.Danesi DT, Arcangeli G, Cruciani E, Altavista P, Mecozzi A, Saracino B, et al. Conservative treatment of invasive bladder carcinoma by transurethral resection, protracted intravenous infusion chemotherapy, and hyperfractionated radiotherapy: Long term results. Cancer. 2004;101:2540–8. doi: 10.1002/cncr.20654. [DOI] [PubMed] [Google Scholar]
- 9.Shipley WU, Kaufman DS, Zehr E, Heney NM, Lane SC, Thakral HK, et al. Selective bladder preservation by combined modality protocol treatment: Long-term outcomes of 190 patients with invasive bladder cancer. Urology. 2002;60:62–8. doi: 10.1016/s0090-4295(02)01650-3. [DOI] [PubMed] [Google Scholar]
- 10.Ikushima H, Iwamoto S, Osaki K, Furutani S, Yamashita K, Kawanaka T, et al. Effective bladder preservation strategy with low-dose radiation therapy and concurrent intraarterial chemotherapy for muscle-invasive bladder cancer. Radiat Med. 2008;26:156–63. doi: 10.1007/s11604-007-0211-x. [DOI] [PubMed] [Google Scholar]
- 11.Terashima Y. CDDP concentration of bladder tumors--comparison between intraarterial infusion and intravenous infusion. Nihon GanChiryo GakkaiShi. 1988;23:859–66. in Japanese. [PubMed] [Google Scholar]
- 12.Okada H, Oguchi N, Uchida J, Mikami O, Matsuda T. Study on platinum concentration in internal iliac venous blood after iliac artery cisplatin infusion for invasive bladder cancer. Hinyokika Kiyo. 1999;45:145–8. in Japanese. [PubMed] [Google Scholar]
- 13.Sumiyoshi Y. Chemoradiotherapy as a bladder-preservation approach for muscle-invasive bladder cancer: Current status and perspectives. Int J Clin Oncol. 2004;9:484–90. doi: 10.1007/s10147-004-0434-0. [DOI] [PubMed] [Google Scholar]
- 14.Caffo O, Fellin G, Graffer U, Valduga F, Bolner A, Luciani L, et al. Phase I study of gemcitabine and radiotherapy plus cisplatin after transurethral resection as conservative treatment for infiltrating bladder cancer. Int J Radiat Oncol Biol Phys. 2003;57:1310–6. doi: 10.1016/s0360-3016(03)00763-6. [DOI] [PubMed] [Google Scholar]
- 15.Galsky MD. The role of taxanes in the management of bladder cancer. Oncologist. 2005;10:792–8. doi: 10.1634/theoncologist.10-10-792. [DOI] [PubMed] [Google Scholar]
- 16.Douple EB, Richmond RC. Platinum complexes as radiosensitizers of hypoxic mammalian cells. Br J Cancer Suppl. 1978;3:98–102. [PMC free article] [PubMed] [Google Scholar]
- 17.Schaake-Koning C, van den Bogaert W, Dalesio O, Festen J, Hoogenhout J, van Houtte P, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med. 1992;326:524–30. doi: 10.1056/NEJM199202203260805. [DOI] [PubMed] [Google Scholar]


