ABSTRACT
In order to develop strategies to prevent HIV-1 (human immunodeficiency virus type 1) transmission, it is crucial to better characterize HIV-1 target cells in the female reproductive tract (FRT) mucosae and to identify effective innate responses. Control of HIV-1 infection in the decidua (the uterine mucosa during pregnancy) can serve as a model to study natural mucosal protection. Macrophages are the main HIV-1 target cells in the decidua. Here we report that in vitro, macrophages and T cells are the main HIV-1 targets in the endometrium in nonpregnant women. As reported for decidual macrophages (dM), endometrial macrophages (eM) were found to have an M2-like phenotype (CD68+ CD163+ CD206+ IL-10high). However, eM and dM may belong to different subpopulations, as they differently express certain markers and secrete different amounts of proinflammatory and anti-inflammatory cytokines. We observed strong expression of the SAMHD1 restriction factor and weak expression of its inactive form (pSAMHD1, phosphorylated at residue Thr592) in both eM and dM. Infection of macrophages from both tissues was enhanced in the presence of the viral protein Vpx, suggesting a role for SAMHD1 in the restriction of HIV-1 infection. This study and further comparisons of the decidua with FRT mucosae in nonpregnant women should help to identify mechanisms of mucosal protection against HIV-1 infection.
IMPORTANCE The female reproductive tract mucosae are major portals of HIV-1 entry into the body. The decidua (uterine mucosa during pregnancy) can serve as a model for studying natural mucosal protection against HIV-1 transmission. A comparison of target cells and innate responses in the decidua versus the endometrium in nonpregnant women could help to identify protective mechanisms. Here, we report for the first time that macrophages are one of the main HIV-1 target cells in the endometrium and that infection of macrophages from both the endometrium and the decidua is restricted by SAMHD1. These findings might have implications for the development of vaccines to prevent HIV-1 mucosal transmission.
INTRODUCTION
One-half of people living with human immunodeficiency virus type 1 (HIV-1) are women (1). Young women (15 to 24 years) are more vulnerable than young men to HIV-1 transmission and account for 22% of new infections (2). Sexual transmission is the most common route of HIV-1 infection.
The precise sites of the female reproductive tract (FRT) involved in establishing the initial infection are poorly known. In the macaque model, the tissue lying at the transition between the ectocervix and endocervix, called the transformation zone, has been identified as the preferential portal for simian immunodeficiency virus (SIV) entry (3). Whether or not HIV-1 can reach the uterine cavity is still controversial. In the macaque model, uterine infection has been observed in one animal 2 days after vaginal inoculation (4). Moreover, human endometrial explants are susceptible to HIV-1 infection (5, 6). Thus, all compartments of the FRT, including the endometrium, might contribute to establishment of the initial infection.
In vitro studies have shown that macrophages and T cells from the cervix and vagina are susceptible to HIV-1 infection (7–13). It was recently reported that endometrial T cells are weakly permissive to HIV-1 infection (14). The permissivity of endometrial macrophages has not been determined. Nevertheless, infected cells with a monocyte/macrophage morphology not belonging to the T cell lineage have been detected in the endometrium of an HIV-1-infected woman (15). In vitro, in the decidua basalis (the uterine mucosa during pregnancy), macrophages are the main HIV-1 target cells (16). Decidual macrophages (dM; CD14+ cells) have an M2-like phenotype and show features of proinflammatory, tolerogenic macrophages (17–19).
It is not known whether decidual and endometrial macrophages (CD14+ cells) have the same phenotype. Macrophages undergo polarization in response to a broad range of environmental signals, and this polarization influences their susceptibility to HIV-1 infection (20). Estrogen and progesterone concentrations are considerably higher during pregnancy than during the different phases of the menstrual cycle (21). It has been shown that estrogen reduces the susceptibility of monocyte-derived macrophages (MDM) to HIV-1 infection (22). In addition, immune cell distribution differs between the decidua and endometrium and changes during the menstrual cycle and pregnancy (23, 24). As the decidual and endometrial environments are different, macrophages from these tissues might have distinct phenotypes and different permissivities to HIV-1 infection.
The decidua is a model of natural mucosal protection against HIV-1 transmission. Mother-to-child HIV-1 transmission is relatively rare during the first trimester of pregnancy, despite the permissivity of the placenta and decidua (25), suggesting that HIV-1 infection is controlled at the maternofetal interface. Soluble factors secreted by decidual cells partially inhibit HIV-1 entry into dM (26). Moreover, viral production by dM is low, suggesting that these cells possess intrinsic viral control mechanisms (16).
In the periphery, the weak susceptibility of myeloid cells to HIV-1 infection is attributed to restriction factors (27). One of these restriction factors is the cellular factor SAMHD1 (sterile alpha motif domain HD domain-containing protein 1) (28, 29). HIV-1 infection is restricted by SAMHD1 in myeloid cells (28, 29) and in resting CD4 T lymphocytes (30, 31). The antiviral activity of SAMHD1 is regulated by phosphorylation of the SAMHD1 Thr592 residue (32), the phosphorylated form being inactive. Vpx, the accessory protein of HIV-2 and some types of SIV, degrades SAMHD1 (28, 29, 33). SAMHD1 restriction is abrogated when MDM are infected in the presence of virus-like particles containing Vpx (VLP-Vpx) (28, 29). To our knowledge, SAMHD1 expression has never been reported for macrophages from FRT mucosae.
Studies of the natural control of HIV-1 infection in the decidua could help to identify mechanisms of mucosal protection. Comparison of immune components in the decidua and FRT mucosae in nonpregnant women could help to identify control mechanisms present in the FRT and those that could be induced to prevent sexual HIV-1 transmission. For accurate comparison of the decidua and endometrium, it is first important to characterize HIV-1 target cells in the endometrium, for which few studies have been conducted.
We therefore characterized HIV-1 target cells in the endometrium in vitro and then compared endometrial macrophages with decidual macrophages. We also analyzed the expression of the restriction factor SAMHD1 in decidual and endometrial macrophages.
MATERIALS AND METHODS
Ethical statement.
All the tissue donors in this study provided their written informed consent. The Biomedical Agency (no. PF508-013), Assistance Publique des Hôpitaux de Paris (no. VAL/2011/06-41/02), and the biomedical research committee of the Institut Pasteur, Paris, France (no. 2005.024) approved the study of the decidua. Comité de protection des personnes (no. 2011, July 12674), Assistance Publique des Hôpitaux de Paris (no. VAL/2011/2011.277/01), the biomedical research committee of the Institut Pasteur, Paris, France (no. 2011.18) and Comité Nationale de l'Informatique et des Libertés (no. DR-2011-491) approved the study of FRT mucosae.
Human decidual tissue collection and isolation of decidual mononuclear cells and CD14+ cells.
Decidual tissues were obtained from healthy women undergoing voluntary termination of pregnancy during the first trimester (8 to 12 weeks of gestation) at Antoine Béclère Hospital (Clamart, France) or Pitié Salpêtrière Hospital (Paris, France). The tissues were minced and digested with 1 mg/ml of collagenase IV (Sigma, St Quentin Fallavier, France) and 250 U/ml of recombinant DNase I (Roche, Meylan, France) for 45 min at 37°C with agitation. The cell suspensions were filtered successively through 100-, 70-, and 40-μm-pore-size sterile nylon cell strainers (BD Biosciences, Le pont de Claix, France). Mononuclear cells were isolated from the cell suspension by Ficoll gradient centrifugation using lymphocyte separation medium (PAA, Les Mureaux, France). CD14+ cells (dM) were purified by positive selection with anti-CD14 magnetic beads (Miltenyi, Paris, France). dM purity was checked by staining and flow cytometry and was 94.2% ± 1.1% (mean ± standard error of the mean [SEM]). Contaminating cells were mainly CD45− cells.
Human endometrial tissue collection and isolation of endometrial mononuclear cells and CD14+ cells.
Endometrial biopsy specimens were obtained from nonmenopausal women aged 24 to 52 years (mean, 42 years) undergoing hysterectomy for nonmalignant disorders at Bicêtre Hospital (Kremlin-Bicêtre, France). Surgery was always performed during the proliferative phase of the menstrual cycle. Histologic analysis of the tissue samples revealed no pathological or tumoral processes. After separation from muscle tissue, the endometrium was minced and digested with 2.5 mg/ml of collagenase IV (Sigma) and 2,000 U/ml of DNase I (Roche) for 1 h at 37°C with agitation. The cell suspensions were filtered successively through 100- and 30-μm-pore-size sterile nylon cell strainers (BD Biosciences and Miltenyi). The mean weight of the endometrial samples was 0.55 g, and 15 × 106 total cells were obtained per gram of tissue. CD14+ cells (eM) were purified by positive selection with anti-CD14 magnetic beads (Miltenyi). eM purity was checked by staining and flow cytometry and was 83.8% ± 1.3% (mean ± SEM). Contaminating cells were mainly CD45− cells.
HIV-1 isolates and infection.
Isolated cells were exposed to HIV-1BaL (R5) or HIV-1 pseudotyped vesicular stomatitis virus G protein (HIV-1/VSV-G). HIV-1BaL was amplified for 11 days in phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMC), depleted of CD8+ cells, from three blood donors. The virus was concentrated by centrifugation on Vivaspin 100,000-kDa columns (Sartorius, Palaiseau, France) at 2,000 × g for 30 min and was titrated on PBMC.
The luciferase-expressing viral pseudotype was produced in HEK-293T cells cotransfected with the NL4-3 HIV-1 and VSV-G plasmids, using the transfection reagent SuperFect (Qiagen, Courtaboeuf, France) (34). pNL4-3ΔEnvLuc+ lacks the nef gene. The resulting pseudotype is nonreplicating. Supernatants were collected 72 h after transfection and titrated on HeLa P4P cells.
Control virus-like particles (VLP) and Vpx-containing VLP were produced in HEK-293T cells cotransfected with pSIV3+ (VLP-Vpx) or pSIV3+ ΔVpx (control VLP) (35–37). Supernatants were collected 24 and 48 h after transfection, then pooled, and concentrated by polyethylene glycol precipitation.
Total decidual or endometrial mononuclear cells or macrophages (CD14+ cells) were incubated for 1 h with HIV-1BaL at a multiplicity of infection (MOI) of 10−3 at 37°C. Cells were spinoculated with the HIV-1/VSV-G pseudotype (600 ng of p24 antigen [Ag]/106 cells) for 30 min at 1,200 × g and then incubated at 30°C for 30 min. Control VLP and VLP-Vpx were added at the same time as the virus or pseudotype. Total mononuclear cells or CD14+ cells were cultured at 106 cells/ml in Ham F-12 medium and Dulbecco modified Eagle medium (DMEM) containing GlutaMAX (Gibco, Cergy Pontoise, France) (50% vol/vol) supplemented with 15% fetal calf serum (PAA; Les Mureaux, France), penicillin (0.1 U/ml), and streptomycin (10−8 g/liter). HIV-1BaL production was measured every 3 or 4 days by enzyme-linked immunosorbent assay (ELISA) quantification (Zeptometrix, Franklin, Massachusetts) of the viral core antigen p24 in culture supernatants. The efficiency of infection by the viral pseudotype was determined by measuring luciferase activity with the luciferase reagent (Promega, Lyon, France) on a Glomax luminometer.
Flow cytometry.
Antibodies used for flow cytometry are listed in Table 1. Intracellular staining of CD68 and p24 Ag was performed with the BD Cytofix/Cytoperm kit. For SAMHD1 staining, cells were fixed with 4% paraformaldehyde for 5 min, followed by permeabilization with phosphate-buffered saline (PBS)–0.5% Triton for 20 min. For pSAMHD1 Thr592 staining, we used an antibody generated and shown to be specific for this form by the laboratory of M. Benkirane (Molecular Virology, Montpellier, France; unpublished data). Cells were fixed with 1% paraformaldehyde for 10 min, followed by permeabilization with 80% methanol for 10 min. An Fc receptor (FcR) blocking reagent (Miltenyi) was added before cell fixation and before pSAMHD1 Thr592 antibody labeling.
TABLE 1.
Antibodies used for flow cytometry analysesa
| Antibody | Clone | Manufacturer or source |
|---|---|---|
| CCR5-APC-Cy7 | 2D7 | BD |
| CD11b-APC-Cy7 | ICRF44 | BD |
| CD11c-PE-Cy7 | 3.9 | eBioscience |
| CD14-Pacific blue | M5E2 | BD |
| CD16-FITC | 3G8 | Beckman Coulter |
| CD163-PE | GHI/61 | BD |
| CD206-APC | 19.2 | BD |
| CD3-PE-Texas red | UCHT1 | Beckman Coulter |
| CD32-APC | FLI8.26 | BD |
| CD4-PE-Cy7 | SK3 | BD |
| CD45-AmCyan | 2D1 | BD |
| CD64-FITC | 10.1 | BD |
| CD68-APC | Y1/82A | Miltenyi |
| CD80-PE | MAB104 | Beckman Coulter |
| CD83-APC | HB15e | BD |
| CD86-PE-Cy7 | FM95 | Miltenyi |
| CXCR4-APC | 12G5 | BD |
| DC-SIGN–PE | 120507 | R&D |
| HLA-DR–PE | Immu-357 | Beckman Coulter |
| p24-FITC | KC57 | Beckman Coulter |
| SAMHD1-A488 | I19-18 | Kindly provided by O. Schwartz (51) |
| Isotype-matched IgG control-A488 | Mouse | Cell Signaling |
| pSAMHD1 Thr592 | Kindly provided by M. Benkirane |
APC, allophycocyanin; PE, phycoerythrin; FITC, fluorescein isothiocyanate.
Samples were analyzed by flow cytometry, using an LSRII 2-Blue 2-Violet 3-Red 5-Yelgr laser configuration (BD Biosciences) and FlowJo 9.1.3 software (Tristar).
Western blotting.
Purified dM (CD14+ cells) were treated with control VLP or VLP-Vpx and lysed after 72 h of culture. Cell extracts were prepared in buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and a protease and phosphatase inhibitor (Roche). Cell extracts were hybridized with anti-SAMHD1 (Abcam, Paris, France) or anti-actin (Sigma) antibodies, followed by secondary anti-mouse antibodies (Cell Signaling). Proteins were revealed on Hyperfilms (Amersham, Courtaboeuf, France) with the SuperSignal West Pico chemiluminescent substrate (Thermo Scientific, Courtaboeuf, France).
Cytokine quantification.
Endometrial and decidual macrophages (CD14+ cells) were cultured at 106 cells/ml. Soluble factors present in the culture supernatant after 72 h were quantified by Luminex assay (cytokine human magnetic 25-plex panel; Invitrogen).
Statistical analyses.
Statistical analyses were performed with GraphPad Prism software version 5.0f. The Mann-Whitney test was used to compare decidual and endometrial samples. The Wilcoxon matched-pairs test was used to analyze the effect of VLP-Vpx on the infection of decidual and endometrial cells.
RESULTS
Viral production kinetics differ between the endometrium and decidua.
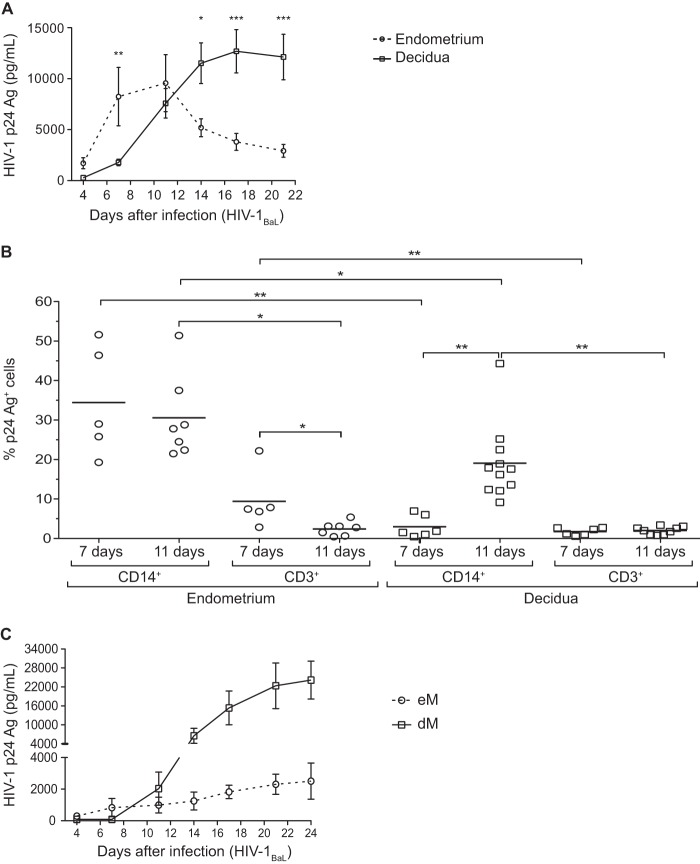
Total cells isolated from the endometrium and decidua were exposed to HIV-1BaL (R5 strain) at an MOI of 10−3. Viral production was monitored by quantifying p24 Ag in culture supernatants (Fig. 1A). In endometrial cells, viral production peaked 11 days after infection. In the decidua, as we have previously reported (16), viral production was maximal around day 17 postinfection. Viral production became detectable and peaked earlier in the endometrium than in the decidua.
FIG 1.
Infection of endometrial and decidual mononuclear cells and macrophages. (A and B) Endometrial and decidual mononuclear cells were exposed to HIV-1BaL at an MOI of 10−3. (A) The HIV-1 p24 Ag was measured by ELISA in the culture supernatants of endometrial (n = 11) and decidual (n = 16) mononuclear cells over time. The mean and the standard error of the mean of the viral production are displayed. (B) Intracellular staining of the p24 Ag was performed on days 7 and 11 postinfection in endometrial and decidual mononuclear cells. The percentage of p24 Ag+ cells among CD14+ cells or CD3+ cells is shown (n = 5 on day 7 and n = 7 on day 11 for the endometrium; n = 6 on day 7 and n = 11 on day 11 for the decidua). Each circle represents one endometrial sample and each square one decidual sample. The means are displayed. *, P < 0.05; **, P < 0.005; ***, P < 0.0005. (C) Endometrial (eM, n = 4) and decidual (dM, n = 17) purified macrophages were exposed to HIV-1BaL at an MOI of 10−3. The HIV-1 p24 Ag was measured by ELISA in the culture supernatants over time.
CD14+ cells and CD3+ cells are HIV-1 targets in both the endometrium and decidua.
Infected cells were identified by intracellular staining and flow cytometry on days 7 and 11 postinfection (Fig. 1B). p24 Ag was detected in both CD14+ cells and CD3+ cells from both the endometrium and the decidua. In both tissues, the percentage of p24 Ag+ cells was higher among CD14+ cells than among CD3+ cells on days 7 and 11.
The percentage of p24 Ag+ cells among endometrial CD14+ cells did not differ significantly between days 7 and 11 (means, 34.4% and 30.6%, respectively) (Fig. 1B), whereas the percentage of p24 Ag+ cells among CD3+ cells fell significantly between day 7 and day 11 (means, 9.4% and 2.7%, respectively) (Fig. 1B).
The percentage of infected cells among dM (CD14+ cells) was lower on day 7 than on day 11 (means, 3.0% and 19.1%, respectively) (Fig. 1B), while the percentage of infected cells among decidual CD3+ cells was stable between days 7 and 11 (means, 1.7% and 2.0%, respectively) (Fig. 1B).
The percentage of infected CD14+ cells was significantly higher among endometrial cells than among decidual cells on days 7 and 11 (Fig. 1B). However, viral production by cells exposed to HIV-1BaL was higher with purified dM than with purified endometrial CD14+ cells (Fig. 1C). The percentage of infected CD3+ cells was also significantly higher among endometrial cells than among decidual cells on day 7 (Fig. 1B), whereas the percentages were similar in cells obtained from both tissues on day 11.
The main HIV-1-infected cells in the endometrium vary with time postinfection.
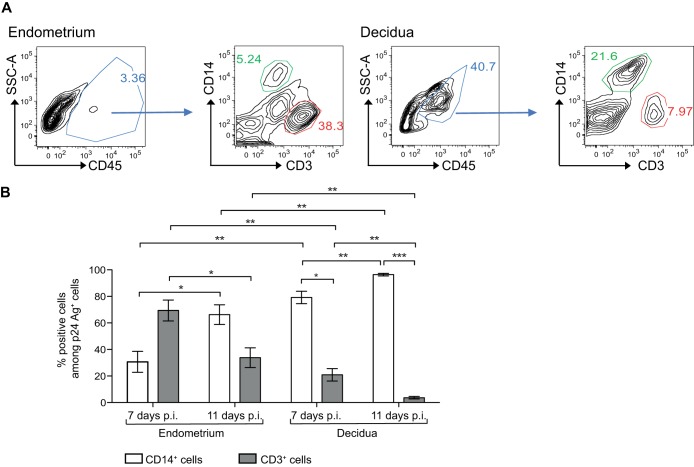
It is important to note that the proportions of CD14+ cells and CD3+ cells differed between the endometrium and decidua. In endometrial tissue used for this study, CD14+ cells accounted for 6.1% of CD45+ cells, whereas CD3+ cells accounted for 31% (mean of 9 samples; see Fig. 2A for a representative sample). In decidual tissues used for this study, CD14+ cells (dM) accounted for 17% of CD45+ cells, while CD3+ cells accounted for 6% (mean of 9 samples; see Fig. 2A for a representative sample).
FIG 2.
Percentage of CD14+ cells and CD3+ cells among endometrial and decidual HIV-1-infected cells. (A) Flow cytometry graphs show the gating strategy for the identification of CD14+ cells (green gate) and CD3+ cells (red gate). (B) Endometrial and decidual mononuclear cells were exposed to HIV-1BaL at an MOI of 10−3. Intracellular staining of the p24 Ag was performed on days 7 and 11 postinfection (p.i.). The mean percentages of CD14+ cells or CD3+ cells among p24 Ag+ cells are displayed (n = 5 on day 7 and n = 7 on day 11 for the endometrium; n = 6 on day 7 and n = 11 on day 11 for the decidua). Bars indicate standard errors of the means. *, P < 0.05; **, P < 0.005.
Thus, on day 7 postinfection, CD14+ cells accounted for 30.6% of infected endometrial cells and CD3+ cells for 69.4% (mean of 5 samples), whereas on day 11 CD14+ cells accounted for 66.2% of infected endometrial cells and CD3+ cells for 33.8% (mean of 7 samples) (Fig. 2B). Among decidual cells, 79.1% of infected cells were CD14+ cells and 20.9% were CD3+ cells on day 7 (mean of 6 samples), while 96.4% of infected cells were CD14+ cells and 3.6% were CD3+ cells on day 11 (mean of 11 samples).
Thus, in the endometrium, T CD3+ cells were the main infected cells on day 7 postinfection, while infected CD14+ cells predominated on day 11. In the decidua, macrophages (CD14+ cells) were the main infected cells at both time points.
Endometrial and decidual CD14+ cells have a macrophage phenotype.
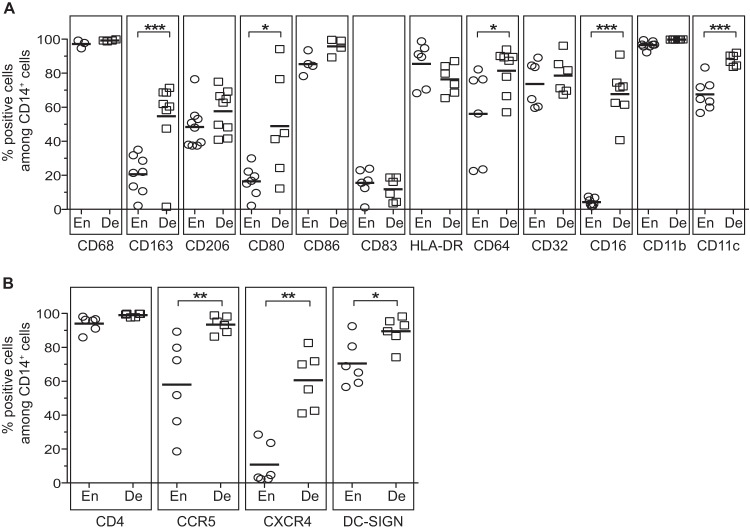
The phenotype of endometrial CD14+ cells is unknown, whereas decidual CD14+ cells are known to be macrophages (dM) with an M2-like phenotype (16–18). We used flow cytometry to compare the phenotypes of endometrial and decidual CD14+ cells. Like dM, endometrial CD14+ cells were CD68+, indicating that they were macrophages (Fig. 3A). The percentage of cells expressing the macrophage scavenger receptor CD163 was significantly lower among endometrial macrophages (eM) than among dM (means, 20.6% and 54.8% of cells, respectively). The macrophage mannose receptor CD206 was similarly expressed on macrophages from both tissues (means, 48.4% of eM and 57.6% of dM). The costimulation markers CD80 and CD86 were also expressed. The percentage of CD80-expressing cells was significantly lower among eM than among dM (means, 16.5% and 48.9%, respectively), while the percentage of CD86-expressing cells was high in the two tissues (means, 85.4% of eM and 95.8% of dM). A small percentage of macrophages expressed the maturation marker CD83 (means, 15.6% of eM and 11.8% of dM). The percentage of HLA-DR+ macrophages was high in both tissues (means, 85.5% of eM and 76.4% of dM). Macrophages from both tissues also expressed the Fcγ receptors CD64 (FcγRI) and CD32 (FcγRII). The percent CD64 expression was significantly lower on eM than on dM (means, 56.1% and 81.5%, respectively). The percentages of CD32-expressing cells were similar in the two tissues (means, 73.6% of eM and 78.6% of dM). The percentage of macrophages expressing CD16 (FcγRIII) was high in the decidua (mean, 67.7%) and low in the endometrium (mean, 4.2%). The percentage of cells expressing CD11b and CD11c was high among both eM and dM, but the percent CD11c expression was significantly lower on eM than on dM (means, 67.6% and 87.9%, respectively).
FIG 3.
Phenotypes of endometrial and decidual CD14+ cells. Expression of antigen-presenting cell markers (A) and of HIV-1 receptor and coreceptors (B) was analyzed on CD45+ CD14+ cells by flow cytometry stainings of total endometrial or decidual cells. The mean percentages of expression of these markers among the CD45+ CD14+ cells from the endometrium (En) or the decidua (De) are depicted. Each circle represents one endometrial sample and each square one decidual sample. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
The percent expression of the HIV-1 receptor molecule CD4 was high on macrophages from both tissues (means, 94% of eM and 99.1% of dM) (Fig. 3B). The percentages of cells expressing the HIV-1 coreceptors CCR5 and CXCR4 were lower among eM than among dM (means, 58% and 93.5%, respectively, for CCR5 and 10.8% and 60.6%, respectively, for CXCR4). DC-SIGN was also expressed on macrophages from both tissues, although its percent expression was lower on eM (means, 70.5% of eM and 89.5% of dM).
Thus, endometrial CD14+ cells, like decidual CD14+ cells, have a macrophage phenotype. However, eM express CD163, CD80, CD64, CD16, CD11c, CCR5, CXCR4, and DC-SIGN significantly less strongly than dM.
Endometrial and decidual macrophages secrete proinflammatory and anti-inflammatory cytokines and chemokines.
Soluble factors secreted by purified eM and dM during 72 h of culture were analyzed with a multiplex cytokine assay. eM and dM both secreted interleukin 10 (IL-10) and IL-1RA (Fig. 4A), but the concentrations of these anti-inflammatory cytokines were lower in eM supernatants than in dM supernatants (means, 1,207 pg/ml and 2,657 pg/ml, respectively, for IL-10 and 728 pg/ml and 1,821 pg/ml, respectively, for IL-1RA). The proinflammatory cytokines IL-1β and tumor necrosis factor alpha (TNF-α) were weakly secreted by both eM and dM (means, 87 pg/ml and 291 pg/ml, respectively, for IL-1β and 46 pg/ml and 606 pg/ml, respectively, for TNF-α) (Fig. 4B). IL-12 was weakly secreted by dM (mean, 118 pg/ml) and was not detected in eM supernatants. The proinflammatory cytokine IL-6 was strongly secreted by both eM and dM (means, 14,740 pg/ml and 15,320 pg/ml, respectively). eM and dM also secreted the cytokines IL-2RA, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-15, and alpha interferon (IFN-α) (data not shown).
FIG 4.
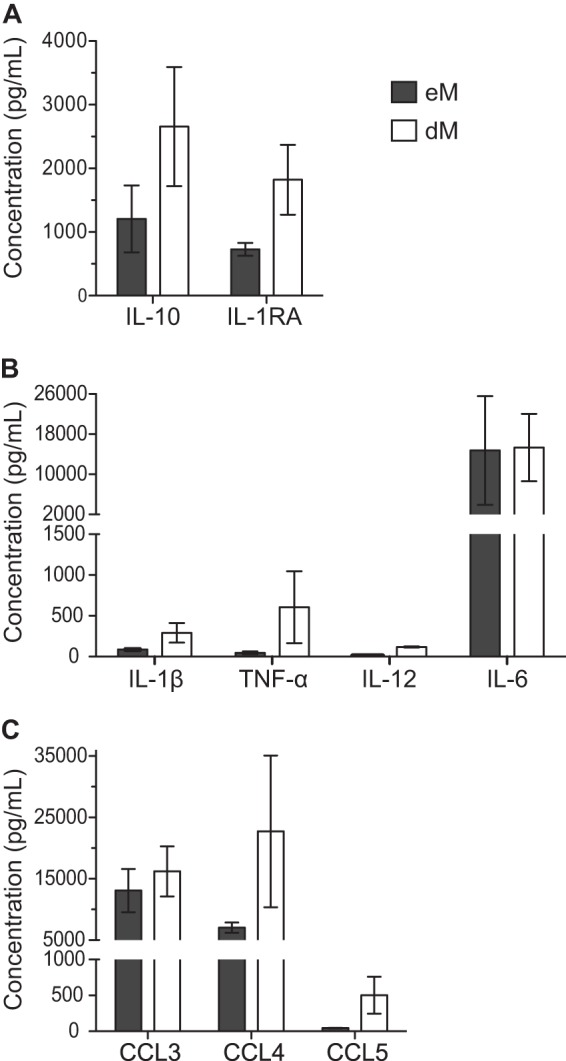
Cytokine and chemokine secretion by purified endometrial and decidual macrophages. Anti-inflammatory cytokine (A), proinflammatory cytokine (B), and chemokine (C) concentrations were analyzed by a Luminex assay in supernatants from purified eM and dM after 72 h of culture. The means of 3 samples and the standard errors of the means are depicted.
The chemokines CCL3 and CCL4 were secreted by both eM and dM (Fig. 4C). CCL3 was secreted at similar levels by eM and dM (means, 13,071 pg/ml and 16,209 pg/ml, respectively). CCL4 was less strongly secreted by eM than by dM (means, 7,043 pg/ml and 22,727 pg/ml, respectively). The chemokine CCL5 was also secreted, but less strongly than CCL3 and CCL4 (means, 45 pg/ml in eM supernatants and 501 pg/ml in dM supernatants). eM and dM also secreted the chemokines CXCL8, CXCL9, and CXCL10 (data not shown).
Thus, eM and dM secrete the same cytokines and chemokines but in different amounts. In particular, eM secrete smaller amounts of proinflammatory and anti-inflammatory cytokines and CCL4 than dM.
SAMHD1 and pSAMHD1 expression in endometrial and decidual macrophages.
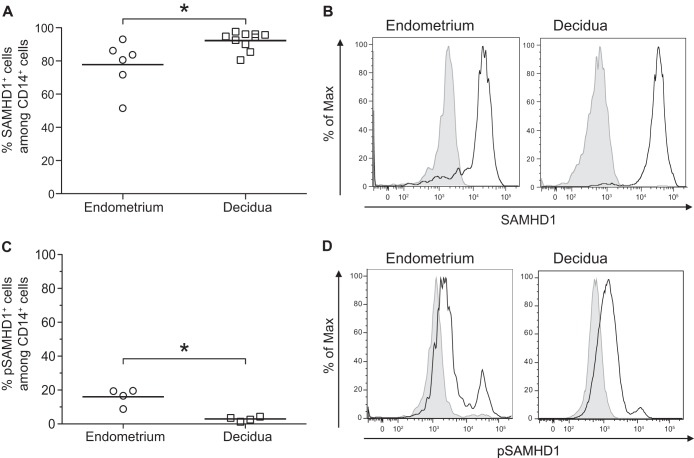
SAMHD1 expression was analyzed by flow cytometry. As shown in Fig. 5A and B, a high percentage of eM and dM were SAMHD1+. The percent SAMHD1 expression was slightly lower in eM than in dM (means, 77.8% and 92.3% of cells, respectively). The Thr592-phosphorylated form of SAMHD1 (pSAMHD1) was expressed at a higher level by eM than by dM (means, 18.0% and 2.9%, respectively) (Fig. 5C and D).
FIG 5.
Expression of SAMHD1 in decidual and endometrial macrophages. The expression of SAMHD1 and the phosphorylated form of SAMHD1 at residue Thr592 (pSAMHD1) was analyzed on CD45+ CD14+ cells by flow cytometry staining of total endometrial or decidual cells. An isotype-matched IgG control was used to confirm the specificity of the anti-SAMHD1 and the anti-pSAMHD1 Thr592. The mean percentages of SAMHD1 (A) and pSAMHD1 (C) expression among the CD14+ cells are displayed. Each circle represents one endometrial sample (n = 6 for SAMHD1 and n = 4 for pSAMHD1) and each square one decidual sample (n = 10 for SAMHD1 and n = 4 for pSAMHD1). *, P < 0.05. (B and D) A representative histogram is shown for one endometrial sample and for one decidual sample. The black line represents SAMHD1 (B) or pSAMHD1 (D) expression and shaded gray the isotype-matched control IgG.
Vpx enhances HIV-1 infection of endometrial and decidual macrophages.
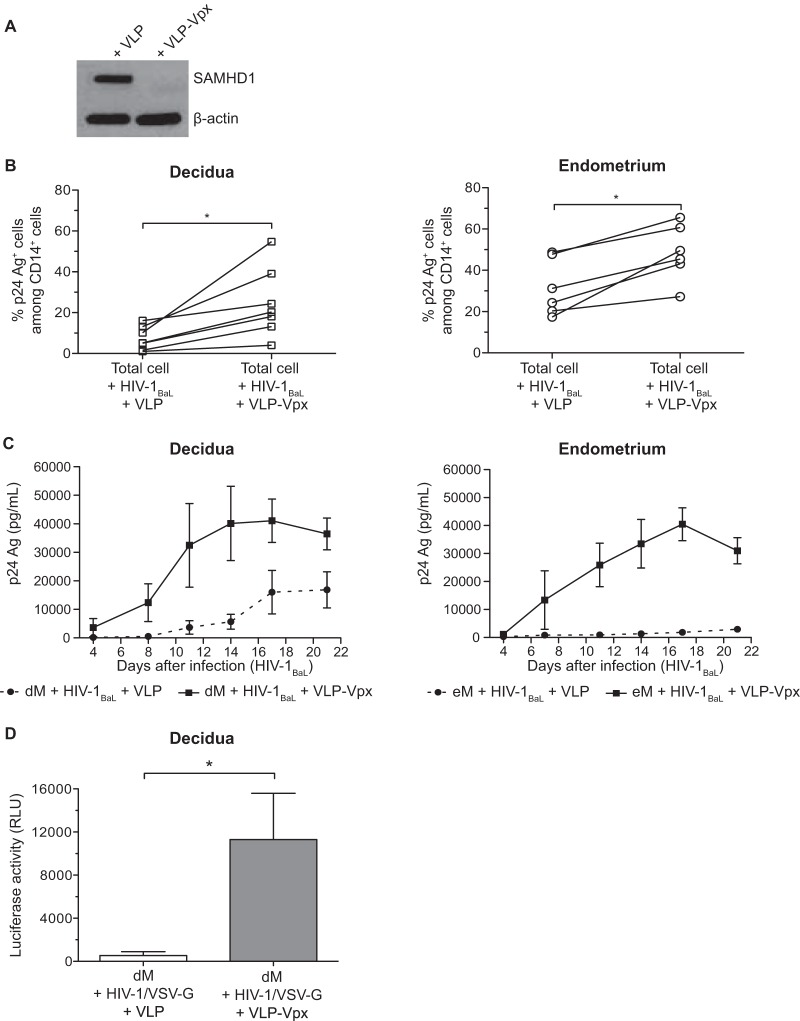
The viral protein Vpx degrades SAMHD1 (28, 29). To determine whether Vpx also degrades SAMHD1 in mucosal macrophages, purified decidual macrophages were treated with virus-like particles containing Vpx (VLP-Vpx) or with control virus-like particles (VLP) and then analyzed for SAMHD1 expression by Western blotting (Fig. 6A). SAMHD1 expression was lower with VLP-Vpx than with control VLP, indicating that Vpx indeed degrades SAMHD1 in decidual macrophages.
FIG 6.
SAMHD1 degradation and infection of decidual and endometrial cells in the presence of VLP-Vpx. (A) Control VLP or VLP-Vpx was added to purified decidual CD14+ cells, and after 72 h, SAMHD1 expression was analyzed by Western blotting. (B) Decidual and endometrial cells were exposed to HIV-1BaL at an MOI of 10−3 in the presence of control VLP or VLP-Vpx. Intracellular staining of the p24 Ag was performed on day 11 postinfection. The percentage of p24 Ag+ cells among CD14+ cells of each sample (n = 6 for the endometrium and n = 7 for the decidua) is displayed. (C) Purified eM and dM were exposed to HIV-1BaL at an MOI of 10−3 in the presence of control VLP (dashed line) or VLP-Vpx (full line). Values represent the means of the p24 Ag concentrations over time (n = 3 for the endometrium and n = 4 for the decidua). (D) Purified dM were exposed to the HIV-1/VSV-G pseudotype (600 ng of p24 Ag/106 cells) in the presence of control VLP or VLP-Vpx. The mean luciferase activity is given for 6 samples, in relative light units (RLU). Bars indicate standard errors of the means. *, P < 0.05.
To determine whether HIV-1 infection is restricted by SAMHD1 in eM and dM, total cells were exposed to HIV-1BaL at an MOI of 10−3 in the presence of VLP-Vpx or control VLP. Eleven days after infection, the percentage of infected cells was determined by intracellular staining of p24 Ag (Fig. 6B). The presence of VLP-Vpx increased the percentage of infected macrophages from both tissues (means, 31.6% with control VLP to 48.6% with VLP-Vpx in the endometrium and 5.8% with control VLP to 17% with VLP-Vpx in the decidua). Macrophage infection was enhanced more strongly by Vpx in the decidua than in the endometrium (3.0-fold versus 1.5-fold).
Decidual and endometrial purified macrophages were then exposed to HIV-1BaL at an MOI of 10−3 in the presence of VLP-Vpx or control VLP (Fig. 6C). Viral production was monitored by quantifying p24 Ag in the supernatants. Viral production by purified eM and dM was higher with VLP-Vpx than with control VLP. The increase in viral production was larger with eM than with dM (23.8-fold and 7.0-fold, respectively, on day 14 postinfection).
Decidual macrophages were then exposed to the HIV-1/VSV-G pseudotype (Fig. 6D), and infection was monitored by quantifying luciferase activity. As observed with HIV-1BaL, VLP-Vpx enhanced dM infection by the HIV-1/VSV-G pseudotype: luciferase activity was 21-fold higher with VLP-Vpx than with control VLP (11,300 relative light units [RLU] and 540 RLU, respectively; mean of 6 samples).
Thus, a high percentage of eM and dM expresses SAMHD1, whereas only a small percentage expresses its phosphorylated form. In addition, Vpx degrades SAMHD1 and thereby increases eM and dM susceptibility to HIV-1 infection.
DISCUSSION
We show that among endometrial mononuclear cells, HIV-1 R5 target cells in vitro are macrophages (CD14+ CD68+ cells) and T lymphocytes (CD3+ cells). This is in keeping with the detection of infected cells with a monocyte/macrophage morphology in the endometrium of an HIV-1-infected woman (15).
T cells were the main infected endometrial cells on day 7 postinfection, while macrophages were the main infected cells on day 11. Several studies have shown that macrophages and T cells are both HIV-1 R5 target cells in the female lower reproductive tract (7–9, 11, 38, 39), and most showed that T cells were the main infected cells. However, infected cells were analyzed no more than 6 days postinfection in all these previous studies.
We found that viral production by endometrial total cells fell after 11 days of culture, possibly owing to the death of infected CD3+ cells. Indeed, the viability of uninfected T cells was better than that of their infected counterparts on days 7 and 14 postinfection. In contrast, the viability of uninfected eM was similar to or worse than that of infected eM (data not shown).
We have previously shown that macrophages are the main target cells of HIV-1 R5 in the decidua (16). In this study, when we infected total endometrial and decidual cells, the percentage of infected cells was significantly lower among dM than among eM. In contrast, when purified cells were infected, viral production was higher in dM than in eM. This difference could be due to different cellular distributions in the endometrium and decidua. In particular, NK cells are more abundant in the decidua than in the endometrium and might control HIV-1 infection, as reported for the periphery (40). Soluble factors could also explain this difference. In fact, it has been reported that decidual soluble factors partially inhibit dM HIV-1 infection (26). The lower viral production by purified eM than by purified dM could also be due to interindividual variability. Indeed, in 3 of the 17 decidual samples, the level of viral production by dM was similar to that of the 4 eM samples examined. This difference could also be explained by the lower percentage of eM than dM that expressed the HIV-1 coreceptor CCR5.
Like dM (17, 41, 42), eM express CD206 and CD163 (markers of M2 macrophages) and secrete proinflammatory and anti-inflammatory cytokines. The strong secretion of IL-10 and weak secretion of IL-12 by eM, together with these cells' CD163 and CD206 expression, suggest that eM also have an M2-like phenotype. Nevertheless, a lower percentage of eM than dM expressed certain markers. In particular, the lower percentage of CD163 expression on eM might indicate that eM are less differentiated than dM, as strong CD163 expression is one characteristic of mature tissue macrophages. The main difference between eM and dM found in this study was the low expression of the Fc receptor CD16 on eM. Our findings are consistent with previous studies showing that endometrial CD163+ cells and vaginal macrophages express low levels of CD16 (13, 43). However, in contrast to eM and dM, vaginal macrophages express low levels of the HIV-1 receptor CD4 and the coreceptor CCR5 (13). We also show that eM and dM secrete different levels of soluble factors. Together, these data suggest that eM and dM may belong to different macrophage subpopulations.
Whereas T cells are the main immune cells in the endometrium, NK cells are the main immune cells in the decidua. Hormone concentrations also differ between the endometrium and decidua. All the endometrial samples used in this study were collected during the proliferative phase of the menstrual cycle, in which the estrogen concentration is high and the progesterone concentration low. In contrast, the progesterone concentration is high during pregnancy. The different environments of the endometrium and decidua could influence the eM and dM phenotypes. More studies are necessary to determine if the eM phenotype changes during the menstrual cycle and if eM differentiate into dM during pregnancy, or whether dM progenitors are recruited to the decidua.
As macrophages from the decidua and endometrium differently express certain markers, they might have different functions. In particular, the percentage of cells expressing the costimulatory molecule CD80 was lower among eM than dM. As CD80 is involved in the activation or inhibition of T lymphocytes (44), eM could have a lesser capacity to regulate T cell activity. In the decidua, regulation of T cell activity by dM is important for fetal tolerance. In addition, the percentage of cells expressing CD16 and CD64, two Fc receptors involved in phagocytosis, was lower among eM than dM, suggesting that eM could have a lower phagocytic capacity. It has been suggested that dM play a role in the phagocytosis of apoptotic trophoblasts (45) and that eM phagocytose endometrial tissue debris (46).
Soluble factors secreted by eM and dM may also influence their infection. For example, IL-10 inhibits while TNF-α enhances HIV-1 replication in placental macrophages (Hofbauer cells) (47). Moreover, decidual soluble factors, particularly CCL3 and CCL4, control HIV-1 infection of decidual mononuclear cells (26). Whether or not endometrial soluble factors inhibit HIV-1 infection of endometrial cells remains to be determined.
Another possible infection control mechanism is the expression of restriction factors such as SAMHD1. Here we show for the first time that SAMHD1 is expressed by mucosal macrophages. The percentage of cells expressing SAMHD1 was high among both eM and dM, whereas only a small percentage of eM and dM expressed the phosphorylated form of SAMHD1. Vpx degraded SAMHD1 and thereby enhanced the infection of eM and dM. Vpx had a more marked effect on dM infection than on eM infection when total cells were infected, whereas the opposite situation was observed when purified cells were infected. As previously discussed, this difference could be due to the different cellular distribution in the endometrium and decidua. As SAMHD1 was found to be less strongly expressed and pSAMHD1 more strongly expressed by eM than by dM, the stronger effect of Vpx on purified eM than on purified dM is surprising. Nevertheless, it is important to note that the enhancement of infection caused by Vpx could not be due only to SAMHD1 degradation, as it has been shown that Vpx has other functions (48). More particularly, Vpx interacts with APOBEC3A and partially counteracts this antiviral factor (49). Moreover, Vpx interacts with IRF5, a factor involved in the induction of type I IFN and proinflammatory cytokines, and inhibits its function as a transcription activator (50).
SAMHD1 expression by the main HIV-1 target cells in the endometrium and decidua might participate in the control of HIV-1 infection in the endometrium in nonpregnant women and also in the prevention of mother-to-child transmission. However, SAMHD1 could also promote viral dissemination by preventing activation of the immune response. Indeed, SAMHD1 limits the ability of monocyte-derived dendritic cells (MDDC) to sense the virus and to trigger an innate immune response (51). Moreover, infection of MDDC in the presence of Vpx activates the antiviral immune response of MDDC, including type I IFN secretion (52). It has been suggested that Vpx could be used to improve protection by enhancing immune responses against HIV-1 infection (48), yet it remains to be shown whether SAMHD1 has a beneficial or detrimental role in mucosal control of HIV-1 infection.
In conclusion, we show that macrophages and T cells are the main HIV-1 target cells in the endometrium in nonpregnant women. Like decidual macrophages, endometrial macrophages have an M2-like phenotype, but they could belong to a different subpopulation. Finally, we show that eM and dM both express the restriction factor SAMHD1 and its phosphorylated form and that Vpx enhances the infection of eM and dM. Further comparisons of the decidua and FRT mucosae in nonpregnant women are needed to identify factors able to prevent sexual transmission of HIV-1.
ACKNOWLEDGMENTS
We thank all the women who provided samples; AP-HP (Assistance Publique Hôpitaux de Paris), Claire de Truchis, Séverine Ballan, Mona Rahmati, and clinical personnel for obtaining the samples; Monsef Benkirane for the pSAMHD1 Thr592 antibody; D. Young for critical editing of the manuscript; PIRC (Pole Intégré de Recherche Clinique, Institut Pasteur) for their help with biomedical regulatory aspects of the project; and CIH (Centre d'Immunologie Humaine, Institut Pasteur) for the Luminex assay.
This work was supported by ANRS (financial support and grant 12165/13129 to H.Q. and H.E.C.), Sidaction (financial support and grant AI20-3-01671 to M.D.), the TOTAL Foundation, INSERM, and Institut Pasteur. We acknowledge the SESAME program for LSRII device funding.
We have no financial conflicts of interest to disclose.
REFERENCES
- 1.UNAIDS. 2013. UNAIDS report on the global AIDS epidemic 2013. UNAIDS, Geneva, Switzerland. [Google Scholar]
- 2.UNAIDS. 2010. Women, girls, gender equality and HIV. UNAIDS, Geneva, Switzerland. [Google Scholar]
- 3.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. 2009. Glycerol monolaurate prevents mucosal SIV transmission. Nature 458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joag SV, Adany I, Li Z, Foresman L, Pinson DM, Wang C, Stephens EB, Raghavan R, Narayan O. 1997. Animal model of mucosally transmitted human immunodeficiency virus type 1 disease: intravaginal and oral deposition of simian/human immunodeficiency virus in macaques results in systemic infection, elimination of CD4+ T cells, and AIDS. J Virol 71:4016–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howell AL, Edkins RD, Rier SE, Yeaman GR, Stern JE, Fanger MW, Wira CR. 1997. Human immunodeficiency virus type 1 infection of cells and tissues from the upper and lower human female reproductive tract. J Virol 71:3498–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asin SN, Eszterhas SK, Rollenhagen C, Heimberg AM, Howell AL. 2009. HIV type 1 infection in women: increased transcription of HIV type 1 in ectocervical tissue explants. J Infect Dis 200:965–972. doi: 10.1086/605412. [DOI] [PubMed] [Google Scholar]
- 7.Cummins JE Jr, Guarner J, Flowers L, Guenthner PC, Bartlett J, Morken T, Grohskopf LA, Paxton L, Dezzutti CS. 2007. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob Agents Chemother 51:1770–1779. doi: 10.1128/AAC.01129-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol 74:5577–5586. doi: 10.1128/JVI.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saba E, Grivel JC, Vanpouille C, Brichacek B, Fitzgerald W, Margolis L, Lisco A. 2010. HIV-1 sexual transmission: early events of HIV-1 infection of human cervico-vaginal tissue in an optimized ex vivo model. Mucosal Immunol 3:280–290. doi: 10.1038/mi.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. 2000. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med 6:475–479. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- 11.Gupta P, Collins KB, Ratner D, Watkins S, Naus GJ, Landers DV, Patterson BK. 2002. Memory CD4+ T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol 76:9868–9876. doi: 10.1128/JVI.76.19.9868-9876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. 2007. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen R, Richter HE, Clements RH, Novak L, Huff K, Bimczok D, Sankaran-Walters S, Dandekar S, Clapham PR, Smythies LE, Smith PD. 2009. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J Virol 83:3258–3267. doi: 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Garcia M, Barr FD, Crist SG, Fahey JV, Wira CR. 2014. Phenotype and susceptibility to HIV infection of CD4 Th17 cells in the human female reproductive tract. Mucosal Immunol 7:1375–1385. doi: 10.1038/mi.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peuchmaur M, Emilie D, Vazeux R, Pons JC, Delfraissy JF, Lemaigre G, Galanaud P. 1989. HIV-associated endometritis. AIDS 3:239–241. doi: 10.1097/00002030-198904000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Marlin R, Nugeyre M-T, de Truchis C, Berkane N, Gervaise Al Barré-Sinoussi F, Menu E. 2009. Antigen-presenting cells represent targets for R5 HIV-1 infection in the first trimester pregnancy uterine mucosa. PLoS One 4:e5971. doi: 10.1371/journal.pone.0005971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houser BL, Tilburgs T, Hill J, Nicotra ML, Strominger JL. 2011. Two unique human decidual macrophage populations. J Immunol 186:2633–2642. doi: 10.4049/jimmunol.1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J. 2011. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol 187:3671–3682. doi: 10.4049/jimmunol.1100130. [DOI] [PubMed] [Google Scholar]
- 19.Gustafsson C, Mjosberg J, Matussek A, Geffers R, Matthiesen L, Berg G, Sharma S, Buer J, Ernerudh J. 2008. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One 3:e2078. doi: 10.1371/journal.pone.0002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cassol E, Cassetta L, Alfano M, Poli G. 2010. Macrophage polarization and HIV-1 infection. J Leukoc Biol 87:599–608. doi: 10.1189/jlb.1009673. [DOI] [PubMed] [Google Scholar]
- 21.Robinson DP, Klein SL. 2012. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav 62:263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Garcia M, Biswas N, Patel MV, Barr FD, Crist SG, Ochsenbauer C, Fahey JV, Wira CR. 2013. Estradiol reduces susceptibility of CD4+ T cells and macrophages to HIV-infection. PLoS One 8:e62069. doi: 10.1371/journal.pone.0062069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hel Z, Stringer E, Mestecky J. 2010. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev 31:79–97. doi: 10.1210/er.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kourtis AP, Read JS, Jamieson DJ. 2014. Pregnancy and infection. N Engl J Med 371:1075–1077. [DOI] [PubMed] [Google Scholar]
- 25.Quillay H, Marlin R, Duriez M, Nugeyre MT, Menu E. 2013. The control of HIV-1 in utero transmission at the materno-fetal interface by immunological determinants, p 827–855 InChaouat G, Sandra O, Lédée N (ed), Immunology of pregnancy 2013. Bentham Science Publishers, Bussum, The Netherlands. [Google Scholar]
- 26.Marlin R, Nugeyre MT, Duriez M, Cannou C, Le Breton A, Berkane N, Barre-Sinoussi F, Menu E. 2011. Decidual soluble factors participate in the control of HIV-1 infection at the maternofetal interface. Retrovirology 8:58. doi: 10.1186/1742-4690-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergamaschi A, Pancino G. 2010. Host hindrance to HIV-1 replication in monocytes and macrophages. Retrovirology 7:31. doi: 10.1186/1742-4690-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. 2011. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. 2011. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldauf HM, Pan X, Erikson E, Schmidt S, Daddacha W, Burggraf M, Schenkova K, Ambiel I, Wabnitz G, Gramberg T, Panitz S, Flory E, Landau NR, Sertel S, Rutsch F, Lasitschka F, Kim B, Konig R, Fackler OT, Keppler OT. 2012. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. Nat Med 18:1682–1687. doi: 10.1038/nm.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Descours B, Cribier A, Chable-Bessia C, Ayinde D, Rice G, Crow Y, Yatim A, Schwartz O, Laguette N, Benkirane M. 2012. SAMHD1 restricts HIV-1 reverse transcription in quiescent CD4(+) T-cells. Retrovirology 9:87. doi: 10.1186/1742-4690-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cribier A, Descours B, Valadao AL, Laguette N, Benkirane M. 2013. Phosphorylation of SAMHD1 by cyclin A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep 3:1036–1043. doi: 10.1016/j.celrep.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Ahn J, Hao C, Yan J, DeLucia M, Mehrens J, Wang C, Gronenborn AM, Skowronski J. 2012. HIV/simian immunodeficiency virus (SIV) accessory virulence factor Vpx loads the host cell restriction factor SAMHD1 onto the E3 ubiquitin ligase complex CRL4DCAF1. J Biol Chem 287:12550–12558. doi: 10.1074/jbc.M112.340711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akkina RK, Walton RM, Chen ML, Li QX, Planelles V, Chen IS. 1996. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J Virol 70:2581–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berger G, Durand S, Goujon C, Nguyen XN, Cordeil S, Darlix JL, Cimarelli A. 2011. A simple, versatile and efficient method to genetically modify human monocyte-derived dendritic cells with HIV-1-derived lentiviral vectors. Nat Protoc 6:806–816. doi: 10.1038/nprot.2011.327. [DOI] [PubMed] [Google Scholar]
- 36.Goujon C, Riviere L, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL, Cimarelli A. 2007. SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology 4:2. doi: 10.1186/1742-4690-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gramberg T, Sunseri N, Landau NR. 2010. Evidence for an activation domain at the amino terminus of simian immunodeficiency virus Vpx. J Virol 84:1387–1396. doi: 10.1128/JVI.01437-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saba E, Origoni M, Taccagni G, Ferrari D, Doglioni C, Nava A, Lisco A, Grivel JC, Margolis L, Poli G. 2013. Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal Immunol 6:1081–1090. doi: 10.1038/mi.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen R, Richter HE, Smith PD. 2011. Early HIV-1 target cells in human vaginal and ectocervical mucosa. Am J Reprod Immunol 65:261–267. doi: 10.1111/j.1600-0897.2010.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jost S, Altfeld M. 2013. Control of human viral infections by natural killer cells. Annu Rev Immunol 31:163–194. doi: 10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- 41.Heikkinen J, Mottonen M, Komi J, Alanen A, Lassila O. 2003. Phenotypic characterization of human decidual macrophages. Clin Exp Immunol 131:498–505. doi: 10.1046/j.1365-2249.2003.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lidström C, Matthiesen L, Berg G, Sharma S, Ernerudh J, Ekerfelt C. 2003. Cytokine secretion patterns of NK cells and macrophages in early human pregnancy decidua and blood: implications for suppressor macrophages in decidua. Am J Reprod Immunol 50:444–452. doi: 10.1046/j.8755-8920.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 43.Jensen AL, Collins J, Shipman EP, Wira CR, Guyre PM, Pioli PA. 2012. A subset of human uterine endometrial macrophages is alternatively activated. Am J Reprod Immunol 68:374–386. doi: 10.1111/j.1600-0897.2012.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharpe AH. 2009. Mechanisms of costimulation. Immunol Rev 229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mor G, Abrahams VM. 2003. Potential role of macrophages as immunoregulators of pregnancy. Reprod Biol Endocrinol 1:119. doi: 10.1186/1477-7827-1-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thiruchelvam U, Dransfield I, Saunders PT, Critchley HO. 2013. The importance of the macrophage within the human endometrium. J Leukoc Biol 93:217–225. doi: 10.1189/jlb.0712327. [DOI] [PubMed] [Google Scholar]
- 47.Johnson EL, Chakraborty R. 2012. Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child transmission (MTCT) by induction of immunoregulatory cytokines. Retrovirology 9:101. doi: 10.1186/1742-4690-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaller T, Bauby H, Hue S, Malim MH, Goujon C. 2014. New insights into an X-traordinary viral protein. Front Microbiol 5:126. doi: 10.3389/fmicb.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berger G, Durand S, Fargier G, Nguyen XN, Cordeil S, Bouaziz S, Muriaux D, Darlix JL, Cimarelli A. 2011. APOBEC3A is a specific inhibitor of the early phases of HIV-1 infection in myeloid cells. PLoS Pathog 7:e1002221. doi: 10.1371/journal.ppat.1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng X, Ratner L. 2014. HIV-2 Vpx protein interacts with interferon regulatory factor 5 (IRF5) and inhibits its function. J Biol Chem 289:9146–9157. doi: 10.1074/jbc.M113.534321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puigdomènech I, Casartelli N, Porrot F, Schwartz O. 2013. SAMHD1 restricts HIV-1 cell-to-cell transmission and limits immune detection in monocyte-derived dendritic cells. J Virol 87:2846–2856. doi: 10.1128/JVI.02514-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. 2010. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature 467:214–217. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]