ABSTRACT
Tulane virus (TV), the prototype of the Recovirus genus in the calicivirus family, was isolated from the stools of rhesus monkeys and can be cultivated in vitro in monkey kidney cells. TV is genetically closely related to the genus Norovirus and recognizes the histo-blood group antigens (HBGAs), similarly to human noroviruses (NoVs), making it a valuable surrogate for human NoVs. However, the precise structures of HBGAs recognized by TV remain elusive. In this study, we performed binding and blocking experiments on TV with extended HBGA types and showed that, while TV binds all four types (types 1 to 4) of the B antigens, it recognizes only the A type 3 antigen among four types of A antigens tested. The requirements for HBGAs in TV replication were demonstrated by blocking of TV replication in cell culture using the A type 3/4 and B saliva samples. Similar results were also observed in oligosaccharide-based blocking assays. Importantly, the previously reported, unexplained increase in TV replication by oligosaccharide in cell-based blocking assays has been clarified, which will facilitate the application of TV as a surrogate for human NoVs.
IMPORTANCE Our understanding of the role of HBGAs in NoV infection has been significantly advanced in the past decade, but direct evidence for HBGAs as receptors for human NoVs remains lacking due to a lack of a cell culture method. TV recognizes HBGAs and can replicate in vitro, providing a valuable surrogate for human NoVs. However, TV binds to some but not all saliva samples from A-positive individuals, and an unexplained observation of synthetic oligosaccharide blocking of TV binding has been reported. These issues have been resolved in this study.
INTRODUCTION
Noroviruses (NoVs), an important cause of nonbacterial acute gastroenteritis in humans, constitute the genus Norovirus of the calicivirus (CV) family. Other genera include Sapovirus, which also causes acute gastroenteritis in humans, Lagovirus, Vesivirus, Nebovirus, and the recently proposed genera Recovirus and Valovirus, which cause a wide variety of diseases in different animal species (1–3). NoVs are further divided into over 30 genotypes classified into five genogroups; two of these (GI and GII) cause major epidemics of acute gastroenteritis in humans, while the remaining three (GIII to GV) mainly infect animals. Both GI and GII human NoVs recognize the human histo-blood group antigens (HBGAs) in a strain-specific manner as potential receptors or attachment factors (4–7), and a number of patterns of binding to different ABO, secretor, and Lewis antigens have been described (8). Human NoVs are difficult to study due to the lack of an in vitro culture method and animal models (9).
Tulane virus (TV) is the prototype of the genus Recovirus and was originally isolated from the stools of rhesus monkeys (10). The TV can be cultivated in monkey kidney-derived cell lines, including LLC MK-2, Vero, and MA104. Recoviruses are genetically closest to the NoVs among the known genera in the calicivirus family (10). A cryo-electron microscopy (cryo-EM) study showed that the TV capsid exhibits a typical icosahedral symmetry with unique conformational flexibility among C/C dimers of the protruding (P) domain compared with the A/B dimers of the TV capsid (11). A challenge of rhesus monkeys with in vitro-cultivated TV resulted in intestinal infection with mild gastroenteritis, indicating that TV is an enteric pathogen (12). Because the TV also recognizes the HBGAs like NoVs (13), it has been used as a surrogate for human NoVs (14–18).
HBGAs are complex carbohydrates distributed abundantly on mucosal epithelia of the respiratory, genitourinary, and digestive tracts and on the surfaces of red blood cells. These antigens are synthesized from various disaccharide precursors through sequential addition of monosaccharides catalyzed by specific glycosyltransferases that are encoded by three major HBGA gene families, including the ABO, secretor (H), and Lewis families.
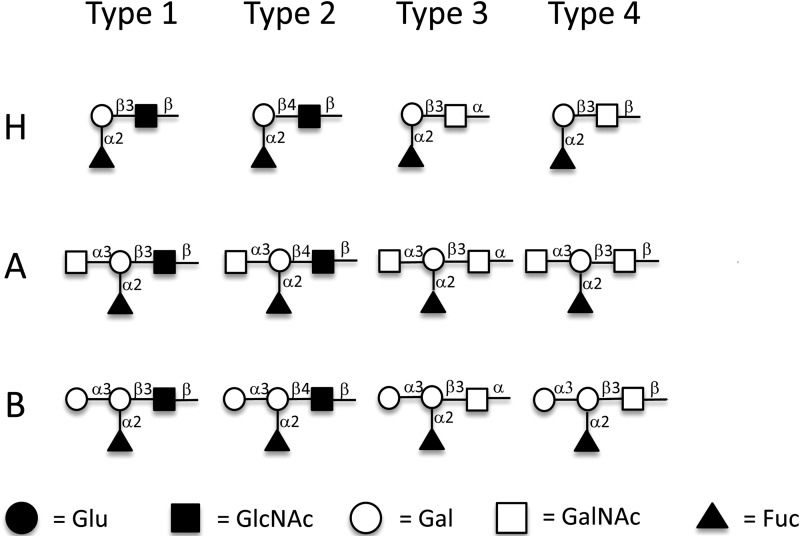
Depending on the oligosaccharide residue and their linkages of the disaccharide precursors, HBGAs can be divided into six types (19). For example, the A, B, and H antigens can be synthesized on type 1 [β-Gal(1→3)β-GlcNAc], type 2 [β-Gal(1→4)β-GlcNAc], type 3 [β-Gal(1→3)α-GalNAc], type 4 [β-Gal(1→3)β-GalNAc], type 5 [β-Gal(1→3)β-Gal], and type 6 [β-Gal(1→4)β-Glc] chains, differing in the linkages or residues of the disaccharide precursors (19). The structures of type 1 to 4 antigens that were studied here are shown in Fig. 1. The H antigens are produced by the addition of a fucose in an α(1→2) linkage to the β-galactose of the six different core chains. Both the A and B antigens are produced from the six types of H antigens by the addition of an N-acetylgalactosamine (A) or a galactose (B) in an α(1→3) linkage to the terminal β-galactose. All six types of ABH antigens can be found on erythrocyte and tissue surfaces; of these six, types 1 to 4 are considered the most important (19), and procedures to synthesize these glycans in vitro are available (20, 21).
FIG 1.
Structures of H type 1 to 4, A type 1 to 4, and B type 1 to 4 antigens conjugated to BSA. Synthetic H type 1 to 4, A type 1 to 4, and B type 1 to 4 antigens were coupled to BSA through the reducing end. Gal, galactose; GalNAc, N-acetylgalactosamine; GlcNAc, N-acetylglucosamine; Fuc, fucose. Symbolic nomenclature used is that developed by the Consortium for Functional Glycomics.
The binding of HBGAs by TV has been demonstrated by various binding and blocking assays; however, the precise structures of HBGAs responsible for TV binding remain unclear. A recent study showed that TV recognizes both type A and B saliva with variations among saliva samples in each type (13).We also observed differences of TV binding to type B versus type A saliva, in which all type B saliva samples but only a subset of type A saliva samples were reactive to TV. These results indicate that further study to define the binding patterns of TV to human HBGAs is necessary. In addition, unexplainable results regarding the role of HBGAs in TV replication were reported: while significant reduction of TV replication in cell cultures was observed following treatments of the TV with type A and B saliva samples, treatments with bovine serum albumin (BSA)-conjugated A and B oligosaccharides increased TV titers (13). Thus, our understanding of the roles of HBGAs in TV replication and host range remains limited.
In this study, we performed further binding and blocking experiments using reagents specific to different types of the ABH antigens to clarify these discrepancies. We demonstrated that the TV bound all four tested types (types 1 to 4) of the B antigens, consistent with the results of TV binding of all types B and AB HBGAs in the saliva-binding assays. However, TV bound only the A type 3 antigen among four types (types 1 to 4) of A oligosaccharide conjugates tested, suggesting that the lack of binding or weak binding of type A saliva samples to TV may be due to a lack of expression or low expression of the A type 3 antigens in these saliva. Accordingly, we showed that the blocking activities of saliva samples on TV replication in cell culture correlated with the binding signals by a monoclonal antibody (MAb) recognizing the A type 3/4 antigens. Finally, we found the reason for the previously described increase of TV replication by BSA-conjugated HBGA oligosaccharides and demonstrated the expected inhibition by these oligosaccharides. Together, our new data support the notion that TV recognizes HBGAs as attachment factors and that such recognition is required for TV infection and replication.
MATERIALS AND METHODS
Virus, MAbs, and synthetic oligosaccharides.
Plaque-purified, low-passage-number TV stock was used for the plaque assay; the H type 3/4-specific MAb MBr1 (to globo-H) was purchased from Enzo Life Sciences, Inc. (Farmingdale, NY), and A type 3/4-specific MAb HE-10 was purchased from Sigma-Aldrich (St. Louis, MO). Polyacrylamide (PAA)-conjugated type A and B trisaccharides of approximately 30 kDa containing 20 mol% carbohydrate were purchased from GlycoTech Corporation, Rockville, MD; bovine serum albumin (BSA)-conjugated type A and B trisaccharides with 20 to 25 carbohydrate chains attached per BSA molecule were purchased from Glycorex AB (Lund Sweden). The H type 1 to 4, A type 1 to 4, and B type 1 to 4 BSA conjugates were prepared as described previously (22), using antigens synthesized as reported previously (20, 21). Briefly, the antigens were synthesized with an alkene at the reducing end that was converted to the corresponding amine salt using a photochemically induced thiol-ene reaction with cysteamine. The amine was then coupled with di-p-nitrophenyl adipate to generate an activated ester derivative (23). These activated derivatives were reacted (22) with bovine serum albumin to give the neoglycoconjugates used the analyses. The structures of these different antigens are shown in Fig. 1; antigen loadings on BSA ranged from 11 to 16, as determined by matrix-assisted laser desorption ionization (MALDI) mass spectrometry.
Purification of TV and preparation of TV specific antibodies.
Rhesus monkey kidney LLC-MK2 cells (ATCC, Manassas, VA) were cultured in rolling bottles with medium 199 (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS), 200 U/ml penicillin, and 200 μg/ml streptomycin. Cells with 80 to 90% confluence were inoculated with 10 ml (multiplicity of infection [MOI] = 0.4)) of TV inoculum for 1 h. Then the inoculum was removed, and 50 ml medium without FBS was added and cultured for 72 h. A total of 600 ml of TV-infected LLC-MK2 cell culture medium was concentrated in a dialysis tube against polyethylene glycol (PEG) 8000. Then the sample was adjusted for density (refractive index of 1.365) with cesium chloride and centrifuged at 288,000 × g (41,000 rpm, SW41Ti rotor; Beckman) for 40 h. The gradient fractions were collected by bottom puncture. TV virions in the fractions were identified and quantified by transmission electron microscopy (TEM), SDS-PAGE, Western blotting, and reverse transcription-PCR (RT-PCR). The CsCl-purified TVs were used for generation of antibody against TV.
For generation of an anti-TV antibody, female BALB/c mice (n = 4; Harlan-Sprague-Dawley, Indianapolis, IN) at 3 to 4 weeks of age were immunized with purified TV three times through intraperitoneal injection with adjuvant in 2-week intervals. Blood was collected by retro-orbital capillary plexus puncture before each immunization and 4 weeks after the final immunization. Sera were processed from blood via a standard protocol. The antisera to TV were evaluated by enzyme-linked immunosorbent assay (ELISA) and Western blotting analyses. The antibody specifically recognized the intact TV and its VP-1 protein. The antibody could also block TV binding to HBGAs and neutralized TV replication in LLC-MK-2 cells.
HA and HAI.
Type A1, A2, B, and O human red blood cells (RBCs) from Immucor, Inc. (Norcross, GA, USA) were used for hemagglutination (HA) and hemagglutination inhibition (HAI) for TV. In addition, RBCs from 24 type A-positive individuals from the Memorial Blood Center (St. Paul, MN, USA) were tested for the role of the A type 3 in causing HA of RBCs by TV. The RBCs were packed by diluting the cells in 0.01 M phosphate-buffered saline (PBS) (without Ca2+ or Mg2+; pH 7.2) (Invitrogen, Carlsbad, CA) and centrifuging for 15 min at 500 × g. The HA of TV to the type B RBCs was performed in 96-well V-bottom plates. Fifty microliters of 0.5% human RBCs in 0.01 M PBS (pH 7.2) and 8 hemagglutination units (HAUs) of TV (50 μl of TV containing 5 × 102 PFU in PBS) were mixed and incubated for 60 to 100 min at 4 to 8°C. Due to the weak interaction of TV with A type antigen, a method reported by Morokutti et al. was modified (24). The HA of TV to the type A RBCs was performed in 96-well flat-bottom plates. Eight HAUs (2 μl of TV containing 2 × 103 PFU in PBS) of TV was added to 20 μl of 0.5% human type A RBCs in 0.01 M PBS (pH 7.2) and mixed. After incubation for 60 to 100 min at 4 to 8°C, agglutination was examined by microscopy. HA inhibition of TV with the type B RBCs by synthetic HBGAs was performed by the same procedures with an additional step of preincubation of TV with serial dilutions of the inhibitors.
Saliva binding assay of TV.
A panel of 86 human saliva samples representing different HBGA types collected in our previous studies (8) was used. The saliva samples were boiled for 10 min to denature potential antibodies that might influence the assay. The levels of the A, B, H type 1, H type 2, and Lewis antigens in the samples were determined previously (8) using MAbs BG-4 (anti-H type 1), BG-6 (anti-Leb), and BG-8 (anti-Ley) (Signet Laboratories Inc. Dedham, MA) and MAbs BCR 9031 (anti-H type 2), BCR 9010 (anti-A), and BCRM 11007 (anti-B) (Accurate Chemical & Scientific Corporation, Westbury, NY). The content of the H type 3/4 and A type 3/4 antigens was determined in this study by ELISA using the MAbs MBr1 (Enzo Life Sciences, Inc., Farmingdale, NY) and HE-10 (Sigma-Aldrich, St. Louis, MO), respectively. To test saliva binding by TV, saliva samples at 1:1,000 were used to coat plates in PBS at 4°C overnight and then incubated with TV at 1 × 104 PFU/ml. The bound TVs were detected by mouse anti-TV serum (1:3,500) and then by a goat anti-mouse IgG conjugated to horseradish peroxidase (HRP) (1:5,000). Color signals were developed with TMB (3,3′,4,4′-tetramethylbenzidine) and read at 450 nm.
Binding of TV with synthetic ABH tri- and tetrasaccharides.
The synthetic ABH type 1 to 4 oligosaccharide-BSA conjugates in PBS were used to coat plates at 4°C overnight, which were blocked with 5% milk at room temperature for 1 h and then rinsed with 200 μl of PBS. Next, 100 μl (1 × 103 PFU) of purified TV was added, and plates were incubated at 4°C overnight and rinsed with PBS three times. The bound TVs were detected by mouse anti-TV serum as described above.
Inhibition of TV replication by A type 3/4- and B-positive saliva and oligosaccharide conjugates in cell culture.
Inhibition of TV replication was assessed by plaque reduction assay of TV in LLC-MK2 cells in 6-well plates. Type A saliva samples with different levels of the A type 3/4 antigen were tested for their ability to block TV replication, while type B saliva was used as a positive control. Saliva samples were boiled and clarified by centrifugation at 10,000 × g for 10 min. The TV inoculum was incubated with saliva starting at a dilution of 1:300 in Dulbecco's modified Eagle medium (DMEM) with 6-fold serial dilutions. The tubes used were precoated with 5% BSA. After 1 h incubation at 37°C, the TV-saliva mixture was inoculated into LLC-MK2 cells and incubated at 37°C for 1.5 h. The plates were overlaid with 0.8% agarose. After a 2-day incubation, the plates were stained with crystal violet, and plaques were counted. The blocking rate (%) of the saliva was calculated by the reduction in plaque numbers in the wells treated with saliva relative to the number in untreated control wells. Blocking rate (expressed as a percentage) was calculated as follows: (1 − PFU of test well/PFU of control well) × 100.
Procedures similar to those used for the plaque reduction assay were also used to determine the roles of the A type 3 and B oligosaccharide conjugates in blocking TV infection in cell cultures. Synthetic oligosaccharide (20 μg/well) was preincubated with TV inoculum and then transferred to 6-well plates. The test tubes used for incubation of TVs with oligosaccharides were precoated with a BSA solution to avoid potential nonspecific binding of TVs to the test tubes.
RESULTS
Binding of TV to type A saliva correlated with the A type 3/4 antigens in saliva.
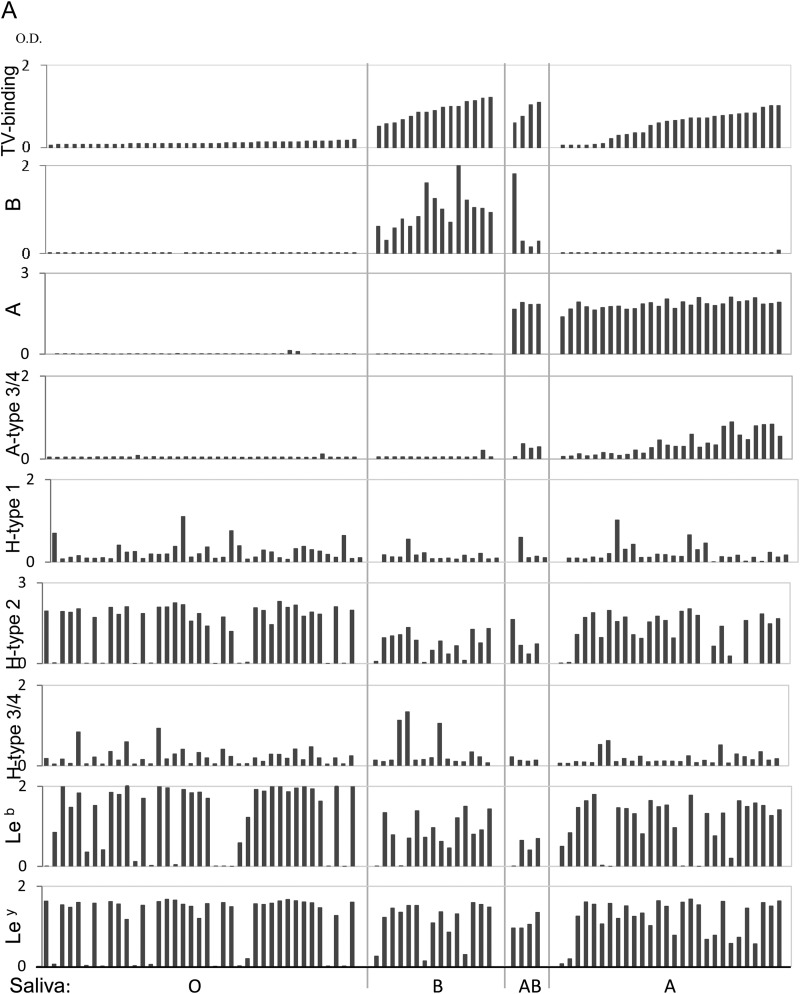
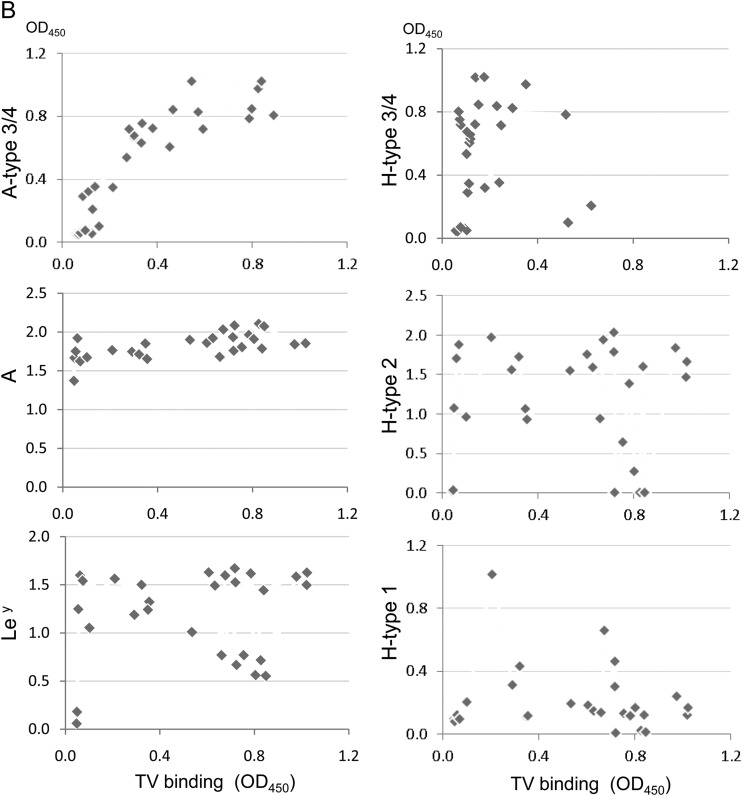
To test type specificity of human HBGAs recognized by TV, we first performed a saliva binding assay on a panel of 86 saliva samples representing different ABH and Lewis types (8). TV bound strongly to all 15 type B and four type AB saliva samples, but the binding signals among the 28 type A saliva samples varied: 17 bound strongly (optical density [OD] > 0.5), five bound weakly to moderately (OD 0.2 to 0.5), and the remaining six did not bind or bound poorly (OD < 0.2) (Fig. 2A). The TV binding signals correlated with neither the A antigens nor other HBGAs, except for the A type 3/4 antigens (r = 0.85, P < 0.05) (Fig. 2B), suggesting that A type 3 and/or A type 4 antigens could be responsible for the observed TV binding signals. Subsequent oligosaccharide binding assays further showed that TV binds the A type 3 but not the A type 4 antigen (see below). None of the 39 type O saliva samples revealed binding signals with TV, indicating that TV does not interact with any of the H type and Lewis antigens in human saliva.
FIG 2.
Binding of TV to saliva detected by ELISA. (A) The 86 saliva samples were sorted according to their binding signals to TV within each of the four blood types: O, B, AB, and A. The levels of B type, A type, A type 3/4, H type 1, H type 2, H type 3/4, Leb, and Ley antigens in the saliva samples were determined by ELISA with corresponding specific MAbs. (B) Correlation analysis of binding activity of TV with HBGA levels in the saliva samples was performed. A significant positive correlation (r = 0.85; P < 0.05) between TV binding and the A type 3/4 antigen in the type A saliva was found.
Specificity of binding of TV to the A type 3 and B oligosaccharides.
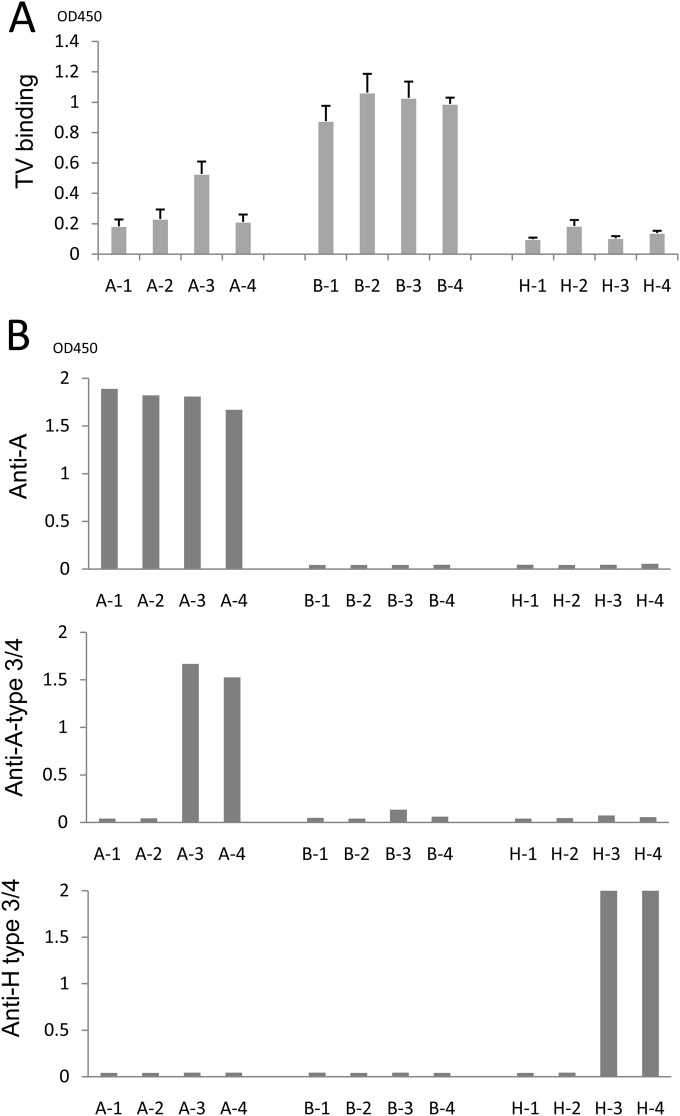
To further determine the binding targets of TV, we performed oligosaccharide-based binding assays using BSA-conjugated oligosaccharides representing four types (types 1 to 4) of the A, B, and H antigens). The results indicated that TV strongly bound all four types of the B antigen but only A type 3 among the four A type antigens (Fig. 3A). The binding signals of TV to the A type 3 oligosaccharide were weaker than those to the B antigens (types 1 to 4). TV did not bind to the H antigens (Fig. 3A), consistent with the lack of TV binding to any type O saliva (Fig. 2A). The antigenic specificities of these oligosaccharides were also validated by corresponding MAbs (Fig. 3B). The A type 3/4 MAb (HE-10) recognized the A type 3 and 4 but not the A type 1 or 2 antigens. This result indicates that MAb HE-10 recognizes an epitope of the A antigens differing from that recognized by TV, in which the former is shared by A types 3 and 4, while the latter is distinct between the two types. Similarly, the H type 3/4 MAb (MBrL) also recognized H type 4 antigens. Finally, MAb (BG2) bound all four types of the A antigens, indicating that a further distinct epitope on the A antigens is recognized by this MAb (Fig. 3A).
FIG 3.
Binding of TV to synthetic HBGA oligosaccharides. (A) Binding of TV to four subtypes of the A, B, and H oligosaccharides. (B) Validation of synthetic ABH oligosaccharides by MAb-based binding assays. Three MAbs, anti-A type 3 (HE-10), anti-globo-H (H type 3/4) (MBr1), and anti-A (BG2), were used. A-1 to A-4, B-1 to B-4, and H-1 to H-4 are A type 1 to 4, B type 1 to 4, and H type 1 to 4 BSA conjugates, respectively.
Roles of the A type 3/4 antigens in hemagglutination (HA) and HA inhibition (HAI) with TV.
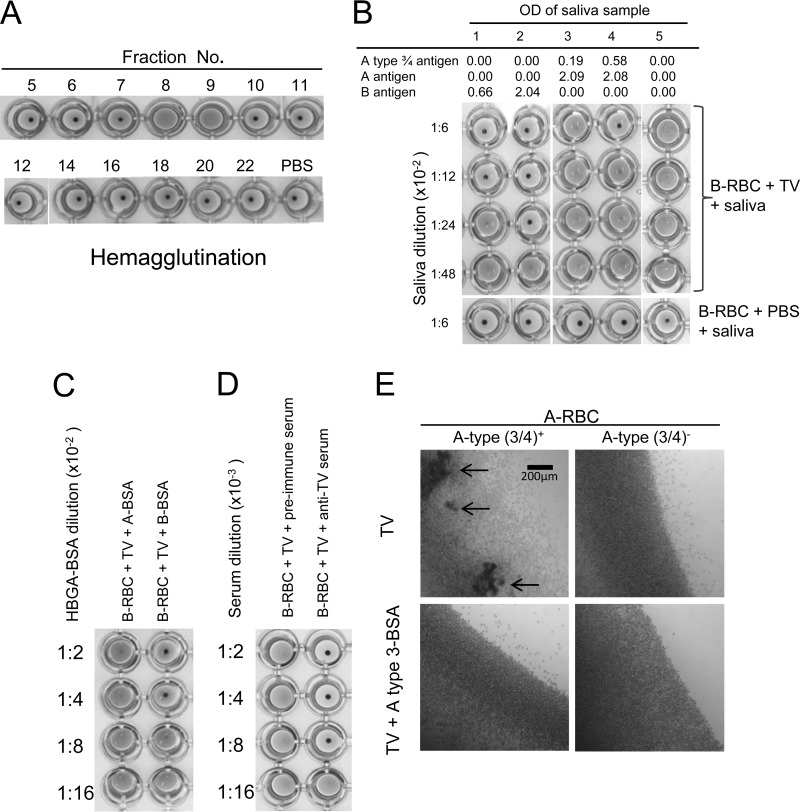
HA and HAI on TV were performed to further prove the specific interaction of TV with human HBGAs. TVs from different fractions of CsCl gradient centrifugation were analyzed by HA and HAI, revealing that the fractions (fractions 7 and 8) containing intact TVs hemagglutinated RBCs specifically (Fig. 4A). In our initial study, V-shaped plates were used, which resulted in clear HA for type B RBCs but only marginal HA for the A type 3/4 RBCs (Fig. 4B). To increase the sensitivity of the HA assay, flat-bottom plates were used, and the HA results were read under a microscope (Fig. 4E). The presentation of A type 3/4, H type 3/4, and Lewis y antigens on the surface of the RBCs was determined by HA using MAbs to these antigens. Among RBCs from 24 A-positive individuals, 15 resulted in HA with A type 3/4 MAb and 13 (87%) of the 15 RBCs revealed HA with TV (Table 1), while no HA by TV was observed in the other 9 A type 3/4-negative RBCs. Although most A-positive individuals are Lewis y antigen positive, no significant difference was seen between Lewis y-positive and -negative individuals (52% versus 67%).
FIG 4.
Hemagglutination of human red blood cells (RBCs) by TV. (A) Hemagglutination of type B human RBCs by TVs from CsCl gradient fractions. Only fractions 8 and 9 contained intact TVs, which was determined by electron microscopy followed by ELISA and detection by PCR. (B) Inhibition of TV-induced hemagglutination by saliva containing A type 3/4 and B antigens. Strong inhibition by the type B saliva (saliva samples 1 and 2) which correlated with the salivary B antigens was observed. Inhibition was also observed for the A type 3/4-positive saliva (saliva samples 3 and 4), but inhibition was not seen for the type O saliva (saliva sample 5). (C and D) Inhibition of TV induced hemagglutination by type B oligosaccharide and by antibody to TV. (E) Inhibition of TV induced hemagglutination by A type 3 oligosaccharide using flat-bottom plates followed by reading of the results under a microscope. Weak agglutination of A type 3/4-positive RBCs developed, and the hemagglutination could be blocked by a synthetic A type 3 oligosaccharide conjugate. Arrows indicate typical agglutinations of RBCs.
TABLE 1.
Hemagglutination of type A RBCs by TV is correlated to the A type 3/4 antigen on individuals' RBCs
| Antigen | No. of individuals (n = 24) with RBC typea | No. (%) of RBC samples with induced HA by TVb |
|---|---|---|
| A type 3/4 | ||
| + | 15 | 13 (87) |
| − | 9 | 0 |
| H type 3/4 | ||
| + | 8 | 3 (38) |
| − | 16 | 10 (63) |
| Lewis y | ||
| + | 21 | 11 (52) |
| − | 3 | 2 (67) |
RBCs of 24 type A-positive individuals were tested. The presence (+) or absence (−) of the A type 3, H type 3, and Lewis y antigens in each individual's RBCs was determined by HA with specific MAbs.
The HA assays were performed in 96-well, flat-bottom plates, and HA was determined by reading the plates under a microscope. A typical TV HA result for A type 3/4 RBCs is shown in Fig. 4E.
The involvement of the B antigens in TV HA was confirmed by blocking of the HA by type B saliva (Fig. 4B), synthetic B type 1 to 4 oligosaccharides (Fig. 4C) and anti-TV serum (Fig. 4D). Both A type 3 and 4 BSA conjugates were also tested in the HAI assay, revealing blocking only by A type 3 BSA conjugate on A type 3/4 RBC HA of TV (Fig. 4E). Interestingly, in the TV HA assay using A type 3/4-positive RBCs, competitive inhibition between the type B antigens and the A type 3/4 antigens occurred, in which the type B saliva completely blocked TV HA of A type 3/4 RBCs (Fig. 4B). This result suggests that the TV most likely uses the same binding site for interaction with both the B and the A type 3 antigens.
Inhibition of TV replication by A type 3/4 and B saliva.
To further determine the biological significance of the A type 3/4 and B HBGAs in TV replication, we performed a plaque reduction assay on TVs in cell culture using human saliva as a blocking reagent. Nearly complete inhibition (∼100%) of TV replication by the type B saliva at 1:300 was observed (Table 2), while variable blocking results were obtained when the type A saliva samples were tested (data not shown). To determine the involvement of the A type 3 antigens in the blocking activities, saliva samples from two individuals with strong (OH45 and OH60) and two individuals with weak (OH32 and OH94) signals for A type 3/4 were tested. High inhibition of TV replication (>20 to 50%) was observed after TVs were incubated with saliva OH45 and OH60 (with strong A type 3/4 signals) at a dilution of 1:1,800. As expected, no blocking or weak blocking (<10%) was seen with saliva OH32 and OH94 (with weak A type 3/4 signals) even when a higher concentration of saliva (1:300) was used (Table 2). All four saliva samples had similar levels of total type A antigens, indicating that the A type 3/4antigen in the saliva is responsible for the inhibition.
TABLE 2.
Inhibition of TV replication in cell culture by A type 3/4 and type B saliva samples
| Dilution of saliva | TV plaque reduction (%) by salivaa |
||||
|---|---|---|---|---|---|
| OH32 | OH45 | OH60 | OH94 | Saliva LY | |
| 1:300 | −2.0 | 90.6 | 67.1 | 2.7 | 100 |
| 1:1,800 | 2.8 | 59.2 | 24.7 | 5.9 | 89.5 |
| 1:10,800 | −0.4 | 21.6 | 15.3 | −3.5 | NT |
| 1:64,800 | −0.2 | 10.6 | 13.7 | −2.0 | NT |
Four type A-positive saliva samples with high (OH45 and OH60, OD > 0.5) or low (OH32 and OH94, OD < 0.2) levels of A type 3/4 antigen were tested, and a type B saliva sample (saliva LY) was also included. NT, not tested.
Synthetic type B-BSA conjugates reduce TV replication in cell culture.
We first repeated the blocking experiments, described in a previous study, that showed that synthetic HBGA-BSA conjugates resulted in an increase in TV infection (13). Using the same conditions, we observed an increase of plaque formation after incubation of TVs with the BSA-conjugated type A and B oligosaccharides compared with the control group without BSA conjugated oligosaccharides. However, it was noted that a similar increase was also seen when BSA alone without type A or B oligosaccharides was used (data not shown), indicating that such an increase of viral titers was nonspecific. After stepwise troubleshooting, we found that the observed “increase” in TV titers was due to nonspecific binding of TV to the incubation tubes, which could be prevented by BSA or the BSA conjugates. Because no additional proteins were included in the control groups, less TV can be removed from the tube to inoculate the cell cultures. As a result, all treatment groups showed more TV plaques after cultivation. This false result can be avoided by precoating of the tubes with BSA.
After resolving this problem, we repeated the oligosaccharide-blocking assay and observed specific inhibition of TV replication by the type B-BSA conjugate, but the blocking efficiency was significantly lower than that of saliva (Table 3). Although no blocking effects on TV replication by other oligosaccharide conjugates were observed, including B-PAA, A-BSA, A type 2-BSA, and A type 3-BSA conjugates, no increase in TV plaques occurred (Table 3). We speculate that the lower blocking efficiency by the oligosaccharide conjugates could be due to their lower valence compared with the multivalent nature of the HBGAs of saliva. Collectively, our data support the notion that HBGAs play an important role in TV replication.
TABLE 3.
Inhibition of TV replication by a plaque reduction assay with synthetic type A and B oligosaccharide conjugates as inhibitors
| Oligosaccharide conjugatea | PFU (avg) | 95% CIb | Plaque reduction (%)c |
|---|---|---|---|
| BSA | 21.5 | 17.4–25.6 | −2.4 |
| B-BSA | 14.5 | 13.2–15.8 | 31.0 |
| B-PAA | 20.3 | 16.4–24.1 | 3.6 |
| A3-BSA | 19.3 | 15.6–22.9 | 8.3 |
| A2-BSA | 17.5 | 13.2–21.8 | 16.7 |
| A-BSA | 17.8 | 13.7–21.8 | 15.5 |
| Control (TV only) | 21.0 |
A- and B-BSA, BSA-conjugated A and B trisaccharides; A2- and A3-BSA, BSA-conjugated A type 2 and 3 tetrasaccharides; B-PAA, polyacrylamide-conjugated B trisaccharide. The control wells were TV not mixed with any oligosaccharides.
95% CI, confidence interval at the 95% confidence level.
Plaque reduction was calculation based on plaque numbers in the test wells compared with that of the control wells.
DISCUSSION
In this study, we verified that the TV recognizes all four types of the B antigens but only the A type 3 of the A antigens. The specificity of TV in recognizing the A type 3 antigens has been demonstrated by binding of TV with A type 3/4-positive saliva, detected with an anti-A type 3/4 MAb. We also obtained direct evidence of TV binding to the A type 3 and B antigens by oligosaccharide-based binding and blocking assays and by HA and HAI with A type 3/4- and B-positive human RBCs and saliva. The biological significance of HBGA as a host factor in TV replication has been demonstrated by specific blocking of TV replication in cell cultures using type B-specific HBGAs and saliva characterized as containing A type 3/4 antigens. Finally, our study clarified an unexplained result reported in the literature regarding the roles of human HBGAs in TV replication. Thus, we conclude that the recognition by TV of the A type 3 and B HBGAs is highly specific and biologically relevant.
The finding that TV recognizes both the A type 3 and B antigens further broadens our understanding of the nature of virus-host interaction of caliciviruses with HBGAs. First, according to the cross-blocking results of TV between the A type 3 and B antigens, we predict that the TV may use a common receptor-binding interface to interact with both the A type 3 and B antigens. This is similar to what was observed in human NoVs that use a common HBGA binding interface to bind different ABH and Lewis antigens. Thus, we further predict that TV may rely on the shared β-galactose in the disaccharide precursors (core chains) and the fucose linked to the β-galactose in the A and B antigens (Fig. 1) as the major binding saccharide for TV to interact with HBGAs. However, since TV does not bind to any types of O saliva, the fucose that linked to the β-galactose of the secretor saliva may be dispensable for the interactions.
In this study, a MAb that recognizes A type 3/4 antigens was used for HBGA typing of human saliva, and a correlation of saliva binding by this MAb and by TV was observed, raising the question of whether the A type 4 antigen in human saliva is also involved in interaction with TV. According to the oligosaccharide-based binding assays, however, TV recognized A type 3 but not the other three types (1, 2, and 4) of the A antigens, indicating that the TV recognizes an epitope on the A antigens differ from that recognized by the MAb. The A antigens contain multiple sugar residues that may form different antigenic epitopes recognized by the MAb and TV, respectively. The recognition of distinct epitopes of human HBGAs in human milk has also been observed between human NoVs and HBGA-specific MAbs (25). Thus, we conclude that TV recognizes the A type 3 antigens specifically, and the cross-reactivity of the MAb to both A types 3 and 4 should not affect our conclusion.
The finding that TV recognizes the A type 3 antigen also helped our understanding of the type specificity of HBGAs recognized by different human NoVs. For example, the prototype Norwalk virus (GI.1) recognizes mainly the type 1 chain of the A and H antigens, while the major epidemic GII.4 NoVs recognize mainly the type 3 chains of the A, B, and H antigens. This type specificity may have a role in clinical infection and prevalence of individual strains of NoVs in different human populations. Future studies to determine the distribution of individual types of the ABH and Lewis antigens in the general population and to explore their roles in the prevalence of individual genotypes of human NoVs are necessary.
The demonstration of the role of human HBGAs in TV replication by the cell culture-based blocking experiments described in our study is highly significant, because such a study is impossible to carry out with human NoVs due to the lack of a cell culture method and an animal model. Similar to results of a previous study (13), significant reduction of TV replication in LLC-MK2 cells was seen after treatment of TVs with the A type 3 and B antigens. As an analogy, we predict that human HBGAs or a carbohydrate receptor also plays an important role in the replication of human NoVs and related caliciviruses (26–29). To look into the report of increased TV infectivity by type A and B oligosaccharides in an earlier study (13), we carefully evaluated the experimental procedures described in the study and identified a potential problem that could cause a false result. We found that TV tends to bind to the test tubes in the preincubation step and that such nonspecific binding can be prevented by precoating of the tube with a protein solution, such as BSA. Our conclusion was strongly supported by a demonstration of an increase in TV titer by preincubation of TV with BSA and by avoiding such false results by precoating the test tubes with a BSA solution. The observed lower blocking efficacy of the oligosaccharide conjugates than human saliva could be due to a lower valence of the oligosaccharide conjugates than of saliva glycans.
ACKNOWLEDGMENTS
The research described in this article was supported by the National Institutes of Health of the United States (R01 AI 089634), the Agriculture and Food Research Initiative Competitive Grants Program of the USDA National Institute of Food and Agriculture (NIFA award 2011-68003-30005), the Alberta Glycomics Centre, and the Canadian Institutes of Health Research (RMF 92091).
REFERENCES
- 1.Green KY, Ando T, Balayan MS, Berke T, Clarke IN, Estes MK, Matson DO, Nakata S, Neill JD, Studdert MJ, Thiel HJ. 2000. Taxonomy of the caliciviruses. J Infect Dis 181(Suppl 2):S322–S330. doi: 10.1086/315591. [DOI] [PubMed] [Google Scholar]
- 2.L'Homme Y, Sansregret R, Plante-Fortier E, Lamontagne AM, Ouardani M, Lacroix G, Simard C. 2009. Genomic characterization of swine caliciviruses representing a new genus of Caliciviridae. Virus Genes 39:66–75. doi: 10.1007/s11262-009-0360-3. [DOI] [PubMed] [Google Scholar]
- 3.Thomas C, Jung K, Han MG, Hoet A, Scheuer K, Wang Q, Saif LJ. 2014. Retrospective serosurveillance of bovine norovirus (GIII.2) and nebovirus in cattle from selected feedlots and a veal calf farm in 1999 to 2001 in the United States. Arch Virol 159:83–90. doi: 10.1007/s00705-013-1795-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan M, Jiang X. 2014. Histo-blood group antigens: a common niche for norovirus and rotavirus. Expert Rev Mol Med 16:e5. doi: 10.1017/erm.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan M, Jiang X. 2011. Norovirus-host interaction: multi-selections by human histo-blood group antigens. Trends Microbiol 19:382–388. doi: 10.1016/j.tim.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan M, Jiang X. 2005. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol 13:285–293. doi: 10.1016/j.tim.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Tan M, Jiang X. 2010. Norovirus gastroenteritis, carbohydrate receptors, and animal models. PLoS Pathog 6:e1000983. doi: 10.1371/journal.ppat.1000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang P, Farkas T, Zhong W, Tan M, Thornton S, Morrow AL, Jiang X. 2005. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J Virol 79:6714–6722. doi: 10.1128/JVI.79.11.6714-6722.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papafragkou E, Hewitt J, Park GW, Greening G, Vinje J. 2013. Challenges of culturing human norovirus in three-dimensional organoid intestinal cell culture models. PLoS One 8:e63485. doi: 10.1371/journal.pone.0063485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farkas T, Sestak K, Wei C, Jiang X. 2008. Characterization of a rhesus monkey calicivirus representing a new genus of Caliciviridae. J Virol 82:5408–5416. doi: 10.1128/JVI.00070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu G, Zhang D, Guo F, Tan M, Jiang X, Jiang W. 2013. Cryo-EM structure of a novel calicivirus, Tulane virus. PLoS One 8:e59817. doi: 10.1371/journal.pone.0059817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sestak K, Feely S, Fey B, Dufour J, Hargitt E, Alvarez X, Pahar B, Gregoricus N, Vinje J, Farkas T. 2012. Experimental inoculation of juvenile rhesus macaques with primate enteric caliciviruses. PLoS One 7:e37973. doi: 10.1371/journal.pone.0037973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farkas T, Cross RW, Hargitt E III, Lerche NW, Morrow AL, Sestak K. 2010. Genetic diversity and histo-blood group antigen interactions of rhesus enteric caliciviruses. J Virol 84:8617–8625. doi: 10.1128/JVI.00630-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirneisen KA, Kniel KE. 2013. Comparing human norovirus surrogates: murine norovirus and Tulane virus. J Food Prot 76:139–143. doi: 10.4315/0362-028X.JFP-12-216. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Ye M, Neetoo H, Golovan S, Chen H. 2013. Pressure inactivation of Tulane virus, a candidate surrogate for human norovirus and its potential application in food industry. Int J Food Microbiol 162:37–42. doi: 10.1016/j.ijfoodmicro.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Shearer AE, Hoover DG, Kniel KE. 2014. Effect of bacterial cell-free supernatants on infectivity of norovirus surrogates. J Food Prot 77:145–149. doi: 10.4315/0362-028X.JFP-13-204. [DOI] [PubMed] [Google Scholar]
- 17.Tian P, Yang D, Quigley C, Chou M, Jiang X. 2013. Inactivation of the Tulane virus, a novel surrogate for the human norovirus. J Food Prot 76:712–718. doi: 10.4315/0362-028X.JFP-12-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Hirneisen KA, Markland SM, Kniel KE. 2013. Survival of murine norovirus, Tulane virus, and hepatitis A virus on alfalfa seeds and sprouts during storage and germination. Appl Environ Microbiol 79:7021–7027. doi: 10.1128/AEM.01704-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mollicone R, Gibaud A, Francois A, Ratcliffe M, Oriol R. 1990. Acceptor specificity and tissue distribution of three human alpha-3-fucosyltransferases. Eur J Biochem 191:169–176. doi: 10.1111/j.1432-1033.1990.tb19107.x. [DOI] [PubMed] [Google Scholar]
- 20.Meloncelli PJ, Lowary TL. 2010. Synthesis of ABO histo-blood group type I and II antigens. Carbohydr Res 345:2305–2322. doi: 10.1016/j.carres.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Meloncelli PJ, West LJ, Lowary TL. 2011. Synthesis and NMR studies on the ABO histo-blood group antigens: synthesis of type III and IV structures and NMR characterization of type I-VI antigens. Carbohydr Res 346:1406–1426. doi: 10.1016/j.carres.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Wu X, Ling C-C, Bundle DR. 2004. A new homobifunctional p-nitro phenyl ester coupling reagent for the preparation of neoglycoproteins. Org Lett 6:4407–4410. doi: 10.1021/ol048614m. [DOI] [PubMed] [Google Scholar]
- 23.Dondoni A, Massi A, Nanni P, Roda A. 2009. A new ligation strategy for peptide and protein glycosylation: photoinduced thiol-ene coupling. Chemistry 15:11444–11449. doi: 10.1002/chem.200901746. [DOI] [PubMed] [Google Scholar]
- 24.Morokutti A, Redlberger-Fritz M, Nakowitsch S, Krenn BM, Wressnigg N, Jungbauer A, Romanova J, Muster T, Popow-Kraupp T, Ferko B. 2013. Validation of the modified hemagglutination inhibition assay (mHAI), a robust and sensitive serological test for analysis of influenza virus-specific immune response. J Clin Virol 56:323–330. doi: 10.1016/j.jcv.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Huang P, Morrow AL, Jiang X. 2009. The carbohydrate moiety and high molecular weight carrier of histo-blood group antigens are both required for norovirus-receptor recognition. Glycoconj J 26:1085–1096. doi: 10.1007/s10719-009-9229-x. [DOI] [PubMed] [Google Scholar]
- 26.Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, Lindblad L, Stewart P, LePendu J, Baric R. 2003. Human susceptibility and resistance to Norwalk virus infection. Nat Med 9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 27.Nystrom K, Le Gall-Recule G, Grassi P, Abrantes J, Ruvoen-Clouet N, Le Moullac-Vaidye B, Lopes AM, Esteves PJ, Strive T, Marchandeau S, Dell A, Haslam SM, Le Pendu J. 2011. Histo-blood group antigens act as attachment factors of rabbit hemorrhagic disease virus infection in a virus strain-dependent manner. PLoS Pathog 7:e1002188. doi: 10.1371/journal.ppat.1002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuart AD, Brown TD. 2007. Alpha2,6-linked sialic acid acts as a receptor for feline calicivirus. J Gen Virol 88:177–186. doi: 10.1099/vir.0.82158-0. [DOI] [PubMed] [Google Scholar]
- 29.Zakhour M, Maalouf H, Di Bartolo I, Haugarreau L, Le Guyader FS, Ruvoen-Clouet N, Le Saux J C, Ruggeri FM, Pommepuy M, Le Pendu J. 2010. Bovine norovirus: carbohydrate ligand, environmental contamination, and potential cross-species transmission via oysters. Appl Environ Microbiol 76:6404–6411. doi: 10.1128/AEM.00671-10. [DOI] [PMC free article] [PubMed] [Google Scholar]