ABSTRACT
Most alphaviruses are mosquito-borne and exhibit a broad host range, infecting many different vertebrates, including birds, rodents, equids, humans, and nonhuman primates. This ability of most alphaviruses to infect arthropods and vertebrates is essential for their maintenance in nature. Recently, a new alphavirus, Eilat virus (EILV), was described, and in contrast to all other mosquito-borne viruses, it is unable to replicate in vertebrate cell lines. Investigations into the nature of its host range restriction showed the inability of genomic EILV RNA to replicate in vertebrate cells. Here, we investigated whether the EILV host range restriction is present at the entry level and further explored the viral factors responsible for the lack of genomic RNA replication. Utilizing Sindbis virus (SINV) and EILV chimeras, we show that the EILV vertebrate host range restriction is also manifested at the entry level. Furthermore, the EILV RNA replication restriction is independent of the 3′ untranslated genome region (UTR). Complementation experiments with SINV suggested that RNA replication is restricted by the inability of the EILV nonstructural proteins to form functional replicative complexes. These data demonstrate that the EILV host range restriction is multigenic, involving at least one gene from both nonstructural protein (nsP) and structural protein (sP) open reading frames (ORFs). As EILV groups phylogenetically within the mosquito-borne virus clade of pathogenic alphaviruses, our findings have important evolutionary implications for arboviruses.
IMPORTANCE Our work explores the nature of host range restriction of the first “mosquito-only alphavirus,” EILV. EILV is related to pathogenic mosquito-borne viruses (Eastern equine encephalitis virus [EEEV], Western equine encephalitis virus [WEEV], Venezuelan equine encephalitis virus [VEEV], and Chikungunya virus [CHIKV]) that cause severe disease in humans. Our data demonstrate that EILV is restricted both at entry and genomic RNA replication levels in vertebrate cells. These findings have important implications for arbovirus evolution and will help elucidate the viral factors responsible for the broad host range of pathogenic mosquito-borne alphaviruses, facilitate vaccine development, and inform potential strategies to reduce/prevent alphavirus transmission.
INTRODUCTION
Arthropod-borne viruses (arboviruses) such as dengue virus, Chikungunya virus, West Nile virus, and Rift Valley fever virus are important causes of human and animal disease worldwide (1, 2). Arboviruses encompass diverse virus families, including Togaviridae, Bunyaviridae, Flaviviridae, Rhabdoviridae, Orthomyxoviridae, Reoviridae, and Asfarviridae, and are maintained in nature primarily through biological transmission between susceptible vertebrate hosts and hematophagous arthropods such as ticks, mosquitoes, and other biting flies (1, 2). This ability to infect diverse vertebrate and arthropod hosts enables arboviruses to persist in nature; however, the viral factors that facilitate their broad host range are poorly understood.
Mostly during the past decade, many “insect-only” or arthropod viruses have been discovered in several arbovirus families: Sigma and Moussa viruses in Rhabdoviridae; Gouleako virus in Bunyaviridae; cell-fusing agent virus, Kamiti River virus, Aedes flavivirus, Culex flavivirus, Quang Binh, Calbertado, Nounané, Lammi, and Marisma mosquito viruses in Flaviviridae (3–17). Based on phylogenetic findings, these arthropod flaviviruses can be classified into two groups: the first consists of viruses distantly related to human and animal pathogens (Sigma, Moussa, Gouleako, cell-fusing agent, Kamiti River, Aedes flavivirus, Culex flavivirus, Quang Binh, and Calbertado viruses), while the second, including Nounané, Lammi, and Marisma mosquito viruses, falls within the mosquito-borne flavivirus clade that includes many human pathogens such as dengue and yellow fever viruses (3–17). The phylogenetic placement of the later arthropod virus group suggests that these arthropod flaviviruses have evolutionarily lost the ability to infect vertebrates. These viruses could therefore yield significant insights into viral factors that facilitate the broad host range of pathogenic flaviviruses. However, the determinants of host range restriction of these flaviviruses remain to be elucidated.
Another major taxon of arboviruses is the genus Alphavirus in the family Togaviridae, which also encompasses a very broad host range. The genus is comprised of small, spherical, enveloped viruses with genomes consisting of a single-strand, positive-sense RNA approximately 11 to 12 kb in length (18). Alphaviruses comprise 30 recognized species classified into 10 complexes based on antigenic and/or genetic similarities (19–21). The two aquatic alphavirus complexes (Southern elephant seal virus [SESV] and salmon pancreas disease virus [SPDV]) are not known to utilize arthropods in their transmission cycles, whereas all the remaining complexes (Barmah Forest, Ndumu, Middelburg, Semliki Forest, Venezuelan equine encephalitis [VEE], Eastern equine encephalitis [EEE], Western equine encephalitis [WEE], and Trocara) consist of arboviruses that almost exclusively utilize mosquitoes as insect vectors (18–24). Mosquito-borne alphaviruses can infect mosquito species encompassing at least eight genera (Aedes, Culex, Anopheles, Culiseta, Haemagogus, Mansonia, Verrallina, and Psorophora spp.) and many vertebrate taxa, including equids, birds, amphibians, reptiles, rodents, pigs, as well as human and nonhuman primates (18, 25–27).
Recently, a host-restricted alphavirus, Eilat virus (EILV), which is serologically distinct but phylogenetically groups within the mosquito-borne clade as a sister to the WEE complex, was described (28). In contrast to all other mosquito-borne alphaviruses, EILV is unable to infect and replicate in vertebrate cells, but it can readily replicate to high titers (>108 PFU/ml) in mosquito cells (28). Investigations into the nature of this host range restriction demonstrated that EILV RNA is incapable of replicating in vertebrate cells, even after electroporation of genomic RNA directly into the cytoplasm (28). Here, we investigated whether the EILV host range is also restricted at the entry level and further characterized viral factors responsible for lack of replication in vertebrate cells.
MATERIALS AND METHODS
Cells.
Vero, BHK-21, HEK-293, DEF, NIH 3T3, and C7/10 cell lines were obtained from the American Type Culture Collection (Bethesda, MD). Cell lines were propagated at 37°C or 28°C with 5% CO2 in Dulbecco's minimal essential medium (DMEM) containing 10% (vol/vol) fetal calf serum (FCS) (NIH 3T3 only), 10% fetal bovine serum (FBS) (vol/vol), sodium pyruvate (1 mM), and penicillin (100 U/ml) and streptomycin (100 μg/ml). C7/10 medium was the same as above and was additionally supplemented with 1% (vol/vol) tryptose phosphate broth (Sigma, St. Louis, MO).
Generation of cDNA clones and rescue of infectious EILV and SINV clones.
SINV-eGFP (Sindbis virus [SINV] labeled with enhanced green fluorescent protein [eGFP]) was generated from strain Tr-339, and chimeras were generated utilizing standard cloning methods (29). All SINV and EILV constructs contained eGFP or enhanced cherry red fluorescent protein (eRFP) under the control of an additional subgenomic promoter downstream of the nsP4 gene. Full-length cDNA clones were confirmed by ABI PRISM BigDye sequencing (Applied Biosystems).
Transcription and electroporation conditions for virus rescue.
SINV and EILV cDNAs (10 μg) were linearized using an XhoI (SINV-eGFP and SINV/EILV [SINV-eGFP containing the structural polyprotein ORF of EILV] chimeras) or NotI (EILV-eRFP and EILV/SINV [EILV-eRFP containing the SINV structural ORF] chimeras) restriction site engineered immediately after the poly(A) tail. Linearized cDNAs were purified via phenol-chloroform extraction, and ∼1 μg was utilized for each transcription reaction. The transcription reaction mixture contained 0.5 mM ribonucleotide triphosphate (rNTP), 0.5 mM m7G(5′)ppp(5′)G RNA cap (New England BioLabs [NEB]), 0.5 μl RNase inhibitor, and 1.25 μl of SP6 polymerase (Ambion) in a total volume of 25 μl. Transcription was performed for 2 h at 42°C, and then the mixture was placed on ice.
The cells were seeded to achieve ∼70% confluence overnight. Monolayers were either trypsinized (vertebrate cells) or gently scraped (C7/10 cells) into single-cell suspensions, and the cell suspensions were washed 5 times with phosphate-buffered saline (PBS) and resuspended in 450 or 700 (Vero cells only) μl of PBS. The cells were mixed with each transcription mixture (∼10 μg of RNA), placed in 2-mm or 4-mm (Vero cells only) electroporation cuvettes, and immediately electroporated (BTX ECM-830 Electro Square Porator; Harvard Apparatus Inc.) using the following conditions: 680 V; pulse length, 99 μs; interval between pulses, 200 ms; number of pulses, five. Electroporation conditions for Vero cells only were as follows: 250 V; pulse length, 10 ms; interval between pulses, 1 s; number of pulses, three. Expression of eGFP or eRFP was observed with fluorescence microscopy, and supernatants containing virus were harvested at 48 h postelectroporation (hpe) and stored at −80°C. All SINV and EILV (parental and chimeras) expressing fluorescent reporters were rescued in C7/10 cells, and virus supernatants were utilized in subsequent in vitro and in vivo analysis.
Coelectroporation of genomic viral RNAs.
All SINV and EILV (parental and chimeras) were linearized and transcribed in vitro as described above. After transcription, RNAs were mixed via pipetting, and ∼60 μg (∼30 μg each) of RNA was electroporated into BHK-21, Vero, HEK-293, and C7/10 cells. Rescue of phenotype was monitored via fluorescence microscopy and/or reverse transcription-PCR (RT-PCR). Fluorescence micrographs were taken 12 (BHK-21) or 20 hpe (C7/10).
RT-PCR to detect EILV negative-strand RNA.
Duplicate wells of cells coelectroporated with genomic RNAs were harvested 6 hpe, and RNA was isolated. Reverse transcription was performed with primers targeting the EILV negative strand within the E1 gene, followed by PCR with primers targeting the EILV nsP3 hypervariable domain. PCR products were analyzed via gel electrophoresis, and the limit of detection of the PCR assay was 5 × 103 genome copies.
Plaque assay.
Virus titration was performed on ∼80% confluent C7/10 cell monolayers seeded overnight in six-well plates. Duplicate wells were infected with 0.1-ml aliquots from serial 10-fold dilutions in growth medium, then 0.4 ml of medium was added to each well to prevent cell desiccation, and virus was adsorbed for 1 h. Following incubation, the virus inoculum was removed, and cell monolayers were overlaid with 3 ml of a 1:1 mixture of 2% tragacanth and 2× minimum essential medium (MEM) with 5% fetal bovine serum (FBS), 2% tryptose phosphate broth solution, penicillin (200 U/ml), and streptomycin (200 μg/ml). The cells were incubated at 28°C with 5% CO2 for 2 days (SINV-eGFP and SINV/EILV-eGFP chimeras) or 3 days (EILV-eRFP and EILV/SINV-eRFP) for plaque development, the overlay was removed, and monolayers were fixed with 3 ml of 10% formaldehyde in PBS for 30 min. The cells were stained with 2% crystal violet in 30% methanol for 5 min at room temperature; excess stain was removed under running water, and plaques were counted.
One-step replication kinetics.
Replication kinetics were assessed on representative vertebrate and insect cell lines in triplicate on 70% confluent monolayers in T-25 (25-cm2) tissue culture flasks. Three replicates of virus infection were performed to achieve a multiplicity of infection (MOI) of 10 PFU/cell, and virus was absorbed for 2 h at 28°C (insect cells) or 37°C (vertebrate cells). Following incubation, the inoculum was removed, monolayers were rinsed five times with room temperature DMEM to remove unbound virus, and 5 ml of growth medium was added to each flask. Aliquots of 0.5 ml were taken immediately afterward as a “time zero” sample and replaced with 0.5 ml of fresh medium. Flasks were incubated at 28°C, and further samples were taken at 12, 24, 48, 72, and 96 h postinfection (hpi). All samples were flash frozen in dry ice/ethanol bath, and stored at −80°C.
Imaging viral infections.
For infection experiments, 30% confluent monolayers were infected with EILV or SINV chimeras at an MOI of 20 PFU/cell. For electroporation experiments, ∼20 to 60 μg of RNA was electroporated into ∼107 cells via the conditions stated above. Following infection or electroporation, eGFP or eRFP expression was monitored 12 to 96 hpi. Phase-contrast and fluorescence field photographs were taken at various time points postinfection.
Intrathoracic mosquito infections.
Mosquito eggs from colonies at the University of Texas Medical Branch (UTMB) were hatched and reared using standard methods (30). Cohorts of 30 to 50 adult females collected 5 or 6 days after emergence from the pupal stage were cold anesthetized and inoculated with ∼1 μl of 107 to 104 PFU/ml of SINV-eGFP, SINV/EILV 3′ untranslated genome region (UTR), or SINV/EILV structural open reading frame (ORF) via the intrathoracic (IT) route. Mosquitoes were given 10% sucrose and held for an extrinsic incubation period of 7 days at 28°C. Whole mosquitoes were placed in 1 ml of DMEM, 20% FBS, penicillin (200 U/ml), streptomycin (200 μg/ml), and 2.5 μg/ml amphotericin B and stored at −80°C. Samples were triturated using a Mixer Mill 300 (Retsch, Newtown, PA) and centrifuged at 18,000 × g for 5 min, and supernatants from each sample were analyzed. Mosquito samples were screened for SINV-eGFP and SINV/EILV 3′ UTR for cytopathic effect (CPE) and eGFP expression using fluorescence microscopy of inoculated Vero cells. SINV/EILV structural ORF samples were screened for CPE and eGFP expression on C7/10 cells.
Mouse infections.
Mouse infection studies were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (31) and approved by the Institutional Animal Care and Use Committee at UTMB. One-day-old newborn CD-1 outbred mice were obtained from pregnant females purchased from Charles River (Wilmington, MA). Animals were injected with 10 μl of SINV-eGFP and SINV/EILV chimeras via the intracranial route at 105, 104, and 103 PFU/animal. Animals were observed daily for signs of illness and were euthanized at the onset of neurological disease.
RESULTS
Host range restriction at entry level: plaque size and in vitro growth kinetics in C7/10 cells.
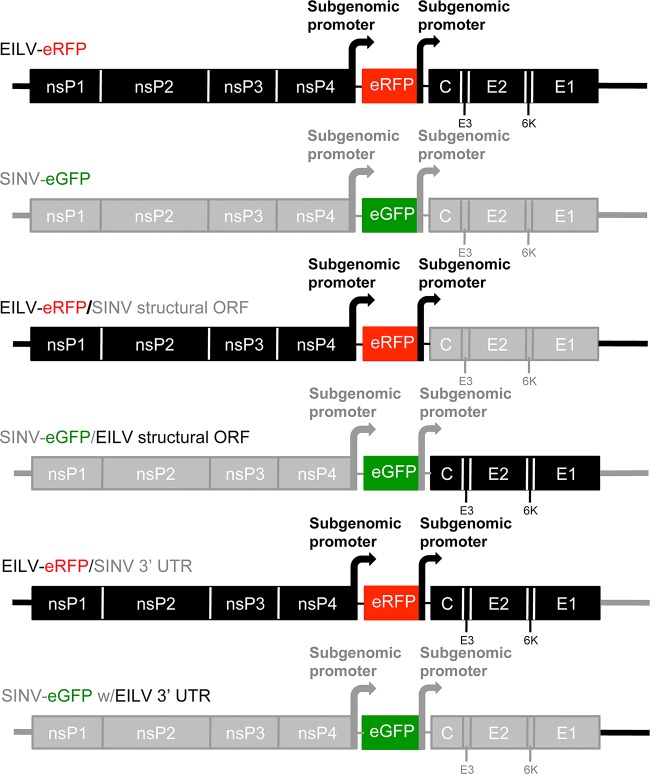
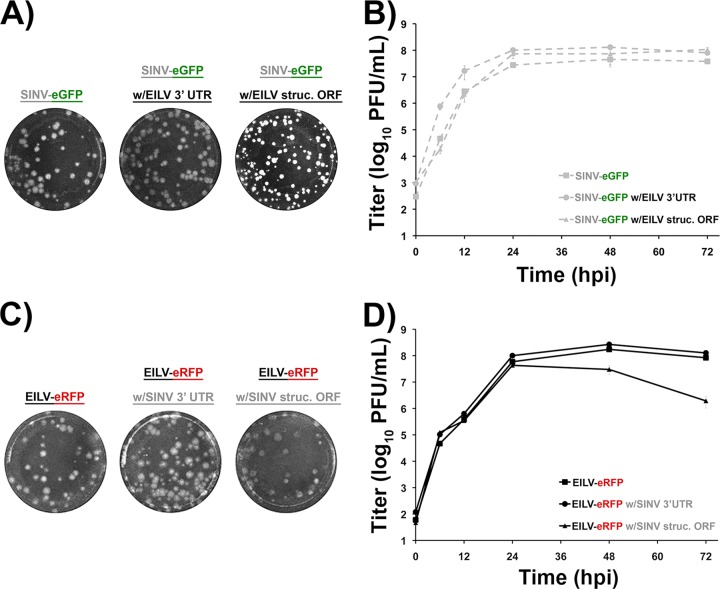
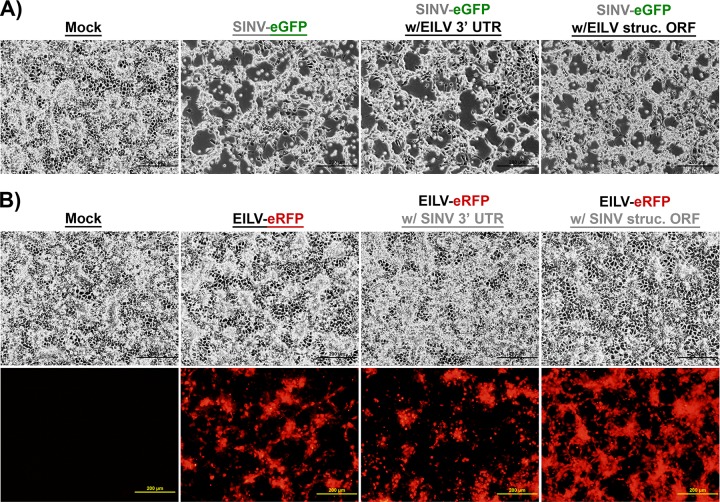
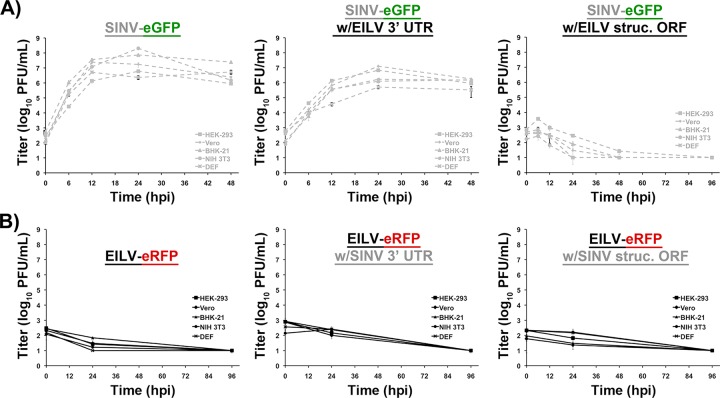
To investigate whether EILV host range restriction is manifested at the entry level, a SINV-eGFP clone containing the structural polyprotein ORF of EILV (SINV/EILV) was generated (Fig. 1). In addition, to investigate any genetic incompatibilities in generating structural ORF chimeras, the reciprocal EILV-eRFP containing the SINV structural ORF (EILV/SINV) was also generated (Fig. 1). Similar to the parental viruses (SINV-eGFP and EILV-eRFP), both chimeras were readily rescued in C7/10 cells and produced plaques similar to those produced by the parental viruses, ∼3 mm in size, 2 (SINV/EILV) or 3 (EILV/SINV) days postinfection (dpi) (Fig. 2A and C). To compare one-step replication kinetics, infections were performed at an MOI of 10 in C7/10 cells. Both chimeras displayed replication kinetics similar relative to those of the parental viruses, with peak virus titers of ∼5 × 107 PFU/ml (Fig. 2B and D). However, the EILV/SINV chimera titer declined ∼50-fold by 72 hpi, suggesting reduced stability of infectious virus particles (Fig. 2D). Infection of C7/10 cells with SINV/EILV produced cytopathic effects in C7/10 cells by 36 hpi; in contrast, no overt cytopathic effects were observed following EILV/SINV infection (Fig. 3A and B).
FIG 1.
Schematic diagrams of SINV and EILV chimeras.
FIG 2.
Plaque phenotype and replication kinetics of SINV and EILV chimeras in C7/10 cells. (A and C) C7/10 cells were infected with chimeras at 2 dpi (SINV-eGFP and SINV/EILV chimeras) (A) or 3 dpi (EILV-eRFP and EILV/SINV chimeras) (C). (B and D) Replication kinetics of SINV-eGFP and SINV/EILV chimeras (B), and EILV-eRFP and EILV/SINV chimeras (D) were performed at an MOI of 10. Each infection was performed in triplicate. Average titers ± standard deviations (SD) (error bars) are shown.
FIG 3.
Infection of C7/10 cells with SINV (A) and EILV (B) chimeras. C7/10 cells were infected at an MOI of 10. Phase-contrast (top row) and fluorescence micrographs (bottom row) were taken at 36 hpi.
Host range restriction at entry level in vertebrate cell lines.
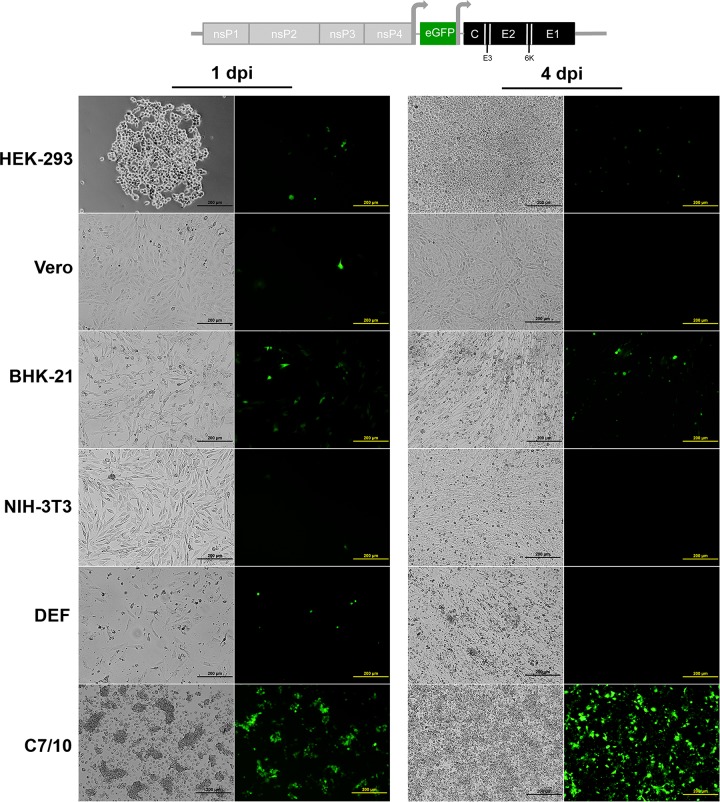
To determine whether EILV host range restriction is manifested at the entry level, cell lines representing various alphavirus vertebrate hosts were infected with SINV/EILV. MOIs of 10 and 20 were utilized to highlight possible inefficiency in virus entry. Mosquito and vertebrate cell lines were infected at an MOI of 20 PFU/cell, and the efficiency of entry and RNA replication was visualized via eGFP expression. Most of the C7/10 cells expressed eGFP by 1 dpi, indicating that SINV/EILV was able to efficiently enter and replicate (Fig. 4). In contrast, minimal eGFP expression was detected in vertebrate cells lines 1 dpi and was completely undetectable in Vero, NIH 3T3, and DEF cells through 4 dpi. However, some eGFP expression was detected in BHK-21 and HEK-293 cells 4 dpi. The inefficiency of vertebrate infection by SINV/EILV was presumably due to inefficient attachment and entry, as eGFP could be readily detected after electroporation (Fig. 5). This conclusion was further corroborated by one-step replication curves in vertebrate cells at an MOI of 10 (Fig. 6A). SINV/EILV inocula decayed by 24 hpi, and virus titers were slightly above or below the limit of detection (101 PFU/ml) by 48 hpi. These results suggest that the EILV structural proteins do not mediate efficient binding and/or entry into vertebrate cells.
FIG 4.
Infection of vertebrate and mosquito cell lines with SINV-eGFP with EILV structural ORF. Each cell line was infected at an MOI of 20, and phase-contrast (left) and fluorescence (right) micrographs were taken at 1 and 4 days postinfection (dpi).
FIG 5.
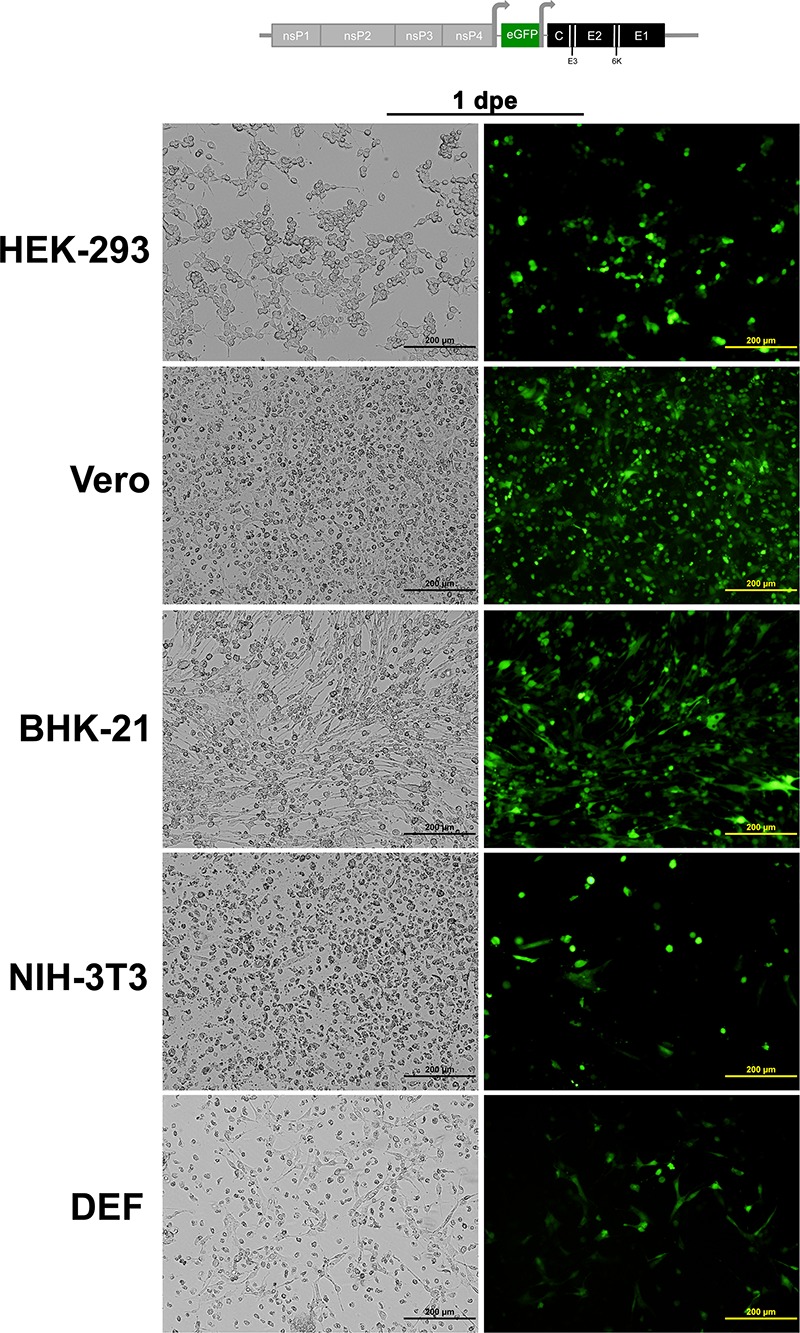
Electroporation of vertebrate cell lines with SINV-eGFP with EILV structural ORF. RNA was transcribed in vitro, and each cell line was electroporated with ∼10 μg of RNA, and phase-contrast (left) and fluorescence (right) micrographs were taken at 1 day postelectroporation (dpe).
FIG 6.
Replication kinetics of SINV (A) and EILV (B) chimeras in vertebrate cell lines. Infections were performed in triplicate at an MOI of 10. Average titers ± SD are shown.
In contrast, the reciprocal EILV/SINV chimera was completely unable to infect and replicate in vertebrate cell lines (Fig. 6B and 7). No eRFP expression was observed in vertebrate cells after infection at an MOI of 10 or after electroporation of viral RNA up to 4 dpi or 4 days postelectroporation (dpe), respectively (Fig. 7). One-step replication curves confirmed these results; EILV/SINV was unable to replicate, and the inoculum decayed to undetectable levels by 96 hpi (Fig. 6B). These data further support the findings that EILV genomic RNA, even when packaged into virions using the SINV structural proteins capable of initiating entry, is incapable of replication in vertebrate cells.
FIG 7.
Infection of vertebrate and mosquito cell lines with EILV-eRFP with SINV structural ORF. Each cell line was infected at an MOI of 10 or electroporated with 10 μg of genomic RNA. Phase-contrast (left) and fluorescence (right) micrographs were taken at 1 dpi (C7/10 cells) or 4 dpi (vertebrate cells).
Host range restriction at entry level in vivo.
To further investigate the host range restriction of SINV/EILV, infections of mosquitoes and newborn mice were performed. Adult female Culex quinquefasciatus, selected because Culex mosquitoes are the main SINV vectors and are susceptible to EILV, were injected via the intrathoracic route with SINV-eGFP and SINV/EILV at doses ranging from ∼10 to 104 PFU/mosquito to determine the mosquito 50% infectious dose (ID50), and at 7 dpi, whole mosquitoes were analyzed via eGFP expression and CPE assays for the presence of virus. C. quinquefasciatus infections with both viruses yielded similar results at all doses, with infection rates ranging from 73% to 100% even after the smallest doses; consequently, an ID50 could not be determined (Table 1).
TABLE 1.
Intrathoracic infection of C. quinquefasciatus with SINV-eGFP and SINV/EILV chimerasa
| Virus | Dose (log10 PFU/μl) | % infected (no. of infected mosquitoes/total no. of mosquitoes) (7 dpi) |
|---|---|---|
| SINV-eGFP | 3.9 | 100 (12/12) |
| 3.5 | 100 (15/15) | |
| 2.2 | 86 (12/14) | |
| 1.4 | 77 (10/13) | |
| SINV-eGFP with EILV 3′ UTR | 3.8 | 100 (15/15) |
| 3.4 | 100 (15/15) | |
| 2.4 | 87 (13/15) | |
| 1.4 | 80 (12/15) | |
| SINV-eGFP with EILV structural ORF | 4.0 | 100 (15/15) |
| 3.6 | 100 (15/15) | |
| 2.5 | 80 (12/15) | |
| 1.4 | 73 (11/15) |
C. quinquefasciatus mosquitoes were injected with ∼104 to 101 PFU/mosquito via the intrathoracic route, and at 7 dpi, the presence of infectious virus was detected by cytopathology and eGFP expression.
Infection of newborn mice, the most permissive and sensitive in vivo infection model for most alphaviruses, was assessed by performing 50% lethal dose (LD50) studies. Newborn mice were injected intracranially (IC) with SINV-eGFP and SINV/EILV at doses ranging from ∼105 to 103 PFU/animal. All animals injected with SINV-eGFP showed signs of illness within 24 hpi. Animals that received 105 PFU became moribund within 72 to 96 hpi and were euthanized. Some of the animals that received lower doses recovered; however, the majority of the animals, 67% and 56% of the animals in the groups receiving doses of 104 and 103 PFU/animal, respectively, met euthanization criteria (Table 2). The LD50 for SINV-eGFP was estimated to be <103 PFU. In contrast, none of the animals injected with SINV/EILV, even at a dose of 105 PFU/animal, showed any signs of illness, and all remained indistinguishable from those in the PBS control group (Table 2).
TABLE 2.
Intracranial infection of 1-day-old newborn mice with SINV-eGFP and SINV/EILV chimerasa
| Virus | Dose (log10 PFU/animal) | Lethalityb |
|---|---|---|
| SINV-eGFP | 4.7 | 9/9 (100) |
| 4.3 | 6/9 (67) | |
| 3.3 | 5/9 (56) | |
| SINV-eGFP with EILV 3′ UTR | 4.8 | 1/7 (14) |
| 4.1 | 0/6 (0) | |
| 3.3 | 0/7 (0) | |
| SINV-eGFP with EILV structural ORF | 5.3 | 0/6 (0) |
| 4.6 | 0/6 (0) | |
| 3.8 | 0/7 (0) | |
| None (PBS control) | 0/7 (0) |
Mice were injected with ∼105 to 103 PFU/animal and were observed for clinical disease.
The number of mice that died or were sick and met euthanization criteria to the total number of infected mice. The values in parentheses are the percentages.
Viral factors responsible for host range restriction at the genomic RNA replication level: plaque size and in vitro replication kinetics in C7/10 cells.
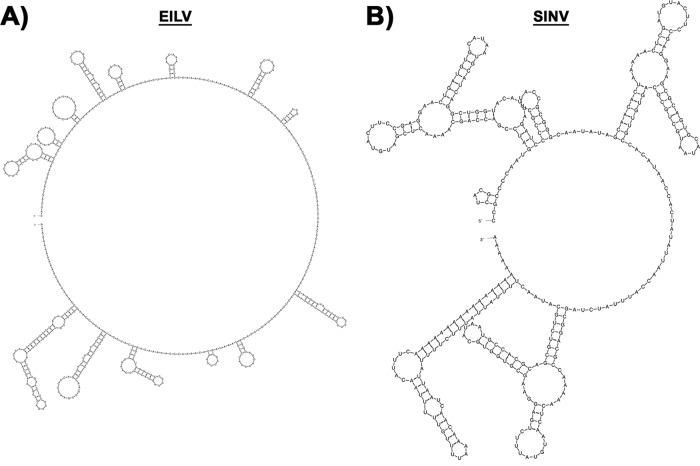
The alphavirus 3′ UTR contains a 19-nucleotide (nt) conserved sequence element (CSE) just upstream of the poly(A) tract that serves as a promoter for initiation of negative-strand synthesis and includes binding sites for mosquito and vertebrate proteins that likely facilitate virus replication (32–36). A previous study showed that the EILV 3′ UTR CSE displays considerable sequence identity to that of other mosquito-borne alphaviruses (28). In contrast, the remainder of the EILV 3′ UTR shows little sequence or structural identity to SINV (Fig. 8; also data not shown). To extend our previous findings, which demonstrated that the EILV host range restriction was manifested at the genomic RNA replication level, we investigated the role of the 3′ UTR. EILV and SINV chimeras (SINV/EILV 3′ UTR and EILV/SINV 3′ UTR) were engineered to contain reciprocal 3′ UTRs (Fig. 1). Similar to the structural chimeras described above, both viruses were readily rescued in C7/10 cells and produced plaques similar to the parental viruses, ∼3 mm in diameter either 2 dpi (SINV/EILV 3′ UTR) or 3 dpi (EILV/SINV 3′ UTR) (Fig. 2A and C). One-step replication curves showed kinetics and peak virus titers of ∼108 PFU/ml that were similar to those of parental viruses (Fig. 2B and D). SINV/EILV 3′ UTR produced cytopathic effects in C7/10 cells at 36 hpi, comparable to those produced by SINV (Fig. 3A). In contrast, no overt cytopathic effects were observed in C7/10 cells infected with EILV/SINV 3′ UTR (Fig. 3B).
FIG 8.
Predicted secondary structure of EILV (A) and SINV (B) 3′ UTRs. Minimal free energy structures were generated using M-fold at 37°C.
Host range restriction at genomic RNA replication level in vertebrate cell lines.
Mosquito and vertebrate cell lines were infected with SINV/EILV 3′ UTR at an MOI of 10, and virus replication was detected via eGFP expression and one-step replication kinetics utilizing infectious assays. SINV/EILV 3′ UTR infection in vertebrate cells produced cytopathic effects by 24 hpi, similar to SINV-eGFP (Fig. 6 and 9A). The chimeric virus displayed replication kinetics similar to those of SINV-eGFP; however, the peak titers were reduced ∼10-fold (Fig. 6A). In contrast, EILV/SINV 3′ UTR was unable to infect vertebrate cell lines as indicated by a lack of eRFP expression even after electroporation of genomic RNA (Fig. 9B). One-step replication kinetics confirmed this finding, with no productive infection detected and decay of the virus inoculum to below the limit of detection by 96 hpi (Fig. 6B). These results suggest that the 3′ UTR is not a major determinant of EILV RNA replication restriction in vertebrate cells.
FIG 9.
Infection of vertebrate and mosquito cell lines with SINV-eGFP with EILV 3′ UTR (A) and EILV-eRFP with SINV 3′ UTR (B). Each cell line was infected at an MOI of 10 with SINV-eGFP with EILV 3′ UTR or electroporated with 10 μg of genomic EILV-eRFP with SINV 3′ UTR RNA. Phase-contrast (left) and fluorescence (right) micrographs were taken at 1 dpi or 4 dpe.
Host range restriction at genomic RNA replication level in vivo.
To further investigate the host range of SINV/EILV 3′ UTR, infections of mosquitoes and newborn mice were performed. Similar to infection of C. quinquefasciatus with SINV-eGFP and the SINV/EILV structural chimera, the majority of mosquitoes became infected with all doses of SINV/EILV 3′ UTR. Infection rates ranged from 80% to 100%, and an ID50 was not determined (Table 1). Surprisingly, infection of newborn mice produced minimal clinical disease and little lethality. Only one animal at the 105 PFU dose developed neurological signs and was euthanized (Table 2). These data demonstrated that SINV/EILV 3′ UTR was at least 100-fold less virulent than the parental SINV-eGFP virus, indicating that the 3′ UTR is a major virulence determinant in the infant mouse model.
Roles of nsPs in EILV host range restriction.
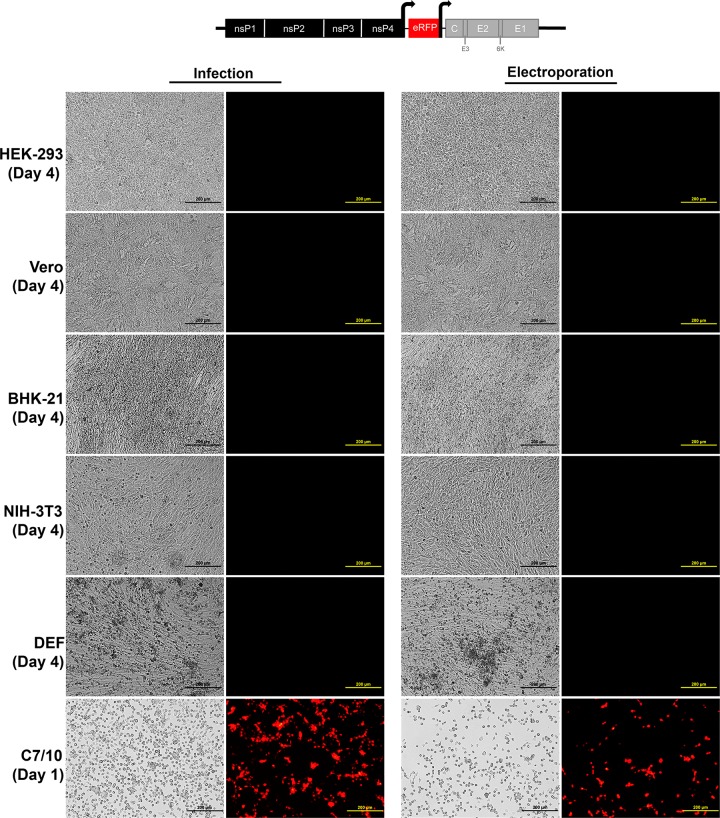
To investigate the roles of individual nonstructural protein (nsP) genes in host range restriction, SINV chimeras containing all four individual EILV nonstructural proteins (nsPs) were generated (Fig. 10A). However, none of the chimeras could be rescued in the C7/10, HEK-293, Vero, or BHK-21 cell line, suggesting incompatibilities between EILV and SINV nsPs. However, this lack of replication was overcome by trans-complementation with electroporated SINV genomic RNA; eGFP could be observed in BHK-21 and C7/10 cells coelectroporated with SINV/EILV and SINV RNAs (Fig. 10B and data not shown).
FIG 10.
(A) Schematic diagrams of SINV-eGFP with EILV nsP chimeras. (B) Electroporation or coelectroporation of SINV and/or SINV/EILV nsP chimera genomic RNAs. Fluorescence micrographs were taken at 12 (BHK-21) or 20 (C7/10) hpe.
Finally, we investigated whether similar trans-complementation could be observed by coelectroporating vertebrate cells with SINV and EILV-eRFP genomic RNAs. EILV negative-strand RNA could be detected by RT-PCR 6 hpe in BHK-21, HEK-293, and Vero cells (Fig. 11A). In addition, some eRFP expression could be detected in BHK-21, HEK-293, and Vero cells 12 to 18 hpe (Fig. 11B). These data demonstrate that SINV nsPs either aided and/or initiated EILV RNA replication, further supporting the hypothesis that the EILV nsPs are unable to form functional replicative complexes in vertebrate cells.
FIG 11.
Detection of EILV negative-strand RNA in vertebrate cell lines. (A) Coelectroporation of SINV and/or EILV-eRFP genomic RNAs into BHK-21, Vero, HEK-293, C7/10 cells and 6 hpe EILV negative-strand RNA was detected via RT-PCR. (B) Phase-contrast (left) and fluorescence (right) micrographs of BHK-21 cells were taken at 12 hpe.
DISCUSSION
In order to propagate, viruses must enter susceptible cells to deliver their genome, hijack cellular machinery to facilitate their replication, and package new genomes to produce infectious progeny (37). Each of these steps constitutes a fundamental challenge that viruses must overcome in order to replicate and be transmitted in nature. Arboviruses must overcome these obstacles in diverse arthropod and vertebrate hosts in order to be maintained in natural transmission cycles; failure to replicate in either host would result in extinction. Thus, arthropod or “insect-only” viruses that group with the vertebrate-pathogenic arboviruses in some taxa represent powerful tools that can be utilized to study viral factors responsible for the broad host range of arboviruses.
We investigated the nature of EILV host range restriction at the levels of entry and RNA replication. The host range of a SINV chimera containing EILV structural polyprotein ORF was unaltered in mosquitoes, both in vitro and in vivo. In contrast, a SINV/EILV chimera displayed a considerable reduction of host range in vertebrates. The altered host range was not due to a complete inability of SINV/EILV RNA to replicate in vertebrate cell lines, as eGFP expression could be detected in all cell lines after electroporation. The SINV/EILV vertebrate host range block was presumably a consequence of poor attachment and/or entry efficiency. This poor efficiency was indicated by detecting very few cells expressing eGFP and an absence of eGFP/virus spread. These data demonstrate that EILV host range is restricted at the entry level.
To extend our previous findings, viral factors responsible for EILV host range restriction at the genomic RNA replication level were investigated (28). The lack of reporter expression following electroporation of synthetically capped EILV genomes suggested a potential role for the 3′ UTR, which is critical to alphavirus RNA replication. However, the EILV 3′ CSE was able to function as a promoter for SINV negative-strand RNA synthesis, and the UTR was able to facilitate virus replication in vertebrate cells. Consequently, the EILV vertebrate host restriction appears to be independent of 3′ UTR. These data suggest that the host restriction is determined by one or more EILV nsPs. This hypothesis was further supported by detection of EILV replication in vertebrate cells upon trans-complementation with SINV nsPs. However, our investigation into the roles of individual EILV nsPs in host restriction was confounded by the inability of various SINV/EILV nsP chimeras to replicate in either mosquito and vertebrate cells. The sequential cleavage of alphavirus nonstructural polyprotein results in formation of negative-strand and positive-strand replicase complexes. As the nsPs interact with each other in these complexes, it is likely that lineage- or complex-specific mutations in one or more alphavirus nsPs select for a compensatory mutation(s) in other nsPs, and therefore, correctly paired nsPs are probably required for the formation of functional replicative complexes. Accordingly, the SINV nsP chimeras containing one or more EILV nsPs were unable to form functional complexes. Viable nsP chimeras have been generated for two closely related members of Semliki Forest complex, Chikungunya and O'nyong-nyong viruses; however, nsP chimeras between members of different alphavirus complexes have not been reported, presumably due to nsP incompatibilities (71). Taken together, our data suggest that the electroporation of capped, genomic RNA of EILV-eRFP, EILV-eRFP with SINV structural ORF, or 3′ UTR chimeras can initiate the virus replication cycle by the translation of the nsP ORF. However, improper interactions of EILV nsPs and/or RNA with vertebrate cell cofactors may render replicative complexes incapable of proceeding to negative-strand synthesis and expression of the subgenomic RNA.
Last, SINV-EILV chimeras produced cytopathic effects in C7/10 cells as demonstrated previously, suggesting a role for the nsPs, likely nsP2 (38). This hypothesis, which is supported by previous studies demonstrating that the induction of cytopathic effects in vertebrate cells by Old World alphaviruses such as SINV is due to expression of nsP2, requires further investigation.
EILV host range restriction.
The need to infect both vertebrates and mosquitoes exerts evolutionary pressure on arboviruses by constraining their adaptation to a single host, presumably due to fitness tradeoffs for replication in vertebrates versus invertebrates (40–45). In some cases, once the requirement for replication in one of the hosts is eliminated, these evolutionary constraints are greatly reduced, and adaptation to the retained host accelerates, coinciding with a loss of fitness for infection of the abandoned host (40–45). EILV appears to be an example of this host-specific adaptation. Its phylogenetic placement suggests that an ancestor of EILV was capable of replication in both vertebrates and insects but subsequently lost its ability to infect vertebrate cells as it specialized for infection of mosquitoes only. Our results support this hypothesis, as EILV's vertebrate host range restriction is present at both attachment/entry and genomic RNA replication levels.
Previous studies investigating host range alteration of alphaviruses demonstrated that various deletions or mutations in CSEs, nsPs, and structural proteins (sPs) result in loss or gain of fitness in either vertebrate or insect hosts; however, none of these mutations examined results in complete loss of replication competence in either host (46–66). The most medically important changes in alphavirus host range elucidated thus far involve Chikungunya virus (CHIKV) and Venezuelan equine encephalitis virus (VEEV) (62–66). Single point mutations in the E1 and E2 proteins of CHIKV dramatically increase fitness for infection of a new urban vector, Aedes albopictus, contributing to an epidemic affecting millions of people in Asia, Africa, and Europe (62–64). Similarly, single E2 mutations in VEEV dramatically increase infectivity for the epizootic bridge vector Aedes (Ochlerotatus) taeniorhynchus, as well as equine amplification competence (65, 66). Our investigation into EILV host restriction suggests that the host range determinants are multigenic, involving mutations in one or more nsP and sP genes required for infection of vertebrate hosts. Pinpointing these mutations will yield insights into viral factors involved in vertebrate host range and viral pathogenesis.
Evolutionary implications of EILV host range restriction.
The presence of EILV vertebrate host range restriction at attachment/entry as well as genomic RNA replication is extraordinary because this virus groups phylogenetically with mosquito-borne viruses that possess broad vertebrate host range and cause severe disease. Thus, EILV's phylogenetic placement implies that alphaviruses can lose or gain the ability to infect either the vertebrate or insect host. This raises the intriguing possibility that EILV descended from a member of the WEE complex that lost the ability to infect vertebrates as the virus adapted unilaterally to its mosquito host. Several lines of evidence support this hypothesis: (i) antigenic cross-reactivity of EILV with members of WEE complex, SINV, WEEV, and Aura virus (AURAV); (ii) the phylogenetic placement based on the nsP ORF groups EILV within the WEE complex, basal to Whataroa virus (WHATV); and (iii) EILV nsPs and sPs display greater amino acid identity to SINV, WHATV, and AURAV than to other mosquito-borne viruses: nsP1 (71 to 73%), nsP2 (60 to 65%), nsP4 (74 to 77%), capsid (50 to 53%), E1 (49% to 50%), and E2 (40% to 42%) (28). The proposed hypothesis provides an interesting interpretation of these results (28).
Alternatively, EILV and other undiscovered mosquito-only alphaviruses may represent greatly undersampled ancestral lineages, a few of which gained the ability to infect vertebrates and gave rise to dual-host alphaviruses. Currently, no data are available to support this hypothesis for alphaviruses, as no other “mosquito-only” alphaviruses have been discovered. However, in the family Flaviviridae, genus Flavivirus, most of the mosquito-only flaviviruses are basal to the main branch of mosquito- and tick-borne pathogenic vertebrate viruses, suggesting that the pathogenic flaviviruses evolved from mosquito-only members of the genus (67). In addition, as insect species far outnumber vertebrate species, ∼16:1, they likely have larger viromes (68). Thus, insect-only viruses may vastly outnumber the dual-host arboviruses. More sampling and experimental work are required to investigate this hypothesis.
Regardless of whether EILV represents a lineage that lost or gained host range, our findings have several important implications. First, the gain or loss of host range may be common among alphaviruses as well as other arboviruses. This is supported by the utilization of swallow bugs, Oeciacus vicarious, as insect vectors by two WEE complex members, Fort Morgan virus (FMV) and its variant Buggy Creek virus (BCV) (69, 70). These viruses represent the only exception to the utilization of mosquitoes as vectors within the genus. Experimental infection of Culex and Culiseta mosquitoes with FMV showed that both species are refractory to oral infection and can support minimal replication only after intrathoracic injection (25, 39). The phylogenetic placement of FMV and BCV within the mosquito-borne clade suggests that an ancestral WEE complex alphavirus adapted to another insect order (order Hemiptera), which subsequently reduced its fitness in the donor mosquito host (order Diptera) (28). Second, our work implies the presence of two yet undiscovered, single-host groups within the alphavirus genus: (i) viruses similar to EILV that will group in the mosquito-borne clade and (ii) a group of arthropod viruses outside the main arbovirus clade. This would be consistent with the presence and phylogenetic placement of insect-only and no-known-vector viruses within the larger genus Flavivirus. The insect-only flaviviruses are present within both the mosquito-borne clade as well as in a separate, basal group within the genus, whereas the no-known-vector flavivirus group is basal to the main branch of mosquito- and tick-borne pathogenic vertebrate viruses (67). Third, adaptation to one host over time leads to host specialization or niche adaptation. EILV exhibits multigenic host range restriction and thus may not be able to gain the ability to infect vertebrates. Such host-specific adaptation can facilitate rapid sequence divergence over time, resulting in phylogenetic placement of such viruses outside the main mosquito-borne clade. Thus, insect-only viruses such as cell-fusing agent virus and other flaviviruses not only represent an ancestral lineage from which viruses gained the ability to infect vertebrates but also may serve as examples of ancestral flaviviruses that have undergone such adaptation. Consequently, our data suggest that EILV likely represents an evolutionary “snapshot” of a virus with such adaptation. Further investigation into EILV and other insect-only viruses across the arbovirus families is required to determine the evolutionary relationships of such viruses.
ACKNOWLEDGMENTS
We thank Jing H. Huang for technical assistance with mosquito experiments. We also thank Robert L. Seymour and Jesse Erasmus for technical assistance with mouse experiments.
This work was supported by National Institutes of Health contract HHSN272201000040I/HHSN27200004/D04 (to R.B.T.).
REFERENCES
- 1.Griffin DE. 2007. Alphaviruses, p 1023–1068 InKnipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed.Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Weaver SC, Reisen WK. 2010. Present and future arboviral threats. Antiviral Res 85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peleg J, Pecht M. 1978. Adaptation of an Aedes aegypti mosquito cell line to growth at 15 degrees C and its response to infection by Sindbis virus. J Gen Virol 38:231–239. doi: 10.1099/0022-1317-38-2-231. [DOI] [PubMed] [Google Scholar]
- 4.Stollar V, Thomas VL. 1975. An agent in the Aedes aegypti cell line (Peleg) which causes fusion of Aedes albopictus cells. Virology 64:367–377. doi: 10.1016/0042-6822(75)90113-0. [DOI] [PubMed] [Google Scholar]
- 5.Crabtree MB, Sang RC, Stollar V, Dunster LM, Miller BR. 2003. Genetic and phenotypic characterization of the newly described insect flavivirus, Kamiti River virus. Arch Virol 148:1095–1118. doi: 10.1007/s00705-003-0019-7. [DOI] [PubMed] [Google Scholar]
- 6.Hoshino K, Isawa H, Tsuda Y, Sawabe K, Kobayashi M. 2009. Isolation and characterization of a new insect flavivirus from Aedes albopictus and Aedes flavopictus mosquitoes in Japan. Virology 391:119–129. doi: 10.1016/j.virol.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Kim DY, Guzman H, Bueno R Jr, Dennett JA, Auguste AJ, Carrington CV, Popov VL, Weaver SC, Beasley DW, Tesh RB. 2009. Characterization of Culex flavivirus (Flaviviridae) strains isolated from mosquitoes in the United States and Trinidad. Virology 386:154–159. doi: 10.1016/j.virol.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 8.Haddow AD, Guzman H, Popov VL, Wood TG, Widen SG, Haddow AD, Tesh RB, Weaver SC. 2013. First isolation of Aedes flavivirus in the Western Hemisphere and evidence of vertical transmission in the mosquito Aedes (Stegomyia) albopictus (Diptera: Culicidae). Virology 440:134–139. doi: 10.1016/j.virol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Crabtree MB, Nga PT, Miller BR. 2009. Isolation and characterization of a new mosquito flavivirus, Quang Binh virus, from Vietnam. Arch Virol 154:857–860. doi: 10.1007/s00705-009-0373-1. [DOI] [PubMed] [Google Scholar]
- 10.Tyler S, Bolling BG, Blair CD, Brault AC, Pabbaraju K, Armijos MV, Clark DC, Calisher CH, Drebot MA. 2011. Distribution and phylogenetic comparisons of a novel mosquito flavivirus sequence present in Culex tarsalis mosquitoes from western Canada with viruses isolated in California and Colorado. Am J Trop Med Hyg 85:162–168. doi: 10.4269/ajtmh.2011.10-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junglen S, Kopp A, Kurth A, Pauli G, Ellerbrok H, Leendertz FH. 2009. A new flavivirus and a new vector: characterization of a novel flavivirus isolated from Uranotaenia mosquitoes from a tropical rain forest. J Virol 83:4462–4468. doi: 10.1128/JVI.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huhtamo E, Putkuri N, Kurkela S, Manni T, Vaheri A, Vapalahti O, Uzcátegui NY. 2009. Characterization of a novel flavivirus from mosquitoes in northern Europe that is related to mosquito-borne flaviviruses of the tropics. J Virol 83:9532–9540. doi: 10.1128/JVI.00529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vázquez A, Sánchez-Seco MP, Palacios G, Molero F, Reyes N, Ruiz S, Aranda C, Marqués E, Escosa R, Moreno J, Figuerola J, Tenorio A. 2012. Novel flaviviruses detected in different species of mosquitoes in Spain. Vector Borne Zoonotic Dis 12:223–229. doi: 10.1089/vbz.2011.0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.L'Heritier PH, Teissier G. 1937. Une anomalie physiologique héréditaire chez la Drosophile. C R Hebd Seances Acad Sci Paris 231:192–194. [Google Scholar]
- 15.Longdon B, Obbard DJ, Jiggins FM. 2010. Sigma viruses from three species of Drosophila form a major new clade in the rhabdovirus phylogeny. Proc Biol Sci 277:35–44. doi: 10.1098/rspb.2009.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan PL, Junglen S, Tashmukhamedova A, Conlan S, Hutchinson SK, Kurth A, Ellerbrok H, Egholm M, Briese T, Leendertz FH, Lipkin WI. 2010. Moussa virus: a new member of the Rhabdoviridae family isolated from Culex decens mosquitoes in Côte d'Ivoire. Virus Res 147:17–24. doi: 10.1016/j.virusres.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marklewitz M, Handrick S, Grasse W, Kurth A, Lukashev A, Drosten C, Ellerbrok H, Leendertz FH, Pauli G, Junglen S. 2011. Gouleako virus isolated from West African mosquitoes constitutes a proposed novel genus in the family Bunyaviridae. J Virol 85:9227–9234. doi: 10.1128/JVI.00230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn RJ. 2007. Togaviridae: the viruses and their replication, p 1001–1022 InKnipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed.Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 19.Powers AM, Brault AC, Shirako Y, Strauss EG, Kang W, Strauss JH, Weaver SC. 2001. Evolutionary relationships and systematics of the alphaviruses. J Virol 75:10118–10131. doi: 10.1128/JVI.75.21.10118-10131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forrester NL, Palacios G, Tesh RB, Savji N, Guzman H, Sherman M, Weaver SC, Lipkin WI. 2012. Genome-scale phylogeny of the alphavirus genus suggests a marine origin. J Virol 86:2729–2738. doi: 10.1128/JVI.05591-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arrigo NC, Adams AP, Weaver SC. 2010. Evolutionary patterns of Eastern equine encephalitis virus in North versus South America suggest ecological differences and taxonomic revision. J Virol 84:1014–1025. doi: 10.1128/JVI.01586-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Linn M, Gardner J, Warrilow D, Darnell GA, McMahon CR, Field I, Hyatt AD, Slade RW, Suhrbier A. 2001. Arbovirus of marine mammals: a new alphavirus isolated from the elephant seal louse, Lepidophthirus macrorhini. J Virol 75:4103–4109. doi: 10.1128/JVI.75.9.4103-4109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villoing S, Béarzotti M, Chilmonczyk S, Castric J, Brémont M. 2000. Rainbow trout sleeping disease virus is an atypical alphavirus. J Virol 74:173–183. doi: 10.1128/JVI.74.1.173-183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weston JH, Welsh MD, McLoughlin MF, Todd D. 1999. Salmon pancreas disease virus, an alphavirus infecting farmed Atlantic salmon, Salmo salar L. Virology 256:188–195. doi: 10.1006/viro.1999.9654. [DOI] [PubMed] [Google Scholar]
- 25.Karabatsos N. 1985. International catalog of arboviruses including certain other viruses of vertebrates, 3rd ed.American Society of Tropical Medicine and Hygiene, San Antonio, TX. [Google Scholar]
- 26.Jupp PG, McIntosh BM, Dos Santos I, DeMoor P. 1981. Laboratory vector studies on six mosquito and one tick species with Chikungunya virus. Trans R Soc Trop Med Hyg 75:15–19. doi: 10.1016/0035-9203(81)90005-5. [DOI] [PubMed] [Google Scholar]
- 27.Webb CE, Doggett SL, Ritchie SA, Russell RC. 2008. Vector competence of three Australian mosquitoes, Verrallina carmenti, Verraullina lineata, and Mansonia septempunctata (Diptera: Culicidae), for Ross River virus. J Med Entomol 45:737–740. doi: 10.1603/0022-2585(2008)45[737:VCOTAM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Nasar F, Palacios G, Gorchakov RV, Guzman H, Da Rosa AP, Savji N, Popov VL, Sherman MB, Lipkin WI, Tesh RB, Weaver SC. 2012. Eilat virus, a unique alphavirus with host range restricted to insects by RNA replication. Proc Natl Acad Sci U S A 109:14622–14627. doi: 10.1073/pnas.1204787109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice CM, Levis R, Strauss JH, Huang HV. 1987. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol 61:3809–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen L, Gubler D. 1974. The use of mosquitoes to detect and propagate dengue viruses. Am J Trop Med Hyg 23:1153–1160. [DOI] [PubMed] [Google Scholar]
- 31.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed.National Academies Press, Washington, DC. [Google Scholar]
- 32.Kuhn RJ, Hong Z, Strauss JH. 1990. Mutagenesis of the 3′ nontranslated region of Sindbis virus RNA. J Virol 64:1465–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pardigon N, Strauss JH. 1992. Cellular proteins bind to the 3′ end of Sindbis virus minus-strand RNA. J Virol 66:1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pardigon N, Lenches E, Strauss JH. 1993. Multiple binding sites for cellular proteins in the 3′ end of Sindbis alphavirus minus-sense RNA. J Virol 67:5003–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garneau NL, Sokoloski KJ, Opyrchal M, Neff CP, Wilusz CJ, Wilusz J. 2008. The 3′ untranslated region of Sindbis virus represses deadenylation of viral transcripts in mosquito and mammalian cells. J Virol 82:880–892. doi: 10.1128/JVI.01205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sokoloski KJ, Dickson AM, Chaskey EL, Garneau NL, Wilusz CJ, Wilusz J. 2010. Sindbis virus usurps the cellular HuR protein to stabilize its transcripts and promote productive infections in mammalian and mosquito cells. Cell Host Microbe 8:196–207. doi: 10.1016/j.chom.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Condit RC. 2007. Principles of virology, p 25–57 InKnipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed.Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 38.Garmashova N, Gorchakov R, Frolova E, Frolov I. 2006. Sindbis virus nonstructural protein nsP2 is cytotoxic and inhibits cellular transcription. J Virol 80:5686–5696. doi: 10.1128/JVI.02739-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brault AC, Armijos MV, Wheeler S, Wright S, Fang Y, Langevin S, Reisen WK. 2009. Stone Lakes virus (family Togaviridae, genus Alphavirus), a variant of Fort Morgan virus isolated from swallow bugs (Hemiptera: Cimicidae) west of the Continental Divide. J Med Entomol 46:1203–1209. doi: 10.1603/033.046.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coffey LL, Vasilakis N, Brault AC, Powers AM, Tripet F, Weaver SC. 2008. Arbovirus evolution in vivo is constrained by host alternation. Proc Natl Acad Sci U S A 105:6970–6975. doi: 10.1073/pnas.0712130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weaver SC, Brault AC, Kang W, Holland JJ. 1999. Genetic and fitness changes accompanying adaptation of an arbovirus to vertebrate and invertebrate cells. J Virol 73:4316–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coffey LL, Vignuzzi M. 2011. Host alternation of Chikungunya virus increases fitness while restricting population diversity and adaptability to novel selective pressures. J Virol 85:1025–1035. doi: 10.1128/JVI.01918-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasilakis N, Deardorff ER, Kenney JL, Rossi SL, Hanley KA, Weaver SC. 2009. Mosquitoes put the brake on arbovirus evolution: experimental evolution reveals slower mutation accumulation in mosquito than vertebrate cells. PLoS Pathog 5:e1000467. doi: 10.1371/journal.ppat.1000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deardorff ER, Fitzpatrick KA, Jerzak GV, Shi PY, Kramer LD, Ebel GD. 2011. West Nile virus experimental evolution in vivo and the trade-off hypothesis. PLoS Pathog 7:e1002335. doi: 10.1371/journal.ppat.1002335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moutailler S, Roche B, Thiberge JM, Caro V, Rougeon F, Failloux AB. 2011. Host alternation is necessary to maintain the genome stability of Rift Valley fever virus. PLoS Negl Trop Dis 5:e1156. doi: 10.1371/journal.pntd.0001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niesters HG, Strauss JH. 1990. Defined mutations in the 5′ nontranslated sequence of Sindbis virus RNA. J Virol 64:4162–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niesters HG, Strauss JH. 1990. Mutagenesis of the conserved 51-nucleotide region of Sindbis virus. J Virol 64:1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fayzulin R, Frolov I. 2004. Changes of the secondary structure of the 5′ end of the Sindbis virus genome inhibit virus growth in mosquito cells and lead to accumulation of adaptive mutations. J Virol 78:4953–4964. doi: 10.1128/JVI.78.10.4953-4964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuhn RJ, Griffin DE, Zhang H, Niesters HG, Strauss JH. 1992. Attenuation of Sindbis virus neurovirulence by using defined mutations in nontranslated regions of the genome RNA. J Virol 66:7121–7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heise MT, Simpson DA, Johnston RE. 2000. A single amino acid change in nsP1 attenuates neurovirulence of the Sindbis-group alphavirus S.A.AR86. J Virol 74:4207–4213. doi: 10.1128/JVI.74.9.4207-4213.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu WY, Fu SH, Wang JL, He Y, Tang Q, Liang GD. 2009. Effects of the nsP2-726 Pro mutation on infectivity and pathogenesis of Sindbis virus derived from a full-length infectious cDNA clone. Virus Res 142:204–207. doi: 10.1016/j.virusres.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 52.Lemm JA, Durbin RK, Stollar V, Rice CM. 1990. Mutations which alter the level or structure of nsP4 can affect the efficiency of Sindbis virus replication in a host-dependent manner. J Virol 64:3001–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vashishtha M, Phalen T, Marquardt MT, Ryu JS, Ng AC, Kielian M. 1998. A single point mutation controls the cholesterol dependence of Semliki Forest virus entry and exit. J Cell Biol 140:91–99. doi: 10.1083/jcb.140.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahn A, Schoepp RJ, Sternberg D, Kielian M. 1999. Growth and stability of a cholesterol-independent Semliki Forest virus mutant in mosquitoes. Virology 262:452–456. doi: 10.1006/viro.1999.9932. [DOI] [PubMed] [Google Scholar]
- 55.Heil ML, Albee A, Strauss JH, Kuhn RJ. 2001. An amino acid substitution in the coding region of the E2 glycoprotein adapts Ross River virus to utilize heparan sulfate as an attachment moiety. J Virol 75:6303–6309. doi: 10.1128/JVI.75.14.6303-6309.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heidner HW, Knott TA, Johnston RE. 1996. Differential processing of Sindbis virus glycoprotein PE2 in cultured vertebrate and arthropod cells. J Virol 70:2069–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levine B, Griffin DE. 1993. Molecular analysis of neurovirulent strains of Sindbis virus that evolve during persistent infection of scid mice. J Virol 67:6872–6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tucker PC, Griffin DE. 1991. Mechanism of altered Sindbis virus neurovirulence associated with a single-amino-acid change in the E2 glycoprotein. J Virol 65:1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levine B, Jiang HH, Kleeman L, Yang G. 1996. Effect of E2 envelope glycoprotein cytoplasmic domain mutations on Sindbis virus pathogenesis. J Virol 70:1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mérour E, Lamoureux A, Bernard J, Biacchesi S, Brémont M. 2013. A fully attenuated recombinant salmonid alphavirus becomes pathogenic through a single amino acid change in the E2 glycoprotein. J Virol 87:6027–6030. doi: 10.1128/JVI.03501-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hernandez R, Sinodis C, Horton M, Ferreira D, Yang C, Brown DT. 2003. Deletions in the transmembrane domain of a Sindbis virus glycoprotein alter virus infectivity, stability, and host range. J Virol 77:12710–12719. doi: 10.1128/JVI.77.23.12710-12719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. 2007. A single mutation in Chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog 3:e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsetsarkin KA, Weaver SC. 2011. Sequential adaptive mutations enhance efficient vector switching by Chikungunya virus and its epidemic emergence. PLoS Pathog 7:e1002412. doi: 10.1371/journal.ppat.1002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsetsarkin KA, Chen R, Yun R, Rossi SL, Plante KS, Guerbois M, Forrester N, Perng GC, Sreekumar E, Leal G, Huang J, Mukhopadhyay S, Weaver SC. 2014. Multi-peaked adaptive landscape for Chikungunya virus evolution predicts continued fitness optimization in Aedes albopictus mosquitoes. Nat Commun 5:4084. doi: 10.1038/ncomms5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brault AC, Powers AM, Ortiz D, Estrada-Franco JG, Navarro-Lopez R, Weaver SC. 2004. Venezuelan equine encephalitis emergence: enhanced vector infection from a single amino acid substitution in the envelope glycoprotein. Proc Natl Acad Sci U S A 101:11344–11349. doi: 10.1073/pnas.0402905101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anishchenko M, Bowen RA, Paessler S, Austgen L, Greene IP, Weaver SC. 2006. Venezuelan encephalitis emergence mediated by a phylogenetically predicted viral mutation. Proc Natl Acad Sci U S A 103:4994–4999. doi: 10.1073/pnas.0509961103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cook S, Moureau G, Kitchen A, Gould EA, de Lamballerie X, Holmes EC, Harbach RE. 2012. Molecular evolution of the insect-specific flaviviruses. J Gen Virol 93:223–234. doi: 10.1099/vir.0.036525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chapman A. 2009. Numbers of living species in Australia and the world, 2nd ed.Australian Biodiversity Information Services, Toowoomba, Australia. [Google Scholar]
- 69.Hayes RO, Francy DB, Lazuick JS, Smith GC, Gibbs EPJ. 1977. Role of the cliff swallow bug (Oeciacus vicarius) in the natural cycle of a Western equine encephalitis-related alphavirus. J Med Entomol 14:257–262. [Google Scholar]
- 70.Rush WA, Francy DB, Smith GC, Cropp CB. 1980. Transmission of an arbovirus by a member of the family Cimicidae. Ann Entomol Soc Am 73:315–318. [Google Scholar]
- 71.Saxton-Shaw KD, Ledermann JP, Borland EM, Stovall JL, Mossel EC, Singh AJ, Wilusz J, Powers AM. 2013. O'nyong nyong virus molecular determinants of unique vector specificity reside in non-structural protein 3. PLoS Negl Trop Dis 7:e1931. doi: 10.1371/journal.pntd.0001931. [DOI] [PMC free article] [PubMed] [Google Scholar]