Abstract
Background:
Frequency of migraine changes at different times of a woman's reproductive cycle because of fluctuation of estrogen levels. Breast cancer has also a link with hormonal changes. Given this fact that both migraine and breast cancer are affected by estrogen, the prevalence of migraine may be different in breast cancer patients compared to the normal population.
Materials and Methods:
In this case–control study, two groups of women with and without breast cancer were compared regarding the prevalence of migraine. Each group consisted of 400 women. The diagnosis of different types of headache was made based on The International Headache Society (IHS) guidelines. Type of headache, type and receptor status of breast cancer, as well as history of taking hormonal medications was recorded. Independent t-test and Chi-square tests were used for data analysis.
Results:
Relative frequency of migraine headache in the normal woman was 38% compared to 19% in the breast cancer group (P < 0.0001). Tension headache was also significantly more prevalent in the normal group (P < 0.001). The frequency of migraine was significantly lower in estrogen receptor (ER)+/progesterone receptor (PR)− women compared to ER−/PR+ (26 and 43 women, respectively; P = 0.04); however, this difference was not significant for tension headache (P = 0.68).
Conclusion:
This study confirmed the lower frequency of migraine, as well as tension headache, in breast cancer sufferers. This could be contributed to several non-hormonal factors, such as a history of long term use of nonsteroidal anti-inflammatory drugs (NSAIDs), and hormonal factors, although only migraine showed a strong link with hormone status.
Keywords: Breast cancer, estrogen, hormonal status, migraine, prevalence
INTRODUCTION
Migraine is a common neurologic disorder which is more frequently found among women aged 25–55 years.[1] Women are approximately two to three times more likely to have migraine than men.[2] This significantly high prevalence of migraine in women has been a clue to investigate the association of female sex hormones and migraine. Investigations showed that a withdrawal state in either endogenous or exogenous estrogen concentration is a significant trigger of migraine in women.[3,4] The physiologic fluctuation of estrogen level during menarche, menses, pregnancy, and perimenopause affects the occurrence of migraine.[5] Among female migraineurs, 60% experience more episodes of severe migraine around the time of menses, when estrogen level is low.[6,7] Conversely, 67% of women with a history of premenopausal migraine report improvement of headache after the menopause. The majority of migraineurs report fewer attacks of migraine or even migraine remission during pregnancy, when they have a high level of estrogen without monthly hormone level fluctuation.[8] One important effect of estrogen on women health is in regard to breast cancer. The correlation between the breast cancer risk and lifetime exposure to estrogen is already proven.[9] Given this fact that both migraine and breast cancer are affected by estrogen, the prevalence of migraine may be different in breast cancer patients compared to the normal population.
The aim of this study is to assess and compare the relative frequency of migraine between breast cancer sufferers and the general population of women in order to find out the relationship of these two conditions.
MATERIALS AND METHODS
This case–control study was performed in 2010 and included a total number of 800 women, aged 20–60 years, divided equally into two groups of case and control.
The case group consisted of 400 women, with a positive history of breast cancer from breast cancer patients, registered at the oncology center of Isfahan University of Medical Sciences, Iran. They were selected by simple random sampling based on the patient's file number. Only patients with histologically confirmed diagnosis of breast cancer were included, whereas those with estrogen receptor (ER) negative/progesterone receptor (PR) positive breast cancer, as well as patients with unknown hormonal receptor status, were excluded from this study. Breast cancer type and hormonal receptor status were recorded for each case.
Women of the control group were enrolled through cluster sampling among women registered in five cultural centers of different areas of Isfahan, Iran. Eighteen women were randomly selected from each health center, and finally, similar to the case group, 400 females entered the study as the control group.
All women in both case and control groups were interviewed by a physician regarding having migraine, type of headache, and history of using exogenous hormones. The diagnosis of different types of headache was made based on The International Headache Society (IHS) guidelines[10] by a neurologist who is an expert in the field of headache.
Baseline characteristics of the patients were recorded in questionnaires as well.
Data were analyzed by SPSS 16.5 software, and independent t-test and Chi-square test were used as indicated. P-values less than 0.05 were considered as the level of significance.
This study is approved by the ethics committee of Isfahan University of Medical Sciences and patients were fully informed regarding the study before interview.
RESULTS
Comparing the two groups regarding baseline characteristics, no significant difference was found (57.38 ± 11.33 vs. 56.72 ± 10.67 for case and control, respectively; (T-test, P value=0.44).
The relative frequency of headache in the case group was significantly lower than the in control group [122/400 (30%) vs. 217/400 (54%), respectively; P < 0.0001, odds ratio 0.37, 95% confidence interval (CI) 0.27–0.49].
Based on the clinical characteristics of headache, 153 patients [100/400 (25%) in the control group and 53/400 (13%) in the case group] were diagnosed with tension headache. Tension headache had also significantly lower prevalence in the breast cancer sufferers than the normal women (P < 0.0001, odds ratio 2.18, 95% CI 1.51–3.15].
For comparing migraineurs to normal population, some parts of data analysis were performed after exclusion of patients with tension headache.
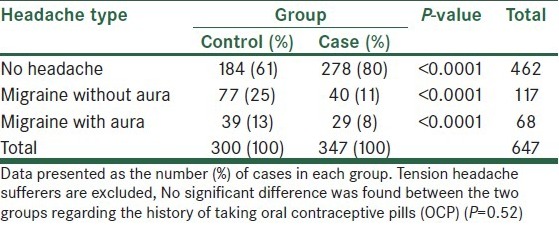
Women in the case group had significantly lower relative frequency of migraine than those in the control group. In contrast to the breast cancer sufferers who had only 19% relative frequency of migraine, women in the control group had a significantly higher relative frequency of migraine at 38% (P < 0.001, odds ratio 2.54, 95% CI 1.78–3.60).
Both migraine with and without aura were significantly more prevalent in the control group (P < 0.0001) [Table 1].
Table 1.
Distribution of different types of headache in women with and without breast cancer
Women with and without history of taking OCP had no significant difference regarding the frequency of migraine, either in the case group or in the control group [Table 2].
Table 2.
Frequency of migraine based on the history of taking OCP in women with and without breast cancer
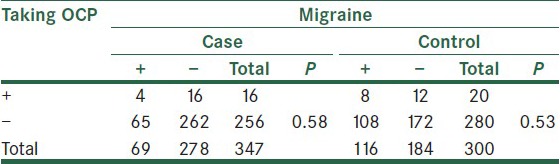
Data presented as the number of cases in each group. Tension headache sufferers are excluded.
Based on the breast cancer type (lobular breast cancer and ductal breast cancer), there was no significant difference in the relative frequency of migraine.
Although comparing the relative frequency of migraine in women with breast cancer showed no s ignificant difference based on the cancer type (P = 0.47), according to the hormone receptor status, migraine was significantly less common in women with a positive hormone receptor status [Table 3].
Table 3.
Frequency of migraine based on the cancer type and cancer hormone receptor status in breast cancer group
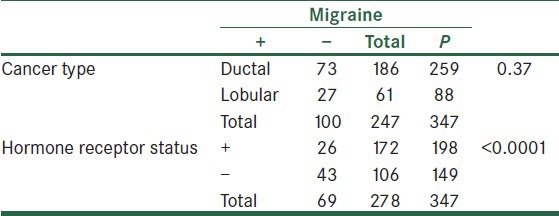
Data presented as the number of cases in each group. Tension headache sufferers are excluded.
In women with breast cancer, the frequency of tension headache showed no significant difference based on the hormone receptor status (25 women with ER+/PR− and 28 with ER−/PR− receptor status, P = 0.68). Conversely, the frequency of migraine was significantly lower in ER+/PR− women compared to ER−/PR+ (26 and 43 women, respectively, P = 0.04).
Regarding the type of migraine, although most of the ER+/PR− migraineurs had migraine with aura (18/26 vs. 8/26), this difference was not statistically significant (P = 0.11).
DISCUSSION
The present study showed that women suffering from breast cancer are less likely to have headache. Consistent with some previous studies, a significantly lower frequency of migraine,[11,12,13] as well as tension headache, was found in women with breast cancer. Though most of the previous studies have investigated the relationship of breast cancer and migraine, we found this relationship attributable to tension headache; hence, it can be inferred that a condition which is common in all headache sufferers may be responsible for this finding. One important similarity between individuals with tension headache and migraine is use of variety of analgesics including nonsteroidal anti-inflammatory drugs (NSAIDs). Many studies indicate that NSAID use may result in diminished risk of breast cancer.[11,14,15] It means that headache sufferers have lesser tendency to this sort of cancer because of long-term use of NSAIDs.
Although we may expect more rate of tension headache in the breast cancer sufferers, we found tension headache to be less prevalent in these patients. This finding may be caused by taking antidepressant medications or analgesics by breast cancer sufferers as a part of breast cancer management; however, it needs more studies to identify the exact cause.
Most of the previous studies have focused on the association of migraine with breast cancer. The primary motivation for probing the possible link between migraine and breast cancer is that both of these conditions are hormonally related.[13] On one hand, prolonged exposure to and higher concentrations of endogenous estrogen are known risk factors for breast cancer;[16] on the other hand, migraine is triggered by decline or fluctuation of the estrogen level.[17] The combination of these conditions is a clue to figure out the role of hormones in this relationship.
This hormonal link is also confirmed by the significantly lower frequency of migraine in ER+/PR− patients than in ER−/PR− ones. This finding supports the role of estrogen in the pathogenesis of migraine. A study performed by Li et al. reported 17% lower risk of ER+/PR− breast cancer in migraineurs; this can explain our finding through reverse causation.[13]
Despite the lower frequency of both tension headache and migraine in breast cancer sufferers, we found a difference between these two variations of headache in the case group: In contrast to migraine that showed a prominent tendency to afflict ER−/PR− women more than ER+/PR− ones, the frequency of tension headache had no difference regarding hormone receptor status.
This indicates that although breast cancer sufferers are less likely to have both migraine and tension headache because of different reasons, the hormonal link between breast cancer and headache may be related to migraine only.
Apart from the aforementioned possible role of long-term use of NSAIDs, some researchers believe that the some researchers believe that migraine is less prevalent in women with breast cancer because migraineurs are less likely to use migraine triggers including alcohol and exogenous hormones, the two known risk factors for breast cancer, and for this reason they have lower risk of developing breast cancer.[12,13,18] In other words, the frequency of migraine is lower among breast cancer sufferers because migraineurs have been kept away from breast cancer risk factors for another reason which is prevention of migraine.
In this study, there was no significant difference between cases and controls in history of taking OCP, and in the study of Li et al., migraine sufferers were more likely to use OCP.[13] This implies that different endogenous hormone status, in addition to less exposure to risk factors, may play role in the relationship of migraine and breast cancer.
Limitations of this study include a relatively small sample size, lack of precise medication history of patients, and not assessing other risk factors of both migraine and breast cancer.
In summary, this study confirmed the lower frequency of migraine, as well as tension headache, in breast cancer sufferers. This could be contributed to several non-hormonal and hormonal factors although only migraine showed a strong link with hormone status. For better clarification of this relationship, additional studies are needed to investigate the potential effective factors more accurately in a prospective design.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64–9. [PubMed] [Google Scholar]
- 2.Nomura M, Akama KT, Alves SE, Korach KS, Gustafsson JA, Pfaff DW, et al. Differential distribution of estrogen receptor (ER)-alpha and ER-beta in the midbrain raphe nuclei and periaqueductal gray in male mouse: Predominant role of ER-beta in midbrain serotonergic systems. Neuroscience. 2005;130:445–56. doi: 10.1016/j.neuroscience.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Scharff L, Turk DC, Marcus DA. The relationship of locus of control and psychosocial-behavioral response in chronic headache. Headache. 1995;35:527–33. doi: 10.1111/j.1526-4610.1995.hed3509527.x. [DOI] [PubMed] [Google Scholar]
- 4.Sulak PJ, Scow RD, Preece C, Riggs MW, Kuehl TJ. Hormone withdrawal symptoms in oral contraceptive users. Obstet Gynecol. 2000;95:261–6. doi: 10.1016/s0029-7844(99)00524-4. [DOI] [PubMed] [Google Scholar]
- 5.Silberstein SD. Sex hormones and headache. Rev Neurol (Paris) 2000;156(Suppl 4):30–41. [PubMed] [Google Scholar]
- 6.HA Z. Hormonal changes throughout life in women. Headache. 2006;46:S49–54. doi: 10.1111/j.1526-4610.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 7.Martin VT. Epidemiology and biology of menstrual migraine. Headache. 2008;48:S124–30. doi: 10.1111/j.1526-4610.2008.01310.x. [DOI] [PubMed] [Google Scholar]
- 8.Melhado EM, Maciel JA, Guerreiro CA. Headache during gestation: Evaluation of 1101 women. Can J Neurol Sci. 2007;34:187–92. doi: 10.1017/s0317167100006028. [DOI] [PubMed] [Google Scholar]
- 9.Dumitrescu RG, Cotarla I. Understanding breast cancer risk - where do we stand in 2005? J Cell Mol Med. 2005;9:208–21. doi: 10.1111/j.1582-4934.2005.tb00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lampl C. [Migraine - diagnostic features, acute therapy and prophylactics] 20110901 DCOM- 20111122 (0040-5930 (Print)) doi: 10.1024/0040-5930/a000202. [DOI] [PubMed] [Google Scholar]
- 11.Li CI, Mathes RW, Bluhm EC, Caan B, Cavanagh MF, Chlebowski RT, et al. Migraine history and breast cancer risk among postmenopausal women. J Clin Oncol. 2010;28:1005–10. doi: 10.1200/JCO.2009.25.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathes RW, Malone KE, Daling JR, Davis S, Lucas SM, Porter PL, et al. Migraine in postmenopausal women and the risk of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:3116–22. doi: 10.1158/1055-9965.EPI-08-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li CI, Mathes RW, Malone KE, Daling JR, Bernstein L, Marchbanks PA, et al. Relationship between migraine history and breast cancer risk among premenopausal and postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2009;18:2030–4. doi: 10.1158/1055-9965.EPI-09-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris RE, Chlebowski RT, Jackson RD, Frid DJ, Ascenseo JL, Anderson G, et al. Breast cancer and nonsteroidal anti-inflammatory drugs: Prospective results from the Women's Health Initiative. Cancer Res. 2003;63:6096–101. [PubMed] [Google Scholar]
- 15.Takkouche BR, Etminan M. Breast cancer and use of nonsteroidal antiinflammatory drugs: A meta-analysis. J Natl Cancer Inst. 2008;100:1439–47. doi: 10.1093/jnci/djn324. [DOI] [PubMed] [Google Scholar]
- 16.Clemons M. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 17.Scharff LT, Marcus DA. Triggers of headache episodes and coping responses of headache diagnostic groups. Headache. 1995;35:397. doi: 10.1111/j.1526-4610.1995.hed3507397.x. [DOI] [PubMed] [Google Scholar]
- 18.Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW, Jr, et al. Alcohol, tobacco and breast cancer: Collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–45. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]


