Abstract
High-risk human papillomavirus (HPV) E6 proteins have a C-terminal PDZ binding motif through which they bind, and target for proteasome-mediated degradation, a number of PDZ-containing cellular targets. Recent studies have suggested that the RING-containing ubiquitin-protein ligase PDZRN3 might also be an HPV E6 target. In this analysis, we show that HPV-16 and HPV-18 E6 can target PDZRN3 in a PDZ- and proteasome-dependent manner and provide a connection between the HPV life cycle and differentiation-related STAT signaling.
TEXT
High-risk human papillomaviruses (HPVs) are the causative agents of cervical cancer, other anogenital cancers, and some head and neck cancers (1, 2). The E6 oncoproteins of high-risk HPV types have a C-terminal PDZ-binding motif (PBM) that is important for a normal viral life cycle and progeny production (for a review, see reference 3). PBM deletion causes viral genome integration, loss of replicative competence, and an increased potential for oncogenic transformation (4–8).
E6 targets several PDZ-containing proteins (9–12), and recently, a proteomic screen of DNA tumor virus interactions (13) and an HPV-16 E6 C-terminal peptide screen of the human PDZome (14) both identified the E3 ubiquitin-protein ligase PDZRN3 (LNX3/SEMCAP3) (15) as a potential HPV-16 E6 binding partner.
This was particularly interesting because HPV replication requires keratinocyte differentiation and PDZRN3 functions in the selection of stem cell differentiation pathways (16–19), inhibiting some but enhancing others and thus playing various roles in diverse differentiation pathways.
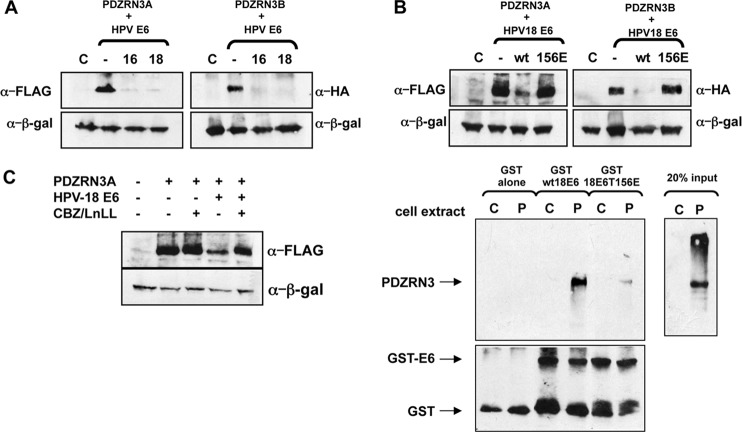
To determine whether PDZRN3 is an E6 target, we transfected 293 cells with pCDNA3:FLAG-PDZRN3A and pCDNA3:HA-PDZRN3B (isoforms of PDZRN3) plus pCDNA3:HPV-16E6 and pCDNA3:HPV-18E6 by Ca2PO4 precipitation (20). After 24 h, total protein was analyzed by SDS-PAGE and Western blotting with anti-hemagglutinin (HA; Roche), anti-FLAG (Stratagene), and anti-β-galactosidase (Promega) antibodies and a horseradish peroxidase-conjugated anti-mouse secondary antibody (Dako). The results in Fig. 1A show that PDZRN3 protein levels are reduced in the presence of HPV-16 and HPV-18 E6.
FIG 1.
(A) HPV-16 and HPV-18 E6 proteins induce degradation of PDZRN3. Plasmids expressing FLAG-tagged PDZRN3A and HA-tagged PDZRN3B were transfected into 293 cells alone or with plasmids expressing HPV-16 or HPV-18 E6. Cell extracts were analyzed by Western blotting with anti-HA and anti-FLAG antibodies; β-galactosidase was included as a control for transfection efficiency. (B) HPV-18 E6-induced degradation of PDZRN3 is PDZ binding dependent. (Top) Plasmids expressing FLAG-tagged PDZRN3A and HA-tagged PDZRN3B were transfected into 293 cells alone or with plasmids expressing wild-type HPV-18 E6 or the PDZ binding-defective HPV-18 E6T156E mutant. Cell extracts were analyzed by Western blotting with anti-HA and anti-FLAG antibodies; β-galactosidase was included as a control for transfection efficiency. (Bottom) Extracts of 293 cells transfected with pCDNA3.1 (lanes C) or pCDNA-FLAG-PDZRN3A (lanes P) were incubated with GST, GST-HPV18E6, or GST-HPV18E6T156E, as indicated. After washing, the bound proteins were analyzed by a Western blot assay probed with anti-PDZRN3 and anti-GST antibodies to confirm equal loading of GST proteins. (C) HPV-18 E6-induced degradation of PDZRN3 is proteasome dependent. A plasmid expressing FLAG-tagged PDZRN3A was transfected into 293 cells alone or with an HPV-18 E6-expressing plasmid. After overnight incubation, the cells were treated with the proteasome inhibitors carbobenzoxy-Leu-Leu-leucinal (CBZ) and N-acetyl-l-leucyl-l-leucyl-l-norleucinal for 3 h before harvesting. Cell extracts were analyzed by Western blotting with anti-FLAG antibody; β-galactosidase was included as a control for transfection efficiency.
To determine whether the interaction is PDZ dependent, we transfected 293 cells with pCDNA3:FLAG-PDZRN3A and pCDNA3:HA-PDZRN3B alone or with pCDNA3:HPV-18E6 or pCDNA3:HPV-18E6T156E (a PBM-defective mutant [9]). After 24 h, the total protein was analyzed as before, and the results in Fig. 1B (upper panels) show that the PBM-defective mutant of HPV-18 E6 cannot induce PDZRN3 degradation. To confirm interaction, extracts from 293 cells transfected with pCDNA3:FLAG-PDZRN3A or pCDNA3 alone were used in a pulldown assay with glutathione S-transferase (GST)-HPV18E6 or GST-HPV18E6T156E. The results in the lower panels show that the PBM mutation abolishes interaction between E6 and PDZRN3.
To determine whether E6-induced degradation of PDZRN3 is proteasome mediated, we transfected 293 cells with pCDNA3:FLAG-PDZRN3A with or without pCDNA3:HPV-18E6. After 24 h, the cells were treated with the proteasome inhibitors carbobenzoxy-Leu-Leu-leucinal (CBZ) and N-acetyl-l-leucyl-l-leucyl-l-norleucinal for 3 h before the proteins were assayed as before. The results in Fig. 1C show that proteasome inhibitors reduce the destabilization of PDZRN3. Thus, HPV-18 E6 targets PDZRN3A for degradation via the proteasome.
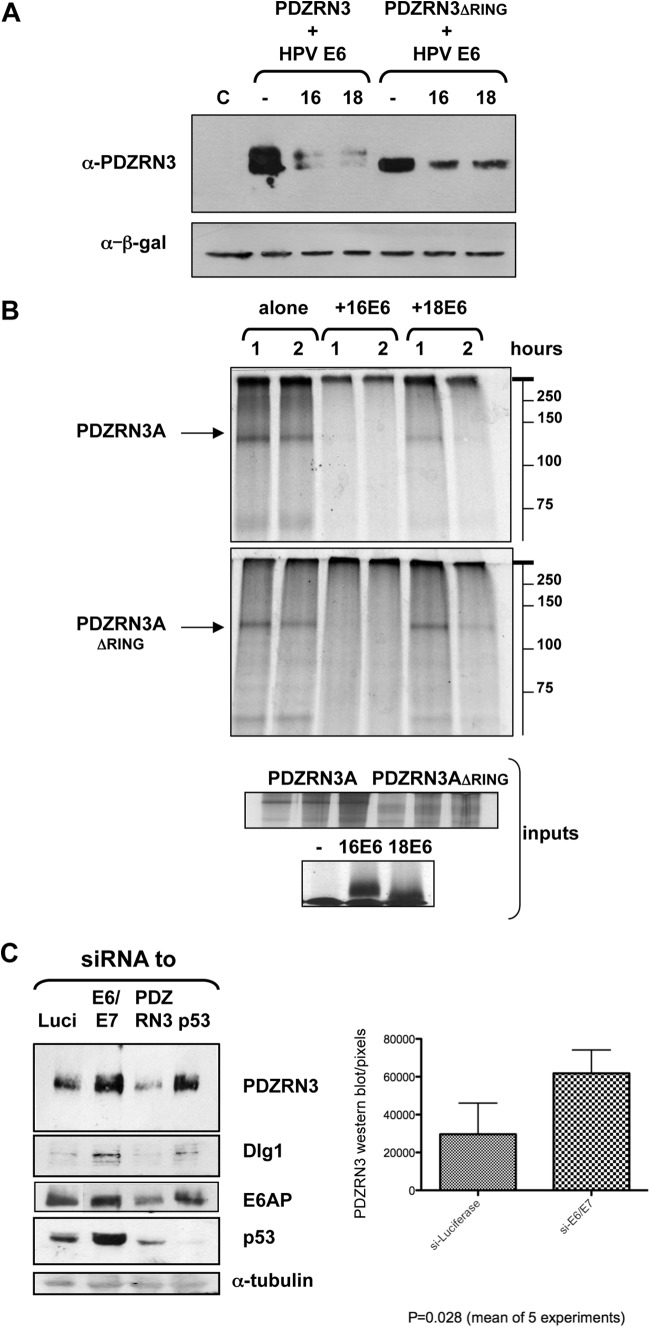
The PDZRN3 RING domain may have a ubiquitin-ligase function; to investigate whether E6 uses this to induce PDZRN3 autodegradation, we transfected 293 cells with plasmids expressing wild-type and ΔRING mutant PDZRN3 with or without pCDNA3:HPV-16E6 or pCDNA3:HPV-18E6, After 24 h, the total protein was analyzed as before with an anti-PDZRN3 antibody (Santa Cruz). Figure 2A shows that mutant PDZRN3ΔRING is degraded efficiently in the presence of HPV-16 and HPV-18 E6, suggesting that the RING domain is not required. Wild-type PDZRN3A appears to run as a doublet (also shown in Fig. 1C), but both bands are degraded equally in the presence of HPV-16 or HPV-18 E6. A degradation assay (Fig. 2B) with the radiolabeled, in vitro-translated PDZRN3 and HPV E6 proteins (TNT kit; Promega), showing that HPV-16 and HPV-18 E6 induces degradation of PDZRN3ΔRING in vitro, confirms that the interaction is robust.
FIG 2.
The RING domain of PDZRN3 is not required for E6-induced degradation. (A) Plasmids expressing PDZRN3A and PDZRN3AΔRING were transfected into 293 cells alone or with plasmids expressing HPV-16 or HPV-18 E6. Cell extracts were analyzed by Western blotting with an anti-PDZRN3 antibody (upper panel); β-galactosidase was included as a control for transfection efficiency (lower panel). (B) In vitro-translated radiolabeled PDZRN3A and PDZRN3AΔRING (arrow) and HPV-16 or HPV-18 E6 were incubated together at 30°C for 1 and 2 h, as indicated, and then analyzed by SDS-PAGE and autoradiography; positions of molecular weight markers are shown on the right. The inputs are shown in the lower panels. (C) Knockdown of HPV-18 E6 in HeLa cells results in increased levels of endogenous PDZRN3. HeLa cells were transfected with siRNA to luciferase (Luci), HPV-18 E6/E7, PDZRN3, or p53 (Dharmacon), and total cell extracts were harvested at 48 h posttransfection. Cell extracts were analyzed by Western blotting for the proteins indicated; α-tubulin was included as a loading control. The histogram shows the collated results of five experiments quantitated with ImageJ software and subjected to statistical analysis by the Prism program.
To determine whether HPV-18 E6 also targets endogenous PDZRN3, HPV-18-transformed HeLa cells, which continuously express E6 and E7, were transfected with small interfering RNAs (siRNAs; Dharmacon) to HPV-18 E6/E7 and PDZRN3, using RNAiMax (Invitrogen), with siRNAs for luciferase and p53 as controls. After 48 h, cell extracts were analyzed by Western blotting as before. The results in Fig. 2C show that HPV-18 E6/E7 knockdown increases the levels of p53, Dlg1, and E6AP (mouse monoclonal antibodies from Santa Cruz and BD Biosystems, respectively).The PDZRN3 levels also increase, indicating that endogenous PDZRN3 is targeted for degradation in HPV-18 E6-positive cells. The results from five such assays were quantitated by ImageJ software and subjected to statistical analysis with the Prism program. The histogram in Fig. 2C shows a statistically significant (P = 0.028) 2-fold increase in PDZRN3 levels in HeLa cells upon treatment with siRNA for HPV-18 E6/E7, relative to those treated with siRNA for luciferase.
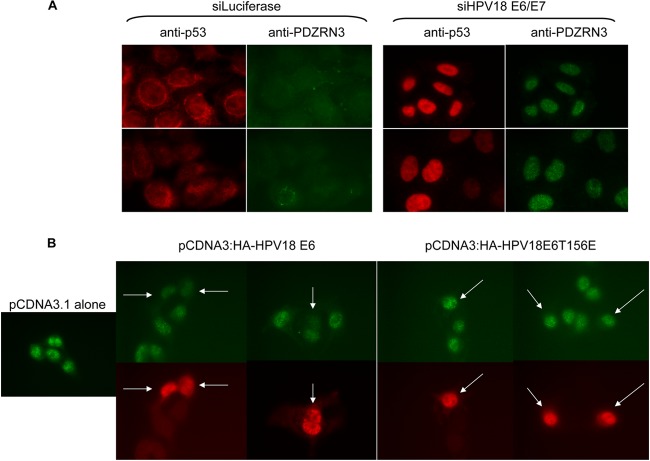
To investigate whether specific pools of the protein are targeted by E6, HeLa cells were transfected with siRNA for luciferase or siRNA for HPV18E6/E7 and, after 48 h, subjected to immunofluorescence analysis, probing for PDZRN3 and p53 as a positive control for HPV18E6/E7 silencing. The secondary antibodies were fluorescein isothiocyanate (FITC) and rhodamine conjugated (Invitrogen). The images in Fig. 3A, obtained with a Leica DMLB fluorescence microscope, show little PDZRN3 staining in HeLa cells transfected with siRNA to luciferase. However, in HeLa cells transfected with siRNA to HPV18E6/E7 that show nuclear p53 staining (indicating HPV-18 E6 knockdown [21]), strong nuclear PDZRN3 staining is also evident. Indeed, the strength of p53 staining, demonstrating the degree of E6 knockdown, also correlates with the strength of nuclear PDZRN3 staining (siRNA for E6/E7, lower panels). To confirm that this effect on the nuclear expression pattern of PDZRNA3 is mediated by the E6 PBM, we transfected HPV-negative C33A cells with either wild-type HA-HPV-18E6 or HA-HPV18E6T156E. The immunofluorescence analysis in Fig. 3B shows that the nuclear localization of PDZRN3 is disrupted by transfection of wild-type HPV18E6 but not by transfection of the T156E mutant.
FIG 3.
(A) Knockdown of HPV-18 E6 in HeLa cells increases nuclear PDZRN3 levels. HeLa cells were seeded onto coverslips and transfected with siRNA for luciferase or HPV-18 E6/E7 (Dharmacon). After 48 h, the cells were fixed, permeabilized, and then probed for p53 (red) and PDZRN3 (green). Each panel shows two different fields of view. (B) In C33A cells, transfection of HPV-18 E6 reduces nuclear PDZRN3 in a PBM-dependent manner. C33A cells were seeded onto coverslips and transfected with pCDNA3.1, pCDNA3:HA-HPV-18E6, or pCDNA3:HA-HPV18E6T156E. After 24 h, the cells were fixed, permeabilized, and then probed for HA (red) and PDZRN3 (green). Arrows indicate cells expressing E6 with a corresponding reduction in nuclear PDZRN3 with wild-type E6 but no change with the T156E mutant.
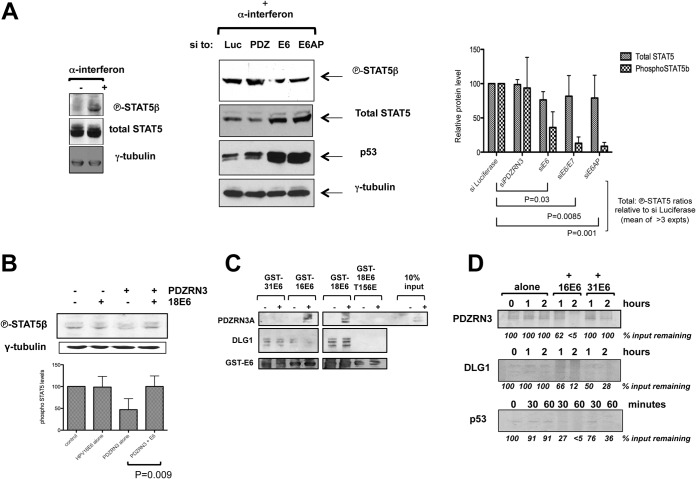
HPV-18 E6 targeting of PDZRN3 suggests that PDZRN3 might inhibit viral replication. The HPV life cycle depends upon keratinocyte differentiation, and PDZRN3 can affect differentiation through the Wnt or STAT5 signaling pathway, respectively (17, 18). Since inhibition of STAT5 signaling has been shown to reduce the genome amplification of HPV-31 (22), we transfected HeLa cells with siRNAs to PDZRN3, HPV-18 E6, and HPV-18 E6/E7 or E6AP to examine the effects on STAT5β activation. In parallel, cells were treated for 5 h with alpha interferon (IFN-α), which has been shown to induce the phosphorylation specifically of STAT5β, but not STAT5α, in HeLa cells (23). The proteins were then analyzed by Western blotting with rabbit monoclonal antibodies specific to phosphorylated (i.e., active) STAT5β and to total STAT5 (Cell Signaling). The left panel of Fig. 4A shows that IFN-α increases the level of phosphorylated STAT5, indicating that it is indeed STAT5β; the right panel shows that knockdown of E6, E6/E7, or E6AP reduces phospho-STAT5β levels, whereas PDZRN3 knockdown modestly increases phospho-STAT5β levels. The results of at least three assays were analyzed as before, and the histogram shows a slight reduction in the total STAT5 levels and a significantly greater reduction in phospho-STAT5β upon E6, E6/E7, or E6AP knockdown (P = 0.03, P = 0.0085, P = 0.001). Figure 4B shows that transfection of PDZRN3 into 293 cells decreases the phospho-STAT5β level by half (P = 0.011), relative to that of vector alone, and this is rescued upon cotransfection of HPV-18 E6 (P = 0.009). Together, these results suggest that one downstream effect of HPV-18 E6-induced degradation of PDZRN3 is an increase in STAT5β activation. In Fig. 2C, the p53 and Dlg1 levels appear to be reduced upon PDZRN3 knockdown; STAT5 activation has been shown to affect p53 function, and it is tempting to speculate that this might be the case here.
FIG 4.
(A) Knockdown of HPV-18 E6 in HeLa cells decreases levels of phosphorylated STAT5β. HeLa cells were transfected with siRNA to luciferase, PDZRN3, HPV18 E6, or E6AP, and total cell extracts were harvested at 72 h posttransfection. (Left panel) IFN-α treatment prior to harvesting specifically induces phosphorylation of STAT5β. (Right panel) Cell extracts were analyzed by Western blotting for the proteins indicated; γ-tubulin was included as a control. The histogram shows the collated results of at least three experiments quantitated with ImageJ software and subjected to statistical analysis by the Prism program. (B) Transfection of PDZRN3 in 293 cells reduces levels of phosphorylated endogenous STAT5β, which is rescued by cotransfection of HPV-18 E6. HEK293 cells were transfected with plasmids expressing PDZRN3 with or without plasmids expressing HPV-18 E6. Empty plasmid was transfected as a control. The cells were extracted in the presence of phosphatase inhibitors, and the extracts were analyzed by SDS-PAGE and Western blotting for the proteins indicated. The histogram shows the collated results of at least three experiments quantitated with ImageJ software and subjected to statistical analysis by the Prism program. (C) HPV-31 E6 binds to PDZRN3 more weakly than HPV-16 or HPV-18 E6. Extracts from 293 cells transfected with pCDNA3.1 (−) or pCDNA-FLAG-PDZRN3A (+) were incubated with GST-HPV31E6, GST-HPV16E6, GST-HPV18E6, or GST-HPV18E6T156E as indicated. After washing, the bound proteins were analyzed by a Western blot assay probed with anti-PDZRN3 and anti-GST antibodies to confirm equal loading of GST proteins. The blot was reprobed for DLG1 to confirm GST-E6 integrity. (D) HPV-31 E6 is less efficient than HPV-16 E6 at inducing PDZRN3 degradation in vitro. In vitro-translated PDZRN3 was incubated at 30°C either alone or with in vitro-translated HPV-16 or HPV-31 E6 as indicated. The remaining PDZRN3 was analyzed by SDS-PAGE and autoradiography. Dlg1 and p53 were included as controls. The values below the lanes are the percentages of the input protein remaining and are the mean results of two assays.
We have shown that high-risk HPV E6 proteins target PDZRN3 for PDZ-dependent, proteasome-mediated degradation, validating previous proteomic analyses (13, 14), and that HPV-18 E6-induced PDZRN3 degradation can lead to an increase in STAT5β activation. In HPV-31, E7 was shown to activate STAT5 directly and E6 was not required (22). However, different HPV types are known to differ in the strategies employed to achieve the same state (24–26). To address this point, extracts from 293 cells transfected with pCDNA3:FLAG-PDZRN3A or pCDNA3 alone were used in a pulldown assay with GST-HPV31E6, GST-HPV16E6, GST-HPV18E6, or GST-HPV18E6T156E. The Western blot analysis in Fig. 4C shows that PDZRN3 binds much more weakly to GST-HPV31E6 than to GST-HPV16 or GST-HPV18E6, whereas the binding to endogenous DLG1 is equivalent, showing that the GST-HPV31E6 PBM is functional. Figure 4D also shows that HPV-31 E6 is much less efficient than HPV-16 E6 at inducing PDZRN3 degradation in vitro.
Thus, it seems possible that different HPV types may activate the PPARγ pathway by activating STAT5β through E7 in some cases and by degrading its inhibitor, PDZRN3, through E6 in others.
ACKNOWLEDGMENTS
We are most grateful to Zhonghua Lu and Guoping Feng at the Department of Neurobiology, Duke University Medical Center, Durham, NC, for the kind gift of the plasmids expressing wild-type, mutant, tagged, and untagged PDZRN3A and PDZRN3B. We are very grateful to Nataša Skoko of the ICGEB Biotechnology Unit for the gift of IFN-α.
This work was supported in part by the Associazione Italiana per la Ricerca sul Cancro and the Wellcome Trust.
REFERENCES
- 1.IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. 2012. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 100(Pt B):1–441. [PMC free article] [PubMed] [Google Scholar]
- 2.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V, WHO International Agency for Research on Cancer Monograph Working Group . 2009. A review of human carcinogens–part B: biological agents. Lancet Oncol 10:321–322. doi: 10.1016/S1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 3.Pim D, Bergant M, Boon SS, Ganti K, Kranjec C, Massimi P, Subbaiah VK, Thomas M, Tomaić V, Banks L. 2012. Human papillomaviruses and the specificity of PDZ domain targeting. FEBS J 279:3530–3537. doi: 10.1111/j.1742-4658.2012.08709.x. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen ML, Nguyen MM, Lee D, Griep AE, Lambert PF. 2003. The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6's induction of epithelial hyperplasia in vivo. J Virol 77:6957–6964. doi: 10.1128/JVI.77.12.6957-6964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee C, Laimins LA. 2004. Role of the PDZ domain-binding motif of the oncoprotein E6 in the pathogenesis of human papillomavirus type 31. J Virol 78:12366–12377. doi: 10.1128/JVI.78.22.12366-12377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolaides L, Davy C, Raj K, Kranjec C, Banks L, Doorbar J. 2011. Stabilization of HPV16 E6 protein by PDZ proteins, and potential implications for genome maintenance. Virology 414:137–145. doi: 10.1016/j.virol.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Delury CP, Marsh EK, James CD, Boon SS, Banks L, Knight GL, Roberts S. 2013. The role of protein kinase A regulation of the E6 PDZ-binding domain during the differentiation-dependent life cycle of human papillomavirus type 18. J Virol 87:9463–9472. doi: 10.1128/JVI.01234-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brimer N, Vande Pol SB. 2014. Papillomavirus E6 PDZ interactions can be replaced by repression of p53 to promote episomal human papillomavirus genome maintenance. J Virol 88:3027–3030. doi: 10.1128/JVI.02360-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardiol D, Kühne C, Glaunsinger B, Lee SS, Javier R, Banks L. 1999. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene 18:5487–5496. doi: 10.1038/sj.onc.1202920. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa S, Huibregtse JM. 2000. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol Cell Biol 20:8244–8253. doi: 10.1128/MCB.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glaunsinger BA, Lee SS, Thomas M, Banks L, Javier R. 2000. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene 19:5270–5280. doi: 10.1038/sj.onc.1203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kranjec C, Banks L. 2011. A systematic analysis of human papillomavirus (HPV) E6 PDZ substrates identifies MAGI-1 as a major target of HPV type 16 (HPV-16) and HPV-18 whose loss accompanies disruption of tight junctions. J Virol 85:1757–1764. doi: 10.1128/JVI.01756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rozenblatt-Rosen O, Deo RC, Padi M, Adelmant G, Calderwood MA, Rolland T, Grace M, Dricot A, Askenazi M, Tavares M, Pevzner SJ, Abderazzaq F, Byrdsong D, Carvunis AR, Chen AA, Cheng J, Correll M, Duarte M, Fan C, Feltkamp MC, Ficarro SB, Franchi R, Garg BK, Gulbahce N, Hao T, Holthaus AM, James R, Korkhin A, Litovchick L, Mar JC, Pak TR, Rabello S, Rubio R, Shen Y, Singh S, Spangle JM, Tasan M, Wanamaker S, Webber JT, Roecklein-Canfield J, Johannsen E, Barabási AL, Beroukhim R, Kieff E, Cusick ME, Hill DE, Münger K, Marto JA, Quackenbush J, Roth FP, DeCaprio JA, Vidal M. 2012. Interpreting cancer genomes using systematic host network perturbations by tumour virus proteins. Nature 487:491–495. doi: 10.1038/nature11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belotti E, Polanowska J, Daulat AM, Audebert S, Thomé V, Lissitzky JC, Lembo F, Blibek K, Omi S, Lenfant N, Gangar A, Montcouquiol M, Santoni MJ, Sebbagh M, Aurrand-Lions M, Angers S, Kodjabachian L, Reboul J, Borg JP. 2013. The human PDZome: a gateway to PSD95-Disc large-zonula occludens (PDZ)-mediated functions. Mol Cell Proteomics 12:2587–2603. doi: 10.1074/mcp.O112.021022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn M, Saha O, Young P. 2011. Molecular evolution of the LNX family. BMC Evol Biol 11:235. doi: 10.1186/1471-2148-11-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko JA, Kimura Y, Matsuura K, Yamamoto H, Gondo T, Inui M. 2006. PDZRN3 (LNX3, SEMCAP3) is required for the differentiation of C2C12 myoblasts into myotubes. J Cell Sci 119(Pt 24):5106–5113. doi: 10.1242/jcs.03290. [DOI] [PubMed] [Google Scholar]
- 17.Honda T, Yamamoto H, Ishii A, Inui M. 2010. PDZRN3 negatively regulates BMP-2-induced osteoblast differentiation through inhibition of Wnt signaling. Mol Biol Cell 21:3269–3277. doi: 10.1091/mbc.E10-02-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honda T, Ishii A, Inui M. 2013. Regulation of adipocyte differentiation of 3T3-L1 cells by PDZRN3. Am J Physiol Cell Physiol 304:C1091–C1097. doi: 10.1152/ajpcell.00343.2012. [DOI] [PubMed] [Google Scholar]
- 19.Lu Z, Je HS, Young P, Gross J, Lu B, Feng G. 2007. Regulation of synaptic growth and maturation by a synapse-associated E3 ubiquitin ligase at the neuromuscular junction. J Cell Biol 177:1077–1089. doi: 10.1083/jcb.200610060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wigler M, Sweet R, Sim GK, Wold B, Pellicer A, Lacy E, Maniatis T, Silverstein S, Axel R. 1979. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell 16:777–785, doi: 10.1016/0092-8674(79)90093-X. [DOI] [PubMed] [Google Scholar]
- 21.Butz A, Ristriani T, Hengstermann A, Denk C, Scheffner M, Hoppe-Seyler F. 2003. siRNA targeting of the viral E6 oncogene efficiently kills human papillomavirus-positive cancer cells. Oncogene 22:5938–5945. doi: 10.1038/sj.onc.1206894. [DOI] [PubMed] [Google Scholar]
- 22.Hong S, Laimins LA. 2013. The JAK-STAT transcriptional regulator, STAT-5, activates the ATM DNA damage pathway to induce HPV 31 genome amplification upon epithelial differentiation. PLoS Pathog 9:e1003295. doi: 10.1371/journal.ppat.1003295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meinke A, Barahmand-Pour F, Wöhrl S, Stoiber D, Decker T. 1996. Activation of different Stat5 isoforms contributes to cell-type-restricted signaling in response to interferons. Mol Cell Biol 16:6937–6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomaić V, Gardiol D, Massimi P, Ozbun M, Myers M, Banks L. 2009. Human and primate tumour viruses use PDZ binding as an evolutionarily conserved mechanism of targeting cell polarity regulators. Oncogene 28:1–8. doi: 10.1038/onc.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas M, Massimi P, Navarro C, Borg JP, Banks L. 2005. The hScrib/Dlg apico-basal control complex is differentially targeted by HPV-16 and HPV-18 E6 proteins. Oncogene 24:6222–6230. doi: 10.1038/sj.onc.1208757. [DOI] [PubMed] [Google Scholar]
- 26.Boon SS, Banks L. 2013. High-risk human papillomavirus E6 oncoproteins interact with 14-3-3ζ in a PDZ binding motif-dependent manner. J Virol 87:1586–1595. doi: 10.1128/JVI.02074-12. [DOI] [PMC free article] [PubMed] [Google Scholar]