Abstract
Background:
The rate of dementia is increasing rapidly. With the recognized high rate of illiteracy among geriatric patients, preparing an appropriate device for special screening among the low-educated elderly seems to be necessary. The aim of this study is to prepare and assess the psychometric properties of the Persian version of the Kimberley Indigenous Cognitive Assessment (KICA) in Iranian adults, in 2012.
Materials and Methods:
One hundred and eighty elders participated in this psychometric study. Ninety patients with dementia according to the Diagnostic and Statistical Manual of Mental Disorders, fourth Edition, Text Revision (DSM-IV-TR) criteria, who had been admitted to Geriatrics and Neurology in some of the private clinics in Esfahan, Iran, in 2012, were selected. The rest of the participants were normal persons with the same demographic characteristics as the dementia group, who were selected from the patients’ acquaintances and from the Retired Personnel Organization. The statistical tools were the KICA scale, Mini-Mental State Examination (MMSE), and Modified Mini-Mental State Examination (3MSE).
Results:
The best clinical cutoff point of the test was 31, with a sensitivity of 92% and specificity of 88%. The Cronbach's alpha coefficient of KICA was 0.93. Among the KICA's subscales, the maximum Cronbach's alpha coefficient belonged to Praxis (α = 0.933) and the minimum one belonged to Delayed Recall (α = 0.927). The correlation coefficients of the KICA score with MMSE and 3MSE were 0.58 and 0.57, respectively.
Conclusion:
The KICA test has been seen to be a reliable and valid tool to assess cognitive impairment in the aged people of Iran. The KICA test can be used as a cognitive assessment test for distinguishing patients with dementia, especially illiterate ones from other healthy people in Iran.
Keywords: 3MSE, geriatrics, Kimberley Indigenous Cognitive Assessment (KICA), MMSE, psychometric properties, reliability, validity
INTRODUCTION
Dementia is a disorder in various cognitive aspects that is at least concomitant with one of the cognitive aspects that include, verbal, Praxis, Gnosis, and executive function, which are associated with considerable disorders in occupational and social functions.[1] The prevalence rate of dementia is increasing rapidly in such a way that the World Health Organization (WHO) calculates that the prevalence rate of the illness will reach from about 0.40% in 2006 to 0.44% in 2015 and 0.56% in 2030.[2] In another study conducted by the Alzheimer Association in 2009, the rate of dementia prevalence in the world will reach from 35.6 million people in 2010 to 65.7 million people in 2030 and 115.4 million people in 2050.[3] The illness causes a decrease in longevity and it is the second cause of mortality in the geriatric population after cancer.[4,5,6] This illness is diagnosed according to the patient's medical history, acquaintances’ complementary history, and clinical observations based on the existence of neurological and neuropsychological features.[7,8]
For the purpose of distinguishing the other cerebral pathologies or diagnosing the dementia subgroup, single-photon emission computed tomography (SPECT), positron emission tomography (PET), magnetic resonance imaging (MRI), and the computed tomography (CT) scan are used.[9] The most rampant type of dementia is Alzheimer's, which commences many years before the patient starts treatment, so diagnosing the cognitive changes in the preclinical stage will lead to diagnosing in the initial stages, wherein the treatment will be more effective.[10,11,12] With respect to early diagnosis, preparing and applying neuropsychological tests is necessary. Some studies have shown that eight years before a patient completes the full criteria of illness, diagnosing with neuropsychological tests is possible.[13]
The most common neuropsychological tests are: Trail Making, Clock Drawing Test (CDT), 3MSE, and MMSE. Among them, MMSE has the most applications in assessing the initial cognitive function of adults.[14,15,16,17,18,19] For most of these tests the examinee should at least have a minimum educational level (e.g., for MMSE the minimum grade is the eighth grade). Unfortunately a great part of aged people in our society do not have the ability to read or write. According to the last reports of the Census Center of Iran, 75% of the Iranian geriatrics had been illiterate in 1996 and conducting these tests on that society has no reliability or validity. Therefore, matching the KICA test with the Iranian society and studying the psychometric properties and clinical cutoff point is necessary.
The Kimberley Indigenous Cognitive Assessment scale has been devised for indigenous illiterate adults in Australia and due to its simplicity it is appropriate for illiterate people and people with elementary education. The test's validity has been reviewed in different rural populations in Australia.[20] The KICA's validity and reliability in assessing and diagnosing dementia has been studied in subjects above 45 years of age. The results show that at a cutoff point of 33.39, with a sensitivity of 93.3% and specificity of 98.4%, KICA-Cog has high discriminate validity and appropriate reliability.[20] The advantage of this test is its ability to assess illiterate adults, as it has been reported by Legollis et al. (2006) that 61% of people, older than 45 years of age and tested with KICA, have had no formal education.[20] The qualification and application of KICA has been reported in a few studies in Southeast Asia. In a study carried out by Smith et al., this test, at a cutoff point of 37, has the best balance of sensitivity and specificity and shows a high correlation with the psychiatric diagnosis.[21] Considering the high population of illiterate geriatrics in Iran, the need for preparing and validating the appropriate criteria for determining the cognitive disorders was felt. The aim of this study is to prepare and determine the psychometric properties of KICA in the Iranian population.
MATERIALS AND METHODS
In this psychometric survey the sample size with respect to the inclusion and exclusion criteria, was 180 people, including 90 people suffering from dementia and 90 normal people in the age range of 45-88 years. All of these samples were assessed by the 3MSE, MMSE, and KICA tests by two psychologists (MS and PhD) and a psychiatric assistant. Ninety participants were admitted to the Adults’ Clinic, Neurology Clinic, and some of the related private clinics in Esfahan, Iran, in 2012, and were diagnosed with dementia according to the valid criteria, such as, DSM-IV-TR. The inclusion criteria included: Persons above 45 years of age, with a clinical diagnosis of dementia, illiterate or with elementary education, and knowledge of the Persian language. The exclusion criteria included: Persons suffering from severe psychiatric disorders, such as, schizophrenia and other psychosis, mental retardation, substance abuse, and substance poisoning.
A normal sample consisted of 90 people from the general population, who were similar to their counterparts in the case group, in terms of age, gender, and education level. They were selected from the patients’ acquaintances and from the Retired Personnel Organization. The data were analyzed by SPSS-20, in terms of correlation methods and discriminate and regression analysis.
INSTRUMENTS
Kimberley indigenous cognitive assessment
This tool has been devised by LoGiudice et al. in the West Australia University.[22] The test includes 17 items, which contain a couple of questions, pictures, and simple objects. The assessment topics include orienting, recalling, recognition, performing, doing simple dynamic skills, and joining parts. Scoring for some items is divalent and for some others it is 3, 5, and 6. The maximum score is 40. Performing and scoring are done within the clinical interview framework. The scale is translated by the investigation team. Bearing in mind the Iranian society, the pictures are substituted and altered. The inter-rater reliability coefficient is obtained with the help of the Kappa coefficient and the Bland Altman method. The difference between the raters is seen to be −0.07, with an SD of 1.83. Other reports show that five subscales (orienting, ability for naming, recording the recall, and free recall) have been able to categorize 91.4% of the subjects successfully. The sensitivity and specificity of the criteria in the clinical cutoff point of 31.32 have been reported to be 91 and 93%, respectively.[23]
Mini-mental state examination
This examination contains 11 cognition task items, which are divided into two parts. The sum of the scores varies from 1 to 30. The first part of the test evaluates some factors such as orientation, memory, and attention; the maximum score in this part is 21. The second part entails an examination in writing and speaking. The maximum score in this part is 9.[24] The reliability of the test in Iran has been reported as 0.73 with a cutoff point of 18; it can distinguish the people with dementia from healthy ones with a sensitivity of 95% and specificity of 97%[25] Although this scale has high reliability and validity, it has a problem with screening illiterate people or those with elementary education.[26] For the purpose of obviating the problems of the scale, an altered form prepared by Tang et al. was used which the score has been changed from 30 to 100 in that.[27]
3MSE test
The 100-point test of 3MSE included additional points on personal information, verbal fluency, abstract-verbal reasoning, and delayed recall. These changes allowed the test to not only distinguish patients with dementia, but also predict the performance of people after Apoplexy.[28,29] In a research conducted by Shanz et al., in Canada, this form was revised to screen people with dementia, to prove the cognitive problems without dementia. A cutoff point of 86.87, with the best sensitivity and specificity was reported. Considering the above-mentioned cutoff point, a sensitivity of 70% and a specificity of 89% was reported for those with cognitive problems and for changing dementia it showed a sensitivity of 67%.[30]
RESULTS
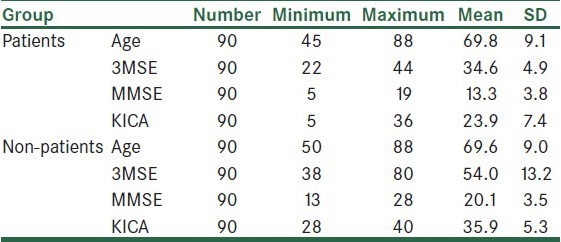
In this study 90 participants with dementia and 90 normal participants were assessed. There were 34 men and 56 women in each group, and 58 participants of both groups were illiterate and 32 participants had elementary education. The mean age of the patient group was 69.8 ± 9.1 years and that of the other group was 69.9 ±.9 years, Among 90 patients, 72 patients had Alzheimer disease, three had vascular dementia, one patient had frontotemporal dementia, and four had multiple etiologies. Table 1 shows the mean, standard deviation, age, and KICA, 3MSE, and MMSE's scores in patients and non-patient participants.
Table 1.
Mean, SD, age, and KICA, 3MSE, and MMSE's scores in the patient and non-patients participants
Reliability of the KICA scale
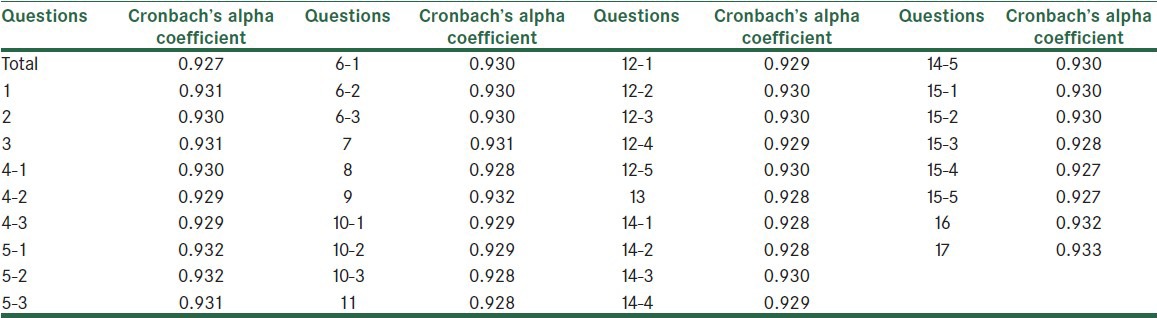
Cronbach's alpha coefficient of the whole scale was 0.93. Cronbach's alpha coefficient for each of the items was calculated and the highest one belonged to question number 17 and the lowest one belonged to question number 15. The information has been presented in Table 2.
Table 2.
Cronbach's alpha coefficient for each KICA question
The internal correlation coefficient was 0.93 and was significant (P < 0.001). confidence interval 0.91-0.94.
Evaluating KICA's validity
Face and Content Validity: The content of this questionnaire's items was assessed by geriatrics, psychiatrists, neurologists, and clinical psychologists in a pilot study that lasted a year in the Isfahan Geriatrics’ Clinic and the items were revised and verified.
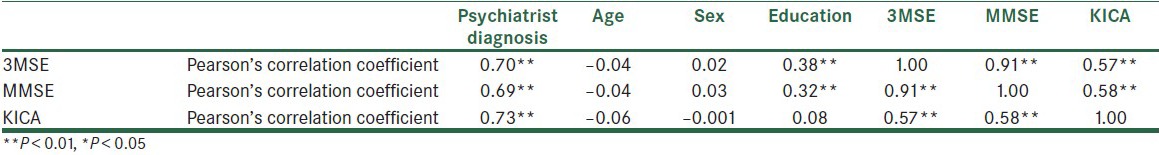
Concurrent and Construct Validity: In Table 3, the correlation between KICA and the gold standard scales has been shown. According to this table, KICA has a correlation of 0.58 and 0.57 with 3MSE and MMSE, respectively. 3MSE and MMSE have a correlation with the education level, while KICA does not.
Discriminate Validity: The results of the discriminate analysis showed that the canonical correlation between the KICA's scores and the expert's diagnosis was r = 0.73 and P < 0.001.
Table 3.
Correlation between KICA, 3MSE, and MMSE scores
Determining the clinical cutoff point and the sensitivity and specificity of the tests
For the purpose of determining the clinical cutoff point and the sensitivity and specificity of the tests, a discriminate analysis was carried out. Table 4 and Figure 1 show the area of the ROC curve. The ROC curve area of KICA equals 0.97, which is a significant amount.
Table 4.
The results of the discriminate analysis and the ROC curve area of KICA, 3MSE, and MMSE
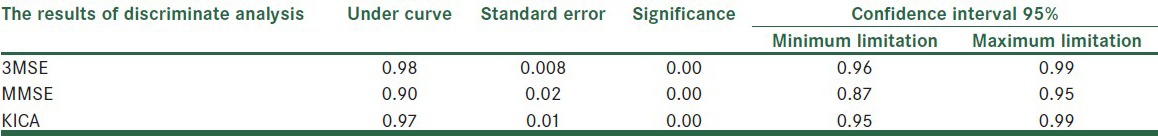
Figure 1.
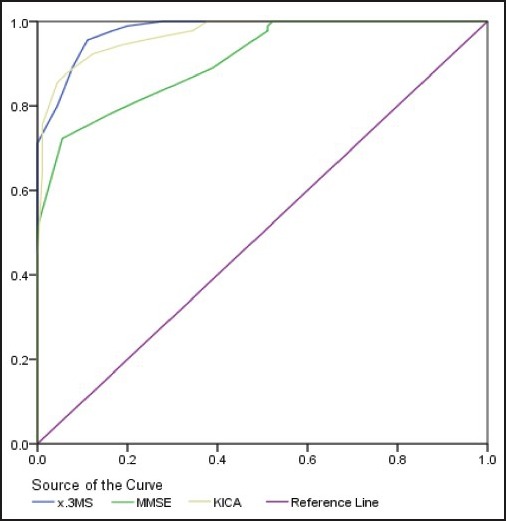
ROC Diagram of the KICA, 3MSE, and MMSE criteria
In Table 5 the cutoff points, sensitivity, and specificity of the three tests have been shown.
Table 5.
Cutoff points, sensitivity, and specificity of KICA, 3MSE, and MMSE tests
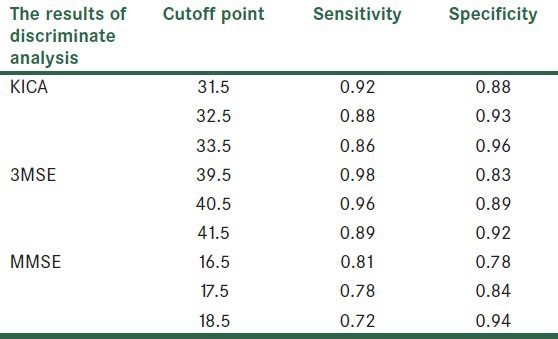
The best clinical cutoff point is sensible in the balance between sensitivity and specificity. The KICA's cutoff point was determined as 31, with a sensitivity of 92% and a specificity of 88%. The 3MSE's cutoff point was determined as 40, with a sensitivity of 96% and a specificity of 89%. The MMSE's cutoff point was determined as 16, with a sensitivity of 81% and a specificity of 78%.
For determining the ability of predicting dementia according to three tests and determining the prediction equation, Logistic Regression Analysis was used. This information has been presented in the Table 6.
Table 6.
Prediction coefficients and the ratio of dementia according to the KICA, 3MSE, and MMSE's scores
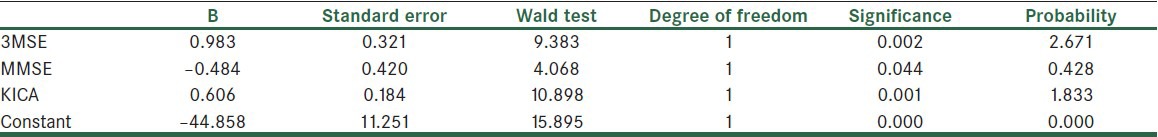
According to the Table 6 per one score reduction in 3MSE, the chance of dementia will become 2.6 times. This number is 1.8 in the case of KICA and 0.4 in the case of MMSE. The formula given below, based on Table 6, is the equation for predicting the possibility of dementia:
Y (group membership) = 0.98 * (3MSE) − 0.84 * (MMSE) + 0.61 * (KICA) − 44.8
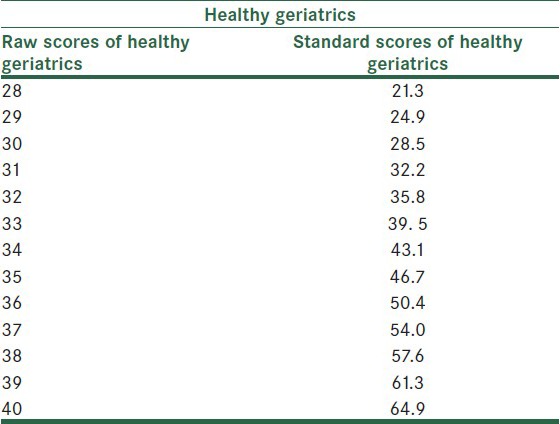
Converting KICA raw scores to standard scores
For the KICA's scores to become more explainable and for determining the status of a person in terms of his/her cognitive performance among other adults, the raw scores were converted to T scores. The results are presented in Tables 7 and 8.
Table 7.
The authors wish to appreciate
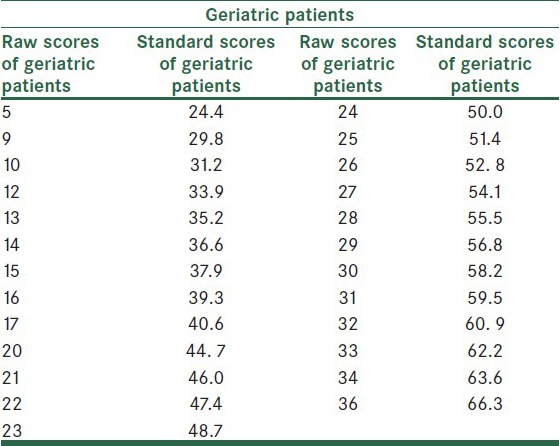
Table 8.
Conversion of KICA scores to T scores in healthy Geriatrics
DISCUSSION
In this study the whole reliability of the KICA scale was calculated by Cronbach's alpha (0.93) and its correlation with 3MSE and MMSE as the gold standard scales, which were 0.58 and 0.57, respectively. KICA's correlation coefficient, with psychiatrist and neurologist diagnosis, was equal to 0.73. LoGiudice et al., obtained the internal correlation as 0.81, which was the same as the result of this study. In another study done by Smith et al., this test showed a high correlation with psychiatrist diagnosis[21] that was very similar to that of the present study. The results of our study showed that the best clinical cutoff point for KICA was 31, with a sensitivity of 92% and a specificity of 88% and the cutoff point above 31 was concomitant with less sensitivity. The ROC curve showed that the area under the curve was 0.97, which was a significant one. This is comparable with the main studies conducted in the Northern Territory by LoGiudice et al., in which the cutoff point was 33.39, with a sensitivity of 93.3% and a specificity of 98.4%.[23] The cause for such a trivial difference may be the nature of society and the method of choosing samples. The present samples were from the geriatrics’ clinics, which may have an overlap of behavioral disorders, while in the LoGiudice et al. study, samples were collected from the rural regions and the KICA scale was developed according to that situation. The area under the curve, cutoff point, sensitivity, and specificity in the present study were the same as those in a study conducted in Australia (except Kimberly), in which the area under the ROC curve was 0.95, sensitivity was 82.3%, specificity was 87.5%, and cutoff point was 31-32. This signified that this instrument had the potential to be applied in the non-Kimberly area.[31] On the other hand, the psychometric properties of KICA in Iranian geriatrics were comparable with the results of Smith et al.'s cross-regional studies.[32] Although, in an indigenous population, the KICA scale had a cutoff point of 33.34, a sensitivity of 93.3%, and a specificity 98.4%, in a similar study, in the Smith et al., studies,[32] a cutoff point of 31.32, with a sensitivity of 82.4% and a specificity 87.5% was obtained. High indices of sensitivity and specificity in the present study showed a high ability of the test to distinguish negative cases (healthy subjects) from positive cases (impaired subjects), and also to signify a high discriminate validity in Iranian illiterate adults and its correlation with psychiatrist and neurologist diagnosis (r = 0.73).
In addition to discriminate validity, the high and significant correlation of the KICA scale with the 3MSE and MMSE scales (0.58 and 0.57, respectively) — which are the common gold standard tools for cognitive assessment of adults — shows the high construct and criteria-concurrent validity in Iran.
The results of the present study showed that the scores of the KICA test have no correlation with the level of education (P > 0.05). One of the most important aims of this study was to prepare and indigenize an instrument for assessing illiterate geriatrics and the ones with elementary education, in Iran. The lack of correlation between the KICA scores and educational level shows that this scale is an appropriate instrument for cognitive assessment in Iranian illiterate geriatric patients. Among the KICA's items the maximum correlation coefficient belonged to item number 17 (apraxia) and the minimum one belonged to item number 15 (delayed recall). Among the items, the maximum intergroup correlation coefficient belonged to item numbers 1, 2, 3 (orientation), 4 (identifying objects), 6 (naming), 9 (verbal fluency), and 16 and 17 (object application). On the other hand, as identifying pictures of an alligator and a kettle had the minimum correlation, replacing these pictures seems necessary. Moreover, due to the fact that the method of sampling was sampling from the available geriatric and neurology clinics, choosing samples from the high wide population would be desirable.
ACKNOWLEDGMENT
The authors wish to appreciate the Director of the Behavioral Sciences Research Center, Director and staff of the Geriatric Patients Clinic, and the Director of the Kashani Neurology Clinic for their warm cooperation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Weimer MF, Lipton AM. United States: American Psychiatric Publishing Inc; 2009. Textbook of Alzheimer Disease and Other Dementias. [Google Scholar]
- 2.World Health Organization. Switzerland: World Health Organization; 2006. Neurological Disorders: Public Health Challenges; pp. 204–7. [Google Scholar]
- 3.Martin Prince, Renata Bryce, Emiliano Albanese, Anders Wimo, Wagner Ribeiro, Cleusa P. Ferri. The global prevalence of dementia: A systematic review and metaanalysis : Alzheimer's & Dementia. 2013;9:63–7. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Mölsä PK, Marttila RJ, Rinne UK. Survival and cause of death in Alzheimer's disease and multi-infarct dementia. Acta Neurol Scand. 1986;74:103–7. doi: 10.1111/j.1600-0404.1986.tb04634.x. [DOI] [PubMed] [Google Scholar]
- 5.Bowen JD, Malter AD, Sheppard L, Kukull WA, McCormick WC, Teri L, et al. Predictors of mortality in patients diagnosed with probable Alzheimer's disease. Neurology. 1996;47:433–9. doi: 10.1212/wnl.47.2.433. [DOI] [PubMed] [Google Scholar]
- 6.Dodge HH, Shen C, Pandav R, DeKosky ST, Ganguli M. Functional transitions and active life expectancy associated with Alzheimer disease. Arch Neurol. 2003;60:253–9. doi: 10.1001/archneur.60.2.253. [DOI] [PubMed] [Google Scholar]
- 7.Mendez MF. The accurate diagnosis of early-onset dementia. Int J Psychiatry Med. 2006;36:401–12. doi: 10.2190/Q6J4-R143-P630-KW41. [DOI] [PubMed] [Google Scholar]
- 8.Klafki HW, Staufenbiel M, Kornhuber J, Wiltfang J. Therapeutic approaches to Alzheimer's disease. Brain. 2006;129:2840–55. doi: 10.1093/brain/awl280. [DOI] [PubMed] [Google Scholar]
- 9.London: (UK) National Institute for Health and Clinical Excellence; 2006. Dementia: Quick reference guide. ISBN 1-84629-312-X. Archived from the original on 27 Feb 2008. [Google Scholar]
- 10.Battistin L, Cagnin A. Vascular cognitive disorder: A biological and clinical overview. Neurochem Res. 2010;35:1933–8. doi: 10.1007/s11064-010-0346-5. [DOI] [PubMed] [Google Scholar]
- 11.Lee AY. Vascular dementia. Chonnam Med J. 2011;47:66–71. doi: 10.4068/cmj.2011.47.2.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeter ML, Stein T, Maslowski N, Neumann J. Neural Correlates of Alzheimer's Disease and Mild Cognitive Impairment. A Systematic and Quantitative Meta-Analysis involving 1,351 Patients. Neuroimage. 2009;47:1196–206. doi: 10.1016/j.neuroimage.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bäckman L, Jones S, Berger AK, Laukka EJ, Small BJ. Multiple cognitive deficits during the transition to Alzheimer's disease. J Intern Med. 2004;256:195–204. doi: 10.1111/j.1365-2796.2004.01386.x. [DOI] [PubMed] [Google Scholar]
- 14.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 15.Tombaugh TN. Trail Making test A and B: Normative Data Stratified by Age and Education. Archives of Clinical Neuropsychology. Arch Clin Neuropsychol. 2004;19:203–14. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 16.Royall D, Cordes J, Polk M. CLOX: An executive clock drawing task”. J Neurol Neurosurg Psychiatry. 1998;64:588–94. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taler V, Phillips NA. Language performance in Alzheimer's disease and mild cognitive impairment: A comparative review. J Clin Exp Neuropsychol. 2008;30:501–56. doi: 10.1080/13803390701550128. [DOI] [PubMed] [Google Scholar]
- 18.Frank EM. Effect of Alzheimer's disease on communication function. J S C Med Assoc. 1994;90:417–23. [PubMed] [Google Scholar]
- 19.Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and circadian rhythms in Alzheimer's disease. Am J Psychiatry. 2001;158:704–11. doi: 10.1176/appi.ajp.158.5.704. [DOI] [PubMed] [Google Scholar]
- 20.LoGiudice D, Smith K, Thomas J, Lautenschlager NT, Almeida OP, Atkinson D, et al. Kimberley Indigenous Cognitive Assessment tool (KICA): Development of a cognitive assessment tool for older Indigenous Australians. Int Psychogeriatr. 2006;18:269–80. doi: 10.1017/S1041610205002681. [DOI] [PubMed] [Google Scholar]
- 21.Smith K, Flicker L, Lautenschlager NT, Almeida OP, Atkinson D, Dwyer A, et al. High prevalence of dementia and cognitive impairment in Indigenous Australians. Neurology. 2008;71:1466–7. doi: 10.1212/01.wnl.0000320508.11013.4f. [DOI] [PubMed] [Google Scholar]
- 22.LoGiudice D. Diagnosis and prevalence of dementia in indigenes Australian. In Symposia S3-03: Diagnosis of Dementia and Alzheimer's disease in Cross Cultural Studies, Developing Countries, and Indigenous Peoples. Australas J Ageing. 2009:14. [Google Scholar]
- 23.LoGiudice D, Smith K, Dwyer A, Thomas J, Lautenschlager N, Almeida O, et al. Kimberley Indigenous Cognitive Assessment tool: Current status of the validity of the cognitive and informant questions. Australas J Ageing. 2005;24(Suppl):A43. [Google Scholar]
- 24.Cockrell JR, Folstein MF. Mini-Mental State Examination (MMSE) Psychopharmacol Bull. 1988;24:689–91. [PubMed] [Google Scholar]
- 25.Bohayraei N. Tehran, Iran: The University of Rehabilitation Sciences; 1379. Evaluation of Mini mental state examination efficiency in the elders with Alzheimer's disease. [Google Scholar]
- 26.Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: A comprehensive review. J Am Geriatr Soc. 1992;40:922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 27.Teng ET, Chui HC. The Modified Mini-mental State(3MS) Examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 28.Tombaugh TN, McDowell I, Kristjansson B. Mini-Mental State Examination (MMSE) and the Modified MMSE (3MS): A psychometric comparison and normative data. Psychol Assess. 1996;8:48–59. [Google Scholar]
- 29.Grace J, Nadler JD, White DA, Guilmette TJ, Giuliano AJ, Monsch AU, et al. Folstein versus Modified Mini-mental State Examination in geriatric stroke: Stability, validity, and screening utility. Arch Neurol. 1995;52:477–84. doi: 10.1001/archneur.1995.00540290067019. [DOI] [PubMed] [Google Scholar]
- 30.Tschanz JT, Welsh-Bohmer KA, Plassman BL, Norton MC, Wyse BW, Breitner JC Cache County Study Group. An Adaptation of the Modified Mini-Mental State Examination: Analysis of Demographic Influences and Normative Data: The cache county study. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:28–38. [PubMed] [Google Scholar]
- 31.Smith K, Flicker L, Almeida O, Lautenschlager N, Thomas J, Waters S, et al. The Kimberley Indigenous Cognitive Assessment (KICA): Results of reliability and validity testing in an Indigenous population. Intern Med J. 2005;35:48–58. [Google Scholar]
- 32.Smith K, Flicker L, Dwyer A, Marsh G, Mahajani S, Almeida O, et al. Assessing cognitive impairment in Indigenous Australians: Re-evaluation of the Kimberley Indigenous Cognitive Assessment in Western Australia and the Northern Territory. Aust Psychol. 2009;44:54–61. [Google Scholar]


