Abstract
Background:
Biodegradable elastomeric materials such as poly glycerol sebacate (PGS) have gained much current attention in the field of soft tissue engineering. The present study reports the synthesis of PGS with molar ratios of 1:1, 2:3, and 3:2 of glycerol and sebacic acid via polycondensation reaction and tests the effect of PGS on human corneal epithelial (HCE) cells viability in vitro.
Materials and Methods:
PGS films were prepared by the casting method. We tried to fabricate PGS with different compositions and various properties as being a viable alternative to the corneal stroma in cornea tissue engineering. The chemical properties of the prepared polymer were investigated by means of attenuated total reflectance – Fourier transform infrared spectroscopy (ATR-FTIR) analysis and the in vitro cytotoxicity was investigated by the Alamarblue method.
Results:
The functional groups observed in the PGS FTIR spectrums of PGS with various molar ratios were the same. However, the main difference was the time of completing the cross-linking reaction. The PGS prepared by 2:3 ratio as a molar ratio had the fastest and the 3:2 ratio had the lowest cross-linking rate because of the higher amount of sebacic acid. Results of the Alamarblue cytotoxicity test assay showed no deleterious effect on HCE cell viability and proliferation.
Conclusions:
PGS is a potentially good candidate material for corneal tissue engineering because of its lack of in vitro HCE cell toxicity.
Keywords: Biodegradation, cornea, poly (glycerol sebacate), tissue engineering
INTRODUCTION
There are a number of inherent immune disorders, diseases and trauma that can cause blindness because of corneal dysfunction. It has been estimated that 45 million people worldwide are bilaterally blind and another 135 million have severely impaired vision because of the loss of corneal transparency.[1]
There are several major tissue engineering and bioengineering challenges to be addressed – how to best deliver cultured cells to the eye, how to ensure long term survival of the cultured cells and how to replace the damaged cornea with a biosynthetic corneal inlay that supports the attachment, proliferation and migration of the cultured cells.[2] In addition, tissue engineering is a promising approach to fulfill the need caused by the chronic shortage of human donors for tissue and organ transplantations. A key element of this approach is the biodegradable substrate that provides support to the seeded cells as well as desired mechanical properties for tissue regeneration and remodeling. The ideal substrate material should be biodegradable and biocompatible and should have physical and chemical properties suitable for this application. Corneal cells should also be able to adhere and grow on the same.[3] To fulfill these requirements, research efforts are directed to synthesizing new biomaterials with improved chemical and biological properties.
Poly (glycerol sebacate) (PGS), a novel biodegradable tough elastomer developed recently, is a material with properties well suited to soft tissue requirements.[4] It is a covalently cross linked, three dimensional network of random coils with hydroxyl groups attached to its backbone; both the cross linking and the hydrogen bonding interactions between the hydroxyl groups contribute to its elastomeric properties. PGS degrades by surface erosion, with simultaneous linear mass and strength loss occurring during its resorption period. The degradation time of PGS can be tailored by varying the degree of cross linking and the ratio of sebacic acid to glycerol.[5]
Some authors have also conducted studies on the preparation of elastomers based on glycerol and sebacic acid, and found that this kind of elastomer exhibited a certain thermal processing performance (e.g., shape molding ability) in some molar ratios of glycerol to sebacic acid and under certain reaction conditions.[6] In other words, the properties of the elastomers could be flexibly adjusted by altering the molar ratio of the reactants. In this study, a specific and systemic report about PGS with different molar ratios is reported. The aim is to find a suitable substitute for corneal stroma tissue engineering.
MATERIALS AND METHODS
Materials
PGS elastomers originating from three prepolymers were prepared first and then the structure and properties were studied. The PGS prepolymer synthesis was adapted from established methods[7] by a polycondensation reaction of glycerol and sebacic acid with different molar ratios as 1:1, 2:3, and 3:2 mixtures of anhydrous glycerol (Merck) and sebacic acid (Merck). Briefly, they were mixed in an airtight glass jar that was partially immersed in a heated silicone bath. The mixture was gradually heated to 120°C under nitrogen gas flow and stirred with a rotor at 50 rpm for 24 h. The reaction was initially carried out at atmospheric pressure, with a low flow of nitrogen across the reaction mixture. The gas flow was then stopped and vacuum (at −20 kPa) was applied for 24 h. Polycondensation results in a highly viscous uncrosslinked pre polymer. PGS films were obtained by curing the pre polymer in a Petri dish in a vacuum oven (at −20 kPa) at set cure times and 135°C.
Characterization of polymers
Prepared PGS samples were analyzed by infrared (IR) spectra with the attenuated total reflectance – Fourier transform infrared spectroscopy (ATR FTIR) spectrometer (Tensor 27, Bruker, Germany) in order to identify the functional groups.
Cell culture
Frozen cultured human corneal epithelial cells (HCE cells) were purchased from CELLnTEC Advanced Cell Systems AG, Bern, Switzerland, and were cultured in the CnT 50 medium (CELLnTEC; Bern, Switzerland) supplemented with 100 U/mL penicillin and streptomycin (Invitrogen, Eugene, OR) and were incubated at 37°C in a humidified 5% CO2 environment. The seeding density was 1 × 105 cells/cm2. The media were changed every 3 days. For the cell viability assay, the HCE cells were plated at a density of 1 × 104 cells per well in 96 well culture plates.
In vitro cytotoxicity
PGS sheets were autoclaved at 20 psi for 20 min and were submerged in 70% ethanol and shaken for 5 min at 100 rpm. The ethanol concentration was gradually reduced to 10% alongside shaking, and the PGS sheets were finally washed with PBS. Polymers were ultimately washed with growth medium prior to use.
Cytotoxicity of the PGS polymer was determined using the Alamarblue (abdSerotec) test assay. Cells were harvested and seeded in a 96 well plate. Twenty four hours later, sterilized PGS sheets were cut into small pieces of 6.5 mm in diameter and placed on cells growing in the plates. After 24 h, 20 μL Alamarblue was added to each well and was incubated for 3 h. Aliquots of 200 μL culture and Alamarblue were transferred into new wells and the absorbance was measured at 570 and 600 nm using a PowerXwave S plate reader. Alamarblue plus cells but no polymer was used as the negative control.
The percent reduction of Alamarblue is typically calculated using the related formula:
Percent reduction of Alamarblue =
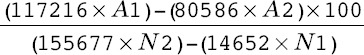
A1 and A2 are the observed absorbances of the test wells at 570 and 600 nm, respectively, and N1 and N2 are the observed absorbances of the negative control.
Alternatively, an online calculator (provided by the Serotec, USA) was used for measuring percent reduction.
Statistical analysis
Statistical analyses were conducted with SPSS version 14. Data are represented as mean ± SD. Differences between groups were analyzed using the one way ANOVA test followed by the Bonferroni post hoc test, and P < 0.05 was considered significant.
RESULTS
Figure 1 shows the FTIR spectra of the PGS with three different molar ratios. All the compositions were almost accordant: The absorption at 2686 cm−1 belonged to the hydroxyl groups in carboxyl, the absorption at 1747 cm−1 corresponded to the ester carbonyl, C = O, and 1700 cm−1 to carboxyl; intense stretches at 1747 cm−1 and 1164 cm−1 were attributed to the formation of ester bonds (C = O and C−O, respectively).
Figure 1.
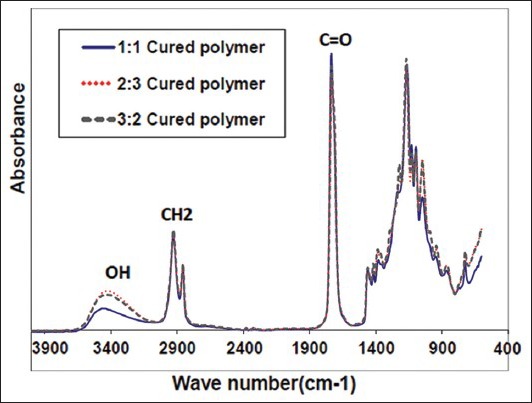
Attenuated total refl ectance – Fourier transform infrared spectroscopy spectra of poly (glycerol sebacate) with different molar ratios
In this study, Alamarblue was used to measure the cytotoxicity of PGS polymer on epithelial corneal cells. Incubating Alamarblue with cells and calculating its percent reduction according to the related formula revealed no significant difference between viability of PGS treated and non treated cells (P ≥ 0.05), indicating that polymer did not pose any toxic effect to the corneal cells Figure 2. Cells in the PGS sample wells were viable and showed normal morphology.
Figure 2.
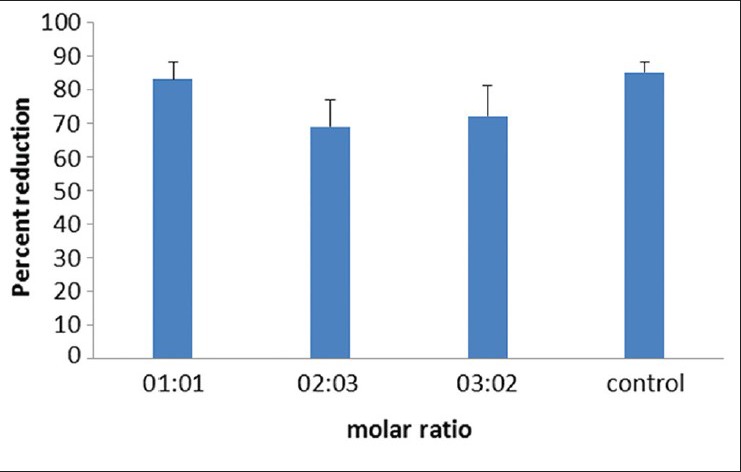
Cell viability evaluated by Alamarblue after 24 h of continuous exposure of cells to poly (glycerol sebacate). There was no significant difference between the groups
DISCUSSION
As can be seen in Figure 1, the absorption at 3461 cm−1 and 940 cm−1 correlated to the hydroxyl groups, which was at lower wave numbers compared with that of the free hydroxyl groups, illustrating the strong action of hydrogen bonding in the elastomers. PGS also exhibits peaks for the alkane group (-CH2) at 2930 cm−1 and 2855 cm−1. All these demonstrated that the three elastomers had similar chemical structures. The absorption peak of the hydroxyl groups was wide, which demonstrated the existence of hydrogen bonding action. Cross linking reactions in PGS with equal molar ratio of glycerol and sebacic acid (1:1) were completed after 96 h at 135°C and −20 KPa, and the others (2:3 and 3:2) were fully cross linked after 72 h and 140 h. This indicates that the absorption of carboxyl carbonyl was strengthened with the increase of sebacic acid in the reaction. Therefore, when more sebacic acid was used (2:3), the esterification degree of glycerol improved and the number of residual hydroxyl groups decreased, although the increase in the number of carboxyl carbonyl groups was attributed mostly to the increasing number of carboxyl groups at the molecular chain ends of the final products.
Alamarblue is a commercially available solution of resazurin salt that is oxidized in the mitochondria of viable cells. Metabolism of the dye is concomitant with a change in color from pink to blue with different light absorbance, and percent reduction of dye is proportional to number of viable cells, i.e., the more viable cells remain after test procedure, the more the dye is reduced to pink. Because of its water solubility, Alamarblue is a suitable substitute for conventional tetrazolium salts such as 3 (4,5 Dimethylthiazol 2 yl) 2,5 diphenyltetrazolium bromide (MTT), allowing continuous monitoring of growth of cultured cells.[8]
In a set of experiments that were assessed by the Alamarblue test assay, no toxicity was found on the corneal epithelium cells. The results of our in vitro studies suggest that PGS with different compositions of 1:1, 2:3, and 3:2 is satisfactorily biocompatible in cells from the cornea. For ophthalmic applications, successful cell survival on the films is useful.
To the best of our knowledge, this is the first report of PGS in vitro cytotoxicity on HCE cells. However, there are several other studies reporting the biocompatibility of PGS.[5,9,10,11,12,13]
For example, Sundback et al. have reported PGS as an excellent candidate material for neural reconstruction applications due to its lack of in vitro Schwann cell toxicity and minimal in vivo tissue response.[5] In other studies, it has been demonstrated that PGS not only has promise for cardiac tissue engineering applications but is also a suitable biomaterial for stem cell based regeneration strategies to restore cardiomyocyte function and regeneration of infarcted myocardium.[9,10,11] Redenti et al. have demonstrated no toxicity of PGS scaffolds on mouse retinal progenitor cells.[12] Furthermore, PGS has shown good properties as a candidate of biodegradable drug carrier.
CONCLUSION
In conclusions, it seems probable that PGS with different molar ratios of glycerol and sebacic acid appears to be biocompatible in vitro and is a potentially suitable biomaterial for cornea regeneration.
ACKNOWLEDGMENT
The authors are grateful for the support received from the Isfahan University of Technology and Isfahan University of Medical Sciences for this research.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Whitcher JP, Scrinivasan M, Upadhyay MP. Prevention of corneal ulceration in the developing world. Int Ophthalmol Clin. 2002;42:71–7. doi: 10.1097/00004397-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Atala A. Tissue engineering of artificial organs. J Endourol. 2000;14:49–57. doi: 10.1089/end.2000.14.49. [DOI] [PubMed] [Google Scholar]
- 3.Martina M, Hutmacher DW. Biodegradable polymers applied in tissue engineering research: A review. Polym Int. 2007;56:145–57. [Google Scholar]
- 4.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002;20:602–6. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 5.Sundback CA, Shyu JY, Wang Y, Faquin WC, Langer RS, Vacanti JP, et al. Biocompatibility analysis of poly (glycerol sebacate) as a nerve guide material. Biomaterials. 2005;26:5454–64. doi: 10.1016/j.biomaterials.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Liu Q, Tian M, Ding T, Shi R, Zhang L. Preparation and characterization of a biodegradable polyester elastomer with thermal processing abilities. J Appl Polym Sci. 2005;98:2033–41. [Google Scholar]
- 7.Jaffar IH, Ammer MM, Jedlicka SS, Pearson RA, Coulter JP. Spectroscopic evaluation, thermal, and thermomechanical characterization of poly (glycerol-sebacate) with variations in curing temperatures and durations. J Mater Sci. 2010;45:2525–9. [Google Scholar]
- 8.Al-Nasiry S, Geusens N, Hanssens M, Luyten C, Pijnenborg R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum Reprod. 2007;22:1304–9. doi: 10.1093/humrep/dem011. [DOI] [PubMed] [Google Scholar]
- 9.Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Sridhar R, Ramakrishna S. Expression of cardiac proteins in neonatal cardiomyocytes on PGS/fibrinogen core/shell substrate for Cardiac tissue engineering. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.04.045. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Chen QZ, Ishii H, Thouas GA, Lyon AR, Wright JS, Blaker JJ, et al. An elastomeric patch derived from poly (glycerol sebacate) for delivery of embryonic stem cells to the heart. Biomaterials. 2010;31:3885–93. doi: 10.1016/j.biomaterials.2010.01.108. [DOI] [PubMed] [Google Scholar]
- 11.Chen QZ, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, et al. Characterisation of a soft elastomer poly (glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008;29:47–57. doi: 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Redenti S, Neeley WL, Rompani S, Saigal S, Yang J, Klassen H, et al. Engineering retinal progenitor cell and scrollable poly (glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials. 2009;30:3405–14. doi: 10.1016/j.biomaterials.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun ZJ, Chen C, Sun MZ, Ai CH, Lu XL, Zheng YF, et al. The application of poly (glycerol-sebacate) as biodegradable drug carrier. Biomaterials. 2009;30:5209–14. doi: 10.1016/j.biomaterials.2009.06.007. [DOI] [PubMed] [Google Scholar]


